Abstract
Learning increases the number of immature neurons that survive and mature in the adult hippocampus (Gould et al., 1999). One week old cells are more likely to survive in response to learning than cells in animals that are exposed to training but do not learn (Shors, 2009). Because neurogenesis is an ongoing and overlapping process, it is possible that learning differentially affects new cells as a function of their maturity. To address this issue, we examined the effects of associative learning on the survival of cells at different stages of development. Training did not alter the number of cells that were produced and present during the training experience. Cells that were 1–2 weeks of age at the time of training remained in the hippocampus several weeks later but cells that were young or older did not. In contrast, cells that were produced during training were less likely to survive when compared to cells in untrained animals. Additionally, the number of cells that were generated after learning in trained animals was not different from untrained animals. Finally, survival was not increased if the association was reacquired and expressed when the cells were about one week old. Together, these results indicate that new neurons are rescued from death by initial acquisition, not the expression or reacquisition, of an associative memory and only during a critical period. Overall, these results suggest the presence of a feedback system, which controls how many new neurons become incorporated into the adult brain in response to learning.
Keywords: adult neurogenesis, Sprague-Dawley, dentate gyrus, BrdU, trace eyeblink conditioning
Introduction
New neurons are produced in the adult hippocampus, a brain region involved in learning and some aspects of memory (Altman & Das, 1965; Squire, 1992; Cameron et al., 1993; Gould et al., 1999). Neurogenesis is an ongoing dynamic process that is influenced by learning. The two types of learning that are known to enhance cell survival include associative learning of the trace eyeblink response and spatial learning during training with the Morris water maze. Indeed, cells that are one week old at the time of training on either task are more likely to survive to become mature neurons (Gould et al., 1999; Shors, 2009). Cells that are much older or younger at the time of spatial maze training were not more likely to survive (Epp et al., 2007). However, the production of younger cells is affected water maze training depending on the phase of learning (Döbrössy et al., 2003; Ambrogini et al., 2004b; Epp et al., 2007). With respect to associative learning, we did not find a difference in the number of cells generated during training (Gould et al., 1999). However, the cells were labeled late in training after many animals had already learned. Therefore, it remains unclear whether trace conditioning increases the number of cells produced during training or only increases the number that survive thereafter. It is conceivable that the effects of trace conditioning on neurogenesis are different from those of spatial maze training. Such differences are not unprecedented. For example, ablation of neurogenesis impairs learning during trace conditioning but has no observable effect on spatial learning (Shors et al., 2001; Shors et al., 2002).
A number of studies report that new neurons in the adult hippocampus respond to exposure of a previously-learned experience (Kee et al., 2007; Trouche et al., 2009). From this, it has been proposed that the new cells are somehow used in aspects of relearning and memory retrieval and/or expression. However, it is unclear whether cell activity in response to memory events influences survival. Because of the growing interest in neurogenesis and its role in memory processes, it is important to describe the effects of associative learning versus those of activation in response to memory expression on the survival of new neurons.
The critical period during which associative learning rescues new neurons from death has not been determined. The effects of learning versus memory of the trace response have also not been determined. In the present study, we used trace eyeblink conditioning to evaluate the effects of associative learning on cells at various stages in their development: 1) when they were being generated, 2) when they were 1–2 weeks old or 3) when they were already mature at > three weeks of age. Second, we examined the effects of associative learning versus memory expression on the survival of cells generated in the adult hippocampus.
Methods and Materials
Subjects
Adult male Sprague-Dawley rats were bred at Rutgers University in the Department of Psychology. After weaning, they were housed in groups of 2–3 in standard plastic “shoebox“ style cages (44.5 cm long ×21.59 cm wide × 23.32 cm high) with unlimited access to water and food. They were kept on a 12 hr light-dark cycle, with lights on at 7am. All handling and experiments were carried out in the light portion of the cycle. When the rats were >60 days of age, they were housed alone. All experiments were conducted with full compliance with the rules and regulation specified by the PHS Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals.
Surgery
Rats were anesthetized with sodium pentobarbital (50 mg/kg, Henry Schein, Indianapolis, IN, USA) and supplied with isoflurane inhalant (Baxter Healthcare, Deerfield, IL, USA) and oxygen during the surgery. Under anesthesia, the head was shaven and a head stage with four electrodes was mounted to the skull. The electrodes were stainless steel wires (0.005 in.) implanted through and around the eyelid. Two electrodes were used to record electromyographic (EMG) activity, to detect eyeblinks, and the other two delivered a periorbital stimulation to the eyelid, which elicits an eyeblink as the unconditioned stimulus (US). Rats were allowed to recover for a minimum of three days before training with the associative learning task.
Trace Eyeblink Conditioning
In general, rats were connected to a cable that allowed free movement within the conditioning chamber. Animals were first acclimated to the conditioning apparatus without the presentation of any stimuli for approximately 40 min (100 twenty-five second trials). During the acclimation period, spontaneous blinks were recorded during a 500 ms period for each trial. After acclimation, rats were returned to their home cage. Training began 24 hr after the initial acclimation period. Spontaneous eyeblinks were again recorded for 30 trials immediately before the first session of training with trace eyeblink conditioning.
During a trial, a 250 ms white noise conditioned stimulus (CS; 82 dB) was followed by a 500 ms trace interval, and then immediately thereafter by the unconditioned stimulus, a 100 ms stimulation of the eyelid (US; .62–.65 mA), which always elicits a behavioral response. The inter-trial interval was 25±5 seconds. Eyeblinks were detected by changes in the eyelid EMG, which were recorded during the trace interval and compared to a 250 ms baseline recording before the onset of the CS. Eyeblinks that occurred during the trace interval, which were larger than 0.5 mV, greater than 2.5 standard deviations above the mean of the baseline recording, and greater than 10 ms in length were considered conditioned responses (CRs).
Experiment 1
To assess cell production and cell survival, all rats were injected once with bromodeoxyuridine (BrdU; 200 mg/kg; i.p.; Sigma, Atlanta, GA, USA) in physiological saline solution. BrdU incorporates into replicating DNA in the S phase of the cell cycle and thereby marks cells that are actively proliferating. BrdU is available in the brain for a period of ~2 hr after a systemic injection (Cameron & McKay, 2001). Groups of rats were injected with BrdU and then trained with the associative learning task at three different time points. The first group (n=6) was injected with BrdU and trained just 30 min later (Fig. 2A). The second group (n=8) was injected with BrdU and trained one week later. The third group (n=7) was injected with BrdU and trained 3 weeks later. All of the rats that were trained were exposed with 150 trials each day (presented in 50-trial blocks) for four consecutive days for a total of 600 trials. These groups were sacrificed four weeks after the BrdU injection, with the exception of one group, which was sacrificed 24 hr after the last day of training (i.e. four days after the BrdU injection).
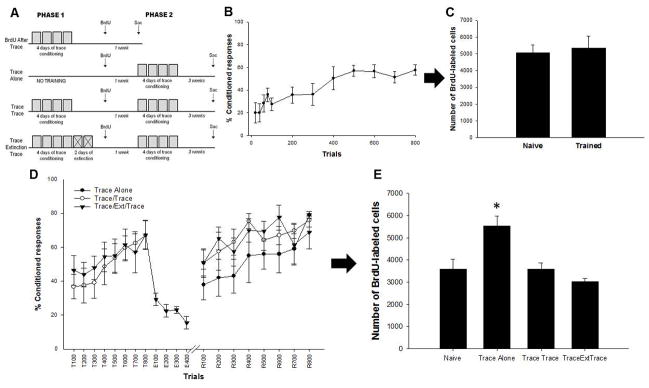
Figure 2. Effects of trace eyeblink conditioning on survival of newly generated neurons.
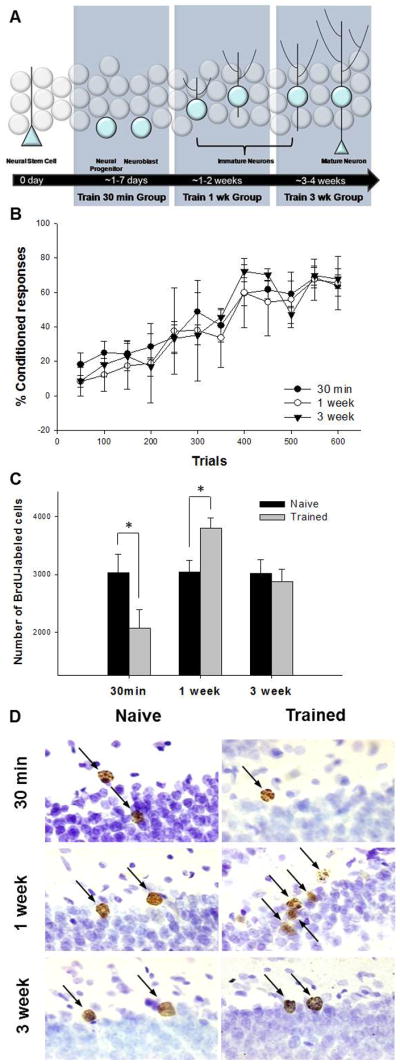
A diagram illustrating the stage of development when BrdU-labeled cells were exposed to training. All animals were injected once with BrdU. They were then trained or not 30min, 1 week or 3 weeks later. To assess the effects of learning on cell survival, animals were euthanized four weeks after the BrdU injection (A). Acquisition of the CR in animals trained 30min, 1 week and 3 weeks after BrdU injection (B). Total number of BrdU-labeled cells in the dentate gyrus in animals trained 30 min, 1 week or 3 weeks after injection compared to naïve animals. Trace conditioning increases the number of BrdU-labeled cells when rats are trained 1 week after injection, but decreases the number that survive when they were trained just 30 min after injection (C). Photomicrographs at 100x of BrdU-positive cells (arrows) from each experimental condition. Cells are located along the infrapyramidal blade in the dorsal hippocampus. Images represent the relative differences in the number of BrdU-labeled cells in response to learning when compared to naïve animals. The granularity in some cells depicts the extended time interval between the BrdU injection and sacrifice (D). * indicates p < 0.05.
Animals that emitted CRs on 60% or more of the trials during a block of trials were considered to have learned. Only rats that reached this learning criterion were used in assessing the effect of learning on the number of BrdU-labeled cells that survived after four weeks. Another group of animals were not trained but injected with BrdU and sacrificed at the same time points. These naïve animals were randomly assigned to one of three groups and matched to the corresponding experimental group (30 min n=7; 1 week n=8; 3 week n=8).
A final group was injected with BrdU and trained 30 min later but sacrificed the day after training rather than three weeks later. These animals were split into “good“ (n=7) or “poor“ (n=7) learners based on whether the animal met the previously described learning criteria. Some animals were injected with BrdU and injected four days later, but not trained (n=9). The BrdU-labeled cell counts from this group were used to assess the number of cells that were being generated during the training experience, as they were compared to the number of cells that survived the learning experience.
Experiment 2
The purpose of this experiment was to determine whether learning would increase the number of cells generated after the training experience had occurred. These groups also served as a control procedure for experiment 2. Two groups of rats were examined. One group was trained with trace conditioning and then injected with BrdU one week later (BrdU After Trace rats; n=6; Fig. 3A). All trained rats in experiment 2 were exposed to 800 trials of trace conditioning, with 200 trials each day for four days (presented in 100-trial blocks) to ensure that the animals learned and learned well. The other group was not trained but injected with BrdU at the same time point (n=13). The groups were sacrificed seven days after the BrdU injection. The number of BrdU cells in these groups was used to determine whether the initial acquisition of the trace memory persistently alters the number of cells that are available to rescue during a second phase of training.
Figure 3. Acquisition but not memory expression increases the number of surviving cells.
A schematic of the experimental design for experiment 2. To assess the effect of learning on the number of cells generated after training, one group of animals were trained and then given a BrdU injection at the time point at which the other groups began phase 2 of training (BrdU After Trace). These animals were euthanized seven days after the BrdU injection. Another group (Trace Alone) animals received no training during phase 1 and were given a BrdU injection seven days before phase 2 trace training. A third group (Trace/Trace) received trace training during phase 1, before the BrdU injection, and were trained with trace conditioning again seven days after the BrdU injection. Another group of animals (Trace/Extinction/Trace) was treated similar to animals in the Trace/Trace condition but received additional extinction trials following phase 1 before the BrdU injection (A). Acquisition of the CR for animals injected with BrdU after trace conditioning (B). The number of BrdU-labeled cells in these animals. Trained and untrained animals have a similar numbers of BrdU-positive cells (C). Acquisition, extinction and reacquisition of the trace memory. Animals in the Trace/Extinction/Trace condition extinguished their responding to the CS but quickly reacquired the CR during retraining. Animals remembered the trace task more than one week after acquisition, regardless of extinction training (D). Animals trained with only a single phase of trace conditioning possessed more BrdU-labeled cells than animals trained again after learning. * indicates p < 0.05.
To examine the effect of memory expression on cell survival, groups of rats were trained with either one or two identical phases of trace eyeblink conditioning (Fig. 3A). All trained groups received one single injection of BrdU one week prior to the start of phase 2 of training. One group of animals (Trace/Trace, n=8) was trained during both phase 1 and phase 2. An additional group of animals (Trace/Ext/Trace, n=6) was trained during both phases, but these animals also received two days of 200 CS-alone extinction trials, which immediately followed phase 1 of training. Once the CR had been extinguished, this group was injected with BrdU. They were then re-exposed to the paired stimuli one week later. Another group of animals (Trace Alone, n=5) was only trained during phase 2. An additional group (Naïve, n=6) remained experimentally naïve. All animals were sacrificed 3 weeks after the BrdU injection. If initial acquisition but not reacquisition or memory expression increases the number of surviving cells, more cells will be present in the animals that were only trained (one week after the BrdU injection. If reacquisition, or the expression, of the CR also increases cell survival, more BrdU cells will be present in the group that was trained again during phase 2 than in animals that were not trained.
BrdU immunohistochemistry and quantification
All rats were deeply anaesthetized with sodium pentobarbital and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were extracted and kept in paraformaldehyde and later transferred to PBS until sectioning. A vibratome was used to cut coronal sections (40 μm) through one hemisphere of the entire DG. The slices were cut in 0.1 M PBS (pH 7.4). Every 12th slide was fixed onto a superfrost glass slide (Fisher, Suwanne, GA, USA) and allowed to air dry. Once dry, slices were stained with peroxidase to visualize the cells that incorporated BrdU. In summary, the slices were pretreated with heated 0.1 M citric acid (pH 6.0). After rinsing with 0.1 M PBS, tissue was incubated in trypsin, followed by 2 N HCl with PBS rinses in between. Slides were kept overnight in primary mouse anti-BrdU (1:200, Becton-Dickinson, Franklin Lakes, NJ, USA) and 0.5% Tween 20 (Vector Laboratories, Burlingame, CA, USA). The next day, tissue was rinsed and incubated for 1 hr in biotinylated anti-mouse antibody (1:200, Vector Labs, Burlingame, CA, USA), then in avidin-biotin-horseradish peroxidase (1:100, Vectastain ABC Kit, Vector Labs, Burlingame, CA, USA), and lastly in diaminobenzidine (DAB SigmaFast tablets, Sigma, Atlanta, GA, USA) with PBS rinses in between. After rinsing one last time, slices were counterstained with 0.1% cresyl violet, dehydrated, cleared and then cover-slipped with Permount (Fisher Scientific, Fair Lawn, NJ, USA).
Quantitative analysis was performed blind to behavioral condition. Estimates of total number of BrdU-labeled cells were determined using a modified unbiased stereology protocol (West et al., 1991; Gould et al., 1999). BrdU-labeled cell in the subgranular zone/granule cell layer and hilus on every 12th unilateral section throughout the entire rostro-caudal extent of the DG were counted at 100x on a Nikon Eclipse E400 light microscope, avoiding cells in the outermost focal plane. The number of cells was multiplied by 24 to obtain an estimate of the total number of BrdU-labeled cells in both hippocampi.
Results
Experiment 1
Learning does not increase the number of cells generated during training
The purpose of this experiment was to evaluate the effects of training and/or learning on the number of new cells generated in the adult hippocampus. A repeated measures ANOVA, with training session as the repeated measure, was used to analyze the learning data. Animals that were trained with paired stimuli emitted more CRs as the number of training trials increased (F(11,132) = 11.41; p < 0.05; Fig. 1A).
Figure 1. Effects of trace eyeblink conditioning on production of newly generated cells.
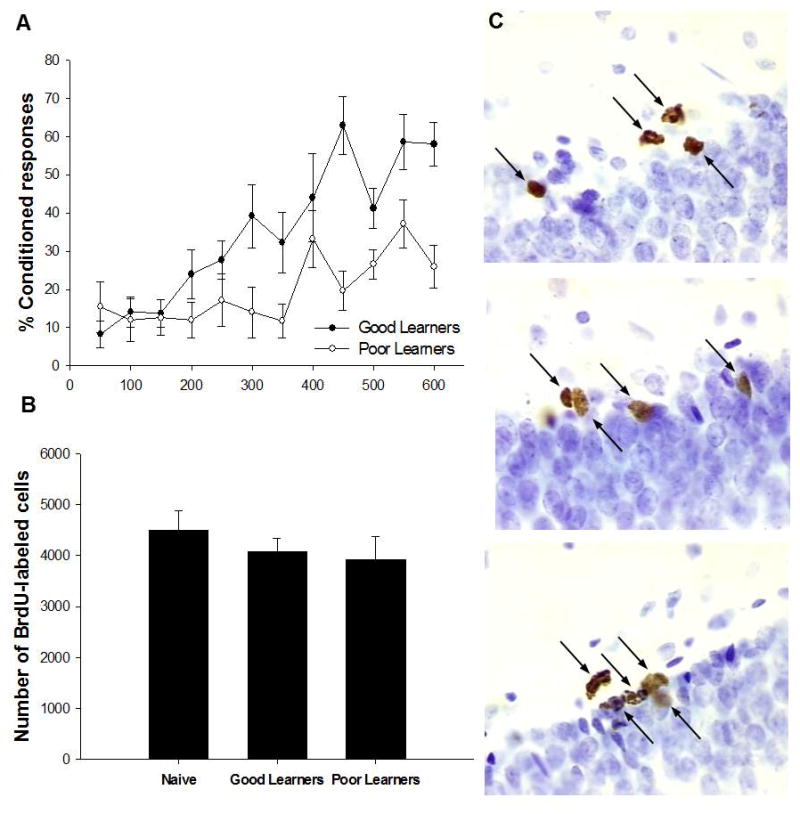
Acquisition of trace eyeblink conditioned responses for the good and poor learners (A). Total number of BrdU-labeled cells when animals were trained 30min and sacrificed four days after injection compared to naïve animals. There was no change in the number of cells labeled with BrdU during the training experience (B).Photomicrographs at 100x of BrdU-positive cells (arrows) from a naïve animal (top panel), a good learner (middle panel), and a poor learner (bottom panel) from similar areas along the infrapyramidal DG blade in the dorsal hippocampus. Images represent the differences in the number of BrdU-labeled cells in response to training when compared to naïves (C).
A one-way ANOVA was used to analyze the effect of training on the number of cells that were being produced during the training experience. Animals which began the training regime just 30 min after the BrdU injection had similar numbers of cells to animals that were untrained, provided that the cells were examined one day after training ended (F(2,20) = 0.68, p = 0.52; Fig. 1B-C). Because cells are still dividing during this time period, at least very early in training since training began shortly after the BrdU injection, these data would suggest that training does not alter the number of cells that are generated or dividing during the training experience.
Animals that emitted 60% CRs in a trial-block were considered “good learners“ and were separated from those that did not and were considered “poor learners“. As expected, the animals that learned to perform the CR had similar numbers of cells as animals that were trained but learned poorly or did not learn. Overall, these data suggest that neither training stimuli nor learning increases the number of cells that are being generated during the training experience.
Learning increases number of cells that are one but not three weeks of age
The purpose of this experiment was to determine whether learning would increase the number of cells that survive if they were exposed to the training experience when they were 30 min of age, one week of age or three weeks of age. Animals that were trained with paired stimuli emitted more CRs as the training trials increased (F(11,198) = 39.62; p < 0.05; Fig. 2B). There was no difference in performance between groups because all animals were treated similarly up to this point in the experiment (F(22,198) = 0.83; p = 0.69).
In previous studies, we found that trace conditioning only increases cell survival in animals that learn to perform the CR and learn it well (Dalla et al., 2007; Waddell & Shors, 2009). Therefore, for this analysis, we only evaluated animals that learned to emit the CR. A two-way ANOVA (trained vs. naïve; time interval after BrdU injection) followed by Newman Keuls post hoc comparisons was used to analyze the effect of learning on cells that were three stages of development. There was a significant interaction between training and developmental stage on the number of cells that remained in the hippocampus three weeks after training had ceased (F(2,38) = 6.25; p < 0.05). The number of BrdU-labeled cells was increased in trained rats compared to naïve rats when training occurred one week after BrdU injection (p < 0.05; Fig. 2C-D). In contrast, the number of cells that survived in animals that began training just 30 min after the injection was reduced (p < 0.05). The number of cells that were present in animals trained three weeks after the BrdU injection was similar to those that were not trained but injected with BrdU at the same time points (p > 0.05). Overall, these data indicate that learning increases the number of surviving cells when they are between one and two weeks of age but not when they are younger (30 min–4 days) or older (3–4 weeks). They also indicate that the youngest cells are actually less likely to survive if they are generated during the training experience.
Experiment 2
Learning does not alter the number of cells generated after training
As expected, animals emitted more CRs across training trials and sessions. A repeated measures ANOVA revealed a significant effect of session, indicating that the groups trained with paired stimuli acquired the conditioned response over the course of training (F(7, 35) = 19.97, p < 0.05, Fig. 3B). There was no difference in the number of BrdU-labeled cells between the trained and the untrained group (t(17) = 0.808, p > 0.05, Fig. 3C). Therefore, successful acquisition of trace memory did not alter the number of cells present one week after the training experience had ceased. These data suggest that associative learning does not persistently increase the number of cells generated in the adult hippocampus.
Acquisition but not reacquisition increases the number of surviving cells
In the second part of this experiment, we evaluated the effects of learning versus memory expression on the number of surviving neurons in the dentate gyrus. During the first phase of training, animals were trained with 800 trials of trace conditioning. By the end of the first phase, most animals learned to accurately predict the occurrence of the US (Fig. 3D). The number of conditioned responses made by animals in the Trace/Trace condition increased over phase 1 (F(7,42) = 3.25; p < 0.01). Similarly, the number of conditioned responses made by animals in the Trace/Extinction/Trace condition increased during the paired stimuli portion of phase 1(F(7,35) = 3.4; p < 0.05). For the two days following acquisition of the task, these animals were further trained with 400 extinction trials during which the CS was presented alone. Animals during extinction quickly ceased responding to the CS (F(3,15) = 8.05, p < 0.01). Thus, at the end of the first phase, both groups of animals had learned the CS-US association, but one group had learned to suppress the CR. Regardless of extinction training, animals remembered the task well, as indicated by a response rate of ~60% CRs upon retraining. Animals in the Trace/Trace condition responded similarly during the 1st trial block of phase 2 as the last trial block of phase 1 (F(1,7) = 3.81; p > 0.05). Animals in the Trace/Extinction/Trace conditions increased their responding significantly from the last trial block in the extinction phase to the 1st trial block in phase 2 (F(1,5) = 21.19; p < 0.01). A third group of animals remained in their home cages during phase 1 and were only trained during phase 2 with paired stimuli one week after one injection of BrdU (Trace Alone rats). These animals also readily learned to accurately predict the US with a well-timed CR (F(3,12) = 3.80; p < 0.05).
A one-way ANOVA revealed that there was a significant effect of training condition on the number of BrdU-labeled cells that survived (F(3,21) = 9.14; p < 0.01; Fig. 3E). As in Experiment 1, animals that received only a single phase of trace eyeblink conditioning (Trace Alone) when the cells were one week of age retained significantly more cells than the naïve controls (p < 0.05). Trace/Trace and Trace/Extinction/Trace animals both had a similar number of BrdU-labeled cells to naïve animals (both p > 0.05). Thus, expression of the memory alone did not increase the number of BrdU cells. These data suggest that memory retrieval and/or expression does not increase the survival of newly generated neurons in the adult dentate gyrus.
Discussion
Neuronal precursor cells in the dentate gyrus (DG) proliferate for approximately a week after their birth, a process that produces thousands if not tens of thousands of new cells per day. However, within weeks, many of these new cells die (Cameron et al., 1993; Cameron & McKay, 2001; Waddell & Shors, 2008). The cells that do survive extend axons, sprout dendritic spines and alter their response properties significantly (Ge et al., 2006; Zhao et al., 2006). Thus, these cells undergo significant changes during the first few weeks after their birth. Neurons that are one week of age at the time of training are more likely to survive if learning is successful (Gould et al., 1999; Waddell and Shors, 2008; Shors, 2009). However, the effects of trace eyeblink conditioning on cells of differing ages has not been determined. Here, we examined the effects of learning on cells at different stages in their maturity. We also examined the effects of memory expression alone on cell survival. The data provide confirmatory evidence as well as novel information. First, animals that began training just 30 min after the BrdU injection did not possess any more cells after training than animals that were not trained. Because the animals started training almost immediately after the BrdU injection were sacrificed only a few days later, these data suggest that training does not significantly increase cell production. Even so, the number of those cells that survived one month later was significantly reduced when training began immediately after the BrdU injection. Thus, while cells that are one to two weeks of age are being rescued from death, those much younger are dying in significant numbers. It would appear that learning retains some neurons at the expense of those that are less mature. We also observed that cells that were about one week of age at the time of the learning experience tended to survive while those that were older did not. Together, these data provide a snapshot of how cells of different ages respond to learning.
Others have found that spatial training with the Morris water maze task increases cell survival but only during a critical period (Epp et al., 2007). Galea and colleagues found that cells which were approximately one week of age at the time of training were more likely to survive whereas those that were older or younger did not. Abrous and colleagues observed that spatial learning increases the number of apoptotic cells in the DG if the cells were 3–4 days old at the time of training (Dupret et al., 2007). Thus, some cells that were exposed to spatial learning are more likely to die whereas others are more likely to survive. The authors suggested a mechanism or system which removes cells that were not selected for integration into the learning network (Dupret et al., 2007). Our data reported here are generally consistent with such a system. Thus, it would appear that both trace eyeblink conditioning and spatial maze learning increase the survival of new neurons when they are about one to two weeks of age while inducing cell death for younger cells. This feedback system might be used to maintain an optimal number of newly generated neurons and ultimately would control the total number of granule cells in the DG.
The current data support the idea of a critical period during which new neurons in the adult hippocampus can be rescued from death by learning. Thus, some cells must possess a characteristic that makes them selectively sensitive to the positive effects of a learning experience. One might suggest NMDA receptor activation, since it is necessary for learning and implicated in the survival of new neurons (Tashiro et al., 2006; Leuner et al., 2007; Tashiro et al., 2007). However, it has been shown that these receptors do not contribute to cell activity until the cells are somewhat older (Ambrogini et al., 2004a). It seems likely that the response to GABA release during the learning experience may be involved. GABA is excitatory very early in neurogenesis and can increase the extent of neuronal differentiation (Overstreet Wadiche et al., 2005; Tonuka et al., 2005). Another candidate is brain-derived neurotrophic factor production, which is elevated during learning and increases neurogenesis (Kesslak et al., 1998; Mizuno et al., 2000; Scharfman et al., 2005). That said, only a small percentage of cells express the this neurotrophin’s Trk receptor during the period when learning increases cell survival (Donovan et al., 2008).
In a second set of experiments, we examined the effects of memory expression on the survival of cells that were not yet present at the time of the initial learning experience, as well as their response to reacquisition after the initial response was extinguished. Training and/or learning, did not persistently increase the number of surviving cells, at least those generated during the week following the training experience. Moreover, cells generated after the initial learning and about one week of age were not rescued from death by expression of the memory alone or by reacquisition of a memory that had been behaviorally extinguished. Therefore, expressing the memory for a task that was learned before the cells were generated does not rescue an entirely new population of cells from death. These data are novel in that they dissociate the effect of learning versus that of memory on the survival of newly generated neurons in the adult hippocampus.
Learning typically occurs once but a memory can be expressed over and over again, admittedly with some new learning each time. It seemed unlikely that new cells would be rescued from death each time a memory is retrieved and generally, the data presented here support this conclusion. Certainly, the cells that were initially rescued by learning might be reactivated during memory retrieval and this has been shown with respect to spatial learning. Trouche and colleagues (2009) found that cells rescued from death during spatial learning were responsive to a memory probe trial and to further training some weeks later. However, it is possible that the cells that are initially rescued from death during learning could respond to the memory experience without being required for recall of the memory. This must certainly be the case with respect to trace conditioning since the hippocampus is not necessary for expression of the memory trace (Kim et al., 1995; Takehara et al., 2003). Conversely, expression of a spatial memory does depend on an intact hippocampus so it many affect survival differently than associative learning (Riedel et al., 1999). In any event, the present data suggest that neither the neuronal activity associated with memory expression nor its rehearsal per se rescue new cells from death – cells that were not yet present at the time of the initial training experience. Indeed, reactivating the now mature previously rescued cells during expression of a memory memory may prevent newly-generated cells from rescue. In general, the data presented here suggest a general system – perhaps a feedback system – that maintains a critical number of neurons in the adult hippocampus as a function of learning and memory.
Acknowledgments
This work was funded by the National Institutes of Health (National Institute of Mental Health-59970) and the National Science Foundation (IOB-0444364).
Abbreviations
- BrdU
bromodeoxyuridine
- CR
conditioned response
- CS
conditioned stimulus
- DG
dentate gyrus
- US
unconditioned stimulus
Contributor Information
Megan L. Anderson, Department of Neuroscience, Rutgers University, 604 Allison Road, Piscataway, NJ, 08854.
Helene M. Sisti, Department of Psychology and Center for Collaborative Neuroscience, Rutgers University, 152 Frelinghuysen Road, Piscataway, NJ, 08854.
Daniel M. Curlik, II, Department of Psychology and Center for Collaborative Neuroscience, Rutgers University, 152 Frelinghuysen Road, Piscataway, NJ, 08854.
Tracey J. Shors, Department of Psychology and Center for Collaborative Neuroscience, Rutgers University, 152 Frelinghuysen Road, Piscataway, NJ, 08854
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. Journal of Comparative Neurology. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell later of adult rat dentate gyrus. Brain Research. 2004a;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Orsini L, Mancini C, Ferri P, Ciaroni S, Cuppini R. Learning may reduce neurogenesis in adult rat dentate gyrus. Neuroscience Letters. 2004b;359:13–16. doi: 10.1016/j.neulet.2003.12.123. [DOI] [PubMed] [Google Scholar]
- Cameron H, Woolley C, McEwen B, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. The Journal of Comparative Neurology. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Dalla C, Bangasser DA, Edgecomb C, Shors TJ. Neurogenesis and learning: Acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiology of Learning and Memory. 2007;88:143–148. doi: 10.1016/j.nlm.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döbrössy MD, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Molecular Psychiatry. 2003;8:974–982. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- Donovan MH, Yamaguchi M, Eisch AJ. Dynamic expression of TrkB receptor protein on proliferating and maturing cells in the adult mouse dentate gyrus. Hippocampus. 2008;18:435–439. doi: 10.1002/hipo.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Döbrössy MD, Panatier A, Rodriguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biology. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp J, Spritzer M, Galea L. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149:273–285. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors T. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nature Neuroscience. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behavioral Neuroscience. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace conditioned responses. Behavioral Neuroscience. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Mirescu C. A critical time for new neurons in the adult hippocampus. The Journal of Neuroscience. 2007;27:5845–5846. doi: 10.1523/JNEUROSCI.1838-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. The Journal of Neuroscience. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. Journal of Neurophysiolology. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nature Neuroscience. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Experimental Neurology. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Saving new brain cells. Scientific American. 2009;300:46–52. doi: 10.1038/scientificamerican0309-46. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Reviews. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. The Journal of Neuroscience. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. Journal of Neuroscience. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Tonuka Y, Fuduka S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Shors TJ. Neurogenesis, learning and associative strength. European Journal of Neuroscience. 2008;27:3020–3028. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. The Anatomical Record. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng E, Summers RJ, Ming G, Gage F. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. Journal of Neuroscience. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]