Abstract
Human maternal and infant biology likely coevolved in a context of close physical contact and some approximation of frequent, “infant-initiated” breastfeeding. Still, mothers and infants commonly sleep apart from one another in many western societies, indicating a possible “mismatch” between cultural norms and infant biology. Here we present data from a 3-night laboratory-based study that examines differences in mother–infant sleep physiology and behavior when mothers and infants sleep together on the same surface (bedsharing) and apart in separate rooms (solitary). We analyze breastfeeding frequency and interval data from the first laboratory night (FN) for 52 complementary breastfeeding mothers and infants (26 total mother–infant pairs), of which 12 pairs were routine bedsharers (RB) and 14 were routine solitary sleepers (RS). RB infants were 12.0 ± 2.7 (SD) weeks old; RS infants were 13.0 ± 2.4 weeks old. On the FN, RB mother–infant pairs (while bedsharing) engaged in a greater number of feeds per night compared to RS (while sleeping alone) (P < 0.001). RB also showed lower intervals (min) between feeds relative to RS (P < 0.05). When we evaluated data from all three laboratory nights (n = 36), post hoc, RB breastfed significantly more often (P < 0.01) and showed a trend towards lower intervals between feeds (P < 0.10). Given the widely known risks associated with little or no breastfeeding, the demonstrated mutually regulatory relationship between bed-sharing and breastfeeding should be considered in future studies evaluating determinants of breastfeeding outcomes.
Keywords: breastfeeding, bedsharing, cosleeping, human evolution
Mother–infant cosleeping is defined as any situation in which a committed mother sleeps within sensory range of an infant (on the same or different surface) permitting mutual monitoring, sensory access, and physiological regulation, including (but not limited to) the delivery and ingestion of breast milk (McKenna et al., 1993). Such close mother–infant proximity during sleep is common across cultures and is nearly ubiquitous among primates (Barry and Paxson, 1971; Anderson, 1984; McKenna et al., 2007). Compared with most other primates, humans are especially vulnerable and dependent, being born with only 25% of adult brain volume, as a result of the anatomical limitations imposed by encephalization and bipedality (Rosenberg and Trevathan, 2005; Robson et al., 2006; Martin, 2007). Consequently, it is highly probable that infants slept near or on their mothers' body to be fed and nurtured over the course of hominin evolution, although evolutionary reconstructions will never be able to fully prove the point, given that maternal behavior does not fossilize or leave an otherwise indelible mark in the archaeological record. Still, whether during wake or sleep, maternal proximity and contact likely maximized the chances of the infant acquiring adequate safety, nutrition, and immunological protection in the challenging ecologies facing our hominin ancestors (McDade 2003; Hart and Sussman, 2005; McKenna et al., 2007).
An anthropological perspective, largely missing in contemporary pediatric sleep research (Anders et al., 1971; Guilleminault and Souquet, 1979; Ferber and Kryger, 1995; Lee-Chiong, 2006), draws on cross-cultural, cross-species, and developmental data to model evolved human norms vis-à-vis mother–infant sleep behaviors. This explanatory framework illustrates how and why maternal breast milk delivery and metabolism (by the infant) combined with mother–infant cosleeping changes almost every important clinical sleep variable for both the mother and infant in potentially positive ways when compared with data derived from solitary sleeping, bottle-fed infants (McKenna et al., 2007). This was initially demonstrated when the first ever study of the behavior and physiology of mother–infant cosleeping was proposed and undertaken 20 years ago to evaluate the appropriateness of conceptualizing solitary sleep as “normative” or “healthy” for the human infant (McKenna et al., 1990). Subsequently, numerous scholars have applied anthropological and evolutionary perspectives to questions of mother–infant sleep practices, especially as related to breastfeeding patterns and health and safety issues in the bedsharing (cosleeping on the same surface in a bed) environment (reviewed in McKenna et al., 2007; Ball and Klingaman 2008). The extent of comparative physiological and behavioral differences between solitary sleeping and cosleeping infants suggests that a significant, possibly harmful, disjuncture may emerge between behavior and biology when solitary infant sleep practices are favored, giving rise to evolutionarily anomalous experiences. For example, not breastfeeding is now known to be an independent risk factor for sudden infant death syndrome (SIDS) (Vennemann et al., 2009), as is an infant sleeping alone in a room by itself (Mitchell and Thompson 1995; Blair et al., 1999; Carpenter et al., 2004).
Although a growing body of evidence from studies in the US, UK, and New Zealand (McKenna et al., 1997; Young 1999; Ball, 2003; McCoy et al., 2004; Ball et al., 2006; Baddock et al., 2007; Ball, 2007) and subsistence-level populations (Super and Harkness 1987; Imong et al., 1989; Sellen, 2001) demonstrate that mother–infant sleep practices relate to day-to-day and overall, long-term breastfeeding patterns, studies examining the contextual factors that influence breastfeeding outcomes generally fail to include sleep location as a relevant variable (e.g., Arora et al., 2000; Alikassifogglu et al., 2001; Ertem et al., 2001; Scott et al., 2001). This may owe, at least in part, to a general paucity of observational, experimental data on the ways in which mothers and infants interact and relate to each other during sleep periods, especially as regards factors influencing nighttime feeding behaviors. While three important studies shed further light on these issues (Young, 1999; Ball et al., 2006; Baddock et al., 2007), a major lacuna still exists and we elaborate on previous work here to help fill it.
To explore the relationship between breastfeeding behavior and routine sleeping arrangements we examine data from three consecutive nights of observed breastfeeding activities, recorded on infrared video in a controlled, laboratory setting. Previous analyses of breastfeeding behavior in this sample compared feed frequency for only the second and third nights in the laboratory and did not assess feed interval data for any night (McKenna et al., 1997). Hundreds of hours of recordings that include data on potential differences and similarities in breastfeeding patterns between solitary infant sleeping and bedsharing were left unanalyzed. The present analysis focuses on the first night data (n = 26, infant age 12.5 ± 2.5 weeks) alone and in combination with the second and third night data (n = 36, age 12.5 ± 2.4 weeks) to test the hypotheses that during nighttime sleep routinely bedsharing mother–infant dyads engage in significantly more breastfeeds and show a shorter interval duration between feeds when compared with routinely solitary sleeping pairs.
MATERIALS AND METHODS
Study population
The methods for this study have been described in detail previously (McKenna et al., 1997). The University of California-Irvine's Human Subjects Review Committee approved this protocol. Subjects were recruited from the Birthing Center at the University of California-Irvine Medical Center. All mother–infant pairs visiting the Birthing Center for postpartum follow-up and meeting the inclusion criteria were asked to participate, and mothers were remunerated for their participation. Inclusion criteria for mothers in the study were as follows: Latina, <38 years old; practice, at least, complementary breastfeeding, according to criteria from WHO/PAHA (2003), as some formula can be used in complementary breastfeeding and, for this study, no more than two 4-ounce bottles of formula per day and none after 3:00 pm so that infants would not be ingesting formula during the study period; have no present or past history of drug or alcohol abuse; have no history of smoking, alcohol, or illicit drug use during pregnancy; have had prenatal care; have had uncomplicated pregnancies, labors, and deliveries; in good health and free of sleep disorders; taking no medications known to affect sleep pattern; have chosen sleeping practice for reasons other than infant temperament (e.g., response to a fussy infant). A physician trained in sleep medicine performed the sleep histories. Routinely bedsharing mothers (RB) were 25.9 ± 6.4 (SD) years of age, and routinely solitary sleeping mothers (RS) were aged 25.9 ± 5.8 years. None of the mothers in this study were obese.
Inclusion criteria for infants were as follows: 7–18 weeks old at the time of the sleep studies; in good health, with normal growth and development; weighed >2,500 g and were >37 weeks' gestational age at delivery; had a 5-min Apgar score ≥8; have no history of SIDS in first degree relatives; no history of prolonged apnea or an apparent life-threatening event.
Mothers and infants were only included in our initial (first night) analysis if they had full breastfeeding data (feed frequency and duration of interval between feeds) available for the first night of the study, resulting in a subsample of 26 mother–infant pairs from the total sample of 36 pairs. Given the strict inclusion criteria, mothers and infants included in this analysis did not differ significantly on demographic, anthropometric, or health variables from those excluded because of missing data. For the first night analysis (n = 26), RB infants were 8 boys and 4 girls, aged 12.0 ± 2.7 (SD) weeks when sleep testing was performed; RS infants were 3 boys and 11 girls and were 13.0 ± 2.4 weeks old. In the full sample (n = 36), RB infants were 14 boys and 6 girls, aged 12.4 ± 2.2 weeks when sleep testing was performed; RS infants were 4 boys and 12 girls and were 12.8 ± 2.6 weeks old.
Two-week daily sleep logs were completed at home just before the laboratory sleep recordings to confirm maternal reports of the infants' usual sleep environment. For the 33 pairs who completed all 14 nights of the log, the mean (±SD) number of bedsharing nights was 13.7 ± 0.5 for the RB group versus 0.6 ± 0.9 for the RS group. Although mothers were recruited based on their self-reports of routine sleep practices, criteria for categorization as RB and RS were established using data from these sleep logs. RB was defined as bedsharing with the mother a minimum of 4 h per night, at least five days per week; RS was defined as bedsharing no more than one night per week for any part of the night. Fathers were excluded from the study protocol, regardless of whether or not they participated in infant bedsharing, to isolate the relationship between maternal-infant bed-sharing on breastfeeding.
Sleep and behavioral measurement
Mother–infant pairs underwent three consecutive nights of laboratory study: an initial night matching the routine home sleeping arrangement, followed by the experimental conditions, a bedsharing night (BN), and a solitary sleeping night (SN), which are experienced in a randomly assigned order. This study focuses, in part, on the first night (FN), the so-called “adaptation night” (Agnew et al., 1966). Sleep studies were performed in the University Medical Center Sleep Disorders Center, a facility accredited by the American Sleep Disorders Association. For solitary sleep, infants slept in a standard crib in the room adjacent to the mother's (within hearing range) with the doors between them ajar. On the bed-sharing night(s), mother–infant pairs shared a twin-size bed, which was the same bed used by the mother on the solitary sleeping night. Infants were maintained on their usual feeding and sleeping schedules, with mothers performing all care taker interventions ad lib. Mothers were blind to all experimental hypotheses and instructed only to prepare and respond to their infants as they would at home. Mothers retired to bed an average of 66.5 ± 24.7 (SD) minutes after their infants (collapsing across groups and conditions). No mother entered the room where her infant slept during this 67-min period. There were no significant differences on any night or experimental condition between RB and RS in total sleep time or total observation time (Mosko et al., 1994; Mosko et al., 1997; McKenna, n.d.). Bed and wake times were consistent with those recorded by mothers in their sleep logs (see Fig. 1).
Fig. 1.

Shown here is a bedsharing mother-infant pair undergoing electro-physiological monitoring of brain wave activity (EEGs and EOGs) to identify transient arousals, awakenings, and sleep stage progression. For each member of this mother-infant dyad nasal air flow, chest movements, heart rate, and oxygen saturation, in addition to breastfeeding behaviors, were continuously recorded and filmed through infrared cameras. Photo taken by Max Aguilera-Hellweg.
Monitoring was initiated when the mother retired and was terminated after mother and infant had awakened the next morning. Observational monitoring included continuous infrared audiovisual recordings as well as the standard, noninvasive polysomnographic measures (including electroencephalograms, electrooculograms, and surface chin electromyogram for determination of sleep-wake stages). A large digital clock placed in the camera field allowed calculation of breastfeeding data to the nearest minute (adapted from McKenna et al., 1997).
Breastfeeding measurement
Breastfeeding was defined as breast attachment, which was usually verifiable through observation of the infrared video recordings. Therefore, identification of breastfeeding did not rely on infant sucking behavior, with the exception of the few occasions when blankets obscured the infant's head; in those instances the sucking artifact, which is readily seen on the chin electromyogram, was used to identify breastfeeding.
Breastfeeding behavior was quantified in terms of breastfeeding sessions (Vitzthum 1994). All breastfeeding sessions were initiated by the mother making her breast available to the infant and defined to capture a single, intentional, and continuous act of breastfeeding on the mother's part. Breastfeeding events began and ended with breast attachment (latching) and detachment, respectively, but sessions also included very short interruptions during which, for example, the mother switched from one breast to the other (adapted from McKenna et al., 1997). These and other interruptions were considered part of the same session if they lasted under 1 min (Vitzthum, 1994).
For infants who fed only once during the observation period (n = 6), lacking a measureable feed interval, occurring between two or more feeds, we documented the duration of time from the beginning of the observation time to the beginning of the feed as well as the duration from the end of the feed to the end of the observation. We then took the longer of the two durations and assigned that value as the interval for these single feed infants. Because infants are likely, but not certain, to feed before nighttime sleep and upon waking (Kent et al., 2006), this represents a conservative estimate of the time between feeds for these infants.
Statistical analysis
All analyses were performed with version 10 of Stata (Stata Corporation, College Station, TX). In statistical analyses, mother–infant pairs were separated into two groups, defined by their habitual, at-home sleeping arrangements, routine solitary (RS), and routine bed-sharing (RB). Overnight infrared video data were used to compute mean number of feeds and mean interval (minutes) between breastfeeding sessions for each group. Analyses were restricted to the time the mothers were in bed each night, from lights out to final morning awakening. Significance was evaluated at P < 0.05. Pearson's chi-square test was used to test whether the sex distribution differed for RS and RB infants. Levene's test for equality of variance was used to compare group variances. All t-tests were two-tailed. Using unpaired Welch's t-tests (for unequal variances), group differences were assessed between RS and RB for these two variables on the FN in the laboratory. Next, to test for “first night effects” (Agnew et al., 1966), paired student's t-tests were used to assess differences in feed frequency and interval between feeds for the FN and the SN for RS (i.e., comparing the two solitary nights for routinely solitary sleepers) and for the FN and BN for RB.
In order to assess potential selection bias involved in focusing only on mother–infant pairs with available data on the FN, feed frequency and intervals on the experimental nights (2 and 3) were compared for pairs with FN data and those missing FN data. Post hoc, new variables were created, combining breastfeeding frequency and interval observations from the FN, BN, and SN nights. Collapsing data across the three nights of observation, including pairs with missing FN data, increased sample sizes for RB (n = 20) and RS (n = 16). Using these expanded samples, unpaired Welch's t-tests were used to test for differences between RB and RS in feed intervals and feed frequency.
RESULTS
The percentage of male and female infants differed for RB and RS on the FN (χ2 = 5.4, P < 0.05). Unpaired student's t-tests demonstrated no significant differences between male and female infants within RS or RB, respectively, for either breastfeeding intervals or feed frequency on the FN (all P > 0.4).
We hypothesized that on the FN RB mother–infant pairs would feed more often with a shorter interval between feeds compared with RS. Results from unpaired Welch's t-tests confirmed these hypotheses, as the groups differed significantly on both variables (Figs. 2 and 3).
Fig. 2.
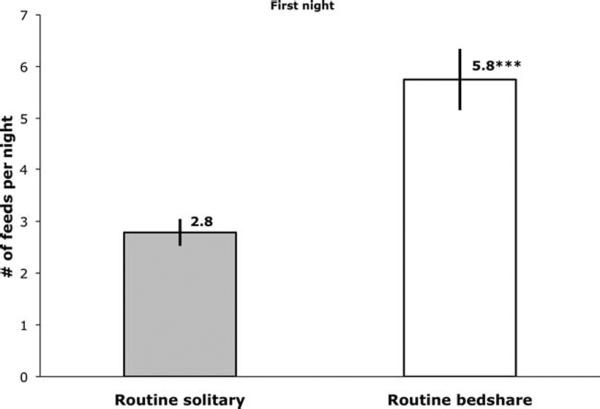
Mean number of breastfeeds per night (with SE) for routine solitary sleepers (while sleeping separately, n = 14) and routine bedsharers (while bedsharing, n = 12) on the first laboratory night. Significant between group difference, ***P < 0.001.
Fig. 3.
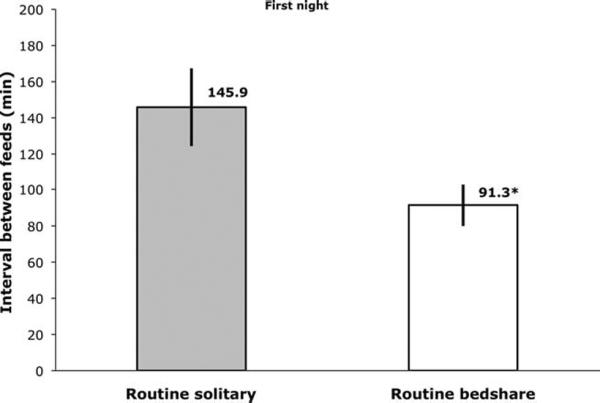
Mean interval between breastfeeds (with SE) for routine solitary sleepers (while sleeping separately, n = 14) and routine bedsharers (while bedsharing, n = 12) on the first laboratory night. Significant between group difference, *P < 0.05.
There is a general assumption in sleep research that an “adaptation night” is necessary to reduce the effects of the novelty of the sleep laboratory on measurable outcomes, “first night effects” (Agnew et al., 1966). Although a previous analysis of physiological data from this sample reported no such first night effects (Richard and Mosko, 2004), we tested for within group differences between the FN and the experimental nights for breastfeeding variables. Mother–infant pairs (n = 17) in this portion of the analysis were limited to those who had full breastfeeding data for all three nights of the study (Table 1). Paired student's t-tests revealed no significant differences between the FN and experimental nights for either breastfeeding variable among RS or RB (Table 1).
TABLE 1.
Testing breastfeeding outcomes for “first night” effects
| Breastfeeding interval (min) | SD | P value ≤c | Breastfeeding frequency | SD | P value ≤c | |
|---|---|---|---|---|---|---|
| RS-FNa | 142.98 | 76.42 | 0.11 | 2.75 | 1.16 | 0.14 |
| RS-SNa | 235.44 | 124.10 | 2.00 | 0.93 | ||
| RB-FNb | 88.59 | 44.10 | 0.58 | 5.78 | 2.17 | 1.00 |
| RB-BNb | 78.41 | 37.66 | 5.78 | 2.05 |
RS, routine solitary sleepers; RB, routine bedsharers; FN, first night; SN, solitary night; BN, bedsharing night.
(n = 8).
(n = 9).
Results of paired t-tests.
In order to test for potential selection bias, mother–infant pairs with FN data were compared to pairs missing FN data for breastfeeding data from the experimental nights 2 and 3 (Table 2). Although the sample size for RS pairs was limited, there were no significant differences between pairs with or without FN data. There were significant differences between RB mothers based on availability of FN data. RB pairs with missing FN data fed significantly less often and showed significantly greater intervals between feeds (Table 2).
TABLE 2.
Testing breastfeeding outcomes for FN sample selection bias
| Breastfeeding interval (min) | SD | P value ≤e | Breastfeeding frequency | SD | P value ≤e | |
|---|---|---|---|---|---|---|
| RS-SNa | 235.44 | 124.10 | 0.16 | 2.00 | 0.93 | 0.24 |
| RS-SNb | 89.25 | 94.40 | 3.00 | 1.41 | ||
| RB-BNc | 78.41 | 37.66 | 0.001 | 5.78 | 2.05 | 0.001 |
| RB-BNd | 159.33 | 27.49 | 2.63 | 0.74 |
RS, routine solitary sleepers; RB, routine bedsharers; SN, solitary night; BN, bedsharing night.
Pairs with FN data (n = 8).
Pairs without FN data (n = 2).
Pairs with FN data (n = 9).
Pairs without FN data (n = 8).
Results of unpaired t-tests.
In light of these significant differences, post hoc, we combined data from all three nights to test for differences between RS and RB for the total sample. The percentage of male and female infants differed for RB and RS (χ2 = 7.2, P < 0.01). Unpaired student's t-tests showed no significant differences between male and female infants within RS or RB, respectively, for either breastfeeding variable (all P > 0.4). When all available data points were considered, it was shown that RB fed significantly more often than RS (see Fig. 4), similar to the comparison on the FN only (see Fig. 2). The difference between RS (on FN + SN) and RB (on FN + BN) for intervals between feeds was no longer significant, although it remained a statistical trend (see Fig. 5).
Fig. 4.
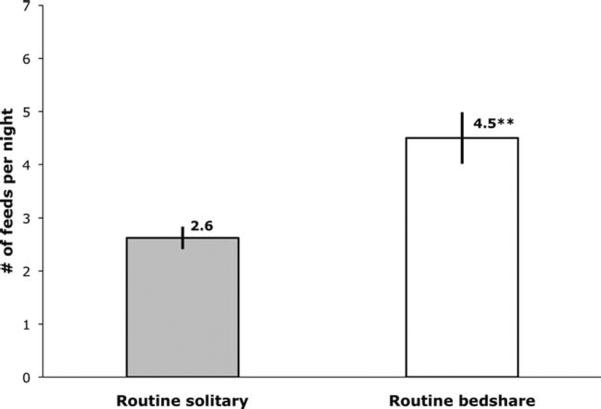
Mean number of breastfeeds per night (with SE) for routine solitary sleepers (while sleeping separately, n = 16) and routine bedsharers (while bedsharing, n = 20), averaged over all three laboratory nights. Significant between group difference, **P < 0.01.
Fig. 5.
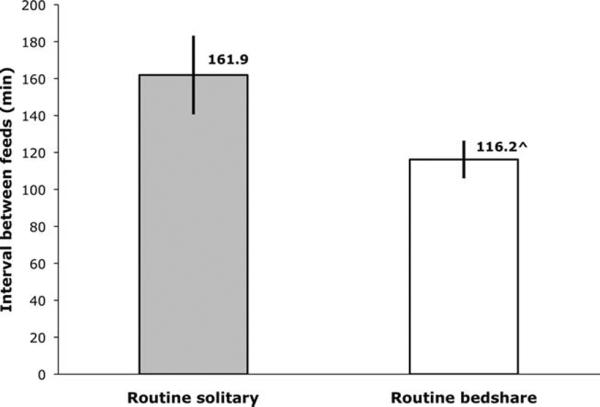
Mean interval between breastfeeds (with SE) for routine solitary sleepers (while sleeping separately, n = 16) and routine bedsharers (while bedsharing, n = 20), averaged over all three laboratory nights. Statistical trend towards between group difference, ^P < 0.10.
DISCUSSION
In this sample of breastfeeding, Latina mother–infant pairs, routine bedsharing (RB) is associated with significantly greater number of feeds per night compared to mother–infant dyads who routinely sleep separate (RS) from one another in different rooms. We hypothesized that RB mother–infant pairs would show significantly shorter intervals between feeds compared with RS, largely owing to greater number of feeds during the night among RB (McKenna et al., 1997). In analyzing previously unpublished data from the FN, we documented results consistent with this expectation (see Fig. 3). Further examination of the combined data from all three laboratory nights showed that RB and RS differed significantly in terms of breastfeeding frequency whereas the difference for intervals between feeds shifted from significant to a statistical trend (Figs. 4 and 5). In total, our findings are consistent those of previous studies documenting greater total nighttime breastfeeds among cosleeping mother–infant dyads (McKenna et al., 1997; Young 1999; Baddock et al., 2007; Ball et al., 2006; Ball and Klingaman, 2008). These results provide further confirmation of the mutually reinforcing relationship between nighttime mother–infant cosleeping and breastfeeding, likely reflecting a coevolutionary history of these behaviors (McKenna et al., 1994; Young, 1999; Ball, 2006; McKenna et al., 2007).
Bedsharing is increasingly more common (or, possibly, merely increasingly reported) in many western industrialized societies, with recent studies showing that, for example, 50–75% of all new parents in the US and UK bring their infants to bed with them for some or all of the night (Ball et al., 1999; Willinger et al., 2003; Blair and Ball, 2004; Lahr et al., 2005), despite some prominent medical and public health recommendations against it (Gettler and McKenna, 2010), particularly in the US (e.g., AAP 2005). It would appear that a combination of underlying biological and/or socio-emotional factors draw breastfeeding mothers, specifically, and infants together at night. This may be especially true regarding the relative ease with which bedsharing facilitates breastfeeding, as 65% of breastfeeding mothers were shown to bedshare in the months following parturition despite 80%–85% reporting no intention of doing so before their babies were born (Ball et al., 1999; McKenna and Volpe, 2007). It has been demonstrated that women who elect to breastfeed are nearly twice as likely to sleep with their babies in the first month after birth than mothers who bottle-feed (McCoy et al., 2004).
Multiple longitudinal studies of mother–infant sleep and breastfeeding outcomes have demonstrated that bed-sharing is positively associated with significantly longer duration (in months) of any breastfeeding and exclusive breastfeeding, particularly in the first six months of life when infants are most immunologically and developmentally vulnerable (McCoy et al., 2004; Ball, 2002, 2003, 2007; Ball and Klingaman, 2008). However, these studies do not include details on the specific associations between sleep ecology and nighttime breastfeeding patterns, which may help explain why bedsharing mothers experience enhanced long-term breastfeeding results. One of the strengths of our study is the recorded, real time observations of mother–infant interactions during the sleep period, allowing for precise quantification of feeds and length of feed intervals. These recordings provide an unbiased record of nighttime breastfeeding structure, avoiding potential errors in maternal recall, which can be less reliable than observations of mother–infant interactions, especially among cosleeping mothers (McKenna et al., 1994; Vitzthum, 1994; Sellen, 2001). Consequently, our laboratory-based, observational data provide a crucial complement to longitudinal studies of breastfeeding and infant sleep, in confirming that routine mother–infant bedsharing is positively related to nighttime breastfeeding frequency for bedsharers compared to solitary sleepers.
To our knowledge, prior laboratory or observational studies of breastfeeding behavior have not reported nighttime feed interval differences based on routine sleeping practices. Our novel finding that RS infants show a different breastfeeding structure, feeding significantly less often than RB with a slightly longer (~45 min) feed interval (Figs. 4 and 5) suggests that RS may experience extended portions of the night without feeding, a pattern, which, for example, may be suboptimal for infant weight gain and maintenance of long-term breastfeeding (De Carvalho et al., 1983; Hornell et al., 1999). Our finding that RS experience fewer feeds but do not differ significantly in their feed intervals relative to RB may be explained by a group of 4 statistical outliers among RS, who each fed twice but with an interval between feeds ranging from 10.5 to 22.5 min (evaluated against a mean of 197.4 min for the rest of RS). By comparison, the two shortest intervals recorded among RB on any night were 40.8 min and 49.4 min, both of which resulted from the infants feeding eight times. We hypothesize that these RS infants may not have returned to sleep after their first feed and may have called their mothers back a second time because of separation distress and need for soothing, rather than for an explicit need for additional feeding (Christensson et al., 1995). Excluding these outliers, the feed interval (±SD) comparison between RS (on FN + SN) and RB (on FN + BN) is highly significant (RS: 197.37 ± 59.02; RB: ± 116.21 ± 45.62; P < 0.001) and the interval difference is nearly doubled (45 min vs. 81 min).
Along with these feed interval differences, RB infants from this sample have been shown to feed more often, as we document here, and also for longer per feeding session and for a greater duration of total time over the course of the night, relative to RS (McKenna et al., 1997). Moreover, it has been demonstrated that ingested breast milk volume is positively related to total nursing time and positively associated with nighttime feeding (Imong et al., 1989; Dewey et al., 1991; Kent et al., 2006), and it is also estimated that many US breastfed infants fail to consume enough calories to meet their daily energetic budget (Dewey et al., 1991). Consequently, it seems likely that mother–infant nighttime proximity and its relationship to greater duration and frequency of feeding are important in determining whether infants fully derive the energetic and immunological benefits of their mothers' milk, particularly in societies or ecologies where infants have reduced access to their mothers during the daytime (Scariati et al., 1997; Dewey et al., 1995; Bener et al., 2001; Chen and Rogan, 2004; Vennemann et al., 2009).
The observation that nighttime proximity is associated with higher breastfeeding frequency dovetails with existing data on infant feeding and sleeping across cultures and primate species. Relative to maternal milk of species in which young are left unattended for extended periods of time (“nested” or “cached”) (Ben Shaul 1962; Jenness, 1986), breast milk in humans is high in carbohydrates and low in fat and proteins, leading to shorter bouts of infant satiation and requiring frequent feeding, as seen in other primate species where mothers carry their infants and provide “infant-initiated” (i.e., on-demand) feeding (Tilden and Oftedal, 1997; Czank et al., 2007). Across all primates, because of the anatomical constraints imposed by bipedalism and encephalization (the obstetrical dilemma), human infants are among the most neurologically, physically immature at birth, making them especially reliant on mother–infant proximity, maternal regulation, and breastfeeding for normal growth and development, especially in the brain (Tilden and Oftedal 1997; Rosenberg and Trevathan 2005; Martin, 2007). This implies that the human mother–infant biological relationship likely evolved in a context of closeness, contact, and high feeding demands (Blurton Jones, 1974; McKenna et al., 2007; Schön and Silvén, 2007).
These observations are consistent with data from contemporary subsistence-level populations in which young infants are carried by or are in close proximity to their mothers for the majority of the 24-hour day with regular bouts of nursing, as was likely normative for the majority of hominin evolution (Lozoff and Brittenham, 1979; Konner and Worthman, 1980; Wood et al., 1985; Super and Harkness, 1987; Imong et al., 1989; Vitzthum, 1989; Panter-Brick, 1991; Worthman et al., 1993; Gray, 1994; Sellen, 2001). For example, in Konner and Worthman's (1980) seminal study of nursing frequency in the !Kung (Botswana), infants nursed an average of 4.06 times per hour during daytime observations. Largely reflecting the challenges of gathering accurate nighttime breastfeeding data, few studies of nighttime feeding patterns among nonindustrialized or subsistence-level populations are available. Sellen (2001) reports that infants breastfed an average of 4.2 times per night among Datoga pastoralists (Tanzania), which corresponds with our finding that routinely bedsharing infants show a mean of 4.5 feeds per night. In a study of (cosleeping) rural Thai mother–infant pairs, Imong (1989) observed that an average of 53% of infant breast milk intake (over 24 h) occurred during the nighttime period. Collectively, these data provide further confirmation that nighttime mother–infant sleep proximity is key factor influencing nighttime breastfeeding across cultures.
An integrated evolutionary, anthropological approach applied to assessing the settings within which contemporary infant sleep and feeding practices find expression draws on a diverse array of comparative cross-cultural, cross species, and infant developmental data. Such a perspective provides the necessary contextualization and background for understanding the essential role of mother–infant proximity (including during sleep) in influencing both short- and long-term breastfeeding patterns and outcomes. Still, many studies intended to capture relevant factors related to breastfeeding initiation and duration and/or the adoption of exclusive breastfeeding fail to incorporate sleep practices as a predictor variable (e.g., Arora et al., 2000; Alikassifogglu et al., 2001; Ertem et al., 2001; Scott et al., 2001). The uniqueness of our analysis is that it is one of a handful in which the data emerge under controlled conditions wherein the experimental design and hypotheses are inspired and tested using a well-developed evolutionary model (McKenna et al., 1994; Young, 1999; Ball and Klingaman 2008). Our findings further confirm the potentially important immediate consequences that culturally guided decisions, such as solitary sleep, play in influencing infant health and development. Human infant biology may lack the capability to accommodate the relatively rapid pace of culturally based shifts in care, which may deviate from practices designed by natural selection specifically to maximize both proximate and long-term benefits. When considered in combination with studies of longitudinal breastfeeding patterns and mother–infant sleep, our findings suggest that future research aimed at explaining variation in breastfeeding patterns should consider the likely evolved interrelationships between mother–infant sleep proximity and nighttime feeding.
Limitations
Our study is limited by its cross-sectional design, preventing us from drawing causal inferences based solely on these data. However, Ball et al. (2006) conducted a random-assignment, nonblinded trial of new mothers on a maternity ward in the UK and showed that close mother–infant sleep proximity leads to higher rates of breastfeeding frequency compared with when mothers and infants sleep apart.
The sample used in this study is also relatively homogenous by design in order to control for confounding issues such as feeding practice, maternal or infant health, or cultural differences in beliefs about bedsharing. While we cannot make definitive statements regarding the generalizability of our data, we are confident that these findings contribute to our understanding of the ways in which breastfeeding and various forms of mother–infant cosleeping are intimately connected across cultural and ecological settings.
Some of our analyses are somewhat constrained by limited sample sizes. However, given the cost and labor intensive nature of combining polysomnographic recording, respiratory and heart rate monitoring, and videotaped behavioral observation for a total of 72 mothers and infants (36 dyads), as was done in the larger study of which this analysis is a part, our sample size meets or exceeds the standards for sampling in this research domain (e.g., Scher et al., 1992).
Finally, the finding that RB with and without FN data differed significantly on their breastfeeding intervals and feed frequency suggests that there may be a form of selection bias in the FN data in studies with a design such as ours (Table 2). The absence of FN data suggests that, at least for some dyads, something went awry with the protocol, precluding a full night's observation. However, that RB infants with FN data showed comparable feeding patterns on the FN and BN indicates that their FN data were not anomalous (Table 1). Moreover, our post hoc analyses demonstrate a significant difference in number of breastfeeds for RS and RB when all data points were considered, speaking to the robustness of the relationship between sleep proximity and breastfeeding.
ACKNOWLEDGMENTS
Dr. McKenna would like to thank his coresearchers Drs. Sarah Mosko, Chris Richard, and Sean Drummond who played critical roles in designing and conducting this research. We wish to extend our gratitude to the efforts of all the mothers (and their infants) who weathered three consecutive nights in a sleep laboratory and who made possible whatever insights we have gained to benefit other mothers and infants. Finally, especial appreciation is given to Dr. Ruff, the rest of the editorial staff at AJPA, and our two anonymous reviewers. All of these scholars provided careful and diligent insights and suggestions that vastly improved and enhanced the usefulness of our contribution. This research was conducted with funds from the National Institutes of Child Health and Human Development RO1 27482 and a Shannon Award.
Grant sponsor: NICHD RO1; Grant number: 27482; Grant sponsors: Northwestern University graduate fellowship, National Science Foundation Graduate Research Fellowship.
LITERATURE CITED
- AAP American Academy of Pediatrics (AAP) Task Force on Sudden Infant Death Syndrome. The changing concept of Sudden Infant Death Syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245–1255. doi: 10.1542/peds.2005-1499. [DOI] [PubMed] [Google Scholar]
- Agnew HWJ, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- Alikassifogglu M, Erginoz E, Gur ET, Baltas Z, Beker B, Arvas A. Factors influencing the duration of exclusive breastfeeding in a group of Turkish women. J Hum Lact. 2001;17:220–226. doi: 10.1177/089033440101700305. [DOI] [PubMed] [Google Scholar]
- Anders T, Emde R, Parmelee A. UCLA Brain Information Sciences. Neurological Network; Los Angeles: 1971. A manual of standardized terminology, techniques, and criteria for scoring of states of sleep and wakefulness in newborns. [Google Scholar]
- Anderson J. Ethology and ecology of sleep in monkeys and apes. In: Rosenblatt JS, Beer C, Busnel MC, Slater PJ, editors. Advances in the study of behavior. Academic Press; Orlando: 1984. pp. 166–216. [Google Scholar]
- Arora S, McJunkin C, Wehrer J, Kuhn P. Major factors influencing breastfeeding rates: mother's perception of father's attitude and milk supply. Pediatrics. 2000;106:e67. doi: 10.1542/peds.106.5.e67. [DOI] [PubMed] [Google Scholar]
- Baddock SA, Galland BC, Taylor BJ, Bolton DP. Sleep arrangements and behavior of bed-sharing families in the home setting. Pediatrics. 2007;119:e200–e207. doi: 10.1542/peds.2006-0744. [DOI] [PubMed] [Google Scholar]
- Ball H. Parent-infant bed-sharing behavior: effects of feeding type and presence of father. Hum Nat. 2006;17:301–318. doi: 10.1007/s12110-006-1011-1. [DOI] [PubMed] [Google Scholar]
- Ball HL. Reasons to bed-share: why parents sleep with their infants. J Reprod Infant Psychol. 2002;20:207–221. [Google Scholar]
- Ball HL. Breastfeeding, bed-sharing, and infant sleep. Birth: Issues Perinat Care. 2003;30:181–188. doi: 10.1046/j.1523-536x.2003.00243.x. [DOI] [PubMed] [Google Scholar]
- Ball HL. Bed-sharing practices of initially breastfed infants in the first 6 months of life. Infant Child Dev. 2007;16:387–401. [Google Scholar]
- Ball HL, Hooker E, Kelly PJ. Where will the baby sleep? Attitudes and practices of new and experienced parents regarding cosleeping with their newborn infants. Am Anthropol. 1999;101:143–151. [Google Scholar]
- Ball HL, Klingaman KP. Breastfeeding and mother-infant sleep proximity: implications for infant care. In: Trevathan W, Smith EO, McKenna JJ, editors. Evolutionary medicine and health: new perspectives. Oxford University Press; New York: 2008. pp. 226–241. [Google Scholar]
- Ball HL, Ward-Platt MP, Heslop E, Leech SJ, Brown KA. Randomised trial of infant sleep location on the postnatal ward. Arch Dis Child. 2006;91:1005–1010. doi: 10.1136/adc.2006.099416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry H, Paxson LM. Infancy and early childhood: cross-cultural codes 2. Ethnology. 1971;10:466–508. [Google Scholar]
- Ben Shaul DM. The composition of the milk of wild animals. Int Zoo Yearb. 1962;4:333–342. [Google Scholar]
- Bener A, Denic S, Galadari S. Longer breast-feeding and protection against childhood leukaemia and lymphomas. Eur J Cancer. 2001;37:234–238. doi: 10.1016/s0959-8049(00)00339-7. [DOI] [PubMed] [Google Scholar]
- Blair P, Fleming P, Bensley D, Smith I, Bacon C, Taylor E, Berry P, Golding J. Where should babies sleep—alone or with parents? Factors influencing the risk of SIDS in the CESDI Study. BMJ. 1999;319:1457–1462. [Google Scholar]
- Blair PS, Ball HL. The prevalence and characteristics associated with parent-infant bed-sharing in England. Arch Dis Child. 2004;89:1106–1110. doi: 10.1136/adc.2003.038067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton Jones N. Comparative aspects of mother-child contact. In: Blurton Jones N, editor. Ethological studies of child behaviour. Cambridge; Cambridge University Press: 1974. pp. 305–328. [Google Scholar]
- Carpenter R, Irgens L, Blair P, Fleming P. Sudden unexplained infant death in 20 regions in Europe: case control study. Lancet. 2004;363:185. doi: 10.1016/s0140-6736(03)15323-8. [DOI] [PubMed] [Google Scholar]
- Chen A, Rogan W. Breastfeeding and the risk of post-neonatal death in the United States. Pediatrics. 2004;113:e435–439. doi: 10.1542/peds.113.5.e435. [DOI] [PubMed] [Google Scholar]
- Christensson K, Cabrera T, Christensson E, Uvnäs-Moberg K, Winberg J. Separation distress call in the human neonate in the absence of maternal body contact. Acta Paediatr. 1995;84:468–473. doi: 10.1111/j.1651-2227.1995.tb13676.x. [DOI] [PubMed] [Google Scholar]
- Czank C, Mitoulas LR, Hartmann PE. Human milk composition- fat; carbohydrates; nitrogen and energy content. In: Hale TW, Hartmann PE, editors. Textbook of human lactation. Hale Publishing; Amarillo, TX: 2007. pp. 49–88. [Google Scholar]
- De Carvalho M, Robertson S, Friedman A, Klaus M. Effect of frequent breast-feeding on early milk production and infant weight gain. Pediatrics. 1983;72:307–311. [PubMed] [Google Scholar]
- Dewey KG, Heinig J, Nommsenrivers LA. Differences in morbidity between breast-fed and formula-fed infants. J Pediatr. 1995;126:696–702. doi: 10.1016/s0022-3476(95)70395-0. [DOI] [PubMed] [Google Scholar]
- Dewey KG, Heinig MJ, Nommsen LA, Lonnerdal B. Maternal versus infant factors related to breast milk intake and residual milk volume: the DARLING Study. Pediatrics. 1991;87:829–837. [PubMed] [Google Scholar]
- Ertem IO, Votto N, Leventhal JM. The timing and predictors of the early termination of breastfeeding. Pediatrics. 2001;107:543–548. doi: 10.1542/peds.107.3.543. [DOI] [PubMed] [Google Scholar]
- Ferber R, Kryger M, editors. Principles and practice in sleep medicine in the child. Saunders; Philadelphia: 1995. [Google Scholar]
- Gettler LT, McKenna JJ. Never sleep with baby? Or keep me close but keep me safe: Eliminating inappropriate “safe infant sleep” rhetoric in the United States. Curr Pediatr Rev. 2010;6:71–77. [Google Scholar]
- Gray SJ. Comparison of effects of breast-feeding practices on birth-spacing in three societies: nomadic Turkana. Gainj, and Quechua. J Biosoc Sci. 1994;26:69–90. doi: 10.1017/s0021932000021076. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Souquet M. Sleep states and related pathologies. In: Guilleminault C, Korobkin R, editors. Advances in perinatal neurology. SP Medical and Scientific Books; New York: 1979. pp. 225–246. [Google Scholar]
- Hart D, Sussman RW. Man the hunted: primates, predators, and human evolution. Westview Press; New York: 2005. [Google Scholar]
- Hornell A, Aarts C, Kylberg E, Hofvander Y, Gebre-Medhin M. Breastfeeding patterns in exclusively breastfed infants: a longitudinal prospective study in Uppsala. Sweden. Acta Paediatr. 1999;88:203–211. doi: 10.1080/08035259950170402. [DOI] [PubMed] [Google Scholar]
- Imong SM, Jackson DA, Wongsawasdii L, Ruckphaophunt S, Tansuhaj A, Chiowanich P, Woolridge MW, Drewett RF, Baum JD, Amatayakul K. Predictors of breast milk intake in rural northern Thailand. J Pediatr Gastroenterol Nutr. 1989;8:359–370. doi: 10.1097/00005176-198904000-00017. [DOI] [PubMed] [Google Scholar]
- Jenness R. Lactational performance of various mammalian species. J Dairy Sci. 1986;69:869–885. doi: 10.3168/jds.S0022-0302(86)80478-7. [DOI] [PubMed] [Google Scholar]
- Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117:e387–395. doi: 10.1542/peds.2005-1417. [DOI] [PubMed] [Google Scholar]
- Konner M, Worthman C. Nursing frequency, gonadal function, and birth spacing among !Kung hunter-gatherers. Science. 1980;207:788–791. doi: 10.1126/science.7352291. [DOI] [PubMed] [Google Scholar]
- Lahr MB, Rosenberg KD, Lapidus JA. Bedsharing and maternal smoking in a population-based survey of new mothers. Pediatrics. 2005;116:E530–E542. doi: 10.1542/peds.2005-0354. [DOI] [PubMed] [Google Scholar]
- Lee-Chiong TL, editor. Sleep: a comprehensive handbook. Wiley-Liss; Hoboken, New Jersey: 2006. [Google Scholar]
- Lozoff B, Brittenham G. Infant care: cache or carry. J Pediatr. 1979;95:478–483. doi: 10.1016/s0022-3476(79)80540-5. [DOI] [PubMed] [Google Scholar]
- Martin RD. The evolution of human reproduction: a primatological perspective. Yearb Phys Anthropol. 2007;50:59–84. doi: 10.1002/ajpa.20734. [DOI] [PubMed] [Google Scholar]
- McCoy RC, Hunt CE, Lesko SM, Vezina R, Corwin MJ, Willinger M, Hoffman HJ, Mitchell AA. Frequency of bed sharing and its relationship to breastfeeding. J Dev Behav Pediatr. 2004;25:141–149. doi: 10.1097/00004703-200406000-00001. [DOI] [PubMed] [Google Scholar]
- McDade TW. Life history theory and the immune system: steps toward a human ecological immunology. Yearb Phys Anthropol. 2003;46:100–125. doi: 10.1002/ajpa.10398. [DOI] [PubMed] [Google Scholar]
- McKenna J, Mosko S, Richard C, Drummond S, Hunt L, Cetel MB, Arpaia J. Experimental studies of infant-parent co-sleeping: mutual physiological and behavioral influences and their relevance to SIDS (Sudden Infant Death Syndrome) Early Hum Dev. 1994;38:187–201. doi: 10.1016/0378-3782(94)90211-9. [DOI] [PubMed] [Google Scholar]
- McKenna J, Thoman E, Anders T, Sadeh A, Schechtman V, Glotzbach S. Infant-parent co-sleeping in evolutionary perspective: implications for understanding infant sleep development and the Sudden Infant Death Syndrome (SIDS) Sleep. 1993;16:263–282. doi: 10.1093/sleep/16.3.263. [DOI] [PubMed] [Google Scholar]
- McKenna JJ, Ball HL, Gettler LT. Mother-infant co-sleeping, breastfeeding and sudden infant death syndrome (SIDS): what biological anthropology has discovered about normal infant sleep and pediatric sleep medicine. Yearb Phys Anthropol. 2007;50:133–161. doi: 10.1002/ajpa.20736. [DOI] [PubMed] [Google Scholar]
- McKenna JJ, Mosko S, Dungy C, McAninch J. Sleep and arousal patterns of co-sleeping human mother infant pairs—a preliminary physiological study with implications for the study of Sudden Infant Death Syndrome (SIDS) Am J Phys Anthropol. 1990;83:331–347. doi: 10.1002/ajpa.1330830307. [DOI] [PubMed] [Google Scholar]
- McKenna JJ, Mosko SS, Richard CA. Bedsharing promotes breastfeeding. Pediatrics. 1997;100:214–219. doi: 10.1542/peds.100.2.214. [DOI] [PubMed] [Google Scholar]
- McKenna JJ, Volpe LE. Sleeping with baby: an internet-based sampling of parental experiences, choices, perceptions, and interpretations in a western industrialized context. Infant Child Dev. 2007;16:359–385. [Google Scholar]
- Mitchell EA, Thompson JMD. Co-sleeping increases the risk of SIDS, but sleeping in the parents' bedroom lowers it. In: Rognum TO, editor. Sudden Infant Death Syndrome: new trends in the nineties. Scandinavian University Press; Oslo, Norway: 1995. pp. 266–269. [Google Scholar]
- Mosko S, Richard C, McKenna J. Maternal sleep and arousals during bedsharing with infants. Sleep. 1994;20:142–150. doi: 10.1093/sleep/20.2.142. [DOI] [PubMed] [Google Scholar]
- Mosko S, Richard C, McKenna J. Infant arousals during mother-infant bed sharing: implications for infant sleep and Sudden Infant Death Syndrome research. Pediatrics. 1997;100:841–849. doi: 10.1542/peds.100.5.841. [DOI] [PubMed] [Google Scholar]
- Panter-Brick C. Lactation, birth spacing and maternal work-loads among two castes in rural Nepal. J Biosoc Sci. 1991;23:137–154. doi: 10.1017/s0021932000019179. [DOI] [PubMed] [Google Scholar]
- Richard CA, Mosko SS. Mother-infant bedsharing is associated with an increase in infant heart rate. Sleep. 2004;27:507–511. doi: 10.1093/sleep/27.3.507. [DOI] [PubMed] [Google Scholar]
- Robson SL, Van Schaik CP, Hawkes K. The derived features of human life history. In: Hawkes K, Paine RR, editors. The evolution of human life history. 1st ed. School of American Research Press; Santa Fe: 2006. pp. 17–45. [Google Scholar]
- Rosenberg K, Trevathan W. Bipedalism and human birth: the obstetrical dilemma revisited. Evol Anthropol. 2005;4:161–168. [Google Scholar]
- Scariati PD, Grummer-Strawn LM, Fein SB. A longitudinal analysis of infant morbidity and the extent of breastfeeding in the United States. Pediatrics. 1997;99:e5. doi: 10.1542/peds.99.6.e5. [DOI] [PubMed] [Google Scholar]
- Scher MS, Steppe DA, Dahl RE, Asthana S, Guthrie RD. Comparison of EEG sleep measures in healthy full term and preterm infants at matched conceptional ages. Sleep. 1992;15:442–448. doi: 10.1093/sleep/15.5.442. [DOI] [PubMed] [Google Scholar]
- Schön RA, Silvén M. Natural parenting: back to basics in infant care. Evol Psychol. 2007;5:102–183. [Google Scholar]
- Scott JA, Landers MCG, Hughes RM, Binns CW. Factors associated with breastfeeding at discharge and duration of breastfeeding. J Paediatr Child Health. 2001;37:254–261. doi: 10.1046/j.1440-1754.2001.00646.x. [DOI] [PubMed] [Google Scholar]
- Sellen DW. Weaning, complementary feeding, and maternal decision making in a rural East African pastoral population. J Hum Lact. 2001;17:233–244. doi: 10.1177/089033440101700307. [DOI] [PubMed] [Google Scholar]
- Super C, Harkness S. The infant's niche in rural Kenya and metropolitan America. In: Adler LC, editor. Issues in cross-cultural research. Academic Press; New York: 1987. pp. 113–162. [Google Scholar]
- Tilden CD, Oftedal OT. Milk composition reflects pattern of maternal care in prosimian primates. Am J Primatol. 1997;41:195–211. doi: 10.1002/(SICI)1098-2345(1997)41:3<195::AID-AJP3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Vennemann M, Bajanowski T, Jorch G, Mitchell E. Does breastfeeding reduce the risk of Sudden Infant Death Syndrome? Pediatrics. 2009;123:e406–410. doi: 10.1542/peds.2008-2145. [DOI] [PubMed] [Google Scholar]
- Vitzthum V. Comparative study of breastfeeding structure and its relation to human reproductive ecology. Yearb Phys Anthropol. 1994;37:307–349. [Google Scholar]
- Vitzthum VJ. Nursing behaviour and its relation to duration of post-partum amenorrhoea in an Andean community. J Biosoc Sci. 1989;21:145–160. doi: 10.1017/s0021932000017843. [DOI] [PubMed] [Google Scholar]
- WHO/PAHO . Guiding principles for complementary feeding of the breastfed child. Pan American Health Organization; Washington, DC: 2003. [Google Scholar]
- Willinger M, Ko CW, Hoffman HJ, Kessler RC, Corwin MJ. Trends in infant bed sharing in the United States, 1993–2000—The National Infant Sleep Position study. Arch Pediatr Adolesc Med. 2003;157:43–49. doi: 10.1001/archpedi.157.1.43. [DOI] [PubMed] [Google Scholar]
- Wood JW, Lai D, Johnson PL, Campbell KL, Maslar IA. Lactation and birth spacing in Highland New Guinea. J Biosoc Sci Suppl. 1985;9:159–173. doi: 10.1017/s0021932000025190. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Jenkins CL, Stallings JF, Lai D. Attenuation of nursing-related ovarian suppression and high fertility in well-nourished, intensively breast-feeding Amele women of Lowland Papua New Guinea. J Biosoc Sci. 1993;25:425–443. doi: 10.1017/s0021932000021817. [DOI] [PubMed] [Google Scholar]
- Young J. Night-time behavior and interactions between mothers and their infants of low risk for SIDS: a longitudinal study of room sharing and bed sharing. University of Bristol; Bristol, UK: 1999. [Google Scholar]


