Abstract
Injured patients stress the transfusion service with frequent demands for uncrossmatched red cells and plasma, occasional requirements for large amounts of blood products and the need for new and better blood products. Transfusion services stress trauma centers with demands for strict accountability for individual blood component units and adherence to indications in a clinical field where research has been difficult, and guidance opinion-based. New data suggest that the most severely injured patients arrive at the trauma center already coagulopathic and that these patients benefit from prompt, specific, corrective treatment. This research is clarifying trauma system requirements for new blood products and blood-product usage patterns, but the inability to obtain informed consent from severely injured patients remains an obstacle to further research.
Keywords: blood bank protocols, clinical blood use, cryoprecipitate transfusion, injury, injury care, plasma transfusion, red cell transfusion, shock resuscitation, transfusion protocols, trauma center protocols
Physical injury is the most common cause of death for people in the developed world aged 1–44 years [1]. As a result, injury is the most common cause of loss of years of productive life for all individuals in these countries. Each year, 93,000 Americans die as a result of physical injuries and 1.2 million are admitted to hospitals [2]. To meet the needs of these patients, there are 1084 trauma centers in the USA and Canada accredited by the American College of Surgeons and hundreds more that are accredited by state agencies. There are 252 hospitals with accident and emergency receiving capability in England and Northern Wales. The aggregate direct cost of trauma care is in excess of US$110 billion a year in the USA, approximately 5% of the entire cost of medical care. Other direct costs of injury, such as disability compensation and family support, are also substantial, and the indirect costs of injury from loss of productive citizens are even higher.
While injuries are best prevented by education and by social and engineering controls, secondary prevention of injury-related death and disability through the provision of emergency medical care and physical and occupational rehabilitation is also cost-effective [3]. Blood products play a major role in this secondary prevention of death and disability as an adjunct to surgical care.
Blood use in trauma care
Most trauma care is for mild and moderate injuries. Lacerations and minor fractures need suturing and splinting; mild concussions need evaluation and observation, and so forth. In any given year, more than 90% of patients admitted directly from the scene of injury to the University of Maryland’s R Adams Cowley Shock Trauma Center (STC) do not require any blood products. Of 5649 such patients admitted to the STC in the calendar year 2000, 514 received a blood product, and 490 of those patients received red blood cells (RBCs) [4]. The other 24 were mostly elderly individuals taking warfarin for atrial fibrillation who fell, sustaining head injuries with intracranial bleeding, and received plasma. Of the entire direct-admission group, 8% received red cells, 5% received plasma, 3% received platelets and 0.1% received cryoprecipitate. The total number of units of blood components given was 5219 units of red cells, 5226 units of plasma, 2892 units of platelets and 64units of cryoprecipitate. Use rates for blood products were thus slightly less than a unit of red cells and plasma per casualty, half a unit of platelets and one hundredth of a unit of cryoprecipitate for each admission. The survival rate for these patients was 97%. For patients with fatal injuries, severe neurologic injury was the most frequent cause of death; uncontrolled hemorrhage was the second most frequent cause; and multiple organ failure was the third. Other causes of trauma-related deaths in the STC were uncommon.
Most of the blood that was used in trauma care was used to support patients with uncontrolled hemorrhage. Of the 5219 units of red cells used to support 5649 trauma patients, three-quarters were given to the 146 patients who received ten units of red cells or more and half were given to 68 patients who received 20 units or more [4]. A recent review of all patients who died of uncontrolled hemorrhage at the STC from 1997 to 2006 showed that, even among patients who survived at least 15 minutes, 42% were dead by 90 min, 64% were dead by 3 h, 81% were dead by 6 h and 90% were dead by 12 h after admission. As a result, 62% of all of the red cells used in the trauma center were used in the first day of care and 11% were used in the first hour of care [3].
In the retrospective analysis by Como and colleagues, initial massive transfusion, defined as receiving more than ten units of red cells in the first 24 h of care, occurred in 90 patients, 1.7% of all admissions, and these patients received almost 40 units of red cells each in the course of their admission [4]. Almost 50% survived, and these patients used 70% of all of the red cells used by the STC. Vaslef and colleagues, from the trauma center at Duke University, have written that the survival of patients who require 75 units of blood products is the same as that of patients who require 50 units, roughly 43% [5]. In our cohort at STC, one out of three patients who received more than 100 units of red cells survived. These data suggest that there is no obvious upper limit to the number of units of red cells that injured patients tolerate in successful transfusion support.
Changing patterns of trauma resuscitation
For more than 20 years, the recommendations of the Advanced Trauma Life Support (ATLS) course of the Committee on Trauma of the American College of Surgeons has defined a practice plan for resuscitations in trauma centers in developed countries and in their militaries [6,7]. These recommendations were based on laboratory studies of controlled hemorrhage (the Wiggers model), on observation of bleeding patients undergoing elective surgery and the thought that trauma patients with ongoing bleeding needed intravascular volume, red cells, plasma and platelets, in that order [8–11]. The ATLS recommendations state that significantly injured patients should have two large-bore IVs placed, and if there is evidence of shock, such as a pulse greater than 100 or a systolic blood pressure less than 100, 2 l of crystalloid fluid should be given. At that point, if signs of shock remain, red cells should be started. Red cells are also advised if signs of shock are corrected but return (‘transient responders’) or if visible bleeding of greater than 100 ml/min is present. If shock persisted, further boluses of crystalloid or RBCs were indicated. Frequent laboratory measures were recommended to guide plasma and platelet therapy.
The ATLS recommendations have probably helped many injured patients, ensuring the early placement of vascular access, creating a sense of urgency about shock and providing basic rules for red cell support [12]. However, the ATLS guidelines were not based on evidence derived from injured unanesthetized patients or uncontrolled hemorrhage models. With the development of animal models of uncontrolled hemorrhage in the 1980s and 1990s, it became apparent that aggressive fluid volume administration in the situation of uncontrolled hemorrhage could exacerbate bleeding and increase mortality. Studies by Bickell and colleagues and Stern and colleagues showed that attempting to resuscitate swine with small aortic tears could increase the mortality of this lesion from 15% untreated to 100% with crystalloid resuscitation [13–15]. The higher blood pressure ‘popped the clot’ and the remaining blood was too dilute to clot again before terminal shock developed. Bickell, working with the Houston ambulance system, then demonstrated that a similar regimen of withholding crystalloid resuscitation during uncontrolled hemorrhage reduced mortality in patients with penetrating truncal injury [16]. Dutton and colleagues at our STC went on to demonstrate that resuscitation titrated to a lower than normal target blood pressure was well tolerated by patients with uncontrolled hemorrage [17].
The result of this work has been a much sharper focus on the injuries and physiology of injured patients. Patients with mild shock need to be evaluated quickly, their sources of bleeding identified and treated by the least-invasive means. As an example, patients with isolated splenic rupture used to undergo laparotomy and splenectomy but are now identified with ultrasound or computed tomography scanning and are treated with catheter embolization by an interventional radiologist, and more than 90% of them do not undergo surgery or lose their spleen [18,19]. Nonoperative management of splenic, liver and pelvic injuries is facilitated by limiting resuscitation to avoid rebleeding and dilution of the clotting system.
In more severely injured patients, with profound shock and ongoing hemorrhage, the most important intervention is rapid transfer to the operating room. These patients frequently have multiple injuries, including lacerations of major vessels, fractures of solid organs or disruption of a major venous plexus. Bleeding in these circumstances can be extensive and not susceptible to immediate control. In such circumstances, trauma teams now consciously sacrifice normal anatomy to stabilize vital physiology in a set of maneuvers called ‘damage control surgery’ [20–22]. In this process, disruptions of named vessels are shunted, sources of continued oozing are packed, and sites of gut contamination are tied off and urinary contamination drained, with the intention of ending the surgery in less than 40 min. The abdomen is packed and left open to prevent abdominal compartment syndrome, covered with a suction system to monitor blood loss and a plastic vapor barrier to prevent evaporative cooling and tissue drying.
Blood administration during truncal surgery with ongoing uncontrolled massive hemorrhage includes gravity infusion through large-bore lines, forced infusion with blood bag compression devices, the use of high-volume rapid-infusion systems, blood-salvage devices and even emergency cardiopulmonary bypass. The choice of infusion system depends on the resources available, the vascular access, the need for concurrent component mixing and warming and the need for concurrent heart or lung bypass. Conventional gravity-feed blood-administration systems are useful when required rates of flow are less then 100 ml/min. Blood bag compression systems can increase rates of delivery to 100 ml/min/bag. High-volume mechanical blood injectors can effectively deliver volumes up to 500 ml/min and occasionally more, but most cannot achieve full blood warming of previously refrigerated blood in amounts in excess of 500 ml/min. Cardiopulmonary bypass can deliver many liters per minute, depending on the size and location of venous drainage.
Acute coagulopathy of trauma
It has been recognized since the Vietnam War era that a portion of trauma patients present to trauma centers with abnormal coagulation tests, even in the absence of significant resuscitation [23–25]. Presumably, the extent of injury is great enough in these patients to lead to massive consumption of coagulation factors, either directly or through activation of the protein C pathway. More recently, in series published by Brohi and colleagues and MacLeod and colleagues, a quarter of all direct trauma admission had abnormal coagulation tests at admission [26,27].
In our own trauma center, we have reviewed the relationship between injury severity, abnormal coagulation tests and mortality. Among more that 35,000 patients admitted directly from the scene of injury in the years 2000–2006, 23,000 had an injury severity score (ISS) of 5 or greater. The prevalence of an abnormal prothrombin time, expressed as an international normalization ratio (INR), increased in a stepwise manner from 5 to 45% as the injury severity score increased from 5–9 to over 45. At the same time, the mortality associated with increased injury severity and an abnormal INR increased from less than 1% for moderate injury and normal INR to more than 80% for severe injury and a significantly prolonged INR of 2.2 or greater. Similar but less frequent were abnormalities of other coagulation tests, such as the partial thromboplastin time, the fibrinogen concentration and the platelet count. However, each of these coagulation abnormalities, when they occurred, was similarly associated with major increases in mortality.
Support of initial massive transfusion
Patients with uncontrolled hemorrhage requiring immediate transfusion as part of their initial care are the major test of a trauma center transfusion service. Patients present and can die within minuntes. To survive, the most critically injured need red cells and plasma within a very short time and in adequate quantities. Major trauma centers have worked out several different systems to meet this need. Each potential solution is a compromise between the safety and the availability of blood products, and each requires discussion and agreement between the trauma service and transfusion service physicians.
Group O red cells
To make uncrossmatched group O red cells immediately available in the trauma receiving unit (TRU) may require interim storage of some units in the trauma center. The number of such units and the fraction of Rh-D-negative units among them will depend on the proximity of the supporting transfusion service. Our transfusion service is located 300 m from the TRU, so we keep ten units of group O Rh-D-positive red cells and two units of group O Rh-D-negative cells in the TRU. In our review of the use of uncrossmatched group O red cells in our TRU from 1999 to 2003, 802 individuals received 2581 units of RBC and 408 of these patients survived to hospital discharge [28]. Among the 49% of patients who received one-to-two units, 64% survived; of the 27% who received three-to-four units, 49% survived; of the 18% who received five-to-eight units, 31% survived. Only 5% received nine or more units and 10% of these survived. Several times in the 5-year period, requests for additional quantities of uncrossmatched group O red cells were made, and one patient actually received 14 units, but died despite this effort [26].
The relatively low proportion of Rh-D-negative red cells in our protocol is based on the following factors.: three-quarters of our admissions requiring uncrossmatched red cells are male; a third of women who need immediate transfusion are over 50 years old; and more than 85% of all our trauma patients are Rh-D-positive. Furthermore, half of all patients who receive uncrossmatched red cells in the trauma center receive only one or two units, and the survival of individuals who receive three or more units of uncrossmatched red cells over a recent 5-year period was 30% [28]. Given our population, therefore, each year we expose one Rh-D-negative female of pre- or active child-bearing age who will survive and possibly go on to D alloimmunization. In reviewing 5 years experience with this system, we were able to identify one woman of child-bearing age who was alloimmunized to Rh-D. At the same time, two other women of childbearing age were alloimmunized to other common red cell antigens, one with anti-E and a second with anti-K and anti-Fy(a). This unusually low rate of recognized alloimmunization probably reflects a combination of the immunosuppression associated with massive injury, as discussed below, and the lack of long-term follow-up that is characteristic of the US trauma system. The experience also suggests that we have probably pushed the chances of Rh-D alloimmunization down to the background rate for all alloimmunization. In a typical year, we use approximately 600 units of uncrossmatched group O red cells, of which 140 are Rh-D negative.
Our experience has been that multiple simultaneous casualties occasionally stress but do not overwhelm this system. In an episode used for national blood donor recruiting by the American Red Cross, a pair of sisters involved in a serious vehicular collision presented simultaneously with massive liver injuries and were successfully resuscitated, treated and recovered. This is in keeping with national experience, where only four mass casualty situations occurred between the years 1975 and 2000 that required more than 100 units of RBCs to treat all of the casualties in the first 24 h of their care [29]. The largest of these incidents, the bombing of the Murrah Federal Building in Oklahoma City in 1996, left 83 people hospitalized and required 131 units of red cells for the first 24 h of their care. Our transfusion service typically has 200–300 units of group O red cells on hand. This is part of our standard inventory of 600 red cell units used to support an average daily use of almost 100 units a day at our institution.
The safety of using uncrossmatched red cells is occasionally questioned because of the possibility of group O donors having high titers of anti-A or anti-B. While reactions have been reported, the incidence and severity of such complications is less than that of not receiving blood or the risk of receiving red cells of the wrong ABO type in emergency situations. During the Vietnam War, the US Army used more than 100,000 units of uncrossmatched group O red cells without a single fatal hemolytic transfusion reaction, and noted that, at the same time, all nine fatal hemolytic transfusion reactions observed in the war occurred with the misidentification of recipients of crossmatched units of red cells or whole blood [30].
AB or low-titer A plasma
The recognition of the acute coagulopathy of trauma and the dangers of massive use of crystalloid fluids in resuscitation has led to the suggestion that the prophylactic administration of plasma along with RBCs for initial resuscitation is appropriate in a small number of the most severely injured patients. Such patients can be identified on the basis of severe injury and rapid ongoing bleeding. They constitute approximately 3% of all direct trauma center admissions. As noted earlier, a quarter to a half of such patients present to the hospital with an abnormal INR that would justify giving plasma by conventional transfusion guidelines and any individual bleeding at a rate of 100 ml/min will be coagulopathic within 25 min based on the volume-for-volume replacement of 2500 ml blood lost with five units of plasma-poor packed RBCs. As it is difficult to correct coagulopathy once it is established, it appears to be better to prevent it. This approach has been called ‘damage-control resuscitation’ [31,32].
The core of damage-control resuscitation is beginning resuscitation with a 1:1 ratio of plasma to red cells in additive solution. This concept developed as a way of treating the massively injured patients seen in the combat support hospital in Baghdad, Iraq, where it appeared to markedly reduce coagulopathic bleeding, allowing surgeons to operate in less blood-obscured field and to reduce the tissue swelling and organ failure seen postoperatively. A retrospective review of this experience showed that, among massively transfused casualties, it was associated with a reduction in mortality from 66 to 19% [33]. A group of academic trauma centers have now confirmed this marked improvement in injury mortality in retrospective reviews of their own experience with massive transfusion in a combined series of more than 400 patients [34]. Although the optimal proportions of RBCs to plasma has not yet been proven, the concept that the most severely injured trauma patients should be resuscitated from the outset with a mix of red cells and plasma that more nearly resembles whole blood is gaining wide acceptance [35–37].
Providing AB ‘universal donor’ plasma for immediate use in the trauma center is a major burden on the blood system. AB donors are only 4% of the donor population, and AB plasma is needed for AB patients, fetal exchange transfusions, infants and other situations where plasma free of anti-A and anti-B is desirable. The recent decision to remove plasma from female donors to prevent transfusion-related acute lung injury (TRALI) has made the supply tighter [38]. It would be possible to target AB donors for the apheresis donation of double units of AB plasma and collect them up to 24-times a year, but only units collected on functionally closed systems are approved for 5-day thawed storage as are other units of plasma in the USA. As a result of the newly increased demand, a shortage has developed and the price of AB plasma was recently doubled by our blood supplier.
In an attempt to speed the delivery of plasma to the severely injured, we have placed four units of thawed AB plasma in our TRU blood refrigerator. We replace them when they are used or remove them on the fifth day and issue the unused units as type-compatible thawed plasma to other hospital patients. Over the course of the first year, we issued approximately 700 AB units to the TRU refrigerator, 345 were used in the TRU, and 180 went to patients who received at least four units of uncrossmatched group O RBCs at the same time. Overall, 30 thawed units did not find a recipient. Observation on cumulative outcome is still in process.
If AB plasma becomes scarcer, we would consider supplementing the limited supply with low-titer anti-B group A units. Hemolytic reactions in recipients of type-incompatible platelets are uncommon – said to occur in one in 9000 out-of-group apheresis platelet transfusions – and usually occur when group O platelets with high anti-A titers are given to group A or AB recipients. A unit of apheresis platelets typically contains more plasma, approximately 300 ml, than a unit of plasma, approximately 200 ml.
Platelets
Owing to the requirements for continuous agitation, the narrow range of storage temperatures and the very limited number of group AB units, there is no easy way to maintain platelets outside of the blood bank itself. Moreover, the optimal replacement protocol for platelets is not clear. Early work on blood component resuscitation from the Harborview group in Seattle suggested that platelets were not a significant issue in resuscitation in hemorrhagic shock [39]. The recognition of the role of hypothermia in the coagulopathy of trauma and the documented sensitivity of platelet function to temperature may mean that active prevention of hypothermia during resuscitation often provides sufficient time to obtain a platelet count and blood type prior to adding platelets to the early transfusion mix [40–43]. This is supported by the recognition that even among severely injured patients with an ISS of greater than 15, the prevalence of an admission platelet count of less than 100 × 109/l is less than 3% in our center, and the prevalence of an admission platelet count of less than 50 × 109/l is less than 1%.
Old work on dilutional coagulopathy suggested that many patients required platelets after transfusion of two blood volumes. However, reviews of groups of seriously injured patients, such as that of Cosgriff, suggest that patients who receive more platelets early in care have lower rates of coagulopathy and mortality [40]. Our standard issue of blood products in the face of ongoing massive transfusion is a basin with ten units of type-compatible RBCs, ten units of type-compatible plasma and one bag of apheresis platelets. If an initial platelet count was available and less than 50 × 109/l, suggesting that administering a single apheresis bag would not raise the count above 100 × 109/l, a second bag may be issued.
Cryoprecipitate
As noted earlier, in the past we rarely used cryoprecipitate. In our recent review of conventional coagulation test results at admission for severe trauma, the prevalence of an admission fibrinogen less than 1 g/l was less than 3%. With such patients now receiving plasma in general earlier and in greater proportions than in the past, dilutional hypofibrinogenemia should also become less common. However, recent work by Fries and colleagues in Austria in animal and in vitro models suggests that increasing plasma fibrinogen concentration leads to denser and stronger clots [44,45]. Our review of conventional coagulation testing, cited above, revealed that, in severely injured patients, even among those with fibrinogen concentrations in the normal range at admission, those with relatively higher admission fibrinogen levels had relatively lower mortality.
Over the past 2 years, we have used increasing amounts of cryoprecipitate in one very specific clinical situation: the patient with profound, ongoing hemorrhage, severe acidosis and established coagulopathy. This is the patient in whom efforts to prevent coagulopathy by the early use of plasma have failed, either because of overwhelming anatomic injury and profound shock (e.g., a stab wound to the heart) or pre-existing coagulation defects (e.g., end-stage cirrhosis). In these circumstances, the concentration of critical clotting factors in plasma is too dilute to reverse coagulopathy before the patient succumbs to hemorrhagic shock. In most circumstances, this patient is moribund, and further efforts at resuscitation are not justified. In some cases, however, when the surgeons have successfully controlled the primary anatomic source of hemorrhage, an effort to ‘jumpstart’ the coagulation system may be justified. Our empiric approach to this patient at present is the rapid and near-simultaneous administration of 6–12 units of cryoprecipitate, one-to-two pheresis packs of platelets and 100 μg/kg of recombinant human clotting factor VIIa, providing the essential ingredients for clotting in a concentrated form. While prospective, randomized evidence of the benefits of this approach is obviously lacking, our anecdotal experience has been favorable, and further study of this empiric ‘clotting cocktail’ is currently underway.
Our ability to study this approach has been facilitated by discussions between the trauma service and the Blood Bank. In the past, when cryoprecipitate was requested for an acute trauma patient, it frequently took several hours to determine a blood type, and unpackage, thaw and pool a standard ten-unit dose. These events typically occurred on the evening or night shifts, when transfusion service staffing was less, and the need to thaw plasma and issue products took precedence, creating even more delay. We have now begun using prepooled cryoprecipitate, frozen in six-unit aliquots, which markedly speeds the process of unpacking, thawing and issuing this component in an emergency.
Coordination between the TRU & the blood bank
The aforementioned example of our changes in the form and handling of cryoprecipitate to deliver the product faster demonstrates the importance of ongoing efforts at continuous quality improvement both within transfusion services and in relation to their associated trauma centers. Such efforts start with the identification of a problem, the exploration of potential solutions, the development of protocols, the practicing of procedures and the evaluation of products, processes and outcomes.
Our particular experience at the STC may be illustrative. In the past, we had a small satellite blood bank in the trauma center. This was inactive most of the time and inadequate when really needed. The single assigned technician could not perform rapid ABO typing and issue emergency red cell units at the same time or rapidly issue large numbers of RBC units. The transfusion service now maintains a self-service emergency blood refrigerator in the TRU – the only one in the hospital. Each morning, transfusion service personnel inventory the products, document the refrigerator temperatures and recover any paperwork. Nurses in the TRU call the blood bank when products are removed or the refrigerator alarm sounds. Transfusion center personnel restock the refrigerator after every use.
The TRU blood refrigerator is a large, seven-shelf, monitored blood bank model. On the top shelf are the ten units of group O Rh-positive red cells; on the second shelf, the two units of group O Rh-negative red cells; and on the third shelf, four units of AB plasma. The lower shelves are used for products issued to specific patients undergoing evaluation. Each of the uncrossmatched units has a card attached by a rubber band that identifies what the unit is and with a space for the nurse to write the name and number of the patient to whom the blood was given and the time of issue. When the card is filled out, it is placed back in the refrigerator. There is also an inventory sheet on a clipboard, initially filled in by the transfusion service and annotated by trauma center personnel with recipient name and administration time in the documentation phase of an acute admission. The attending trauma surgeon is asked to complete the request for emergency release of uncrossmatched blood products form after the fact.
As a blood type is typically available in 15–30 min and antibody screen becomes available approximately 45 min after admission, type-specific or -compatible blood products are issued as rapidly as possible to reduce the demand for group O red cells and AB plasma. In cases of massive uncontrolled hemorrhage, the blood request from the TRU is usually for ten units of red cells, ten units of plasma and ten units of platelets. Blood bank training routines include drills for the rapid issue and assembly of type-compatible red cell units, available units of type-compatible plasma and a unit of apheresis platelets. The units are brought to the front desk, a barcode sticker is removed from each unit and placed on a blank sheet of paper, the units are placed in a plastic washtub and the washtub is issued to the TRU blood courier. The units are issued in the computer by reading the barcode labels from the issue sheet. If insufficient units of thawed plasma were available to fill the order they are thawed and sent in a second courier run. At the same time, senior blood bank technologists consult with TRU nursing supervisors regarding ongoing supply issues, such as the need to thaw additional plasma and plan for additional bulk issues.
If the number of RBC units issued impinges on the supply of group O units, or type-compatible red cells are in short supply, a blood bank Medical Director is consulted by the technologists. It is possible to give type-incompatible red cells in the middle of a massive transfusion, but we have not done so in 30 years. This process was more common 25 years ago when liver transplants occasionally required 300 units of red cells. Dzik has described his experience of the medical aspects of this process at the Beth Israel Hospital in Boston (MA, USA), and Susan Butch of the University of Michigan (MI, USA) blood service has reviewed the administrative requirements [46,47]. Finally, in times of profound regional shortages of group O red cells, deferral of elective surgeries and triage of trauma patients may be necessary. In July 2003, our normal inventory of more than 260 units of group O red cells fell briefly at one point to nine units.
The transfusion service must do its best to ensure the safety and availability of blood products and provide expert advice on product administration without interfering with or delaying patient care. This can create a point of conflict with the treating physicians who may have more or less expertise on the details of the management of massive transfusion, especially in coping with the coagulopathy of trauma. The sense can easily evolve that the blood bank is blocking patient care from home or behind a desk, while the person in the trauma bay is fighting to keep a dying patient alive. Clear published guidelines are essential, with active and ongoing education of the physician and nursing staff as to what is indicated and when. In addition, the active involvement of the transfusion service Medical Director at the bedside aids greatly in the care of these patients and fosters a team mentality.
Blood support of trauma patients after initial resuscitation
Some patients do not achieve definitive control of hemorrhage for many days. A not uncommon situation involves the packing of the liver to limit massive hemorrhage and having the patient rebleed when the liver is unpacked several days later, as it must be to limit granulation of the peritoneal surfaces in response to the packing and to remove bacterially contaminated gauze. Ideally, such patients are maintained in intensive care units, warmed, and otherwise prepared to optimize their coagulation and other physiologic functions for the next surgery. However, in the most severely injured, multiple surgical procedures cascade one into another. A damage-control laparotomy performed to limit life-threatening hemorrhage may be followed by several orthopedic procedures on long bones, pelvis and spine just to stabilize a patient to allow nursing care, and be followed in turn by repairs of facial or distal extremity injuries.
Complications of alloexposure & alloimmunization
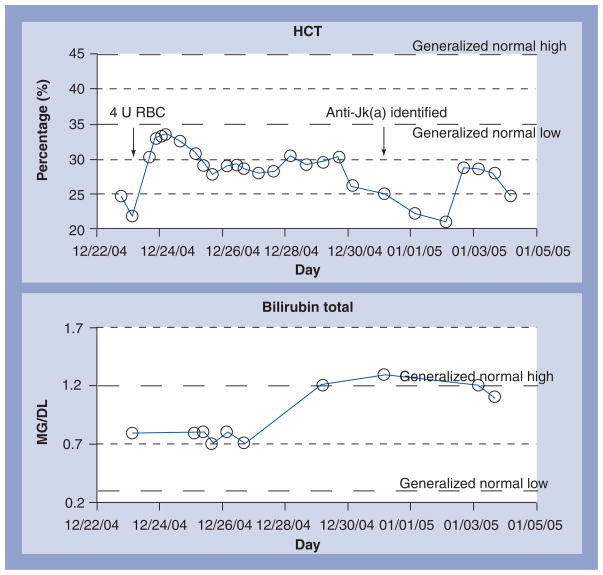
In this time period, multiple transfusion-related problems may also need to be addressed. If Rh-D-positive red cells or platelets are given to a woman of childbearing age, it may be possible to prevent alloimmunization with the administration of Rh immunoglobulin. If she is clinically stable and heavily exposed, it may even be appropriate to perform red cell exchange by apheresis before administration of Rh immunoglobulin [48]. If alloantibodies were detected in the initial antibody screen and ignored in an initial urgent or massive transfusion, the possibility of a delayed hemolytic transfusion reaction must be considered and the care team notified. This situation also arises with secondary immune responses to alloantibodies that did not show at presentation. Figure 1 shows the course of the hematocrit of a young man admitted after trauma and transfused four units of red cells. On the sixth day, he manifested a Jk(a) alloantibody and sustained a brisk delayed hemolytic transfusion reaction of the three Jk(a) antigen-positive units. Prompt feedback from the transfusion service to the clinicians caring for this man prevented an extensive workup for a possible new source of blood loss.
Figure 1. Time course of serial hematocrit measurements of a trauma patient who presented with a major scalp laceration and abdominal injuries and received four units of red cells during the initial phase of treatment.
At 6 days later, his hematocrit again decreased and a new anti-Jk(a) red cell alloantibody was noted. Approximately three-quarters of Caucasian donors are Jk(a) antigen-positive and retained segments of the blood bag tubing tested positive for Jk(a) in three of the four units administered. The patient hemolyzed these three units with a nine-point drop in his hematocrit. He was then uneventfully transfused with Jk(a) antigen-negative red cells.
RBC: Red blood cell; U: Unit.
Microchimerism
The trauma and transfusion groups at the University of California, Davis (CA, USA), have shown that transfused trauma patients have a significant incidence of at least a transient transfusion-related microchimerism that can range from 10 to more than 50% of populations they have examined and appears to have the potential to persist in approximately 5% of affected individual for up to a period of decades [49–54]. Microchimerism appears to occur when donor and recipient have closely matched HLA alleles. At this point, there do not appear to be chronic immunologic sequella of this finding, and the incidence of scleroderma is not higher in well-characterized patients so far [55].
Limiting blood product exposure
Blood transfusion is not without risks and, while lifesaving in the acute phase of trauma resuscitation in moderately and severely injured patients, should be avoided when not required. The Transfusion Requirements in Critical Care trial showed that hemodynamically stable intensive care patients, including many trauma patients, did not need red cell transfusion and did not appear to benefit from it [56]. Many retrospective studies suggest a relationship between the administration of blood products and the incidence of multiple organ failure but are unable to distinguish statistically between the possible adverse effects of the blood itself and transfusion as a marker of worse injury [57–59]. However, there is no question that misidentification of donors, recipients with acute hemolytic transfusion reactions, administration of bacterially contaminated blood products and TRALI are all associated with transfusion-related deaths each year in the USA and Britain [60,61]. How to assess the balance points between dilutional coagulopathy and the risk of rebleeding, the cardiovascular consequences of traumatic anemia and the risk of transfusion-related adverse events remains both difficult and obscure. Currently, the best hope for significant advances around this problem may be the early evaluation and management of the coagulopathy of trauma resulting in decreasing the need for massive transfusion and its attendant risks.
Role of laboratory testing
The common laboratory tests used in the management of transfusion decisions in trauma patients are hemoglobin or hematocrit, platelet count, prothrombin time (often expressed as INR), partial thromboplastin time and fibrinogen concentration. These tests are widely available, fast, reproducible and cheap. As noted above, abnormal values at admission are correlated with injury severity and predictive of in-hospital mortality. Subsequent values over the course of care can be observed for trends and used in conventional ways as guides for transfusion. Transfusion triggers in actively bleeding patients or those who have recently bled are higher for platelets, and probably also for plasma, than for stable patients or those undergoing common elective surgeries. The mortality for brain injury and neurosurgery increases rapidly with modest increases in prothrombin time.
Thromboelastometry is also used to monitor coagulation function in the injured, but is slower, less reproducible and more costly than the conventional battery of laboratory tests [62]. Newer versions of the device appear to have better performance characteristics [63]. It can be useful as a test of platelet function or fibrinolysis in the face of a normal screen with the conventional tests, particularly in patients with normal platelet counts but who are taking aspirin, clopidogrel or other antiplatelet agents prior to the traumatic event. This test has been shown to be effective in limiting platelet transfusion in massive transfusion after cardiac surgery; however, its role in trauma has yet to be elucidated [64,65].
Advances in intravascular volume management
‘The golden hour’ described by R Adams Cowley, the founder and namesake of our trauma center, is a metaphor for the importance of speed in the diagnosis and treatment of trauma patients. The desire for speed in treatment was the basis for the ATLS guidelines – goal-directed therapy with aggressive fluid resuscitation and red cell support to reverse shock – and, subsequently, the recognition that this approach may oversimplify complex physiologic considerations such as coagulation. In addition, dilutional therapy necessarily decreases the hematocrit and increases cardiac work. Beyond the issues of coagulopathy detailed above, knowing when the golden hour has ended and the time has come to titrate fluids, including transfusion, to optimize cardiac function is difficult with currently available tools. Although the anesthesiologist often has a strong feeling for the moment when hemorrhage is substantially controlled (because of continuous feedback from the patient’s response to anesthetizing agents), it is harder to transfer this management paradigm to the intensive care unit. The phenomenon of ‘occult hypoperfusion syndrome’ remains common in young trauma patients reaching the intensive care unit [66]. This has become more obvious as pulmonary artery catheters are used less and less often because of research showing them to be ineffective in improving outcome in a variety of patient populations [67,68]. As a result, some patients are grossly over-resuscitated while others are under-resuscitated. Small arterial catheters that use pulse contour analysis to estimate cardiac output and intravascular volume status may help to partially fill this void. Severe trauma in older patients may be an exception to this general rule [69].
Abdominal sonography and echocardiography both have the potential to provide additional critical information. This has been established for abdominal ultrasound in the form of the focused abdominal sonography for trauma (FAST) exam [70–72]. In this process, a surgical attending or fellow examines the epigastrum, flanks and pelvis of a patient with blunt abdominal trauma to look for free fluid (blood) and major solid organ fracture. The combination of free fluid and shock is an indication for immediate laparotomy in most major trauma centers, and indicates the need for ongoing transfusion. However, shock alone can have many causes in trauma patients beyond bleeding, such as pericardial effusion or myocardial stunning. Following the work carried out in both children and adults, our center is working to develop a focused rapid echocardiographic evaluation (FREE) to help titrate fluid management and optimize cardiac function in severely injured, resuscitated patients [73–75]. This would allow for the identification of patients with the well functioning but relatively empty heart or, conversely, the full but poorly functioning heart; the former would be treated with transfusion and fluid, the latter with inotrophic support and perhaps diuresis. The information provided by arterial catheters, the FAST and the FREE are turning the decision to transfuse trauma patients from an educated guess into an information-rich decision, which is progress.
Expert commentary
Only 2% of civilian trauma patients and 8% of military casualties are likely to require massive transfusion and therefore are likely to benefit from greater exposure to plasma and other blood products. Severe injury, shock and evidence of rapid or uncontrollable bleeding are the clinical indicators that suggest the need for the early use of plasma to prevent coagulopathy. After acute resuscitation, conservative blood guidelines apply. Better tools for hemorrhage control and physiologic assessment are needed.
Five-year view
A world with more people, more vehicles and more guns will produce more injuries, but there is also work ongoing to improve methods of care for the injured in the field and the hospital that will reduce the use of blood components. Modern tourniquets are now recognized as safe for the field control of extremity bleeding without compromising extremity function if transportation to care is rapid and the injuries are treated promptly [76]. Hemorrhage-control bandage technology has improved rapidly over the last decade. Such topical hemostats are now saving lives on the battlefield, and implantable and absorbable versions for general surgical use are in development and testing [77,78]. New and more concentrated plasma products will improve the control of coagulopathic bleeding and reduce exposure to the undesirable biologic response modifiers in random donor plasma. Erythropoietic and thrombopoietic drugs will help overcome injury-induced marrow suppression and lessen the requirements for transfusion in the recovery phase. The number of elderly with pre-existing medical conditions, including cardiac dysfunction, will increase; this will further increase the need for data-guided fluid management. However, in the absence of safe and effective substitutes for blood cells and many plasma proteins, the requirement for blood transfusion will remain.
Communication between transfusion medicine specialists and those who provide direct care to the injured will remain important for the foreseeable future. This will involve both mutual teaching, shared responsibility in patient care, and shaping the goals and forms of research. There is plenty of work for all.
Key issues.
Injuries will increase as the population increases and ages.
There is an acute coagulopathy of trauma.
The association between an acute coagulopathy of trauma and mortality will increase demand for AB plasma.
Implantable hemorrhage-control bandages are coming. They will reduce mortality and blood product use, but they are several years away.
Rapid laboratory test turnaround can guide blood product use even in the acute phase of hemorrhage control.
Once hemorrhage control is obtained, lower transfusion triggers are desirable.
Better tools for physiologic assessment will allow better blood management.
Transfusion triggers will depend on the rapid availability of rescue blood products.
Communication between the Transfusion Service and the Trauma Service remains critical.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
RP Dutton and JR Hess have served as consultants to NovoNordisk over the last year. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Sarah B Murthi, University of Maryland School of Medicine, Baltimore, MD, USA.
Richard P Dutton, University of Maryland School of Medicine, Baltimore, MD, USA.
Bennett B Edelman, University of Maryland School of Medicine, Baltimore, MD, USA.
Thomas M Scalea, University of Maryland School of Medicine, Baltimore, MD, USA.
John R Hess, Email: jhess@umm.edu, Professor of Pathology and Medicine, University of Maryland School of Medicine, Baltimore, MD, USA and c/o Blood Bank, N2W50a, University of Maryland Medical Center, 22 South Greene Street, Baltimore, MD 21201, USA, Tel.: +1 410 328 3834, Fax: +1 410 328 6816.
References
Papers of special note have been highlighted as:
• of interest
• of considerable interest
- 1.Mathers CD, Lopez A, Stein C, et al. Working paper 18, Disease Control Priorities Project, Evidence and Information for Policy Group. World Health Organization; Switzerland, Geneva: Jan, 2005. Deaths and disease burden by cause: global burden of diseases estimates for 2001 by World Bank Country Groups. [Google Scholar]
- 2.Branas CC, MacKenzie EJ, Willians JC, et al. Access to trauma centers in the United States. JAMA. 2005;293:2626–2633. doi: 10.1001/jama.293.21.2626. [DOI] [PubMed] [Google Scholar]
- 3•.Mackenzie EJ, Rivara FP, Jurkovich GJ, et al. The National Study on Costs and Outcomes of Trauma. J Trauma. 2007;63(Suppl 6):S54–S67. doi: 10.1097/TA.0b013e31815acb09. Very large study demonstrating the cost–effectiveness and social value of trauma centers. [DOI] [PubMed] [Google Scholar]
- 4•.Como JJ, Dutton RP, Scalea TM, Edelman BB, Hess JR. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44:809–813. doi: 10.1111/j.1537-2995.2004.03409.x. Describes day-to-day rates of blood use in a large urban trauma center; totals are modest, but episodically large and rapid. [DOI] [PubMed] [Google Scholar]
- 5.Vaslef SN, Knudsen NW, Neligan PJ, Sebastian MW. Massive transfusion exceeding 50 units of blood products in trauma patients. J Trauma. 2002;53:291–295. doi: 10.1097/00005373-200208000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Arnold JL, Dickinson G, Tsai MC, Han D. A survey of emergency medicine in 36 countries. Can J Emerg Med. 2001;3:109–118. doi: 10.1017/s1481803500005340. [DOI] [PubMed] [Google Scholar]
- 7.Collicott PE, Hughes I. Training in advanced trauma life support. JAMA. 1980;43:1156–1159. [PubMed] [Google Scholar]
- 8.Wiggers HC, Goldberg H, Roemhild F, Ingraham RC. Impending hemorrhagic shock and the course of events following administration of dibenamine. Circulation. 1950;2:179–185. doi: 10.1161/01.cir.2.2.179. [DOI] [PubMed] [Google Scholar]
- 9.Shearburn EW, 3rd, Craig WD, Maitland CL, Howard PL, McCoy S, Drucker WR. Hemodynamic and metabolic alterations in peripheral tissue during hemorrhagic shock. Am Surg. 1975;41:696–703. [PubMed] [Google Scholar]
- 10.Zollman W, Culpepper RD, Turner MD, Hardy JA, Hardy JD. Hemorrhagic shock in dogs. Comparison of treatment with shed blood alone versus shed blood plus Ringer’s lactate: intravascular pressures, cardiac output, oxygen consumption, arteriovenous oxygen differences, extracellular fluid PO2, electrolyte changes, and survival rates. Am J Surg. 1976;131:298–305. doi: 10.1016/0002-9610(76)90121-5. [DOI] [PubMed] [Google Scholar]
- 11.Collins JA. Massive transfusion and current blood banking practices. In: Chaplin H Jr, Jaffe ER, Lenfant C, Valeri CR, editors. Preservation of Red Blood Cells. National Academy of Sciences; Washington, DC, USA: 1973. pp. 39–40. [Google Scholar]
- 12.Carrico CJ, Canizaro PC, Shires GT. Fluid resuscitation following injury: rationale for the use of balanced salt solutions. Crit Care Med. 1976;4:46–54. [PubMed] [Google Scholar]
- 13.Bickell WH, Bruttig SP, Millnamow GA, O’Benar J, Wade CE. The detrimental effects of intravenous crystalloid after aortotomy in swine. Surgery. 1991;110:529–536. [PubMed] [Google Scholar]
- 14.Bickell WH, Bruttig SP, Wade CE. Hemodynamic response to abdominal aortotomy in the anesthetized swine. Circ Shock. 1989;28:321–332. [PubMed] [Google Scholar]
- 15.Stern SA, Wang X, Mertz M, et al. Under-resuscitation of near-lethal uncontrolled hemorrhage: effects on mortality and end-organ function at 72 h. Shock. 2001;15:16–23. doi: 10.1097/00024382-200115010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Bickell WH, Wall MJ, Jr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 17.Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52:1141–1146. doi: 10.1097/00005373-200206000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Davis KA, Fabian TC, Croce MA, et al. Improved success in nonoperative management of blunt splenic injuries: embolization of splenic artery pseudoaneurysms. J Trauma. 1998;44:1008–1013. doi: 10.1097/00005373-199806000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Haan JM, Biffl W, Knudson MM, et al. Western Trauma Association Multi-Institutional Trials Committee. Splenic embolization revisited: a multicenter review. J Trauma. 2004;56:542–547. doi: 10.1097/01.ta.0000114069.73054.45. [DOI] [PubMed] [Google Scholar]
- 20.Rotondo MF, Schwab CW, McGonigal MD. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375–383. [PubMed] [Google Scholar]
- 21.Rotondo MF, Zonies DH. The damage control sequence and underlying logic. Surg Clin North Am. 1997;77:761–777. doi: 10.1016/s0039-6109(05)70582-x. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro MB, Jenkins DH, Schwab CW, Rotondo MF. Damage control: collective review. J Trauma. 2000;49:969–978. doi: 10.1097/00005373-200011000-00033. [DOI] [PubMed] [Google Scholar]
- 23.Hardaway RM, McKay DG. The syndromes of disseminated intravascular coagulation. Rev Surg. 1963;20:297–328. [PubMed] [Google Scholar]
- 24.Innes D, Sevitt S. Coagulation and fibrinolysis in injured patients. J Clin Pathol. 1964;17:1–13. doi: 10.1136/jcp.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardaway RM., 3rd The significance of coagulative and thrombotic changes after injury. J Trauma. 1970;10:354–357. [PubMed] [Google Scholar]
- 26••.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;4:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. A fraction of the most severely injured arrive at the trauma center with coagulopathy even before significant resuscitation or hypothermia. [DOI] [PubMed] [Google Scholar]
- 27.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 28.Dutton RP, Shih D, Edelman BB, Hess J, Scalea TM. Safety of uncrossmatched type-O red cells for resuscitation from hemorrhagic shock. J Trauma. 2005;59:1445–1449. doi: 10.1097/01.ta.0000198373.97217.94. [DOI] [PubMed] [Google Scholar]
- 29••.Schmidt PJ. Blood and disaster – supply and demand. N Engl J Med. 2002;346:617–620. doi: 10.1056/NEJM200202213460813. The four largest disasters in the USA over a 25-year period used a maximum of 131 units of red blood cells (RBCs) to care for patients in the first 24 h. [DOI] [PubMed] [Google Scholar]
- 30.Camp FR, Dawson RB. Prevention of injury to multiple casualties requiring resuscitation following blood loss. Milit Med. 1974;139:893–898. [Google Scholar]
- 31.Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46:685–686. doi: 10.1111/j.1537-2995.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 32.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 33•.Borgman MA, Spinella PC, Perkins JG, et al. The ration of blood products transfused affects mortality in patient receiving massive transfusions in a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. Retrospective study of casualties in Baghdad, suggesting that giving a high ratio of plasma to RBC units has a major impact on improving survival in the massively injured. [DOI] [PubMed] [Google Scholar]
- 34••.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to RBC ratios improves outcome in 466 massively transfused civilian trauma patients. Am Surg. 2008 doi: 10.1097/SLA.0b013e318185a9ad. In Press. Confirmation of the findings above in a second retrospective cohort with data provided on massively transfused patients from 16 academic trauma centers. [DOI] [PubMed] [Google Scholar]
- 35.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60(Suppl 6):S91–S96. doi: 10.1097/01.ta.0000199549.80731.e6. [DOI] [PubMed] [Google Scholar]
- 36.Hess JR, Dutton RB, Holcomb JB, Scalea TM. Giving plasma at a 1:1 ratio with red cells in resuscitation: who might benefit? Transfusion. 2008;48(8):1763–1765. doi: 10.1111/j.1537-2995.2008.01743.x. [DOI] [PubMed] [Google Scholar]
- 37.Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1182. doi: 10.1097/TA.0b013e31816c5c80. [DOI] [PubMed] [Google Scholar]
- 38.Eder AF, Herron R, Strupp A, et al. Transfusion-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion. 2007;47(4):599–607. doi: 10.1111/j.1537-2995.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 39.Counts RB, Haisch C, Simon TL, Maxwell NG, Heimbach DM, Carrico CJ. Hemostasis in massively transfused trauma patients. Ann Surg. 1979;190:91–99. doi: 10.1097/00000658-197907000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42:857–861. doi: 10.1097/00005373-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Hess JR, Lawson JH. The coagulopathy of trauma versus disseminated intravascular coagulation. J Trauma. 2006;60(Suppl 6):S12–S19. doi: 10.1097/01.ta.0000199545.06536.22. [DOI] [PubMed] [Google Scholar]
- 42.Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 2006;60:S51–S58. doi: 10.1097/01.ta.0000199432.88847.0c. [DOI] [PubMed] [Google Scholar]
- 43.Kermode JC, Zheng Q, Milner EP. Marked temperature dependence of the platelet calcium signal induced by human von Willebrand factor. Blood. 1999;94:199–207. [PubMed] [Google Scholar]
- 44.Fries D, Innerhofer P, Reif C, et al. The effect of fibrinogen substitution on reversal of dilutional coagulopathy: an in vitro model. Anesth Analg. 2006;102:347–351. doi: 10.1213/01.ane.0000194359.06286.d4. [DOI] [PubMed] [Google Scholar]
- 45.Fries D, Krismer A, Klingler A, et al. Effect of fibrinogen on reversal of dilutional coagulopathy: a porcine model. Br J Anaesth. 2005;95:172–177. doi: 10.1093/bja/aei160. [DOI] [PubMed] [Google Scholar]
- 46.Dzik WH. Massive transfusion in the adult patient: lessons from liver transplantation. In: Jefferies LC, Brecher ME, editors. Massive Transfusion. American Association of Blood Banks; Bethesda, MD, USA: 1994. [Google Scholar]
- 47.Butch SH. Administrative aspects of managing massive transfusion. In: Jefferies LC, Brecher ME, editors. Massive Transfusion. American Association of Blood Banks; Bethesda, MD, USA: 1994. [Google Scholar]
- 48.Werch J, Todd C. Resolution by erythrocytapheresis of the exposure of an Rh-negative person to Rh-positive cells: an alternative treatment. Transfusion. 1993;33:530–532. doi: 10.1046/j.1537-2995.1993.33693296819.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee TH, Paglieroni T, Ohto H, Holland PV, Busch MP. Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long-term microchimerism in severe trauma patients. Blood. 1999;93:3127–3139. [PubMed] [Google Scholar]
- 50.Utter GH, Owings JT, Lee TH, et al. Blood transfusion is associated with donor leukocyte microchimerism in trauma patients. J Trauma. 2004;57:702–707. doi: 10.1097/01.ta.0000140666.15972.37. [DOI] [PubMed] [Google Scholar]
- 51.Lee TH, Wen L, Montalvo L, et al. Minimum conditions of major histocompatibility complex compatibility and recipient immune compromise required to establish donor white blood cell persistence in a murine transfusion model. Transfusion. 2005;45:301–314. doi: 10.1111/j.1537-2995.2005.04223.x. [DOI] [PubMed] [Google Scholar]
- 52.Lee TH, Paglieroni T, Utter GH, et al. High-level long-term white blood cell microchimerism after transfusion of leukoreduced blood components to patients resuscitated after severe traumatic injury. Transfusion. 2005;45:1280–1290. doi: 10.1111/j.1537-2995.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 53.Dunne JR, Lee TH, Burns C, Cardo LJ, Curry K, Busch MP. Transfusion-associated microchimerism in combat casualties. J Trauma. 2008;64(Suppl 2):S92–S97. doi: 10.1097/TA.0b013e318160a590. [DOI] [PubMed] [Google Scholar]
- 54.Gill RM, Lee TH, Utter GH, et al. The TNF (−308A) polymorphism is associated with microchimerism in transfused trauma patients. Blood. 2008;111:3880–3883. doi: 10.1182/blood-2007-08-107144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Utter GH, Reed WF, Lee TH, Busch MP. Transfusion-associated microchimerism. Vox Sang. 2007;93:188–195. doi: 10.1111/j.1423-0410.2007.00954.x. [DOI] [PubMed] [Google Scholar]
- 56.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 57.Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129:39–45. doi: 10.1001/archsurg.1994.01420250051006. [DOI] [PubMed] [Google Scholar]
- 58.Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54:898–905. doi: 10.1097/01.TA.0000060261.10597.5C. [DOI] [PubMed] [Google Scholar]
- 59.MacLeod J, Lynn M, McKenney MG, Jeroukhimov I, Cohn SM. Predictors of mortality in trauma patients. Am Surg. 2004;70:805–810. [PubMed] [Google Scholar]
- 60•.Holness L, Knippen MA, Simmons L, Lachenbruch PA. Fatalities caused by TRALI. Transfus Med Rev. 2004;18:184–188. doi: 10.1016/j.tmrv.2004.03.004. Data from the US FDA on the rising rate of fatal transfusion-related acute lung injury identified in the last few years. [DOI] [PubMed] [Google Scholar]
- 61.Stainsby D, Jones H, Asher D, et al. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273–282. doi: 10.1016/j.tmrv.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Coakley M, Reddy K, Mackie I, Mallett S. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. J Cardiothorac Vasc Anesth. 2006;20:548–553. doi: 10.1053/j.jvca.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 63.Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5:289–295. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 64.Anderson L, Quasim I, Soutar R, Steven M, Macfie A, Korte W. An audit of red cell and blood product use after the institution of thromboelastometry in a cardiac intensive care unit. Transfus Med. 2006;16:31–39. doi: 10.1111/j.1365-3148.2006.00645.x. [DOI] [PubMed] [Google Scholar]
- 65.Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. doi: 10.1111/j.1365-2257.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 66.Blow O, Magliore L, Claridge JA, Butler K, Young JS. The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 h improves outcome from major trauma. J Trauma. 1999;47:964–969. doi: 10.1097/00005373-199911000-00028. [DOI] [PubMed] [Google Scholar]
- 67.Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 68.Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005;294:1664–1670. doi: 10.1001/jama.294.13.1664. [DOI] [PubMed] [Google Scholar]
- 69.Friese RS, Shafi S, Gentilello LM. Pulmonary artery catheter use is associated with reduced mortality in severely injured patients: a National Trauma Data Bank analysis of 53,312 patients. Crit Care Med. 2006;34:1597–1601. doi: 10.1097/01.CCM.0000217918.03343.AA. [DOI] [PubMed] [Google Scholar]
- 70.McKenney MG, Martin L, Lentz K, et al. 1,000 consecutive ultrasounds for blunt abdominal trauma. J Trauma. 1996;40:607–610. doi: 10.1097/00005373-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 71.Rozycki GS, Ballard RB, Feliciano DV, Schmidt JA, Pennington SD. Surgeon-performed ultrasound for the assessment of truncal injuries: lessons learned from 1540 patients. Ann Surg. 1998;228:557–567. doi: 10.1097/00000658-199810000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thourani VH, Pettitt BJ, Schmidt JA, Cooper WA, Rozycki GS. Validation of surgeon-performed emergency abdominal ultrasonography in pediatric trauma patients. J Pediatr Surg. 1998;33:322–328. doi: 10.1016/s0022-3468(98)90455-9. [DOI] [PubMed] [Google Scholar]
- 73.Moore CL, Rose GA, Tayal VS, Sullivan DM, Arrowood JA, Kline JA. Determination of left ventricular function by emergency physician echocardiography of hypotensive patients. Acad Emerg Med. 2002;9:186–193. doi: 10.1111/j.1553-2712.2002.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 74.Randazzo MR, Snoey ER, Levitt MA, Binder K. Accuracy of emergency physician assessment of left ventricular ejection fraction and central venous pressure using echocardiography. Acad Emerg Med. 2003;10:973–977. doi: 10.1111/j.1553-2712.2003.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 75.Pershad J, Myers S, Plouman C, et al. Bedside limited echocardiography by the emergency physician is accurate during evaluation of the critically ill patient. Pediatrics. 2004;114:667–671. doi: 10.1542/peds.2004-0881. [DOI] [PubMed] [Google Scholar]
- 76.Kragh JF, Jr, Walters TJ, Baer DG, et al. Practical use of emergency tourniquets to stop bleeding in major limb trauma. Trauma. 2008;64(Suppl 2):S38–S49. doi: 10.1097/TA.0b013e31816086b1. [DOI] [PubMed] [Google Scholar]
- 77.Rhee P, Brown C, Martin M, et al. QuikClot use in trauma for hemorrhage control: case series of 103 documented uses. J Trauma. 2008;64:1093–1099. doi: 10.1097/TA.0b013e31812f6dbc. [DOI] [PubMed] [Google Scholar]
- 78.Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS. An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med. 2008;15:74–81. doi: 10.1111/j.1553-2712.2007.00009.x. [DOI] [PubMed] [Google Scholar]