Abstract
Objective
This experiment tested the hypothesis that exercise would improve executive function.
Design
Sedentary, overweight 7- to 11-year-old children (N = 171, 56% female, 61% Black, M ± SD age 9.3 ± 1.0 yrs, body mass index (BMI) 26 ± 4.6 kg/m2, BMI z-score 2.1 ± 0.4) were randomized to 13 ± 1.6 weeks of an exercise program (20 or 40 minutes/day), or a control condition.
Main outcome measures
Blinded, standardized psychological evaluations (Cognitive Assessment System and Woodcock-Johnson Tests of Achievement III) assessed cognition and academic achievement. Functional magnetic resonance imaging measured brain activity during executive function tasks.
Results
Intent to treat analysis revealed dose response benefits of exercise on executive function and mathematics achievement. Preliminary evidence of increased bilateral prefrontal cortex activity and reduced bilateral posterior parietal cortex activity due to exercise was also observed.
Conclusion
Consistent with results obtained in older adults, a specific improvement on executive function and brain activation changes due to exercise were observed. The cognitive and achievement results add evidence of dose response, and extend experimental evidence into childhood. This study provides information on an educational outcome. Besides its importance for maintaining weight and reducing health risks during a childhood obesity epidemic, physical activity may prove to be a simple, important method of enhancing aspects of children’s mental functioning that are central to cognitive development. This information may persuade educators to implement vigorous physical activity.
Keywords: cognition, aerobic exercise, obesity, antisaccade, fMRI
Executive function appears more sensitive than other aspects of cognition to aerobic exercise training (Colcombe & Kramer, 2003). Executive function constitutes supervisory control of cognitive functions to achieve a goal and is mediated via prefrontal cortex circuitry. Planning and carrying out action sequences that make up goal directed behavior requires allocation of attention and memory, response selection and inhibition, goal setting, self-control, self-monitoring, and skillful and flexible use of strategies (Eslinger, 1996; Lezak, Howieson, & Loring, 2004). The executive function hypothesis was proposed based on evidence that aerobic exercise selectively improves older adults’ performance on executive function tasks and leads to corresponding increases in prefrontal cortex activity (Colcombe et al., 2004; Kramer et al., 1999). Children’s cognitive and neural development may be sensitive to physical activity (Diamond, 2000; Hillman, Erickson, & Kramer, 2008; Kolb & Whishaw, 1998). Theoretical accounts of the links between motor behavior and cognitive development during childhood have ranged from hypothesized brain networks to the construction of perception-action representations (Rakison & Woodward, 2008; Sommerville & Decety, 2006).
A meta-analysis of exercise studies in children showed improved cognition with exercise; however, randomized trial results were inconsistent (Sibley & Etnier, 2003). A selective effect of exercise on executive function may explain mixed experimental results obtained in children (Tomporowski, Davis, Miller, & Naglieri, 2008). Studies utilizing cognitive tasks requiring executive function showed benefits of exercise (Davis et al., 2007; Tuckman & Hinkle, 1986), while those using less sensitive measures did not (Lezak et al., 2004, pp. 36, 611–612; e.g., Ismail, 1967; Zervas, Apostolos, & Klissouras, 1991). A preliminary report from this study, with a smaller sample, showed a benefit of exercise on executive function (Davis et al., 2007). The final results are presented here.
In children, vigorous physical activity has been associated with better grades (Coe, Pivarnik, Womack, Reeves, & Malina, 2006; Taras, 2005), physical fitness with academic achievement (Castelli, Hillman, Buck, & Erwin, 2007; Dwyer, Sallis, Blizzard, Lazarus, & Dean, 2001; Wittberg, Northrup, Cottrell, & Davis, accepted), and overweight with poorer achievement (Castelli et al., 2007; Datar, Sturm, & Magnabosco, 2004; Dwyer et al., 2001; Shore et al., 2008; Taras & Potts-Datema, 2005). The strongest conclusion to be drawn regarding the effect of physical activity on academic achievement, however, is that it does not impair achievement, even when it takes away classroom time (Dwyer, Coonan, Leitch, Hetzel, & Baghurst, 1983; Sallis et al., 1999; Shephard et al., 1984). Because overweight is a marker of chronic inactivity (Must & Tybor, 2005), overweight, sedentary children may be more likely to benefit from exercise than lean children.
The primary hypothesis of this study was that sedentary, overweight children assigned to exercise would improve more than children in a control condition on executive function, but not other cognitive processes such as resistance to distraction, spatial and logic processes, and sequencing. A secondary hypothesis was that a dose response relation would be observed between exercise and cognition. Effects on academic achievement were explored. Based on previous studies in adults showing exercise related changes in brain function, effects on activity in prefrontal cortex circuitry were explored using functional magnetic resonance imaging (fMRI) in a subgroup of participants.
Method
Main Study
Participants
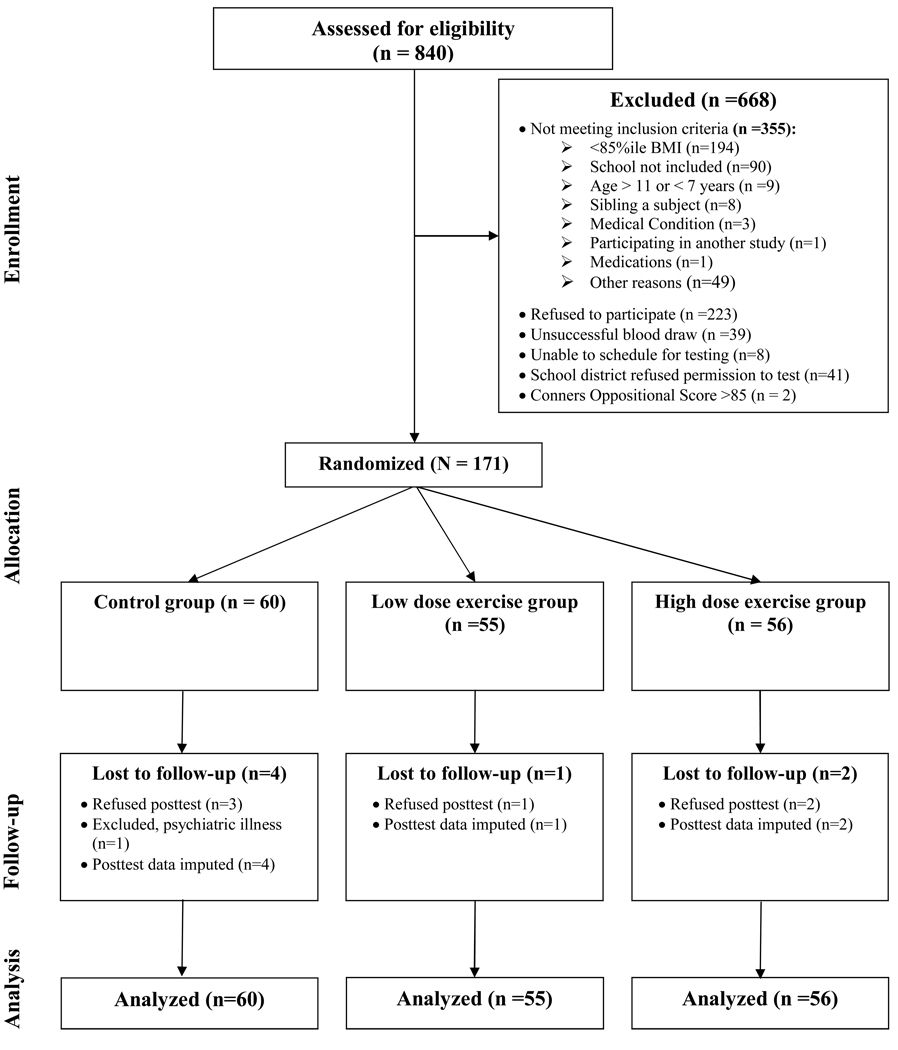
Students were recruited from schools during 2003–2006 for a trial of aerobic exercise on children’s health. Children were eligible if they were overweight (≥85th percentile BMI) (Ogden et al., 2002), inactive (no regular physical activity program >1 hr/wk), and had no medical condition that would affect study results or limit physical activity. One hundred and seventy-one children 7–11 years of age were randomized (56% female, 61% Black, 39% White, M ± SD age 9.3 ± 1.0 yrs, body mass index (BMI) 26.0 ± 4.6 kg/m2, BMI z-score 2.1 ± 0.4, parent (i.e. primary caregiver) education level 5.0 ± 1.1, where 1 = less than 7th grade, 2 = 8th or 9th, 3 = 10th or 11th, 4 = high school graduate, 5 = some college, 6 = college graduate, 7 = postgraduate). One child was excluded from posttest due to a psychiatric hospitalization that occurred after randomization. Children were encouraged to posttest regardless of adherence to the intervention. Eleven children taking medication for attention deficit disorder were included (and took their medication as usual; n = 4 in control, n = 4 in low dose, and n = 3 in high dose group) to maximize generalizability. Children and parents completed written informed assent and consent. The study was reviewed and approved by the Institutional Review Board of the Medical College of Georgia. Testing and intervention occurred at the Medical College of Georgia. The participant flow diagram is presented in Fig. 1.
Fig. 1.
Participant flow diagram.
Study Design
Children were assigned randomly by the statistician to low dose (20 minutes/day) or high dose (40 minutes/day) aerobic exercise, or to a no exercise control. Randomization was stratified by race and sex. Assignments were concealed until baseline testing was completed, then communicated to the study coordinator, who informed the subjects. The control condition did not provide any after school program or transportation. The exercise conditions were equivalent in intensity, and differed only in duration (i.e., energy expenditure). Five cohorts participated in the study over 3 years.
Aerobic Exercise Intervention
Children assigned to exercise were transported to an after school exercise program each school day (student:instructor ratio about 9:1). The emphasis was on intensity, enjoyment, and safety, not competition nor skill enhancement. Activities were selected based on ease of comprehension, fun, and eliciting intermittent vigorous movement, and included running games, jump rope, and modified basketball and soccer (Gutin, Riggs, Ferguson, & Owens, 1999). The program handbook is available on request. Heart rate monitors (S610i; Polar Electro, Oy, Finland; 30 second epoch) were used to observe the dose. Each child’s average heart rate during the sessions was recorded daily, and points awarded for maintaining an average >150 beats per minute. Points were redeemed for weekly prizes. Children assigned to the high dose condition completed two 20 minute bouts each day. Children in the low dose condition completed one 20 minute bout, and then a 20 minute period of sedentary activities (e.g. board games, card games, drawing) in another room. No tutoring was provided during this period. Each session began with a five minute warm up (moderate cardiovascular activity, static and dynamic stretching). Bouts ended with a water break, light cool down cardiovascular activity, and static stretching.
During the 13 ± 1.6 weeks of intervention (13 ± 1.5, 13 ± 1.7 in low and high dose conditions, respectively), attendance was 85 ± 13% (85 ± 12, 85 ± 14). Average heart rate was 166 ± 8 beats per minute (167 ± 7, 165 ± 8). Children achieved an average heart rate > 150 beats per minute on most days (87 ± 10% overall; 89 ± 8, 85 ± 12 in low and high dose conditions, respectively). The duration of the intervention period, average attendance, heart rate, and proportion of the time the heart rate goal was achieved were similar across exercise conditions, and the time between baseline and posttest was similar across all experimental conditions (19 ± 3.3, 18 ± 2.6, 18 ± 2.5 weeks in control, low, and high dose conditions, respectively).
Measures
A standardized psychological battery assessed cognition and achievement at baseline and posttest. Most children (98%) were evaluated by the same tester, at the same time of day, and in the same room at baseline and posttest. Testers were unaware of the child’s experimental condition. Standard scores were analyzed. Altogether, 5 cohorts provided data for cognition and 4 cohorts for achievement. The means fell in the normal range (Table 1).
Table 1.
Cognitivea and achievementb scores (M ± SE) by group at baseline and posttest, and adjusted means at post
| Control | Low Dosec | High Dosed | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Adj. | Adj. | Adj. | |||||||
| Assessment | Pre | Post | Post | Pre | Post | Post | Pre | Post | Post |
| Planning | 99 ± 1.6 | 102 ± 1.7 | 102 ± 1.0 | 98 ± 1.6 | 102 ± 1.7 | 103 ± 1.1 | 101 ± 1.6 | 107 ± 1.4 | 105 ± 1.1e |
| Attention | 97 ± 1.5 | 104 ± 1.5 | 104 ± 1.1 | 96 ± 1.5 | 103 ± 1.7 | 104 ± 1.1 | 100 ± 1.8 | 106 ± 2.0 | 104 ± 1.1 |
| Simultaneous | 101 ± 1.6 | 104 ± 1.5 | 105 ± 1.1 | 101 ± 1.9 | 106 ± 1.6 | 107 ± 1.2 | 106 ± 1.3 | 108 ± 1.7 | 106 ± 1.2 |
| Successive | 101 ± 1.6 | 102 ± 1.6 | 101 ± 0.9 | 98 ± 1.6 | 102 ± 1.4 | 103 ± 0.9 | 102 ± 1.5 | 105 ± 1.6 | 104 ± 0.9 |
| Broad Reading | 98 ± 1.2 | 98 ± 1.4 | 100 ± 0.8 | 100 ± 1.6 | 100 ± 1.6 | 101 ± 0.8 | 103 ± 1.9 | 102 ± 2.1 | 100 ± 0.8 |
| Broad Math | 104 ± 1.7 | 104 ± 1.4 | 104 ± 0.8 | 104 ± 1.8 | 105 ± 1.6 | 105 ± 0.8 | 106 ± 1.6 | 107 ± 1.4 | 107 ± 0.8f |
Note: Covariates included baseline score and sex for Planning and Attention, baseline score and race for Simultaneous and Broad Math, and baseline score and parent education for Planning, Broad Reading, and Broad Math.
Cognitive Assessment System Standard Scores; n in control group = 60, low dose = 55, high dose = 56.
Woodcock-Johnson Test of Achievement III Standard Scores; n in control group = 51, low dose = 45, high dose = 45.
Low Dose = 20 minutes/day aerobic exercise.
High Dose = 40 minutes/day aerobic exercise.
Linear contrast p = .013, contrast comparing control vs. both exercise groups p = .03, control vs. high dose group p = .013.
Linear contrast p = .045, control vs. high dose group p = .045.
A standardized, theory based (Das, Naglieri, & Kirby, 1994; Naglieri, 1999) cognitive assessment with excellent psychometric qualities, the Cognitive Assessment System, was utilized (Naglieri & Das, 1997). The Cognitive Assessment System was standardized on a large representative sample of children aged 5–17 years who closely match the U.S. population on a number of demographic variables (e.g., age, race, region, community setting, educational classification, and parental education). It is strongly correlated with academic achievement (r = .71), though it does not contain achievement-like items (Naglieri & Rojahn, 2004). It is known to respond to educational interventions (Das, Mishra, & Poole, 1995), and it yields smaller race and ethnic differences than traditional intelligence tests, making it more appropriate for the assessment of disadvantaged groups (Naglieri, Rojahn, Aquilino, & Matto, 2005).
The Cognitive Assessment System measures children’s mental abilities defined on the basis of four interrelated cognitive processes: Planning, Attention, Simultaneous, and Successive. Each of the four scales is comprised of three subtests. Only the Planning scale measures executive function (i.e., strategy generation and application, self-regulation, intentionality, and utilization of knowledge; internal reliability r = .88). The Planning scale has better reliability than neuropsychological tests of executive function (Rabbitt, 1997). The remaining scales measure other aspects of cognitive performance, and thus can determine whether the effects of exercise in children are stronger for executive function than for other cognitive processes. The Attention tests require focused, selective cognitive activity and resistance to distraction (internal reliability r = .88). The Simultaneous subtests involve spatial and logical questions that contain nonverbal and verbal content (internal reliability r = .93). The Successive tasks require analysis or recall of stimuli arranged in sequence, and formation of sounds in order (internal reliability r = .93). Preliminary results on this measure have been published (Davis et al., 2007). One child was erroneously administered the 8-yr-old version of the test at baseline when the child was 7 yrs old.
Children’s academic achievement was measured using two interchangeable forms of the Woodcock-Johnson Tests of Achievement III (McGrew & Woodcock, 2001) which were randomly counterbalanced. The Broad Reading and Broad Mathematics clusters were the outcomes of interest. One hundred forty-one children in 4 cohorts provided achievement data.
Statistical Analysis
Intent to treat analysis of covariance tested group differences on cognition and achievement at posttest, adjusting for baseline score. Analyses were conducted using the last observation carried forward imputation for the 7 children who did not provide posttest data. Covariates (cohort, race, sex, parent education) were included if they were related to the dependent variable. The Planning, Simultaneous, Attention, and Successive scales, as well as Broad Reading and Broad Math clusters, were examined. A priori contrasts testing a linear trend, and comparing the control group to the two exercise groups, were performed, along with orthogonal quadratic and low vs. high dose contrasts. Statistical significance was assessed at α = .05. Significant analyses were repeated excluding the 11 children taking medications for attention deficit disorder, and excluding 18 seven-year-olds, who because of their age were administered a slightly different version of the Cognitive Assessment System. A sample size of 62 subjects per group was estimated to provide 80% power to detect a difference between groups of 6.6 units.
FMRI Substudy
Participants
Twenty children in the last cohort of the study participated in an fMRI pilot study consisting of baseline (control n = 9, exercise n = 11) and posttest (control n = 9, exercise n = 10) brain scans. Left-handed children and those who wore glasses were excluded. One posttest session in the exercise group was refused. There were no significant differences in characteristics between this subset (9.6 ± 1.0 years, 40% female, 40% Black, BMI 25.3 ± 6.0, BMI z-score 1.9 ± 0.46) and the rest of the sample. Low and high dose exercise groups (14 ± 1.7 wks exercise) were collapsed for fMRI analyses.
Design and Procedure
Images were acquired on a GE Signa Excite HDx 3 Tesla MRI system (General Electric Medical Systems, Milwaukee, WI). Visual stimuli were presented using MRI compatible goggles (Resonance Technologies, Inc., Northridge, CA), and eye movements were monitored using an eye tracking system which allowed investigators to see that subjects were awake and engaged in the task. Subjects wore ear plugs and their heads were restrained using a vacuum pillow. Prior to the acquisition of MRI data, the magnetic homogeneity was optimized using an automated shimming procedure that determines low order shim values by performing least squares fits of magnetic field maps and automatically applies the low order shim values as direct current offset currents in the X, Y, and Z gradient waveforms. Functional images were obtained using a spoiled gradient echo planar imaging sequence (time of repetition (TR) 2800 ms, echo time (TE) 35 ms, flip angle 90°, field of view (FOV) 280 × 280 mm2, matrix 96 × 96, 34 slices, slice thickness 3.6 mm). Next, structural images were obtained using a 3-dimensional fast spoiled gradient echo sequence (TR 9.0 ms, TE 3.87 ms, flip angle 20°, FOV 240 × 240 mm2, matrix 512 × 512, 120 slices, slice thickness 1.3 mm). The high resolution structural images were used to normalize functional images into a standard stereotaxic space for analyses (Talairach & Tournoux, 1988).
Antisaccade task
Functional imaging data were acquired while subjects completed another measure of executive function, an antisaccade task (McDowell et al., 2002). Correct antisaccade performance requires inhibition of a prepotent response to a visual cue and the generation of a response to the mirror image location of that cue (opposite side, same distance from central fixation). After an initial fixation period (25.2 sec), a block paradigm alternated between baseline (N = 7 blocks; 25.2 sec of a cross presented at central fixation) and experimental (N = 6 blocks; 25.2 sec consisting of 8 antisaccade trials, 48 trials total) conditions (5.46 minute run time; 117 volumes; the first 2 volumes were omitted from analysis to account for magnetization stabilization). During baseline subjects were instructed to stare at the cross. During antisaccade trials subjects were instructed to stare at a central cross until it went off, and then a cue in the periphery signaled subjects to look as quickly as possible to the mirror image location of the cue, without looking at the cue itself. Subjects had two separate practice sessions before each scanner session to ensure they understood instructions. Personnel interacting with the children during the scan were unaware of the child’s assignment.
Image analysis
Analyses were conducted as in previously published data from our laboratory (Camchong, Dyckman, Austin, Clementz, & McDowell, 2008; Camchong, Dyckman, Chapman, Yanasak, & McDowell, 2006; Dyckman, Camchong, Clementz, & McDowell, 2007; McDowell et al., 2002) using AFNI software (Cox, 1996). Briefly, for each session, volumes were registered to a representative volume to correct for minor head movement (and 6 regressors were calculated: 1 each for a) rotational, and b) translational head motion in each of 3 planes). A 4 mm full width at half maximum Gaussian filter was then applied to each dataset. For each voxel, the percent change in blood oxygenation level dependent signal from baseline was calculated for each time point. The resulting percent change across time was detrended for linear drift and correlated with a trapezoidal reference function modeling baseline (fixation) and experimental (antisaccade) conditions, using the 6 motion parameters as noise regressors. Data were then transformed into standardized space based on the Talairach and Tournoux Atlas (Talairach & Tournoux, 1988), and resampled to 4 × 4 × 4 mm voxels.
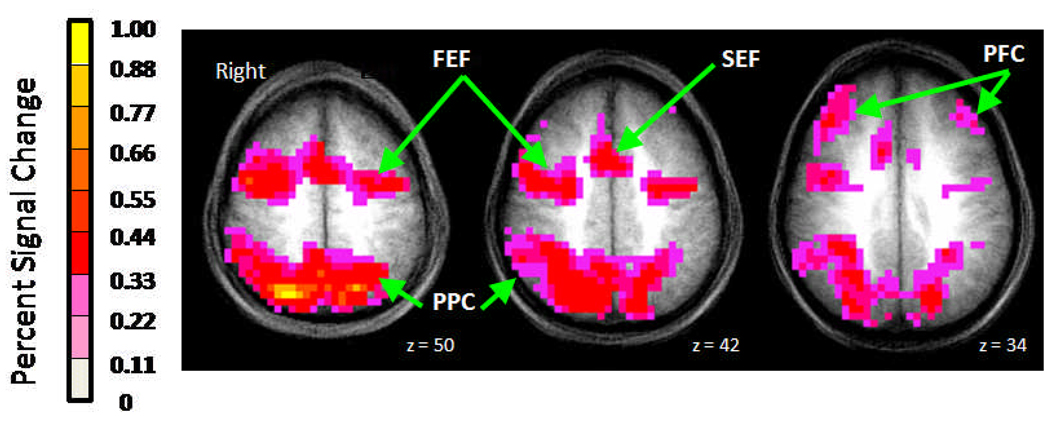
In order to identify the neural circuitry supporting antisaccade performance (Fig. 2), the data were collapsed across groups and time points for analysis of variance. To protect against false positives, a cluster threshold method derived from Monte Carlo simulations (based on the geometry of the data set) was applied to the F map (Ward, 1997). Based on these simulations, the family wise alpha at p = .05 was preserved with an individual voxel thresholded at p = .0005 and a cluster size of 3 voxels (192 µL). The resulting clustered F map was used to identify regional blood oxygenation level dependent signal change.
Fig. 2.
Axial views displaying blood oxygenation level dependent percent signal change associated with antisaccade performance from one-sample analysis at three different levels in the brain. Data from 39 sessions (20 children at baseline, 19 at posttest) are shown radiologically oriented (right hemisphere on left side). Colors from pink to yellow indicate increasing antisaccade-related percent signal change. The background is an anatomical image averaged over 20 participants. FEF, frontal eye field; PPC, posterior parietal cortex; SEF, supplementary eye field; PFC, prefrontal cortex.
Region of interest analyses
For each cortical region that showed significant activity in the clustered F map (frontal eye field, supplementary eye field, prefrontal cortex, posterior parietal cortex), a sphere (radius 8 mm, similar to Kiehl et al., 2005; Morris, DeGelder, Weiskrantz, & Dolan, 2001) was positioned at the center of mass, with bilateral activity collapsed across hemispheres. Mean percent signal changes at baseline and posttest were calculated for each region of interest for each participant, and difference scores analyzed. Because of nonnormal distributions of region of interest values, experimental conditions were compared using the Mann-Whitney U test (exact 2-tailed probabilities).
Results
Psychometric Data
Sex was related to posttest Planning (boys, 101.3 ± 12.1 vs. girls, 105.2 ± 12.7, t = −2.0, p = .044) and Attention (99.8 ± 12.2 vs. 107.5 ± 12.5, t = −4.1, p < .001) scores. Race was linked with posttest Simultaneous (White, 109.3 ± 13.6 vs. Black, 104.0 ± 10.9, t = 2.9, p = .004) and Broad Math (109.0 ± 9.3 vs. 102.0 ± 10.1, t = 4.2, p < .001) scores. Parent education was correlated with posttest Planning (r = .18, p = .02), Broad Reading (r = .27, p = .001) and Broad Math (r = .27, p = .001) scores. These covariates were included in corresponding analyses.
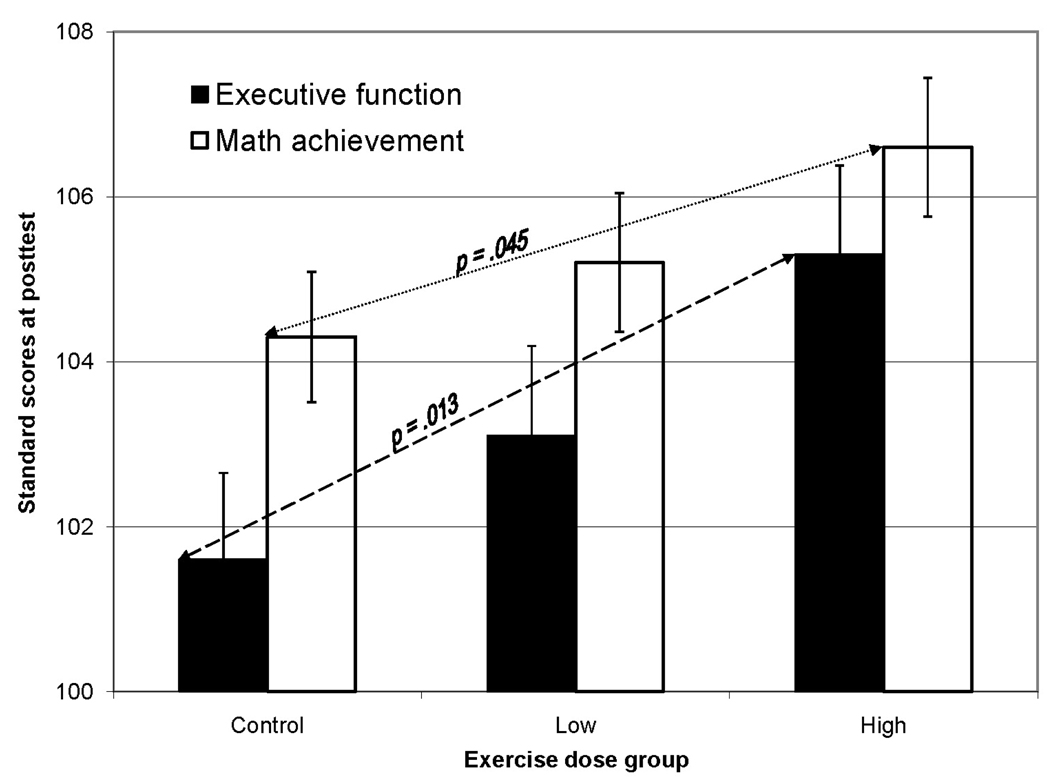
A statistically significant a priori linear contrast indicated a dose response benefit of exercise on executive function (i.e. Planning, Fig. 3; L = 2.7, 95% confidence interval (CI) 0.6 to 4.8, t(165) = 2.5, p = .013). The a priori contrast comparing the control group to the exercise groups also was significant, showing that exposure to either the low or high dose of the exercise program resulted in higher Planning scores (L = −2.8, CI = −5.3 to −0.2, t(165) = 2.1, p = .03). As expected, no effects were detected on the Attention, Simultaneous, or Successive scales. For the Broad Math cluster, a statistically significant a priori linear contrast indicated a dose response benefit of exercise on mathematics achievement (Fig. 3; L = 1.6, CI 0.04 to 3.2, t(135) = 2.03, p = .045). The contrast comparing the exercise conditions to the control condition was not statistically significant (p = .10). No effects were detected on the Broad Reading cluster.
Fig. 3.
Executive function (Planning) at posttest adjusted for sex, parent education, and baseline score, and math achievement means (SE) at posttest adjusted for race, parent education, and baseline score, showing dose response effects of the aerobic exercise program.
The low and high dose conditions did not differ, and no quadratic trends were detected. Apart from baseline score, the only significant covariates in analyses of cognition or achievement were sex in the Attention analysis (p < .001) and race for Broad Math (p = .03). The results were similar when excluding children with attention deficit disorder (linear contrasts on Planning, t(154) = 2.84, p = .005, Broad Math, t(125) = 2.12, p = .04) and 7-year-olds (Planning, t(147) = 2.92, p = .004, Broad Math, t(117) = 2.23, p = .03).
Neuroimaging Data
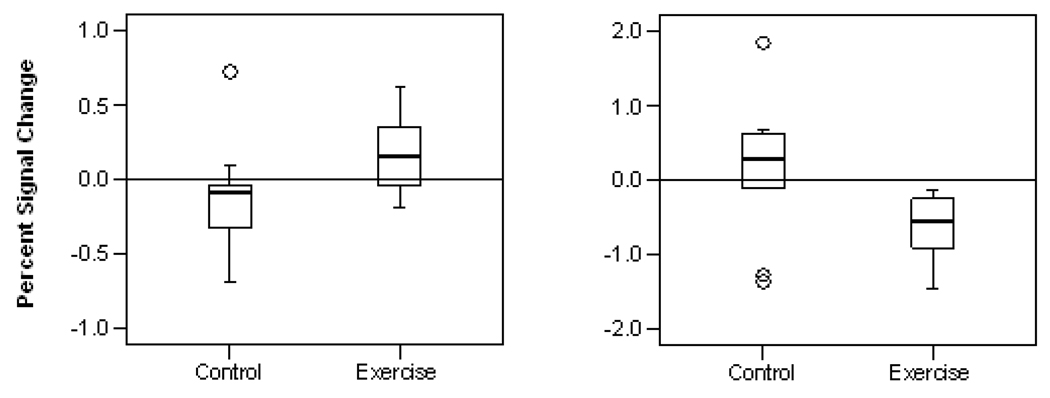
The antisaccade-related blood oxygenation level dependent signal (collapsing across group and time point) revealed cortical saccadic circuitry (including frontal eye fields, supplementary eye fields, posterior parietal cortex, and prefrontal cortex; Fig. 2), which is well defined in adults (Luna et al., 2001; Sweeney, Luna, Keedy, McDowell, & Clementz, 2007). Region of interest analyses demonstrated group differences in signal changes from baseline to posttest that were significant in two regions: bilateral prefrontal cortex (center of mass in Talairach coordinates (x,y,z): right = 36, 32, 31; left = −36, 32, 31) and bilateral posterior parietal cortex (right = 25, −74, 29; left = −23, −70, 22). Specifically, the exercise group showed increased bilateral prefrontal cortex activity (Fig. 4, left panel; U = 20, p = .04) and decreased activity in bilateral posterior parietal cortex (Fig. 4, right panel; U = 18, p = .03) compared to controls. Region of interest analyses of motor regions (frontal and supplementary eye fields) did not show significant differences between groups.
Fig. 4.
Boxplots by experimental condition showing change in activation from baseline to posttest. Left panel: prefrontal cortex. Right panel: posterior parietal cortex.
Discussion
The experiment tested the effect of approximately 3 months of regular aerobic exercise on executive function in sedentary, overweight children using cognitive assessments, achievement measures, and fMRI. This multifaceted approach revealed convergent evidence that aerobic exercise improved cognitive performance. More specifically, blinded, standardized evaluations showed specific dose response benefits of exercise on executive function and math achievement. Increased prefrontal cortex activity and reduced posterior parietal cortex activity due to the exercise program were observed.
In sum, these results are consistent with those in adults regarding demonstrable behavioral and brain activity changes due to exercise (Colcombe et al., 2004; Pereira et al., 2007). They also add evidence of dose response, which is particularly rare in exercise trials with children (Strong et al., 2005), and provide important information on an educational outcome. The high dose condition resulted in mean Planning scores 3.8 points, or a quarter of a standard deviation (σ = 15), higher than the control condition. Demographics did not contribute to the model. Similar results were obtained when children with attention deficit disorder or 7-year-olds were excluded. Therefore the results may be generalized to overweight Black or White 7- to 11-year-olds.
Executive function develops in childhood, and is crucial for adaptive behavior and development (Best, Miller, & Jones, 2009; Eslinger, 1996). In particular, the capacity to regulate one’s behavior (e.g., inhibiting inappropriate responses, delaying gratification) is important for a child to succeed in elementary school (Blair, 2002; Eigsti et al., 2006). This effect may have important implications for child development and educational policy. The finding of improved math achievement is remarkable, given that no academic instruction was provided, and suggests that a longer intervention period may result in more benefit. The improvement observed on achievement was specific to mathematics, with no benefit to reading.
We hypothesize that regular vigorous physical activity promotes children’s development via effects on brain systems that underlie cognition and behavior. Animal studies show that aerobic exercise increases growth factors such as brain derived neurotrophic factor, leading to increased capillary blood supply to the cortex and growth of new neurons and synapses, resulting in better learning and performance (Dishman et al., 2006). Experimental and prospective cohort studies conducted with adults demonstrate that long-term regular physical activity alters human brain function (Colcombe et al., 2004; Weuve et al., 2004). A randomized, controlled experiment revealed that 6 months of aerobic exercise led to improved cognitive performance in older adults (Kramer et al., 1999). An important paper reports clear evidence for the impact of aerobic exercise on brain activity in adults in two studies using fMRI techniques: A cross-sectional comparison of high-fit to low-fit individuals showed that prefrontal cortex activity was related to physical fitness, and an experiment showed that 6 months of aerobic exercise (walking) in sedentary 55- to 77-year-olds increased prefrontal cortex activity and led to improvements on a test of executive function (Colcombe et al., 2004). Interestingly, a meta-analysis found no support for aerobic fitness as a mediator of the effect of physical activity on human cognition (Etnier, Nowell, Landers, & Sibley, 2006). Thus, rather than being mediated by cardiovascular benefits, the cognitive changes due to exercise may be a direct result of neural stimulation by movement. While the case has been made that physical activity may affect children’s cognitive function directly via changes in neural integrity, there are other plausible explanations, such as engagement in goal directed, effortful mental involvement (Tomporowski et al., 2008).
This study has limitations. The results are limited to a sample of overweight Black and White 7- to 11-year-old children. Lean children and those of other ethnicities or age groups may respond differently. It is unknown whether cognitive benefits persist after a period of detraining. If benefits accumulate over time, however, this would be important for child development. There may be sensitive periods during which motor activity would exert a particularly strong effect on the brain (Knudsen, 2004). It remains to be determined whether other types of exercise, such as strength training or swimming, are also effective. Participants and intervention staff could not be blinded to experimental condition or the study hypothesis; however, the recruitment materials emphasized physical health benefits rather than cognitive ones. Another limitation is that the use of a no-intervention control condition does not allow the trial to rule out some alternative explanations (e.g., attention from adults, enjoyment). Psychological changes may occur in children who participate in exercise because of social interactions that occur during the sessions rather than due to exercise per se. The dose response pattern of results belies this explanation, however, because both exercise groups spent equal time at the research facility with instructors and peers.
The study did not find a difference between the exercise dose groups. This does not conflict with the dose response finding, which shows that the exercise intervention caused an improvement in cognition (Hill, 1965). Given that the linear contrast demonstrated a graded effect of treatment, a pairwise dose comparison asks a follow-up question, whether one specific dose is superior to another (Ruberg, 1995). The test of the dose-response benefit to achievement was significant, but the comparison of the control group to the two exercise groups was not, providing partial support to the hypothesis that exercise improves mathematics achievement.
The fMRI results are limited by a small sample size and do not provide a test of dose response, which renders them more subject to alternative explanations. Nevertheless, specific changes were observed, and the direction of changes differed in prefrontal and parietal regions, arguing against a global trend in brain activity. Although antisaccade performance and its supporting brain activity change with age (Luna et al., 2001), this is an unlikely confounder because the groups were of similar age.
These experimental data offer evidence that a vigorous after school aerobic exercise program improved executive function in dose response fashion among overweight children; social factors may have contributed to this effect. Changes in corresponding brain activation patterns were observed. These results also provide partial support of a benefit to mathematics performance. The assignment of conditions was randomized and outcome evaluations blinded, minimizing potential bias or confounding. Overweight children now constitute over a third of U.S. children and are overrepresented among disadvantaged populations. Besides its importance for reducing health risks during a childhood obesity epidemic (Ogden et al., 2006), aerobic activity may prove to be an important method of enhancing aspects of children’s mental functioning that are central to cognitive development (Welsh, Friedman, & Spieker, 2006).
Acknowledgements
C.A. Boyle, C. Creech, J.P. Tkacz, and J.L. Waller assisted with data collection and analysis. Supported by NIH DK60692, DK70922, Medical College of Georgia Research Institute, a State of Georgia Biomedical Initiative grant to the Georgia Center for Prevention of Obesity and Related Disorders, and bridge funding from the Medical College of Georgia and University of Georgia.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea
Contributor Information
Catherine L. Davis, Georgia Prevention Institute, Pediatrics, Medical College of Georgia
Phillip D. Tomporowski, Department of Kinesiology, University of Georgia
Jennifer E. McDowell, Department of Psychology, University of Georgia
Benjamin P. Austin, Department of Psychology, University of Georgia
Patricia H. Miller, Department of Psychology, University of Georgia
Nathan E. Yanasak, Department of Radiology, Medical College of Georgia
Jerry D. Allison, Department of Radiology, Medical College of Georgia
Jack A. Naglieri, Department of Psychology, George Mason University
References
- Best JR, Miller PH, Jones LL. Executive function after age 5: Changes and correlates. Developmental Review. 2009;29(3):180–200. doi: 10.1016/j.dr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. School readiness. Integrating cognition and emotion in a neurobiological conceptualization of children's functioning at school entry. American Psychologist. 2002;57:111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Camchong J, Dyckman KA, Austin BP, Clementz BA, McDowell JE. Common neural circuitry supporting volitional saccades and its disruption in schizophrenia patients and relatives. Biological Psychiatry. 2008;64:1042–1050. doi: 10.1016/j.biopsych.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Dyckman KA, Chapman CE, Yanasak NE, McDowell JE. Basal ganglia-thalamocortical circuitry disruptions in schizophrenia during delayed response tasks. Biological Psychiatry. 2006;60:235–241. doi: 10.1016/j.biopsych.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Castelli DM, Hillman CH, Buck SM, Erwin HE. Physical fitness and academic achievement in third- and fifth-grade students. Journal of Sport and Exercise Psychology. 2007;29:239–252. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- Coe DP, Pivarnik JM, Womack CJ, Reeves MJ, Malina RM. Effect of physical education and activity levels on academic achievement in children. Medicine and Science in Sports and Exercise. 2006;38:1515–1519. doi: 10.1249/01.mss.0000227537.13175.1b. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Das JP, Mishra RK, Pool JE. An experiment on cognitive remediation of word-reading difficulty. Journal of Learning Disabilities. 1995;28:66–79. doi: 10.1177/002221949502800201. [DOI] [PubMed] [Google Scholar]
- Das JP, Naglieri JA, Kirby JR. Assessment of Cognitive Processes. Needham Heights, MA: Allyn & Bacon; 1994. [Google Scholar]
- Datar A, Sturm R, Magnabosco JL. Childhood overweight and academic performance: national study of kindergartners and first-graders. Obesity Research. 2004;12:58–68. doi: 10.1038/oby.2004.9. [DOI] [PubMed] [Google Scholar]
- Davis CL, Tomporowski PD, Boyle CA, Waller JL, Miller PH, Naglieri JA, et al. Effects of aerobic exercise on overweight children's cognitive functioning: a randomized controlled trial. Research Quarterly for Exercise and Sport. 2007;78:510–519. doi: 10.1080/02701367.2007.10599450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Dwyer T, Sallis JF, Blizzard L, Lazarus R, Dean K. Relation of academic performance to physical activity and fitness in children. Pediatric Exercise Science. 2001;13:225–237. [Google Scholar]
- Dwyer T, Coonan WE, Leitch DR, Hetzel BS, Baghurst PA. An investigation of the effects of daily physical activity on the health of primary school students in South Australia. International Journal of Epidemiology. 1983;12:308–313. doi: 10.1093/ije/12.3.308. [DOI] [PubMed] [Google Scholar]
- Dyckman KA, Camchong J, Clementz BA, McDowell JE. An effect of context on saccade-related behavior and brain activity. Neuroimage. 2007;36:774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Eigsti IM, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani MB, et al. Predicting cognitive control from preschool to late adolescence and young adulthood. Psychological Science. 2006;17:478–484. doi: 10.1111/j.1467-9280.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ. Conceptualizing, describing and measuring components of executive functions: A summary. In: Lyon GR, Krasnegor NA, editors. Attention, Memory and Executive Function. Baltimore: Paul H. Brooks Publishing Co; 1996. pp. 367–395. [Google Scholar]
- Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Research Reviews. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gutin B, Riggs S, Ferguson M, Owens S. Description and process evaluation of a physical training program for obese children. Research Quarterly for Exercise & Sport. 1999;70:65–69. doi: 10.1080/02701367.1999.10607731. [DOI] [PubMed] [Google Scholar]
- Hill AB. The Environment and Disease: Association or Causation? Proceedings of the Royal Society of Medicine. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Ismail AH. The effects of a well-organized physical education programme on intellectual performance. Research in Physical Education. 1967;1:31–38. [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. Neuroimage. 2005;25:899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ. Brain plasticity and behavior. Annual Review of Psychology. 1998;49:43–64. doi: 10.1146/annurev.psych.49.1.43. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, et al. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biological Psychiatry. 2002;51:216–223. doi: 10.1016/s0006-3223(01)01204-5. [DOI] [PubMed] [Google Scholar]
- McGrew KS, Woodcock RW. Woodcock-Johnson III: Technical Manual. Itasca, IL: Riverside Publishing Company; 2001. [Google Scholar]
- Morris JS, DeGelder B, Weiskrantz L, Dolan RJ. Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain. 2001;124(Pt 6):1241–1252. doi: 10.1093/brain/124.6.1241. [DOI] [PubMed] [Google Scholar]
- Must A, Tybor DJ. Physical activity and sedentary behavior: a review of longitudinal studies of weight and adiposity in youth. International Journal of Obesity (Lond) 2005;(29 Suppl 2):S84–S96. doi: 10.1038/sj.ijo.0803064. [DOI] [PubMed] [Google Scholar]
- Naglieri JA. Essentials of CAS Assessment. New York: Wiley; 1999. [Google Scholar]
- Naglieri JA, Das JP. Cognitive assessment system: Interpretive handbook. Itasca, IL: Riverside Publishing; 1997. [Google Scholar]
- Naglieri JA, Rojahn J. Construct validity of the PASS theory and CAS: Correlations with achievement. Journal of Educational Psychology. 2004;96:174–181. [Google Scholar]
- Naglieri JA, Rojahn JR, Aquilino SA, Matto HC. Black-white differences in cognitive processing: A study of the planning, attention, simultaneous, and successive theory of intelligence. Journal of Psychoeducational Assessment. 2005;23:146–160. [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA: The Journal of the American Medical Association. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P. Introduction: Methodologies and models in the study of executive function. In: Rabbit P, editor. Methodology of frontal and executive function. Hove, East Sussex, UK: Psychology Press Ltd; 1997. pp. 1–38. [Google Scholar]
- Rakison DH, Woodward AL. New perspectives on the effects of action on perceptual and cognitive development. Developmental Psychology. 2008;44:1209–1213. doi: 10.1037/a0012999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis JF, McKenzie TL, Kolody B, Lewis M, Marshall S, Rosengard P. Effects of health-related physical education on academic achievement: Project SPARK. Research Quarterly for Exercise & Sport. 1999;70:127–134. doi: 10.1080/02701367.1999.10608030. [DOI] [PubMed] [Google Scholar]
- Shephard RJ, Volle M, Lavallee H, LaBarre R, Jequier JC, Rajic M. Required physical activity and academic grades: A controlled longitudinal study. In: Ilmarinen J, Valimaki I, editors. Children and sport. Berlin: Springer Verlag; 1984. pp. 58–63. [Google Scholar]
- Shore SM, Sachs ML, Lidicker JR, Brett SN, Wright AR, Libonati JR. Decreased scholastic achievement in overweight middle school students. Obesity (Silver Spring) 2008;16:1535–1538. doi: 10.1038/oby.2008.254. [DOI] [PubMed] [Google Scholar]
- Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: A meta-analysis. Pediatric Exercise Science. 2003;15:243–256. [Google Scholar]
- Sommerville JA, Decety J. Weaving the fabric of social interaction: articulating developmental psychology and cognitive neuroscience in the domain of motor cognition. Psychonomic Bulletin & Review. 2006;13:179–200. doi: 10.3758/bf03193831. [DOI] [PubMed] [Google Scholar]
- Strong WB, Malina RM, Blimkie CJ, Daniels SR, Dishman RK, Gutin B, et al. Evidence based physical activity for school-age youth. Journal of Pediatrics. 2005;146:732–737. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Keedy SK, McDowell JE, Clementz BA. fMRI studies of eye movement control: investigating the interaction of cognitive and sensorimotor brain systems. Neuroimage. 2007;(36 Suppl 2):T54–T60. doi: 10.1016/j.neuroimage.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-Dimensional proportional system - An approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Taras H. Physical activity and student performance at school. Journal of School Health. 2005;75:214–218. doi: 10.1111/j.1746-1561.2005.00026.x. [DOI] [PubMed] [Google Scholar]
- Taras H, Potts-Datema W. Obesity and student performance at school. Journal of School Health. 2005;75:291–295. doi: 10.1111/j.1746-1561.2005.00040.x. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD, Davis CL, Miller PH, Naglieri J. Exercise and children's intelligence, cognition, and academic achievement. Educational Psychology Review. 2008;20:111–131. doi: 10.1007/s10648-007-9057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckman BW, Hinkle JS. An experimental study of the physical and psychological effects of aerobic exercise on schoolchildren. Health Psychology. 1986;5:197–207. doi: 10.1037//0278-6133.5.3.197. [DOI] [PubMed] [Google Scholar]
- Ward B. Simultaneous inference for FMRI data. Milwaukee, WI: Biophysics Research Institute, Medical College of Wisconsin; 1997. [Google Scholar]
- Welsh MC, Friedman SL, Spieker SJ. Executive functions in developing children: Current conceptualizations and questions for the future. In: McCartney K, Phillips D, editors. Blackwell Handbook of Early Childhood Development. Malden, MA: Blackwell Publishing; 2006. pp. 167–187. [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA: Journal of the American Medical Association. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Wittberg R, Northrup K, Cottrell LA, Davis CL. Aerobic fitness thresholds associated with fifth grade academic achievement. American Journal of Health Education. (Accepted) [Google Scholar]
- Zervas Y, Apostolos D, Klissouras V. Influence of physical exertion on mental performance with reference to training. Perceptual and Motor Skills. 1991;73:1215–1221. doi: 10.2466/pms.1991.72.3c.1215. [DOI] [PubMed] [Google Scholar]