Abstract
The serum response factor (SRF) transcription factor is essential for murine embryogenesis. Srf–/– embryos stop developing at the onset of gastrulation, lacking detectable mesoderm. This developmental defect may reflect cell-autonomous impairment of Srf–/– embryonic cells in mesoderm formation. Alternatively, it may be caused by a non-cell-autonomous defect superimposed upon inappropriate provision of mesoderm-inducing signals to primitive ectodermal cells. We demonstrate that the ability of Srf–/– embryonic stem (ES) cells to differentiate in vitro into mesodermal cells is indeed impaired. However, this impairment can be modulated by external, cell-independent factors. Retinoic acid, but not dimethylsulfoxide, permitted activation of the mesodermal marker gene T(Bra), which was also activated when SRF was expressed in Srf–/– ES cells. Embryoid bodies from Srf–/– ES cell aggregates also activated mesodermal marker genes, but displayed unusual morphologies and impairment in cavitation. Finally, in nude mice, Srf–/– ES cells readily differentiated into mesodermal cells of Srf–/– genotype, including cartilage, bone or muscle cells. We demonstrate that SRF contributes to mesodermal gene expression of ES cells and that Srf–/– ES cells display a non-cell-autonomous defect in differentiation towards mesoderm.
Keywords: embryoid bodies/embryonic stem cells/mesoderm induction/murine embryogenesis/serum response factor
Introduction
Serum response factor (SRF) (Norman et al., 1988), a transcription factor of the MADS-box family (Sommer et al., 1990; Pellegrini et al., 1995; Shore and Sharrocks, 1995), mediates signal-stimulated transcriptional induction of immediate-early genes (IEGs) (Herschman, 1991; Johansen and Prywes, 1995; Morgan and Curran, 1995; Treisman, 1996). It also contributes to cell-type-specific gene control, e.g. in muscle (Vandromme et al., 1992; Firulli and Olson, 1997; Browning et al., 1998) or neuronal cells (Curran and Morgan, 1995; Ghosh and Greenberg, 1995). SRF binds to serum response elements (SREs) that contain the CArG-box DNA core sequence [CC(A/T)6GG] (Treisman, 1986; Pellegrini et al., 1995). SRF interacts with accessory proteins (Shaw et al., 1989; Treisman, 1994; Firulli and Olson, 1997; Wasylyk et al., 1998) to assemble DNA-bound protein complexes, which may serve as nuclear targets for MAP kinase signaling (Gille et al., 1992; Treisman, 1996).
SRF function in gene control has been studied extensively in cultured cells but less is known about SRF activity at the level of the organism. Initial clues regarding SRF function in multicellular organisms were revealed by the two independent Drosophila melanogaster mutations pruned (Guillemin et al., 1996) and blistered (Montagne et al., 1996), two alleles of the gene encoding Drosophila SRF (Affolter et al., 1994). Pruned affects the formation of the tracheal system by preventing terminal tracheal cells from projecting cytoplasmic outgrowths. In wings of blistered flies, there are ectopic wing veins and impaired cell–cell adhesion between epithelia. Mammalian SRF function has been investigated by generating Srf-null mice by homologous recombination (Arsenian et al., 1998). Srf–/– mouse embryos display an embryonic-lethal phenotype. They fail to form detectable mesodermal cells and do not express the mesoderm-associated marker genes T(Bra), Shh and Bmp2. It is not known whether Srf–/– embryonic cells are generally incapable of differentiating into mesodermal cell derivatives or whether Srf–/– embryos do not provide the signals required for the formation of mesoderm.
Murine embryonic stem (ES) cells can serve as in vitro models for in vivo differentiation. ES cells are totipotent descendants of the inner cell mass (ICM) of blastocysts (Evans and Kaufman, 1981; Martin, 1981). They can be maintained undifferentiated in vitro in the presence of leukemia inhibitory factor (LIF) (Smith et al., 1988; Williams et al., 1988; Shen and Leder, 1992). Differentiation is stimulated when cells, grown in monolayer cultures, are cultivated in the absence of LIF. This can be promoted further by addition of differentiation factors to the culture medium. Application of dimethylsulfoxide (DMSO) leads to a rapid differentiation towards cardiac and skeletal muscle cell types (McBurney et al., 1982). Retinoic acid (RA), instead, leads to differentiation towards neuronal cells (Bain et al., 1995; Gajovic et al., 1998; Guan et al., 1999). RA also accelerates cardiac differentiation of ES cells (Wobus et al., 1997). Aggregates of ES cells form embryoid bodies (EBs), which spontaneously differentiate into a wide range of embryonic cell types (Doetschman et al., 1985; Shen and Leder, 1992; Guan et al., 1999). Such EBs contain extraembryonic visceral and parietal endoderm, which arises from primitive endoderm, and mesodermal cell types such as cardiac muscle and blood, which are primitive ectoderm derivatives. Such EBs have also served as models to study the process of proamniotic cavitation (Coucouvanis and Martin, 1995, 1999).
In order to analyze the effect of SRF on mesodermal differentiation, we generated Srf–/– ES cells and studied their in vitro differentiation potential. We show elsewhere that, in comparison with SRF-containing ES cells, the rates of proliferation of Srf–/– ES cells are not severely altered (G.Schratt, B.Weinhold, A.S.Lundberg, S.Schuck, J.Berger, H.Schwarz, R.A.Weinberg, U.Rüther and A.Nordheim, submitted). However, their colony-forming abilities were significantly reduced. Herein we report that the lack of SRF blocks monolayer ES cell differentiation at early (endoderm-like) stages. However, this block can be overcome under certain conditions in vitro and in vivo, demonstrating that SRF is required in a non-cell-autonomous manner for the development of mesodermal cell lineages.
Results
Embryonic stem cells with mutated Srf loci
In order to investigate at the cellular and molecular level the mesodermal differentiation defect of Srf–/– embryos (Arsenian et al., 1998), we made use of the ES cell in vitro differentiation system. We selected homozygous Srf–/– ES cells from the existing heterozygous Srf–/+ ES cell clone (clone 226) by applying elevated geneticin selection (10 mg/ml). Several Srf–/– ES clones were selected and genotyped by PCR and Southern analysis (e.g. clones 226-77, 226-81 and 226-100).
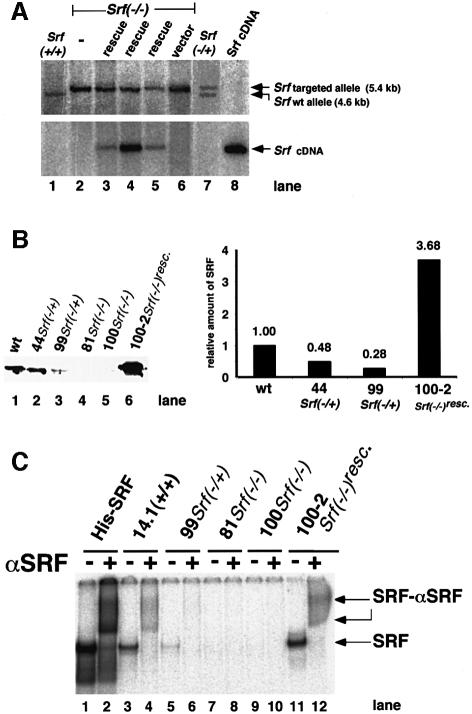
Figure 1A (lane 2) shows the genotyping by Southern blotting of one of the Srf–/– ES clones, 226-100. We confirmed the lack of SRF expression in Srf–/– ES clones by reverse transcription–PCR (RT–PCR) (see, for example, Figure 2A) and by western blot analysis (Figure 1B). Reduced levels of SRF protein were observed in the two heterozygous ES lines 44 and 99. Electrophoretic mobility shift analysis (EMSA) did not detect specific SRF DNA-binding activity in the Srf–/– cells (Figure 1C). The Srf–/– ES cell clones show normal rates of proliferation but their cell morphologies and cell surface activities were altered and their IEG activation response was impaired (G.Schratt, B.Weinhold, A.S.Lundberg, S.Schuck, J.Berger, H.Schwarz, R.A.Weinberg, U.Rüther and A.Nordheim, submitted).
Fig. 1. Genotypes and SRF protein activities of ES cells mutated at the Srf locus. (A) Genotyping by Southern blotting of ES cell lines used in this study. Genomic DNAs from the ES cell lines E14.1 Srf+/+ (lane 1), 226 Srf–/+ (lane 7) and 226-100 Srf–/– (lane 2), 226-100 Srf–/– plus human Srf cDNA expression construct pSGSRF1 (rescue) (lanes 3–5) or 226-100 Srf–/– plus an empty vector (vector) (lane 6) were digested with BglII, electrophoresed and blotted. Filters were successively hybridized with either a murine Srf probe discriminating between wild-type and targeted alleles (top panel) or a human Srf probe detecting the integrated human Srf cDNA (bottom panel). The plasmid pSGSRF1 was used as a hybridization control (Srf cDNA) (lane 8). Positions of the BglII fragments representing the wild-type allele (4.6 kb) or the targeted allele (5.4 kb) are indicated. Lanes 3, 4 and 5 represent the ‘rescue’ lines 226-100-2, 226-100-37 and 226-100-42, respectively. (B) Western analysis of SRF protein in extracts of ES cell lines used in this study. The blot (left) and quantification of the density of the bands on it (right) are shown. No specific SRF western signal was observed in the homozygous Srf–/– ES cell lines. (C) SRF-mediated SRE binding activities in extracts of ES cell lines used in this study. A radiolabeled c-fos SRE oligonucleotide probe was incubated with recombinant His-tagged SRF (lanes 1 and 2) or protein extracts from ES cells of the different genotypes, as indicated. Even-numbered lanes represent extracts with added polyclonal anti-SRF serum. SRF-containing DNA–protein complexes were supershifted by this antiserum. No SRF-containing complexes were detected in extracts from Srf–/– ES cells (lanes 7–10). Lanes 11 and 12 contained 4 µg of protein extract only, whereas 20 µg of protein was used for other cell extracts.
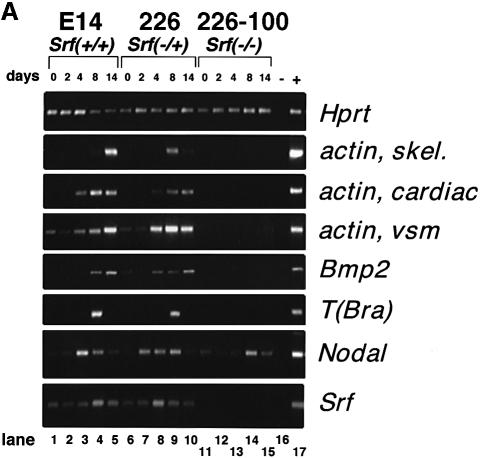
Fig. 2. During in vitro differentiation of monolayers of ES cells, the expression of mesodermal differentiation marker genes is impaired in cells lacking SRF. (A) Expression of differentiation markers in monolayer ES cells of different Srf genotype upon induction of in vitro differentiation by LIF withdrawal (semi-quantitative RT–PCR analysis). The ES lines used were E14.1 (Srf+/+; lanes 1–5), 226 (Srf–/+; lanes 6–10) and 226-100 (Srf–/–; lanes 11–15). RNA expression levels of the genes indicated on the right of the figure were determined by semi-quantitative RT–PCR studies, covering a differentiation period of 14 days, as indicated. Lane 16 is a negative RT–PCR control lane lacking cDNA in the reaction. Lane 17 is a positive control for RT–PCR amplification of each gene. (B) Relative expression levels of differentiation markers in monolayers of ES cells of different Srf genotype upon induction of in vitro differentiation by LIF withdrawal plus simultaneous addition of DMSO (quantitative RT–PCR analysis). ES lines used were 226-99 (Srf–/+; left panel) and 226-100 (Srf–/–; right panel). Expression was followed for the indicated periods of differentiation (days).
SRF expression was rescued in these Srf–/– ES cell clones by the reintroduction of a cytomegalovirus (CMV)-driven expression vector encoding Srf cDNA. Southern blotting of three such Srf–/–rescue ES cell clones (clones 226-100-2, 226-100-37 and 226-100-42) is shown in Figure 1A. These clones have regained SRE-specific SRF DNA-binding activity (Figure 1C) and the ability to activate SRE-regulated target genes (G.Schratt, B.Weinhold, A.S.Lundberg, S.Schuck, J.Berger, H.Schwarz, R.A.Weinberg, U.Rüther and A.Nordheim, submitted). We note that all our Srf–/–rescue ES cell clones tested expressed higher levels of SRF protein than wild-type clones (Figure 1B and C).
In vitro differentiation potential of Srf–/– ES cells is severely impaired
We exploited the ability of ES cells, cultivated as monolayers, to initiate an in vitro differentiation program upon withdrawal of LIF (Shen and Leder, 1992; Keller, 1995; Guan et al., 1999). Using Srf+/+, Srf–/+ and Srf–/– ES clones, we first followed their differentiation by morphological criteria (data not shown). Endoderm-like cells formed rapidly in wild-type or Srf heterozygous ES cells cultivated in the absence of LIF. Depending on the differentiation protocol, fibroblast-like cell types could be observed after 3–6 days. Subsequently, several cell types of mesodermal origin were discernable, e.g. beating cardiomyocytes and blood islands. In contrast, Srf–/– ES cells grown in monolayer culture only formed an endoderm-like cell type, as judged by morphological criteria. Fibroblast-like cells or cells of mesodermal origin, e.g. muscle cells, were never observed, irrespective of the differentiation protocol used (data not shown).
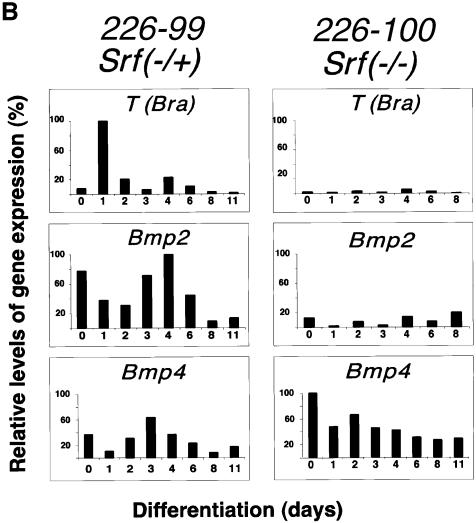
We also followed RNA expression levels for various differentiation marker genes using semi-quantitative RT–PCR (Figure 2A; differentiation assessed every other day for 14 days) or quantitative RT–PCR (Figure 2B; differentiation assessed every day for 6 days). Differentiation was induced either by LIF withdrawal alone (Figure 2A) or by LIF withdrawal plus addition of DMSO (Figure 2B). Expression levels of Hprt and Srf served as internal controls. Bmp2, Bmp4, Nodal, T(Bra) and the muscle actin genes (α-actins) were expressed at different times of differentiation in SRF-containing cells (Figure 2A, lanes 1–10; Figure 2B). Srf RNA levels stayed fairly constant during the course of differentiation. As expected for these cells, LIF withdrawal plus addition of DMSO led to an earlier induction of the mesodermal markers T(Bra) and Bmp2 than did LIF withdrawal alone (compare Figure 2B, first and second panels from top, with Figure 2A, lanes 1–10). None of these genes, however, was activated in Srf–/– ES cells, except for Nodal (Figure 2A, lanes 11–15) and Bmp4 (Figure 2B, third panel from top).
Srf–/–rescue cells, ectopically over-expressing SRF, induce expression of mesodermal marker genes
Further substantiation of the regulatory influence of SRF on the expression of differentiation marker genes can be derived from the Srf–/–rescue ES clones that express SRF protein ectopically (Figure 1B). These rescued cells exhibited spontaneous differentiation behavior when grown as monolayers in vitro (not shown) and, at variable levels, displayed elevated RNA expression of the differentiation markers investigated (Figure 3A). This indicates that Srf–/– ES cells have not lost irreversibly their ability to activate mesodermal marker genes spontaneously and that overexpression of SRF alone can poise ES cells to activate mesodermal gene expression programs.

Fig. 3. Monolayers of Srf–/–ES cells activate mesodermal marker genes when ectopically expressing SRF or when treated with RA. (A) Gene expression patterns in ES cells of different Srf genotype, including Srf ‘rescued’ cells. ES lines used were 226-100-2 (Srf–/–rescue; lane 1), 226-100-37 (Srf–/–rescue; lane 2), 226-100-42 (Srf–/–rescue; lane 3), 226-100 (Srf–/–; lane 4) and 226 (Srf–/+; lanes 5 and 6). LIF removal was only done with the cells investigated in lane 6. Analysis was performed as described in the legend to Figure 2A. Lane 7 is a negative RT–PCR control lacking cDNA in the reaction. Of the three Srf–/–rescue ES lines, 226-100-2 expressed the highest levels of SRF (approximately four times the endogenous levels). (B) Expression of differentiation markers in monolayers of ES cells of different Srf genotype upon induction of in vitro differentiation by LIF withdrawal plus simultaneous addition of RA. ES lines used were 226 (Srf–/+; lanes 1–7) and 226-81 (Srf–/–; lanes 8–14). Analysis was performed as described in the legend to Figure 2A.
RA induces expression of mesodermal marker genes in Srf–/– cells grown as monolayers
Similar to the effects of DMSO treatment on gene expression in heterozygous Srf–/+ ES cells grown in monolayer, RA treatment combined with LIF withdrawal induced expression of the mesodermal lineage differentiation marker genes more rapidly than did LIF withdrawal alone (compare Figure 2A, lanes 6–10, with Figure 2B, left panel and Figure 3B, lanes 1–7). Surprisingly, RA was also able to induce the mesodermal T(Bra) gene and the Bmp2 gene, even in Srf–/– ES cells grown in monolayer, albeit in a delayed fashion. A slight induction of the skeletal actin gene was also seen in Srf–/– ES cells (Figure 3B, lanes 8–14). These effects of RA on gene expression profiles of Srf–/– ES clones confirmed that these cells had not completely lost the ability to induce expression of mesodermal genes, but rather exhibited differential gene activation profiles that depended on the nature of the differentiation stimuli.
EBs developing from Srf–/– ES cell aggregates display unusual morphologies
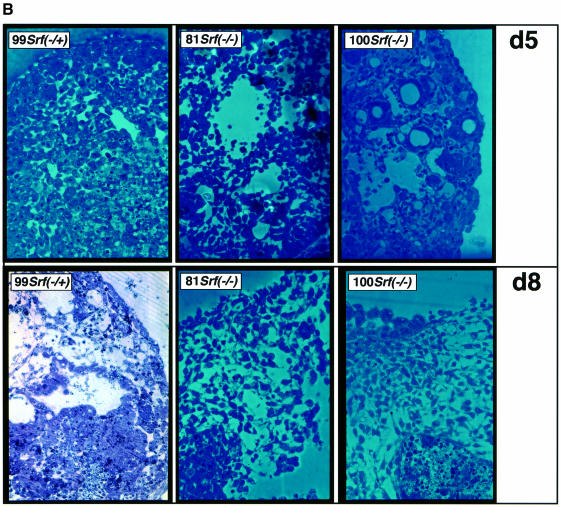
When cultured as cell aggregates, ES cells can differentiate into EBs containing a wide range of embryonic cell types. ‘Hanging drop’ cultivation of ES cells at high density, under conditions of LIF withdrawal, initiates EB differentiation efficiently. We first investigated the influence of the absence of SRF on the morphology of EBs developing over 8 days. On day 2 of the differentiation period, the EBs that had developed from Srf–/+ and Srf–/– ES cells were of comparable size. From day 2 onwards, increasing cell death and cell detachment were observed in Srf–/– EBs (not shown). They were ∼40–70% of the size of corresponding Srf–/+ or Srf+/+ EBs after 6 days of differentiation, with some variability in size distribution. Scanning electron microscopy (SEM) of EBs formed from SRF-containing cells revealed smooth homogeneous surfaces, indicating tight cell–cell interactions (Figure 4A, left). This outer cell layer represents visceral endoderm cells (see below) (Shen and Leder, 1992). In contrast, Srf–/– ES cells formed EBs of irregular structure and heterogeneous surface appearance (Figure 4, middle and right). Such Srf–/– EBs displayed two major types of surface structure: first, regions of rough surface, composed of individual cells each protruding away from neighboring cells and, secondly, smooth patches emerging as large cystic protrusions made up of cells tightly attached to each other. These smooth, cystic protrusions resembled the homogeneous appearance of EBs formed by SRF-containing ES cells. Such cyst-like protruding regions were found with both Srf–/– ES cell clones analyzed, although such regions varied with regard to the degree of smoothness. From this analysis it became apparent that the lack of SRF prevented ES cells from forming EBs with homogeneous and smooth surfaces indicative of tightly interacting cells. The irregular heterogeneous surface appearance of Srf–/– EBs was already discernable on day 5 of EB differentiation (not shown).
Fig. 4. Differentiation of Srf–/– ES cell aggregates leading to the formation of EBs. (A) Scanning electron microscopic depiction of EB morphologies after differentiation in vitro for 8 days. ES lines used were 99 (Srf–/+; left), 81 (Srf–/–; middle) and 100 (Srf–/–; right). The scale bars represent 176 µm (left), 300 µm (middle) and 231 µm (right). Note that fixation does not consistently preserve EB size. (B) Immunofluorescent staining of EBs (after 8 days’ differentiation) for the endodermal surface expression marker laminin using anti-laminin antisera. ES lines used were 99 (Srf–/+; left), 81 (Srf–/–; middle) and 100 (Srf–/–; right). The photographs depict regions of the EBs. Magnification: 400×.
Indirect immunofluorescence staining of day 8 EBs for the presence of the endodermal cell surface marker laminin demonstrated that SRF-containing EBs had differentiated towards the formation of an outer layer of endoderm (Figure 4B), in all likelihood visceral endoderm. In Srf–/– EBs this degree of differentiation was not fully realized, except for the segments representing cyst-like protrusions of smooth appearance. This suggested that the rough surface and lack of tight cell–cell contacts reflected the regional absence of visceral endoderm. A non-uniform differentiation potential within different regions of individual Srf–/– EBs is therefore indicated.
Differentiating EBs develop internal cavities, a process that mirrors proamniotic cavitation of postimplantation mouse embryos (Coucouvanis and Martin, 1995). Therefore, we next sectioned our differentiating EBs and stained them with toluidine in order to investigate whether internal EB morphology was changed as a function of SRF activity. On day 8 of EB differentiation, SRF-containing EBs had not only formed a continuous endodermal layer at the outer rim (see above; Figure 5A, left; arrow) but were also in the process of undergoing internal cavitation, with a layer of columnal ectodermal cells lining the emerging cavity (Figure 5A, left; arrowhead) (Coucouvanis and Martin, 1995). Srf–/– EBs, in contrast, had regional endodermal cells at the periphery in small segments only (Figure 5A, middle and right) and contained more loosely packed cells and hollow segments. None of these holes appeared to be lined with columnar epithelial cells. At higher magnification, the difference in cell packing was already clearly visible on day 5 of differentiation (Figure 5B, top row), accompanied by vacuoles present in SRF-deficient EBs. The difference in cell packing can be seen more clearly in the higher-magnification image of day 8 EBs (Figure 5B, bottom row).
Fig. 5. Arrangements of cells and cavitation within EBs derived from ES cells of different Srf genotypes. EBs of different Srf genotype and at two stages of differentiation (5 or 8 days) were sectioned and stained with toluidine to visualize individual cells. (A) Full views of day 8 EBs from ES cells heterozygous or homozygous for the deleted Srf allele. Loose cell packing inside Srf–/– EBs is apparent. The ES cells used and their genotypes are indicated. Arrows: visceral endoderm cells; arrowhead: columnar ectodermal cells lining part of a cavity inside the Srf–/+ EB. Photographs were taken at 200× magnification. (B) Higher magnification representations of sectioned EBs taken after 5 days (d5; top row) or 8 days (d8; bottom row) of differentiation. Genotypes of ES cells used for EB differentiation were as indicated. The photographs depict regions of the EBs. The bottom row pictures represent sub-regions of the photographs shown in (A). Photographs were taken at 400× magnification.
In summary, at the morphological level, both peripheral and internal cells of EBs displayed altered cell–cell interaction characteristics when formed from ES cells lacking SRF. However, regarding this defect in cell attachment, significant segmental variability was observed within individual Srf–/– EBs, suggesting that this impairment can be overcome regionally. Indeed, peripheral regions of smooth appearance also displayed significant expression levels of the visceral endodermal differentiation marker laminin, which was not expressed in the rough EB segments. This suggests that the cellular environment within EBs may provide a regional differentiation stimulus that helps to overcome locally the differentiation impairment of Srf–/– ES cells.
EBs from Srf–/– ES cells express mesodermal marker genes
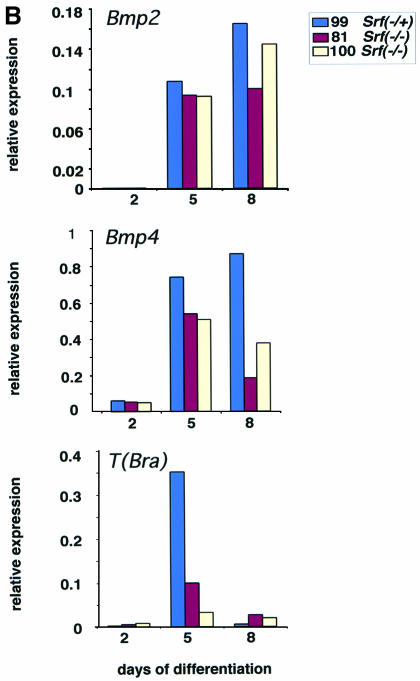
Since both the morphological analysis and the laminin expression data had suggested that Srf–/– EBs were able to undergo some degree of differentiation, we measured the levels of expression of other differentiation marker genes in EBs cultivated for ≤8 days in the absence of LIF. Semi-quantitative RT–PCR measurements revealed expression not only of the endodermal marker genes encoding keratin 18 and α-fetoprotein but also of the mesodermal markers T(Bra), Bmp2, Bmp4 and vimentin (Figure 6A). These findings are corroborated by quantitative RT–PCR measurements of the Bmp2, Bmp4 and T(Bra) genes (Figure 6B). This clearly argues for mesodermal differentiation having taken place to some degree inside Srf–/– EBs.
Fig. 6. Expression of differentiation marker genes in EBs generated from ES cells of different Srf genotype. (A) Semiquantitative RT–PCR. EBs, derived from the indicated ES lines (99 Srf–/+, 81 Srf–/– and 100 Srf–/–) were differentiated for 2 days (lanes 2, 5 and 8), 5 days (lanes 3, 6 and 9) and 8 days (lanes 4, 7 and 10). Lane 1 provides size markers and subjected to RT–PCR analysis. The marker genes investigated were: keratin 18 (a marker of endoderm and trophectoderm), Bmp2 (endoderm, mesoderm), Bmp4 (ectoderm, mesoderm), T(Bra) (mesoderm), vimentin (mesoderm, parietal endoderm) and α-fetoprotein (visceral endoderm). (B) Quantitative RT–PCR. The indicated marker genes, Bmp2, Bmp4 and T(Bra), were analyzed in EBs identical to those described in (A).
Srf–/– ES cells retain the ability to develop into mesodermal cell types in vivo
As demonstrated above (Figure 3B), treatment of monolayer Srf–/– ES cells with RA permitted induction of mesoderm-specific genes like T(Bra), thereby indicating that mesodermal genes could be activated by some but not all stimuli in Srf–/– ES cells cultured in vitro. This indicated that the lack of SRF per se does not prevent mesodermal gene activation in general. EBs derived from Srf–/– ES cells also displayed patchy expression of mesoderm-specific genes (Figure 6). We sought to obtain further independent evidence for the mesodermal differentiation potential of ES cells lacking SRF by examining teratomas formed upon injection of Srf–/– cells under the skin of nude mice. Here we observed the formation of teratocarcinomas that contained various mesodermal lineage tissues (Figure 7). Heterogeneously distributed muscle cells and connective tissue could be identified clearly (Figure 7A), as well as bone marrow with hematopoietic cells (including megakaryocytes) and cartilage with chondrocytes (Figure 7B). Mature and immature cartilage accounted for ∼60% of the tumor masses, whereas muscle tissue varied between 5 and 20%.
Fig. 7. Srf–/– ES cells can give rise to teratomas containing mesodermal cell types. After subcutaneous injection of Srf–/– ES cells into nude mice, the developing teratomas were investigated for the presence of different types of tumor tissue. Several tissues of mesodermal origin, e.g. connective tissue and muscle (A) or cartilage and bone (B) could be identified.
Discussion
During murine early embryonic development, SRF is essential for formation of mesoderm, the third embryonic germ layer (Arsenian et al., 1998). The precise molecular mechanism by which SRF enables mesoderm induction at the onset of gastrulation is not understood. Therefore, we generated Srf–/– ES cells and used the ES cell in vitro differentiation system (Keller, 1995; Guan et al., 1999) to characterize the mesoderm-forming potential of such cells lacking SRF. SRF-deficient ES cells, when induced to differentiate by withdrawal of LIF alone, or together with DMSO treatment, failed to yield mesodermal lineage cells and failed to induce the expression of mesodermal marker genes. However, expression of mesodermal lineage genes could be restored by overexpression of SRF in the SRF-deficient ES cells. In the latter situation, overexpressed SRF may have stimulated the activation of one or several mesodermal switch genes (e.g. MyoD) that, being high up in the regulatory hierarchy, might help differentiation events to be initiated. Together, these data provide strong independent evidence for the requirement for SRF in mesodermal differentiation.
In the present study, we analyzed the mesodermal differentiation potential of SRF-deficient ES cells in three distinctly different cellular environments generated by (i) in vitro culturing of ES cells as cell monolayers; (ii) in vitro culturing of ES cells as cell aggregates, thereby permitting formation of EBs; and (iii) subcutaneous injection of ES cells into nude mice, thereby allowing the in vivo development of ES cell-derived teratocarcinomas.
Morphology of EBs derived from Srf–/– ES cells is severely affected
The morphology of colonies of Srf–/– ES cells cultivated in vitro indicates an impaired ability to establish proper cell–cell interactions (G.Schratt, B.Weinhold, A.S.Lundberg, S.Schuck, J.Berger, H.Schwarz, R.A. Weinberg, U.Rüther and A.Nordheim, submitted). We speculate that impaired interactions of Srf–/– ES cells with each other, and with certain substrata or ECM components, may be caused by changes in the architecture of cytoskeletal substructures in SRF-deficient cells (G.Schratt, U.Philippar, J.Berger, H.Schwarz, O.Heidenreich and A.Nordheim, in preparation.).
EBs derived from differentiating Srf–/– ES cells also displayed reduced cell–cell interaction. This was first noticeable by the extent of free, detached cells found in the vicinity of differentiating EBs. This probably contributed significantly to the apparent, albeit somewhat variable, size reduction of Srf–/– EBs in comparison with their wild-type or heterozygous counterparts. Morphologically, reduced cell–cell affinity was also revealed by SEM analysis, which showed that EBs derived from differentiating Srf–/– ES cells had surface morphologies indicative of impaired interactions of the cells forming the outer layer of these EBs. This impairment was noticeable as a ‘rough’ appearance of the Srf–/– EB surfaces when observed by SEM. Sectioning of such EBs revealed that the cells in the interiors of Srf–/– EBs also established fewer cell–cell contacts. Interestingly, with continued cultivation, Srf–/– EBs did not develop internal cavities with a distinct lining of columnar ectodermal cells (Coucouvanis and Martin, 1995). Should this defect in EB cavitation reflect a potential, hitherto unrecognized defect in proamniotic cavitation of Srf–/– embryos, then this could be a patterning defect that might contribute to the defective mesoderm formation in SRF-deficient embryos.
There was a distinct heterogeneity in surface morphology in Srf–/– EBs. Cyst-like regions protruded from the bodies of EBs with a ‘smooth’ surface, indicative of unimpaired cell–cell interaction. In contrast to the remainder of the Srf–/– EBs, these cystic segments contained cells on their outer surface that expressed differentiation markers indicative of endodermal cell types, as did the wild-type or Srf heterozygous EBs. Therefore, by both morphological and molecular criteria, Srf–/– ES cells display a distinct differentiation defect that can be overcome locally in the context of the EB cellular environment.
Expression of mesodermal marker genes may be regulated by at least two mechanisms
We describe here that the defect in mesoderm formation displayed by Srf–/– embryos (Arsenian et al., 1998) is reflected by a differentiation impairment of Srf–/– ES cells that were cultured in vitro as monolayer cells. In these cells, formation of mesodermal cell derivatives and mesoderm-specific gene induction of T(Bra) and α-actin genes was completely prevented under conditions of LIF withdrawal and addition of DMSO. In contrast, in vitro differentiation of ES cells by the removal of LIF and simultaneous addition of RA led to expression of Bmp2 and T(Bra). This demonstrates that, under monolayer culturing conditions of Srf–/– ES cells, RA-mediated activation of at least some mesodermal marker genes does not require SRF. It further supports the notion that both SRF-dependent and SRF-independent mechanisms of mesodermal lineage gene expression may exist in the ES cell.
Impairment of the ability of Srf–/– ES cells to differentiate into mesodermal cell types can be overcome in a non-cell-autonomous fashion
Several lines of evidence point to the non-cell-autonomous nature of the requirements for mesodermal differentiation. First, the different sensitivities of Srf–/– ES cells to induction of mesodermal differentiation by distinct external stimuli indicates that the mesodermal differentiation defect of these cells is dependent upon conditions of extracellular stimulation. Even in the absence of SRF, ES cells can begin to display markers of mesodermal differentiation. Secondly, the defect in mesodermal differentiation is also partially overcome when Srf–/– ES cells are cultivated in vitro as cell aggregates permitting differentiation as EBs. Under these conditions of cell aggregation, we infer that the cellular environment created is different from that in ES cells cultured as a monolayer. Finally, the non-cell-autonomous nature of the mesodermal differentiation defect of Srf–/– ES cells is fully revealed upon their subcutaneous injection into nude mice, which leads to the formation of teratocarcinomas that contain mesodermal cell types, including cartilage, bone and connective tissue, as well as hematopoetic cells.
Collectively, these data raise the possibility that the early embryonic lethality of Srf–/– embryos may be due to a lack of SRF function in a non-cell-autonomous manner, rather than to a defect in the induction of the mesoderm program within a given cell. In this model the defect may be due to some prior patterning defect resulting in specific mesoderm-inducing signals not being presented appropriately to the receiving primitive ectodermal cells of the embryo. Alternatively, it remains possible that SRF-deficient mesoderm precursor cells are unable to respond adequately to these signals in the specific cellular environment of the Srf–/– embryos. Further work will distinguish between these two possibilities. Toward that aim, the use of our Srf–/– ES cells will be of high value for future use in blastocyst injection experiments leading to the generation of chimeric embryos.
Materials and methods
ES cells
The following ES cells were used in this study: E14.1 Srf+/+, 44 Srf–/+, 226 Srf–/+, 226-77 Srf–/–, 226-81 Srf–/–, 226-100 Srf–/–, 226-100-2 Srf–/–rescue, 226-100-37 Srf–/–rescue, 226-100-42 Srf–/–rescue.
ES cell culture conditions
General growth conditions. ES cells were kept without feeder cells on gelatin-coated dishes in complete medium [Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 g/l glucose and 3.7 g/l NaHCO3, supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM mercaptoethanol, 15% fetal calf serum (FCS) and 1000 U/ml LIF]. Cultivation was at 37°C in a humified atmosphere at 7.5% CO2 and cultures were split every 2–3 days.
Selection of Srf–/– ES cells. Srf–/– ES cells were derived from the Srf–/+ clone (clone 226) that had been used for germline transmission (Arsenian et al., 1998). Srf–/+ cells (3 × 106) were seeded at a density of 3 × 105 per 10 cm dish in complete medium. Two days after seeding, selection was applied by addition of 10 mg/ml G418. Medium was replaced every 2 days. After 21 days, 100 surviving single cell-derived ES cell colonies were picked and propagated. Five of these clones were genotyped by PCR and confirmed by Southern blotting to be Srf–/–. Three clones, named 226-77, 226-81 and 226-100, were used for further analysis. One clone, 226-99 Srf–/+, survived the selection protocol, yet was found to be heterozygous for the mutant Srf allele. This clone may have contained an additional copy of the neomycin resistance gene.
In vitro differentiation conditions. For differentiation experiments, cells were seeded in monolayers on gelatin-coated dishes and kept overnight in complete medium. The first day after seeding was defined as day 0 of differentiation. The medium was then replaced with complete medium lacking LIF or complete medium without LIF, but containing either 0.8% DMSO or 5 × 10–7 M all-trans RA, respectively. Medium was replaced daily and cells were harvested at the indicated time points for RT–PCR analysis. The viability was determined by Trypan blue staining. The viability of detached cells was <5%.
Differentiation of ES cell aggregates to EBs. For differentiation, ES cells were cultivated in hanging drops as described (Rohwedel et al., 1995). Briefly, 600 cells in 20 µl of differentiation medium [Iscove’s Modified Dulbecco’s Medium (Gibco) supplemented with 20% FCS, 2 mM l-glutamine, 100 U/ml penicillin, 100 g/ml streptomycin, 450 µM monothioglycerol and non-essential amino acids] were placed on the lid of bacterial Petri dishes and cultivated as hanging drops for 2 days. EBs were then transferred into 6 cm bacterial dishes supplemented with 4 ml of differentiation medium and cultivation was continued for another 6 days.
Genotyping of ES cells
Cells were harvested and incubated at 56°C overnight in lysis buffer [50 mM Tris–HCl pH 8.0, 100 mM EDTA, 100 mM NaCl, 1% sodium dodecyl sulfate (SDS)] containing 0.7 mg/ml proteinase K. After addition of 5 M NaCl followed by centrifugation, the pellet was discarded and the DNA was precipitated from the supernatant by isopropanol treatment.
For PCR genotyping, the primers A (AGTTCATCGACAACAAGCTGCGG), B (GAGATTTCCACAGAAAGCAACGG) and C (TTGGGAAGACAATAGCAGGCATG) were used for amplification with 35 cycles of: 94°C for 20 s, 65°C for 30 s and 72°C for 45 s, thereby amplifying a 360 bp fragment from the targeted allele and a 610 bp fragment from the wild-type allele.
For Southern blotting, DNA was digested with BglII, electrophoresed, blotted on to nylon membrane and hybridized with a [32P]dCTP-labeled murine Srf fragment, detecting a 4.6 kb wild-type fragment and a 5.4 kb targeted fragment.
Electrophoretic mobility shift assays (EMSAs)
Preparation of whole cell extracts and EMSA studies were performed as described previously (Heidenreich et al., 1999). A 32P-labeled DNA fragment containing the c-fos SRE was used as DNA-binding probe together with ≤50% (v/v) whole cell extract prepared from ES cells. To detect specific binding of SRF to the probe, anti-SRF antiserum (rabbit IgG, Santa Cruz) was included for super-shift analyses.
Western blotting
Preparation of cell extracts was as described for EMSA. Ten to twenty micrograms of protein were resolved by SDS–PAGE on 10% polyacrylamide gels, followed by transfer to polyvinylidene fluoride membrane. The membranes were blocked with 5% non-dry fat milk powder and subsequently probed with polyclonal SRF antibody (1:200 dilution; Santa Cruz) for 1 h and a horseradish peroxidase (HRP)-conjugated goat-anti mouse IgG (1:5000; Amersham) for 30 min. After extensive washing in TST buffer, enzymatic reaction was performed with ECLplus Detection kit (Amersham) according to the manufacturer’s recommendations and the blot was exposed to X-ray film (Kodak). Quantification was done with Image Gauge V3.0 software.
Semi-quantitative RT–PCR
One microgram of total RNA was used for reverse transcription using an Expand reverse transcriptase kit (Boehringer Mannheim) according to the supplier’s instructions. One-twentieth of this reaction was used for PCR amplification with specific primers. The primers and PCR annealing conditions used were as follows Srf: F, AGTTCATCGACAACAAGCTGCGG; R, TACTCTTGAGCACAGTCCCGTTGG; annealing temperature 60°C, generating a 600 bp fragment; T(Bra): F, TGCTGCCTGTGAGTCATAAC; R, TCCAGGTGCTATATATTGCC; annealing temperature 60°C, generating a 950 bp fragment; Goosecoid: F, GCACCATCTTCACCGATGAG; R, AGGAGGATCGCTTCTGTCGT; annealing temperature 55°C, generating a 180 bp fragment; Shh: F, GCCTACAAGCAGTTTATTCCCAAC; R, CAGTGGATGTGAGCTTTGGATTC; annealing temperature 60°C, generating a 400 bp fragment; Bmp2: F, GTTTGTGTTTGGCTTGACGC; R, AGACGTCCTCAGCGAATTTG; annealing temperature 50°C, generating a 720 bp fragment; Bmp4: F, TGTGAGGAGTTTCCATCACG; R, TTATTCTTCTTCCTGGACCG; annealing temperature 50°C, generating a 500 bp fragment; Nodal: F, ACCGTCATTCCTTCTCAGGTCAC; R, GTATCGTTTCAGCAGGCTTCTGG; annealing temperature, 65°C, generating a 500 bp fragment; Hprt: F, CACAGGACTAGAACACCTGC; R, GCTGGTGAAAAGGACCTCT; annealing temperature 60°C, generating a 245 bp fragment.
Quantitative RT–PCR
Preparation of total RNA [using an RNeasy kit (Qiagen)] and first-strand cDNA synthesis (Superscript II; Gibco) were done according to the manufacturers’ protocols. One microgram of total RNA treated with DNase I was used for reverse transcription. One-twentieth of the reverse transcription reaction was included in a 25 µl PCR reaction. For quantitative analysis, SYBR Green PCR technology (Perkin Elmer) was used. Real-time detection of the PCR product was monitored by measuring the increase in fluorescence caused by the binding of SYBR Green to double-stranded DNA with an ABI PRISM 7700 Sequence Detector. For relative quantification, the threshold cycle (Ct), i.e. the cycle at which a statistically significant increase in fluorescence occurs, was derived from the resulting PCR profiles of each sample. Ct is a measure of the amount of template present in the starting reaction. To correct for different amounts of total cDNA in the starting reaction, Ct values for an endogenous control (Hprt) were subtracted from those of the corresponding sample, giving the difference in Ct (ΔCt). The relative quantification value is expressed as 2–ΔCt.
Primers used were as follows: Bmp2: F, CCGCTCCACAAACGAGAAAA; R, TTGCAGCTGGACTTGAGGC; Bmp4: F, GCCAAACGTAGTCCCAAGCAT; R, AATGGCGACGGCAGTTCTT; Hprt: F, GCCTAAGATGAGCGCAAGTTG; R, TACTAGGCAGATGGCCACAGG; T(Bra): F, GCTCCCCTGCACATTACA; R, GAACCAGAAGACGAGGACGTG.
Indirect immunofluorescence
Day 8 EBs were fixed in 4% formaldehyde and permeabilized in 0.2% Triton X-100 in phosphate-buffered saline (PBS). Non-specific binding was blocked by incubating the EBs for 1 h in 1% bovine serum albumin in PBS at 37°C before staining with rabbit anti-laminin (1:30 in PBS; Sigma) for 1 h at 37°C. Secondary antibody was a fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antibody. EBs were washed four times with PBS and once in water; they were then mounted in Moviol. Image acquisition was done with a laser scanning microscope (LSM 510; Zeiss).
Sectioning of EBs and staining with toluidine
EBs were embedded in 2% agarose, dehydrated through a graded series of ethanol and embedded in Epon. Toluidine blue-stained Epon sections of 0.5 or 3 µm thickness were prepared for light microscopy.
Scanning electron microscopy
EBs were fixed with 1.6% glutaraldehyde in 20 mM HEPES, 120 mM NaCl for 5 min at room temperature and for 1 h at 4°C, postfixed with 1% osmium tetroxide in PBS for 1 h on ice, washed with H2O and treated with 1% aqueous uranyl acetate for 1 h at 4°C. For SEM, EBs were dehydrated in ethanol and critical-point-dried from CO2. The samples were sputter-coated with 8 nm gold–palladium and examined at 20 kV accelerating voltage in a Hitachi S-800 field emission scanning electron microscope.
Induction of teratomas and tumor histology
Four-week-old female scid mice were injected subcutanously with 0.5 × 106 to 1 × 107 Srf–/+ ES cells in a total volume of 100 µl of PBS (n = 3) or with 0.7 × 107 to 6 × 107 Srf–/– ES cells (clone 226-100) in 100 µl of PBS (n = 14), respectively. Injection of Srf–/– ES cells led to the formation of teratomas in four mice, of which three were analyzed in detail. Teratomas were dissected and fixed for 2 days in 4% paraformaldehyde. They were subsequently dehydrated, embedded in paraffin, sectioned at 0.3 µm and stained with hematoxylin and eosin.
Acknowledgments
Acknowledgements
We thank Marianne Petry for expert technical assistance. We gratefully acknowledge Dr A. Wobus for teaching G.S. the culturing of ES cells and EBs. We appreciate the useful comments on this manuscript provided by Bill Lundberg and Olaf Heidenreich. This work was supported by grants of the Volkswagen-Stiftung (I/74039 to U.R. and I/74043 to A.N.) and the DFG [NO 120/10-1 and SFB 446 (B7) to A.N.].
References
- Affolter M., Montagne,J., Walldorf,U., Groppe,J., Kloter,U., La Rosa,M. and Gehring,W.J. (1994) The Drosophila SRF homolog is expressed in a subset of tracheal cells and maps within a genomic region required for tracheal development. Development, 120, 743–753. [DOI] [PubMed] [Google Scholar]
- Arsenian S., Weinhold,B., Oelgeschläger,M., Rüther,U. and Nordheim,A. (1998) Serum response factor is essential for mesoderm formation during mouse embryogenesis. EMBO J., 17, 6289–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G., Kitchens,D., Yao,M., Huettner,J.E. and Gottlieb,D.I. (1995) Embryonic stem cells express neuronal properties in vitro. Dev. Biol., 168, 342–357. [DOI] [PubMed] [Google Scholar]
- Browning C.L., Culberson,D.E., Aragon,I.V., Fillmore,R.A., Croissant,J.D., Schwartz,R.J. and Zimmer,W.E. (1998) The developmentally regulated expression of serum response factor plays a key role in the control of smooth muscle-specific genes. Dev. Biol., 194, 18–37. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E. and Martin,G.R. (1995) Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell, 83, 279–287. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E. and Martin,G.R. (1999) BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development, 126, 535–546. [DOI] [PubMed] [Google Scholar]
- Curran T. and Morgan,J.I. (1995) Fos: an immediate-early transcription factor in neurons. J. Neurobiol., 26, 403–412. [DOI] [PubMed] [Google Scholar]
- Doetschman T.C., Eistetter,H., Katz,M., Schmidt,W. and Kemler,R. (1985) The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J. Embryol. Exp. Morphol., 87, 27–45. [PubMed] [Google Scholar]
- Evans M.J. and Kaufman,M.H. (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature, 292, 154–156. [DOI] [PubMed] [Google Scholar]
- Firulli A.B. and Olson,E.N. (1997) Modular regulation of muscle gene transcription: a mechanism for muscle cell diversity. Trends Genet., 13, 364–369. [DOI] [PubMed] [Google Scholar]
- Gajovic S., Chowdhury,K. and Gruss,P. (1998) Genes expressed after retinoic acid-mediated differentiation of embryoid bodies are likely to be expressed during embryo development. Exp. Cell Res., 242, 138–143. [DOI] [PubMed] [Google Scholar]
- Ghosh A. and Greenberg,M.E. (1995) Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science, 268, 239–247. [DOI] [PubMed] [Google Scholar]
- Gille H., Sharrocks,A.D. and Shaw,P.E. (1992) Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature, 358, 414–417. [DOI] [PubMed] [Google Scholar]
- Guan K., Rohwedel,J. and Wobus,A. (1999) Embronic stem cell differentiation models: cardiogenesis, myogenesis, neurogenesis, epithelial and vascular smooth muscle cell differentiation in vitro. Cytotechnology, 30, 211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin K., Groppe,J., Ducker,K., Treisman,R., Hafen,E., Affolter,M. and Krasnow,M.A. (1996) The pruned gene encodes the Drosophila serum response factor and regulates cytoplasmic outgrowth during terminal branching of the tracheal system. Development, 122, 1353–1362. [DOI] [PubMed] [Google Scholar]
- Heidenreich O. et al. (1999) MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J. Biol. Chem., 274, 14434–14443. [DOI] [PubMed] [Google Scholar]
- Herschman H.R. (1991) Primary response genes induced by growth factors and tumor promoters. Annu. Rev. Biochem., 60, 281–319. [DOI] [PubMed] [Google Scholar]
- Johansen F.E. and Prywes,R. (1995) Serum response factor: transcriptional regulation of genes induced by growth factors and differentiation. Biochim. Biophys. Acta, 1242, 1–10. [DOI] [PubMed] [Google Scholar]
- Keller G.M. (1995) In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol., 7, 862–869. [DOI] [PubMed] [Google Scholar]
- Martin G.R. (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA, 78, 7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M.W., Jones-Villeneuve,E.M., Edwards,M.K. and Anderson,P.J. (1982) Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature, 299, 165–167. [DOI] [PubMed] [Google Scholar]
- Montagne J., Groppe,J., Guillemin,K., Krasnow,M.A., Gehring,W.J. and Affolter,M. (1996) The Drosophila serum response factor gene is required for the formation of intervein tissue of the wing and is allelic to blistered. Development, 122, 2589–2597. [DOI] [PubMed] [Google Scholar]
- Morgan J.I. and Curran,T. (1995) Immediate-early genes: ten years on. Trends Neurosci., 18, 66–67. [PubMed] [Google Scholar]
- Norman C., Runswick,M., Pollock,R. and Treisman,R. (1988) Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell, 55, 989–1003. [DOI] [PubMed] [Google Scholar]
- Pellegrini L., Tan,S. and Richmond,T.J. (1995) Structure of serum response factor core bound to DNA. Nature, 376, 490–498. [DOI] [PubMed] [Google Scholar]
- Rohwedel J., Horak,V., Hebrok,M., Fuchtbauer,E.M. and Wobus,A.M. (1995) M-twist expression inhibits mouse embryonic stem cell-derived myogenic differentiation in vitro. Exp. Cell Res., 220, 92–100. [DOI] [PubMed] [Google Scholar]
- Shaw P.E., Schröter,H. and Nordheim,A. (1989) The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell, 56, 563–572. [DOI] [PubMed] [Google Scholar]
- Shen M.M. and Leder,P. (1992) Leukemia inhibitory factor is expressed by the preimplantation uterus and selectively blocks primitive ectoderm formation in vitro. Proc. Natl Acad. Sci. USA, 89, 8240–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore P. and Sharrocks,A.D. (1995) The MADS-box family of transcription factors. Eur. J. Biochem., 229, 1–13. [DOI] [PubMed] [Google Scholar]
- Smith A.G., Heath,J.K., Donaldson,D.D., Wong,G.G., Moreau,J., Stahl,M. and Rogers,D. (1988) Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature, 336, 688–690. [DOI] [PubMed] [Google Scholar]
- Sommer H., Beltran,J.P., Huijser,P., Pape,H., Lonnig,W.E., Saedler,H. and Schwarz-Sommer,Z. (1990) Deficiens, a homeotic gene involved in the control of flower morphogenesis in Antirrhinum majus: the protein shows homology to transcription factors. EMBO J., 9, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. (1986) Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell, 46, 567–574. [DOI] [PubMed] [Google Scholar]
- Treisman R. (1994) Ternary complex factors: growth factor regulated transcriptional activators. Curr. Opin. Genet. Dev., 4, 96–101. [DOI] [PubMed] [Google Scholar]
- Treisman R. (1996) Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol., 8, 205–215. [DOI] [PubMed] [Google Scholar]
- Vandromme M., Gauthier-Rouvière,C., Carnac,G., Lamb,N. and Fernandez,A. (1992) Serum response factor p67SRF is expressed and required during myogenic differentiation of both mouse C2 and rat L6 muscle cell lines. J. Cell Biol., 118, 1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Hagman,J. and Gutierrez-Hartmann,A. (1998) Ets transcription factors: nuclear effectors of the Ras–MAP-kinase signaling pathway. Trends Biochem. Sci., 23, 213–216. [DOI] [PubMed] [Google Scholar]
- Williams R.L. et al. (1988) Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature, 336, 684–687. [DOI] [PubMed] [Google Scholar]
- Wobus A.M. et al. (1997) Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol. Cell Cardiol., 29, 1525–1539. [DOI] [PubMed] [Google Scholar]