Abstract
The 3′-azido-3′-deoxythymidine (AZT)-resistant pheno type of a heavily mutated human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) carrying a dipeptide (Ser-Ser) insertion between codons 69 and 70 as well as other mutations related to resistance to RT inhibitors has been studied. Recombinant virus carrying this variant RT (termed SS RT) showed reduced susceptibility to all nucleoside RT inhibitors in clinical use, particularly to AZT. In the presence of ATP, recombinant SS RT had an increased ability to remove the 3′-terminal nucleotide from AZT- terminated primers and extend the unblocked primer, compared with wild-type HIV-1 RT (BH10 isolate). Insertion of two serines in the sequence context of BH10 RT did not affect the ATP-dependent phosphorolytic activity of the enzyme, and had no influence in resistance to RT inhibitors. However, SS RT mutants lacking the dipeptide insertion or bearing a four-serine insertion showed reduced ATP-dependent phosphorolytic activity that correlated with increased AZT sensitivity, as determined using a recombinant virus assay. Therefore, the insertion appears to be critical to enhance AZT resistance in the sequence context of multidrug-resistant HIV-1 RT.
Keywords: ATP-dependent phosphorolysis/AZT resistance/HIV-1/pyrophosphorolysis/reverse transcriptase
Introduction
Human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) is a heterodimeric enzyme composed of two subunits of 66 and 51 kDa, respectively, which is required to convert the viral genomic RNA into double-stranded DNA that then integrates into the host cell genome. HIV-1 RT is an important target for antiretroviral therapy (for recent reviews see Balzarini, 1999; Jonckheere et al., 2000). Several nucleoside analog RT inhibitors have been approved for clinical treatment of HIV-1-infected patients: 3′-azido-3′de- oxythymidine (AZT; zidovudine), 2′,3′dideoxyinosine (ddI; didanosine), 2′,3′-dideoxycytidine (ddC; zalcitabine), 2′,3′-dideoxy-3′-thiacytidine (3TC; lamivudine), 2′,3′-didehydro-2′,3′-dideoxythymidine (d4T; stavudine) and a dideoxyguanosine derivative (abacavir).
Despite being the first anti-HIV drug to be approved, AZT is still widely used in the clinical setting in combination with other antiretroviral drugs. Its mode of action involves its conversion to AZT triphosphate (AZTTP) in a process mediated by cellular kinases. AZTTP is a competitive inhibitor of RT and once incorporated into DNA it brings about chain termination due to its lack of a 3′-hydroxyl group. Prolonged clinical use of AZT monotherapy invariably results in the appearance of virus resistant to the drug. High-level resistance to AZT is correlated with multiple mutations in the RT, including D67N, K70R, T215F or T215Y and K219Q, and in some cases, M41L and L210W. Virus having the quadruple mutant of RT (D67N, K70R, T215F/Y and K219Q) is >100-fold less sensitive to AZT than the wild-type (WT) virus in cell culture studies (Kellam et al., 1992). The biochemical mechanism of resistance has been difficult to investigate because recombinant RTs having the four mutations related to loss of sensitivity to AZT showed similar kinetics of nucleotide incorporation and inhibitor sensitivity to the WT enzyme in in vitro assays (Lacey et al., 1992; Carroll et al., 1994; Kerr and Anderson, 1997; Krebs et al., 1997). Alterations in template–primer interactions with RT have been implicated in AZT resistance. Thus, it has been reported that the quadruple mutant D67N/K70R/T215Y/K219Q displays higher processivity than the WT RT (Caliendo et al., 1996). This effect appears to be mediated by mutations at codons 215 and 219 (Arion et al., 1998). Since AZT is incorporated in the growing DNA chain, inhibition could be mitigated by an effective mechanism to remove the chain-terminated residue. HIV-1 RT is devoid of a 3′–5′ exonuclease proofreading activity, but recent studies indicate that HIV-1 resistance to AZT involves an increased ability of the mutant viral RT to excise chain-terminating AZT from the primer 3′-terminus.
Removal of AZT-terminating nucleotides can be accomplished by transfer of the 3′ nucleotide from the primer to a nucleoside di- or triphosphate acceptor (ribonucleotide-dependent phosphorolysis) (Meyer et al., 1998, 1999), or by phosphorolytic cleavage of the terminal AZT, mediated by pyrophosphate (PPi) (pyrophosphorolysis) (Arion et al., 1998). The fingers subdomain of RT appears to play a role in AZT removal mediated by both mechanisms, since mutant RTs carrying the amino acid substitutions D67N and K70R showed enhanced ability to extend AZT-terminated primers in the presence of ATP or PPi. AZT-resistant RT binds more tightly to the 3′ end of the AZT-terminated primer compared with the WT RT, allowing a more efficient removal of the blocked primer terminus (Canard et al., 1998).
Treatment with multiple nucleoside inhibitors of RT can lead to accumulation of specific drug resistance mutations, including those described above. However, multiple nucleoside resistance can also appear through the acquisition of a cluster of five mutations in the RT coding region, which include A62V, V75I, F77L, F116Y and Q151M (Shirasaka et al., 1995). Virus harboring these amino acid substitutions is resistant to AZT, ddI, ddC and d4T. In this case, the presence of the substitution Q151M at the dNTP binding site of the RT in the palm subdomain produced an 8-fold increase in the IC50 for AZTTP in assays carried out with recombinant enzyme (Ueno et al., 1995; Ueno and Mitsuya, 1997). The acquisition of additional mutations at positions 62, 75, 77 and 116 led to RTs displaying higher IC50 values for AZT and other nucleoside analog inhibitors. From these studies, it was concluded that unlike the case of the quadruple RT mutants obtained during AZT monotherapy (D67N, K70R, T215F/Y and K219Q), resistance mediated by A62V, V75I, F77L, F116Y and Q151M mutations was related to substrate recognition.
An insertion of two amino acids (often Ser-Ser, Ser-Gly or Ser-Ala) between residues 69 and 70 of HIV-1 RT has recently been described in patients subjected to prolonged therapy with AZT, often together with (or followed by) administration of other nucleoside inhibitors (De Antoni et al., 1997; Tamalet et al., 1998; Winters et al., 1998; Briones and Soriano, 1999; de Jong et al., 1999; Larder et al., 1999; Ross et al., 1999; Sugiura et al., 1999; Briones et al., 2000). The insertion is located in the β3–β4 hairpin loop at the fingers subdomain of the RT, and appears to be associated with multiple amino acid substitutions, including AZT-resistance mutations, such as T215Y. Drug-resistance tests of recombinant patient isolates showed reduced sensitivity to nearly all nucleoside RT inhibitors (Winters et al., 1998; Larder et al., 1999). In this paper, we report the comparison of the catalytic properties and inhibitor susceptibility of an RT derived from a heavily treated patient, and carrying a Ser-Ser insertion between codons 69 and 70, and WT HIV-1 RT (BH10 isolate). A series of mutants involving deletions and insertions at codons 69–70 has been constructed in the sequence background of both BH10 and patient-derived RT, in order to assess the influence of the insertion on drug resistance and polymerase activity. The phenotypic mechanism of AZT resistance displayed by the insertion-containing RT appears to be mediated by an ATP-dependent phosphorolytic mechanism that facilitates the efficient removal of the 3′-terminal nucleotide from AZT-terminated primers.
Results
The RT of 0.8% of HIV-1 isolates from a cohort of patients subjected to prolonged antiretroviral therapy contained a dipeptide insertion between positions 69 and 70 (Briones and Soriano, 1999; Briones et al., 2000). Viral RNA from one of these patients who no longer responded to any drug combination was used as starting material for cloning and expressing the variant RT used in this study. Differences between the RT of this patient (SS RT) and that of isolate BH10 include the insertion of two serines between residues 69 and 70, as well as 44 additional mutations scattered throughout the entire RT coding sequence (Figure 1). Several amino acid substitutions found in the SS RT have been related to drug resistance. M41L, A62V, K70R, L74I, V118I, M184I, L210W, T215Y and G333E have been involved in resistance to nucleoside inhibitors, and Y181C is known to confer high-level resistance to nevirapine and other non-nucleoside inhibitors of RT (Balzarini, 1999 and references therein). The role of the Ser-Ser insertion in RT activity and inhibitor sensitivity was analyzed by engineering replacements in the sequence context of both BH10 and SS RTs. We prepared three mutants of BH10 RT: T69S, T69SSS and T69SSW. The first mutant involves the substitution of Thr69 by Ser, while in the last two cases, Thr69 was replaced by Ser-Ser-Ser or Ser-Ser-Trp, respectively (Figure 1). On the other hand, two mutants of SS RT were obtained (2S0S and 2S4S). The sequences at positions 69–70 of mutants 2S0S and 2S4S were Ser-Arg and Ser-(Ser)4-Arg, instead of the Ser-(Ser)2-Arg sequence found in SS RT (Figure 1).
Fig. 1. Amino acid sequence differences between WT BH10 and SS RTs. Only those amino acid positions where sequence differences are found between both enzymes are indicated. The amino acid sequences around positions 69/70 of mutants used in this work and having insertions, deletions or substitutions are shown above and below the alignment.
Steady-state kinetic constants
Kinetic parameters for the incorporation of dTTP and AZTTP were determined with a 47/25mer template–primer described in Materials and methods. The catalytic efficiency (kcat/Km) of incorporation of dTTP was similar for the WT BH10 RT and for mutants T69S and T69SSS (Table I). The mutant T69SSW displayed 10-fold reduced catalytic efficiency for the incorporation of dTTP. Similar reductions were observed with SS RT, as well as with mutants 2S0S and 2S4S. Since kcat values for the incorporation of dTTP were similar for all tested enzymes, the effects on the catalytic efficiency were attributed to the increased Km values obtained with T69SSW, SS, 2S0S and 2S4S (Table I). WT BH10 RT and mutant 2S0S showed a kcat/Km of nucleotide incorporation that was 2.8-fold higher for dTTP than for AZTTP. This ratio was moderately increased in the case of SS RT and the T69SSS mutant, whose catalytic efficiencies of dTTP incorporation were 7.7- and 13.5-fold higher than their corresponding kcat/Kms of AZTTP incorporation, respectively.
Table I. Steady-state kinetic constants for incorporation of dTTP and AZTTP of wild-type and mutant RTsa.
| Enzyme | Substrate | Km (nM) | kcat (min–1) | kcat/Km (nM–1 min–1) | kcat/Km relativeb |
|---|---|---|---|---|---|
| WT BH10 | dTTP | 102.0 ± 1.2 | 3.19 ± 0.40 | 3.1 × 10–2 | 1 |
| AZTTP | 523 ± 59 | 5.80 ± 0.42 | 1.1 × 10–2 | 0.35 (1) | |
| T69S | dTTP | 78.5 ± 2.7 | 2.59 ± 0.20 | 3.3 × 10–2 | 1.06 |
| T69SSS | dTTP | 184 ± 13 | 5.99 ± 2.32 | 3.3 × 10–2 | 1.06 |
| AZTTP | 2009 ± 792 | 4.91 ± 1.85 | 2.4 × 10–3 | 0.078 (0.22) | |
| T69SSW | dTTP | 807 ± 54 | 2.81 ± 0.16 | 3.4 × 10–3 | 0.11 |
| SS | dTTP | 1011 ± 11 | 5.46 ± 0.83 | 5.4 × 10–3 | 0.17 |
| AZTTP | 6971 ± 982 | 4.90 ± 0.16 | 7.0 × 10–4 | 0.022 (0.063) | |
| 2S0S | dTTP | 613 ± 193 | 2.04 ± 1.15 | 3.3 × 10–3 | 0.11 |
| AZTTP | 6377 ± 882 | 7.75 ± 1.49 | 1.2 × 10–3 | 0.039 (0.11) | |
| 2S4S | dTTP | 1033 ± 402 | 4.06 ± 0.76 | 3.9 × 10–3 | 0.13 |
aD2-47/PG5-25 was used as template–primer. Elongation reactions were incubated for 20 s for the incorporation of T. Data shown are the mean values ± standard deviation obtained from a non-linear least-squares fit of the kinetics data to the Michaelis–Menten equation.
bThe relative catalytic efficiencies were calculated as the ratio between the kcat/Km value obtained with each enzyme and substrate and the kcat/Km value obtained with WT BH10 and dTTP. The values in parentheses are referred to as the catalytic efficiency of incorporation of AZTTP by the WT BH10 enzyme.
Processivity
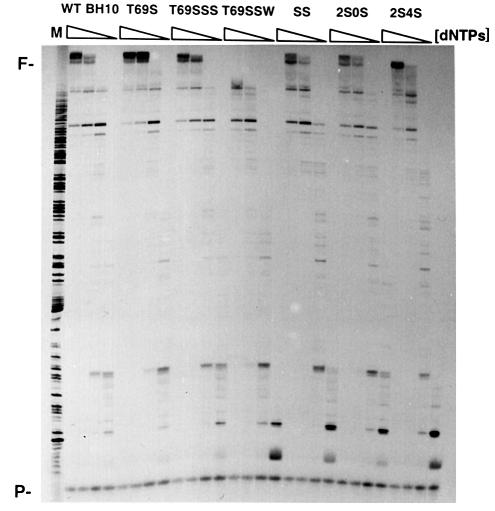
Qualitative measurements of the processivity of RTs were carried out with the 20 nucleotide DNA primer pT, annealed to heteropolymeric M13mp2 single-stranded DNA, in the presence of various dNTP concentrations. All enzymes, except T69SSW, were able to generate long extension products at the highest dNTP concentration (50 µM), used here as a control of maximum DNA elongation for each enzyme. At limiting dNTP concentrations (1 and 0.1 µM), SS RT and mutants 2S0S and 2S4S were less processive than the BH10-related RTs (except T69SSW) (Figure 2). These results are consistent with the kinetic parameters. The lower Km values observed with BH10 RT and mutants T69S and T69SSS could facilitate a more efficient primer extension in the presence of low concentrations of dNTP.
Fig. 2. DNA polymerization processivity of WT BH10 and mutant RTs. DNA synthesis primed by the 20 nucleotide primer pT on M13mp2 ssDNA was carried out in the presence of a mixture of the four dNTPs at 50, 10, 1 and 0.1 µM concentration each. Products synthesized by WT BH10, T69S, T69SSS, T69SSW, SS, 2S0S and 2S4S RTs in the presence of DNA trap were resolved in a denaturing 6% polyacrylamide gel. M, DNA markers; P, primer; F, full-length product.
Effect of sodium PPi on the rate of pyrophosphorolysis
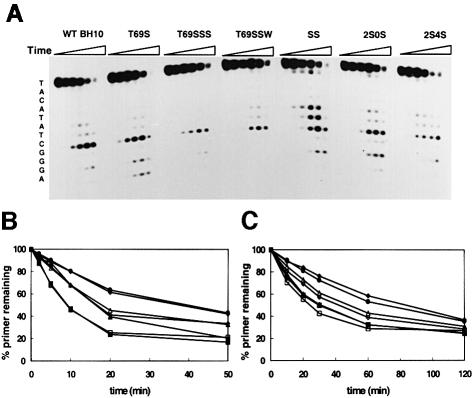
RTs utilized PPi to hydrolyze the 25 nucleotide primer of the template–primer complex D2-47/PG5-25 (Figure 3A). In the absence of added dNTP, the lowest rate of pyrophosphorolysis was observed with mutants T69SSS and T69SSW. The time dependence of pyrophosphorolysis was investigated at two concentrations of sodium PPi. At the highest PPi concentration tested (1 mM), WT BH10 and SS RTs displayed the highest rate of pyrophosphorolysis (Figure 3B), while at 150 µM, the rates of pyrophosphorolysis were similar for SS RT, T69S and WT BH10, and higher than for mutants 2S0S and 2S4S (Figure 3C). The lowest rate of pyrophosphorolysis at both concentrations of sodium PPi was observed with mutants T69SSS and T69SSW (Figure 3B and C). In addition to the relatively small differences in the pyrophosphorolysis rate, the sensitivity to PPi was similar for all tested RTs. The IC50 values for PPi showed <2-fold differences between any pair of studied RTs carried out with the D2-47/PG5-25 duplex using a 100 nM concentration of dNTP. The IC50 values obtained were 536.3 ± 6.5 µM (for WT BH10), 516.4 ± 4.2 µM (for T69S), 593.5 ± 22.9 µM (for T69SSS), 729.5 ± 71.9 µM (for T69SSW), 818.9 ± 18.82 µM (for SS), 720.8 ± 114.4 µM (for 2S0S) and 860.8 ± 34.5 µM (for 2S4S).
Fig. 3. Time course of pyrophosphorolysis catalyzed by the studied RTs. Reactions were carried out in 50 mM HEPES pH 7.0, containing 15 mM NaCl, 15 mM magnesium aspartate, 130 mM potassium acetate, 1 mM DTT and 5% polyethylene glycol 6000, in the absence of dNTP. (A) Results of experiments carried out after addition of sodium PPi to 1 mM. For each enzyme, six lanes are shown, which correspond to aliquots taken after incubating the samples at 37°C for 0, 2, 5, 10, 20 and 50 min, respectively. The sequence of the primer is shown on the left. Plots indicating the amount of the 25 nucleotide primer remaining as a function of incubation time of pyrophosphoro lysis are shown for assays carried out with 1 mM (B) and 150 µM (C) sodium PPi. Filled squares, WT BH10; filled triangles, T69S; filled circles, T69SSS; filled diamonds, T69SSW; open squares, SS; open triangles, 2S0S; open diamonds, 2S4S.
Unblocking the AZT-terminated primer
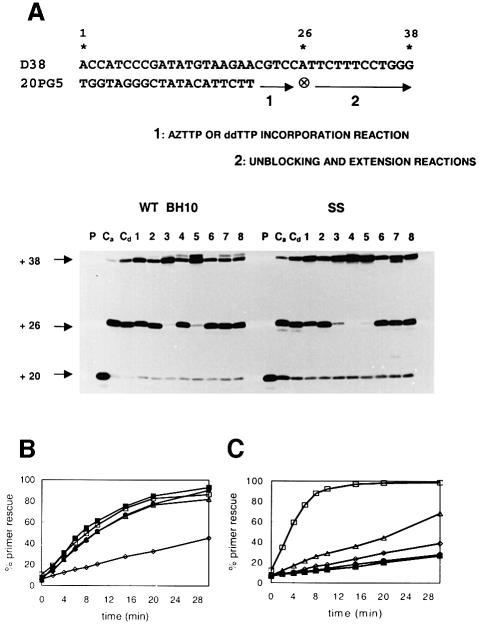
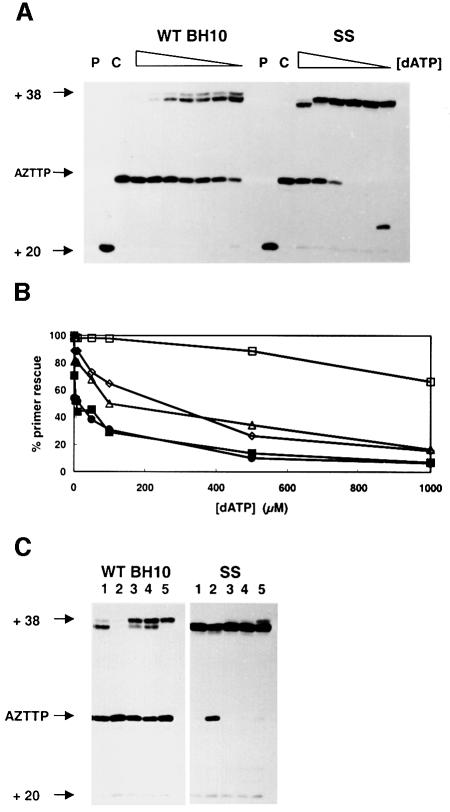
Removal of AZT-monophosphate from a chain-terminated primer has been identified as a critical event in resistance to AZT (Arion et al., 1998; Meyer et al., 1998). We determined the ability of the RTs to rescue AZT- and dideoxythymidine (ddT)-terminated primers in the presence of sodium PPi (150 µM) and/or ATP (3.2 mM). In these experiments, a 38/20mer template–primer (D38/20PG5) with an 18 nucleotide overhang containing only one A located at position +6 was extended to form a 38/26mer template–primer with an AZT- or a ddT-terminated primer (Figure 4A). Incubation of WT BH10 and SS RTs in the presence of dATP, dCTP, dGTP and AZTTP led to the formation of an AZT-terminated product of 26 nucleotides (Figure 4A, lane Ca). Further addition of 100 µM dTTP allowed the removal of a relatively low amount of AZT-monophosphate to complete the extension reaction (Figure 4A, compare lane 1 with lane Ca). This effect was observed with both WT BH10 and SS RTs. In contrast, ddT-terminated primers (Figure 4A, lane Cd) were not efficiently unblocked and extended once dTTP was added to the reaction mixture (compare lane 2 with lane Cd). Furthermore, WT BH10 and SS RTs were not able to catalyze efficiently the formation of full-length product (38 nucleotides) in the presence of physiological concentrations of sodium PPi (Figure 4A, lane 6), ATP (lane 7) or both (lane 8), when removal of ddTMP at the 3′ end of the primer was required to complete the reaction. Interestingly, with the same reaction conditions used for unblocking and extending the ddT-terminated primer, both enzymes were able to rescue >95% of the AZT-terminated primer, in the presence of PPi, ATP or both, except in the case of WT BH10 RT when assays were carried out with 3.2 mM ATP (Figure 4A, lanes 3–5). In the presence of 150 µM sodium PPi, the rate of primer rescue and further extension was similar for all the enzymes assayed except for 2S4S (Figure 4B). In the presence of 3.2 mM ATP, the rates of 38 nucleotide DNA product formation were higher for SS, 2S0S and 2S4S RTs than for the other RTs, SS being the most efficient enzyme (Figure 4C). It is interesting to note that the rescue reaction catalyzed by WT BH10 RT and mutant T69SSS was ∼3-fold more efficient in the presence of PPi than in the presence of ATP, while primer rescue mediated by ATP-dependent phosphorolysis was significantly enhanced in the case of SS RT (compare the corresponding primer rescue reaction kinetics for each enzyme, shown in Figure 4B and C).
Fig. 4. Rescue DNA polymerization initiated from AZT- or ddT-terminated primers. (A) Reactions were carried out with a 38/20mer heteropolymeric template–primer (sequence shown at the top), which contained only one A in the 18mer template overhang (at position +6). AZT- or ddT-terminated primers were obtained after incubating the WT BH10 or SS RTs with the template–primer for 45 min at 37°C, in the presence of dATP, dCTP and dGTP (at 100 µM each), and 25 µM AZTTP (lane Ca) or 25 µM ddTTP (lane Cd) (AZTTP or ddTTP incorporation reaction). Removal of AZT-monophosphate and further extension of the primer were carried out after addition of dTTP to a final concentration of 100 µM, in the absence of sodium PPi and ATP (lane 1), or in the presence of 150 µM sodium PPi (lane 3), 3.2 mM ATP (lane 4), or both 150 µM sodium PPi and 3.2 mM ATP (lane 5) (unblocking and extension reactions). The corresponding rescue DNA polymerization reactions initiated from ddT-terminated primers are shown in lanes 2, 6, 7 and 8, respectively. The positions of the unextended primer (+20), the ddT- or AZT-terminated primer (+26), and the fully extended product (+38) are indicated on the left. P, unextended primer. Time courses of the rescue reactions obtained in the presence of 150 µM sodium PPi and 3.2 mM ATP are given in (B) and (C), respectively. For WT BH10 and SS RTs, the amount of primer rescued after a 30 min incubation in the presence of PPi or ATP is shown in lanes 3 and 4, respectively, in (A). Filled squares, WT BH10; filled circles, T69SSS; open squares, SS; open triangles, 2S0S; open diamonds, 2S4S.
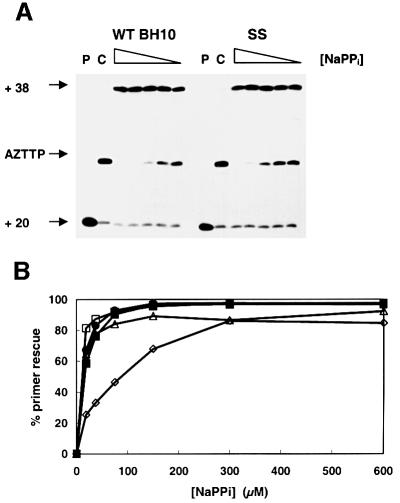
The efficiency of rescue reactions carried out in the presence of PPi was similar for WT BH10 and SS RTs (Figure 5A). WT BH10, T69SSS, 2S0S and SS were able to rescue the AZT-terminated primer very efficiently at PPi concentrations >100 µM (Figure 5B). However, mutant 2S4S showed significantly reduced ability to unblock and extend AZT-terminated primers in the presence of PPi concentrations <200 µM (Figure 5B). The concentration of PPi needed to rescue 50% of the AZT-terminated primer in our assay conditions was 7.7 µM for SS RT, 10.5 µM for mutant 2S0S, 11.3 µM for mutant T69SSS, 12.9 µM for WT BH10 RT and 73.7 µM for mutant 2S4S (Figure 5B).
Fig. 5. Effect of sodium PPi on the efficiency of the AZT-terminated primer rescue reaction. (A) Rescue reactions initiated from AZT-terminated primers by addition of dTTP, in the presence of different concentrations of sodium PPi (300, 150, 75, 37.5 and 18.75 µM). P shows the unextended 20 nucleotide DNA primer and C corresponds to AZT-terminated control reactions. (B) AZT-terminated primer rescue values (as a percentage) for WT BH10 and SS RTs, and their mutants T69SSS, 2S0S and 2S4S, as a function of different concentrations of NaPPi. Filled squares, WT BH10; filled circles, T69SSS; open squares, SS; open triangles, 2S0S; open diamonds, 2S4S.
AZT-terminated primer rescue can be specifically inhibited by the dNTP complementary to the next position on the template (Tong et al., 1997; Meyer et al., 1998). The next complementary base to be incorporated at the 3′ end of the AZT-terminated primer in the 38/26mer complex is A (Figure 4A). Rescue reactions catalyzed by WT BH10 RT in the presence of ATP were ∼1000-fold more sensitive to dATP inhibition than those catalyzed by the SS RT (Figure 6A). The 50% inhibitory concentrations of dATP in the primer rescue reactions were 8.8 µM for WT BH10 RT, 12 µM for mutant T69SSS, 147.4 µM for mutant 2S4S, 185.6 µM for mutant 2S0S and >1 mM for SS RT (Figure 6B). Other dNTPs, such as dCTP, dGTP or dTTP, had no inhibitory effect on the primer rescue reaction (Figure 6C). Unlike in the case of ATP-dependent phosphorolysis, the concentration of dATP required to achieve 50% inhibition of the rescue reaction in the presence of 150 µM PPi was >0.5 mM and similar for both WT BH10 and SS RTs (data not shown). No significant inhibition was observed in reactions carried out in the presence of 100 µM dATP.
Fig. 6. Inhibition of ATP-dependent phosphorolysis by the next complementary nucleotide at the 3′ end of the AZT-terminated primer. (A) Rescue reactions initiated from AZT-terminated primers by addition of dTTP, in the presence of 3.2 mM ATP, and different concentrations of dATP (1000, 500, 100, 50, 10, 5 and 1 µM for WT BH10 RT, and 10 000, 1000, 500, 100, 10 and 1 µM for SS RT). (B) AZT-terminated primer rescue values (as percentages) for WT BH10 and SS RTs, and their mutants T69SSS, 2S0S and 2S4S, in the presence of different concentrations of dATP. Filled squares, WT BH10; filled circles, T69SSS; open squares, SS; open triangles, 2S0S; open diamonds, 2S4S. (C) Inhibitory effect of dNTPs on the rescue reaction mediated by ATP. Twenty-nucleotide primers were AZT-terminated by adding AZTTP at 25 µM, and dATP, dCTP and dGTP at 100 µM final concentrations. Then, AZT-terminated primers were unblocked and further elongated by adding dTTP and ATP (at 100 µM and 3.2 mM final concentration, respectively), in the absence (lane 1), or in the presence of 1 mM dATP (lane 2), 1 mM dCTP (lane 3), 1 mM dGTP (lane 4) or 1 mM dTTP (lane 5).
Viability of recombinant HIV-1 variants and resistance to nucleoside analog inhibitors
WT BH10, SS and their corresponding mutant RTs were introduced in an infectious HIV-1 clone using a recombinant virus assay. Following transfection, virus was cultured and production of p24 antigen was monitored. All virus recovered from transfections, except the one carrying the T69SSW mutation in the RT, was able to infect and replicate in SupT1 cells. Growth curves were almost identical for all viable virus (data not shown). Recombinant HIV-1 clones were assayed to establish the level of resistance to drugs in clinical use (Table II). Interestingly, WT BH10 RT and its mutants T69S and T69SSS were sensitive to AZT and other nucleoside analog inhibitors of RT. In contrast, SS RT and its mutants 2S0S and 2S4S show intermediate to high-level resistance to all tested nucleoside analogs. None of the RTs showed highly significant resistance to phosphonoformic acid (PFA; Foscarnet). IC50 values for this PPi analog were <4.5 times higher for all tested enzymes than for the WT BH10 RT. The highest levels of phenotypic resistance were obtained with AZT in assays carried out with SS RT and mutant 2S0S, which showed a 786- and 143-fold increase in their IC50 values, respectively, compared with the WT BH10 enzyme. Mutant 2S4S showed a small increase in the IC50 value. These results suggest that the insertion of two serines is critical to achieve the high-level resistance to AZT found with SS RT.
Table II. Susceptibility of HIV-1 constructs to nucleoside analog RT inhibitors.
| Enzyme | IC50 (µM)a |
|||||
|---|---|---|---|---|---|---|
| AZT | ddC | ddI | 3TC | d4T | PFA | |
| WT BH10 | 6.6 × 10–3 | 0.23 | 1.26 | 0.56 | 0.24 | 46.2 |
| T69S | 2.4 × 10–3 (0.4×) | 0.12 (0.5×) | 0.51 (0.4×) | 0.22 (0.4×) | 0.54 (2.2×) | 95.8 (2.1×) |
| T69SSS | 2.2 × 10–3 (0.3×) | 0.14 (0.6×) | 1.91 (1.5×) | 1.84 (3.3×) | 0.26 (1.1×) | 198.2 (4.3×) |
| SS | 5.17 (786×) | 1.10 (4.7×) | 13.4 (10.6×) | >20 | 2.23 (9.1×) | 79.9 (1.7×) |
| 2S0S | 0.94 (143×) | 2.86 (12.2×) | 11.3 (8.9×) | >20 | 5.94 (24.2×) | 49.8 (1.1×) |
| 2S4S | 0.024 (3.6×) | 0.38 (1.6×) | 6.27 (5×) | >20 | 0.47 (1.9×) | 130 (2.8×) |
aIC50 values represent the mean of 2–4 tests, each one performed in sextuplicate. The fold increase in IC50 relative to wild-type HXB2 virus control carrying the RT sequence of BH10 is shown in parentheses.
Discussion
One of the major problems for AIDS control is the ability of HIV-1 to develop resistance to antiretroviral compounds used individually or in combination (Balzarini, 1999 and references therein). Sequential monotherapy or combination therapy with several nucleoside RT inhibitors can lead to the appearance of multidrug-resistant strains. These isolates can accumulate resistance mutations specific for AZT (e.g. M41L, D67N, L210W or T215Y) and other RT inhibitors (e.g. K65R for ddC, L74V for ddI, etc.), which arise during monotherapy. Alternatively, multidrug-resistant HIV-1 strains containing the amino acid substitutions A62V, V75I, F77L, F116Y and Q151M may appear under combination therapy with nucleoside RT inhibitors (Shirasaka et al., 1995). Recently, a different multidrug resistance pattern of amino acid substitutions has been observed in a number of patients who were exposed to AZT, ddI, ddC and/or d4T in prolonged therapeutic regimens. Substitution of Thr69 by Ser and insertion of two residues (most frequently, Ser-Ser, Ser-Gly or Ser-Ala) between codons 69 and 70 occurred in the RT of these isolates. The insertion per se did not confer significant resistance to nucleoside RT inhibitors (Winters et al., 1998; Larder et al., 1999; this study). However, in the presence of substitutions T215Y and M41L or L210W, these virus isolates manifested resistance to most nucleoside analog RT inhibitors, and particularly to AZT (Winters et al., 1998; Larder et al., 1999). A survey of 67 published sequences carrying the insertion at codons 69/70 (De Antoni et al., 1997; Tamalet et al., 1998, 2000; Winters et al., 1998; Briones and Soriano, 1999; de Jong et al., 1999; Ross et al., 1999; Sugiura et al., 1999; Briones et al., 2000) reveals that in the vast majority of cases, virus harbored AZT-resistance mutations that had arisen during monotherapy with AZT. The most frequent amino acid changes were T215Y (97%), M41L (60%) and L210W (52%). Other substitutions related to monotherapeutic treatments with other nucleoside RT inhibitors were also frequently found (e.g. the 3TC-specific mutations M184I/V and the ddI-specific mutation L74V, in 40 and 15% of the isolates, respectively). Interestingly, none of the analyzed RTs contained the characteristic AZT-resistance substitution D67N, with Asp, Gly and Glu being the only residues found at this position.
We have cloned, expressed and purified an RT that was obtained from a heavily treated patient. This RT (SS RT) contains a Ser-Ser insertion and a series of accompanying mutations including M41L, K70R, L210W and T215Y, which have been related to AZT resistance. Recombinant virus constructed with the SS RT showed high-level resistance to AZT, remarkable resistance to 3TC, and intermediate resistance to ddI, ddC and d4T, in agreement with previously reported data (Winters et al., 1998; Larder et al., 1999). Here, we show that structural modifications at the insertion region lead to virus with significant alterations in their levels of AZT resistance. Thus, removal of the two serines in SS RT rendered virus showing a 5-fold reduction of the IC50 value for AZT, while the introduction of two additional serines at positions 69–70 almost abrogated AZT resistance (Table II). The nucleotide sequence around positions 69/70 of the SS RT coding region is ‘…GATAGTTCTAGTTCTAGA…’, which corresponds to the amino acid sequence ‘…Asp-Ser-Ser-Ser-Ser-Arg…’. The duplication of the two underlined codons results in the introduction of the two-serine insertion. Although the molecular mechanism leading to the appearance of this duplication is not known, our results clearly indicate that further duplication events (e.g. insertion of two additional serines) would confer a great disadvantage in terms of virus replication in the presence of AZT.
The biochemical mechanism underlying AZT resistance appears to be different for variants arising during monotherapy (e.g. the quadruple mutant D67N/K70R/T215F/K219Q) than for enzymes having the multidrug-resistance marker Q151M. Since Gln151 is part of the dNTP binding site of HIV-1 RT, located close to the sugar ring of the incoming dNTP in the palm subdomain, mutations at this position affect the inhibitor binding properties of the mutated RT (Ueno et al., 1995; Ueno and Mitsuya, 1997), while in the case of the quadruple mutant, kinetic parameters for AZTTP and dTTP incorporation appear to remain unchanged compared with the WT enzyme (Lacey et al., 1992). Our results show that SS RT is slightly less efficient than the WT BH10 RT in incorporating dTTP or AZTTP. As reported for the quadruple mutant D67N/K70R/T215F/K219Q (Lacey et al., 1992; Carroll et al., 1994; Kerr and Anderson, 1997; Krebs et al., 1997), the phenotypic resistance to AZT of SS RT cannot be explained solely on the basis of competition between AZTTP and dTTP for incorporation into DNA primer. Interestingly, neither the substitution of Thr69 by Ser nor the substitution of Thr69 by Ser-Ser-Ser led to significant differences in the kinetic parameters or DNA-dependent DNA polymerase processivity compared with the WT BH10 RT. These results are broadly in agreement with a previous characterization of WT RT mutants having the sequences Ser-Ser-Ser-Arg, Ser-Ser-Gly-Arg, Thr-Ser-Ser-Lys and Thr-Ser-Gly-Lys instead of Thr-Lys at positions 69–70 (Boyer et al., 1999). None of these mutants showed large alterations in the kinetic parameters, in assays carried out with homopolymeric template–primers, with relatively small differences in processivity between the analyzed mutants. Our results show that removal or duplication of the two-serine insertion of SS RT did not produce large alterations of the steady-state kinetic parameters. Although the insertion does not appear to be critical for dNTP recognition, it must be emphasized that the BH10 RT mutant T69SSW has a diminished catalytic efficiency due to its higher Km value for dNTP incorporation. In addition to its low catalytic efficiency of nucleotide incorporation, mutant T69SSW displayed reduced processivity, and virus harboring the sequence Ser-Ser-Trp instead of Thr69 in its RT was not viable, as determined with a recombinant virus assay. Our results suggest that there are structural constraints that limit the acceptability of large residues between codons 69 and 70. The binding of the template–primer and dNTP induces significant conformational changes in the fingers and thumb subdomains of p66, as reported for other polymerases (Sarafianos et al., 1999 and references therein). Conformational changes at the dNTP binding site produced by drug resistance mutations could also mediate in the mechanism of AZT resistance (Ren et al., 1998). The presence of small residues at the tip of the fingers subdomain would be required to maintain the flexibility of this region, which is necessary to preserve RT function.
Alterations in template–primer interactions have been correlated with AZT resistance. Thus, it has been reported that the D67N/K70R/T215F/K219Q mutant displayed an increased processivity of RNA- and DNA-directed DNA synthesis compared with the WT RT (Caliendo et al., 1996; Arion et al., 1998). The increased processivity shown by the quadruple mutant could be a compensatory effect for its increased pyrophosphorolytic activity. In the case of the insertion-containing RTs, we found no differences in DNA polymerase processivity between any of the enzymes described in this work, except for the T69SSW mutant. The reduced processivity observed for SS RT and mutants 2S0S and 2S4S, using limiting concentrations of dNTPs, appears to be related to their reduced efficiency of nucleotide incorporation.
Recently published evidence reveals that phosphorolytic cleavage of the terminal AZT, mediated either by PPi (Arion et al., 1998, 2000) or by ATP (Meyer et al., 1998, 1999), constitutes the primary mechanism of HIV-1 resistance to AZT. Mutant RTs harboring the substitutions D67N/K70R and D67N/K70R/T215F/K219Q showed enhanced sensitivity to PPi as well as an increased rate of phosphorolysis mediated by PPi or ATP in comparison with mutant T215F/K219Q and WT RTs (Arion et al., 1998; Meyer et al., 1999). In our study, all tested RTs except 2S4S were able to rescue AZT-terminated primers with a similar efficiency in the presence of physiological concentrations of PPi (150 µM), and primer rescue reactions catalyzed by WT BH10 or SS RTs were not significantly inhibited by concentrations of dATP as high as 100 µM. In addition, we did not observe large differences between WT BH10 and SS RTs in their pyrophosphorolytic activity and sensitivity to PPi inhibition.
Our results suggest that ribonucleotide-mediated pyro phosphorolysis is the most important mechanism underlying AZT resistance in the case of the multidrug-resistant SS RT. Unblocking and subsequent extension of the primer in the presence of ATP were much more efficiently catalyzed by the heavily mutated SS RT than by the WT BH10 RT. When AZT-terminated primer rescue reactions were carried out in the presence of different concentrations of the next complementary dNTP (in our assay conditions, dATP), SS RT was able to eliminate the inhibitor at a 100-fold higher dATP concentration than the WT BH10 RT. Interestingly, removing the two-serine insertion at codons 69/70, or increasing its size by adding two additional serines, dramatically reduced both the efficiency of the ATP-dependent phosphorolytic reaction and the sensitivity of this reaction to dATP. These processes appear to be specific for AZT-terminated primers, since removal of ddTMP and further extension of the unblocked primer were not observed with the ddT-terminated primer and any of the assayed enzymes. The observed differences between ddT- and AZT-terminated primer rescue reactions could be explained either by assuming that ddTMP is not removed as efficiently as AZTMP, or alternatively, by assuming that the ddT-terminated primer rescue reaction is more sensitive to dATP inhibition than the AZT-terminated primer rescue reaction.
The data presented in this paper underscore the importance of ribonucleotide-related phosphorolysis as a general AZT-resistance mechanism that plays a significant role in the acquisition of multidrug resistance by HIV-1 variants that carry a two-amino acid insertion at positions 69/70. In addition, our results support the role of pyrophosphorolysis as a natural AZT-resistance mechanism shown by all RTs, which operates during the elongation phase of reverse transcription. Pyrophosphorolysis is very inefficient during the initiation of reverse transcription, since WT RT is unable to unblock the AZT-terminated primer in the presence of PPi concentrations as high as 8 mM (Rigourd et al., 2000). It is still not known whether variant RTs displaying high-level resistance to AZT are able to use ATP to unblock AZT-terminated primers during initiation of reverse transcription. It has been shown that certain mutations in the RT palm subdomain (e.g. A114S, M184V) could reduce the RT’s ability to carry out ATP- or PPi-mediated phosphorolytic removal of chain-terminating AZT, in the sequence context of AZT-resistant RTs bearing the mutations D67N, K70R, T215F and K219Q (Arion et al., 2000; Götte et al., 2000). However, our results point to the flexible loop between the β3 and β4 structures of the RT fingers subdomain as a major structural determinant of the phosphorolytic reaction required for rescue of AZT-terminated primers. This loop contains several residues that mutate upon treatment with other nucleoside RT inhibitors (e.g. K65R, T69D, L74V). It will be interesting to assess the influence of these mutations on resistance to AZT, as well as the effect of phosphorolysis on the development of resistance to other nucleoside analog inhibitors.
Materials and methods
Clinical samples
Plasma samples were obtained in 1998 at the Hospital Carlos III (Instituto de Salud Carlos III, Madrid, Spain) from a 37-year-old man infected with HIV-1. This patient was identified together with another three individuals in the context of a large study involving 475 patients and aimed at the identification of virus harboring a dipeptide insertion between codons 69 and 70 of HIV-1 RT (Briones and Soriano, 1999; Briones et al., 2000). The patient had a complex history of treatments, starting with nucleoside inhibitors of RT including AZT, then combinations of nucleoside inhibitors and protease inhibitors, and finally a combination of five drugs, including nucleoside and non-nucleoside inhibitors of RT (ddI, d4T and nevirapine) and protease inhibitors (saquinavir and nelfinavir). At the time of sample collection, the plasma viral load was 96 000 HIV-1 RNA copies/ml, and the patient was considered to be failing therapy.
Plasmid constructions
Viral RNA was extracted using the silica-based method (Boom et al., 1990). The pol region of the RNA that includes the RT coding region was reverse transcribed and amplified by RT–PCR. The PCR-amplified DNA was digested with the restriction enzymes BamHI and HindIII, and cloned in plasmid pGEM3zf(+) (Promega). Nucleotide sequences of PCR-amplified and plasmid DNA were determined with the ABI Prism Dye Termination Cycle Sequencing kit with Ampli Taq polymerase FS (Applied Biosystems). Plasmids pRT6 and pT51H, which contain the coding regions of p66 and p51 subunits of subtype B (BH10) HIV-1 RT, were used as expression vectors (Martín-Hernández et al., 1996; Menéndez-Arias, 1998). Plasmid DNA containing the RT coding region was digested with MscI and KpnI to obtain two fragments of 1537 and 1208 bp, which were then cloned at the appropriate sites of pRT6 and pT51H, respectively. The 1537 bp fragment corresponds to the sequence comprising residues 25–536 of p66, and the 1208 bp fragment extends from position 25 to 427 of p51.
Mutagenesis
Site-directed mutagenesis was carried out with the Altered Sites in vitro mutagenesis system kit (Promega), following the manufacturer’s instructions. The single-stranded DNA (ssDNA) template used in the mutagenesis reaction was obtained from Escherichia coli DH5αF′ cultures harboring a pALTER-derived construct containing the coding sequence of the 66 kDa subunit of HIV-1 BH10 RT (Martín-Hernández et al., 1996). RT mutations and oligonucleotides used in the mutagenesis reaction were T69S, 5′-TTTTCTCCATTTCGAACTGTCTTTTTTC-3′; T69SSS, 5′-TTTCTCCATTTCGAACTACTCGAGTCTTTTTTCTTTATGGC-3′; and T69SSW, 5′-TTTCTCCATTTCCAACTACTCGAGTCTTTTTTCTTTATGGC-3′. Mutations were also introduced in the RT of virus found in the patient (SS RT). The nucleotide sequence of the SS RT was cloned in a derivative of pALTER-1, obtained by introducing a 10 base phosphorylated linker containing an NcoI site (New England Biolabs), at the SmaI site of the plasmid. The plasmid used for generating the template ssDNA of the mutagenesis reaction was then obtained after cloning the SS RT sequence in the NcoI and EcoRI sites of the modified pALTER-1. RT mutations and oligonucleotides used to obtain SS RT mutants were: 2S0S, 5′-GTCATAAAGAAAAAAGATAGTTCTAGATGGAGAAAA-3′; and 2S4S, 5′-GTCATAAAGAAAAAAGATTCAAGTAGTTCTAGTTCTAGATGGAGA-3′. In all cases, the introduced mutations were confirmed by DNA sequencing, and inserts containing the appropriate mutations were cloned in the expression vectors pRT6 and pT51H, by following previously described procedures (Martín-Hernández et al., 1996; Menéndez-Arias, 1998).
Protein expression and purification
WT BH10 and mutant RTs were purified after independent expression of their subunits (p66 and p51; Martín-Hernández et al., 1996). The 51 kDa subunit was obtained with an extension of 14 amino acid residues at its N-terminal end, which includes six consecutive histidine residues to facilitate its purification by metal chelate affinity chromatography. All RTs were purified as p66–p51 heterodimers, and were at least 95% pure as judged by SDS–PAGE.
Template–primers
Polyacrylamide gel-purified oligonucleotides pT (5′-GGATTTTAGACAGGAACGGT-3′), PG5-25 (5′-CCAGAATGCTGGTAGGGCTATACAT-3′) and 20PG5 (5′-TGGTAGGGCTATACATTCTT-3′) were labeled at their 5′ termini with [γ-32P]ATP and T4 polynucleotide kinase (Promega). The phosphorylated primers were then annealed to templates. The templates used were: M13mp2 ssDNA, for primer pT; D2-47 (5′-GGGATTAAATAAAATAGTAAGAATGTATAGCCCTACCAGCATTCTGG-3′), for primer PG5-25; and D38 (5′-GGGTCCTTTCTTACCTGCAAGAATGTATAGCCCTACCA-3′), for primer 20PG5. The templates and their corresponding primers were annealed in 150 mM NaCl and 150 mM magnesium acetate, as previously described (Martín-Hernández et al., 1997). The template–primer molar ratio was adjusted to 1:1.
Kinetic studies
Single-nucleotide incorporation assays were performed in 10 µl of 50 mM HEPES pH 7.0 buffer, containing 15 mM NaCl, 15 mM magnesium aspartate, 130 mM potassium acetate, 1 mM dithiothreitol (DTT) and 5% polyethylene glycol 6000. The template–primer (D2-47/PG5-25) concentration was 30 nM, and the active enzyme concentration in these assays was 3–5 nM. Reactions were initiated by incubating the enzyme with the corresponding annealed template–primer in the absence of dNTP (10 min at 37°C), followed by the addition of dTTP (Amersham Pharmacia) or AZTTP (Moravek Biochemicals, Brea, CA) at various concentrations. The reaction mixtures were incubated for 20 s at 37°C, and then the reactions were stopped by adding 8 µl of 10 mM EDTA in 90% formamide containing 3 mg/ml xylene cyanol FF and 3 mg/ml bromophenol blue. The extension products resulting from the incorporation of one nucleotide at the 3′ end of the primer were resolved by electrophoresis in 20% polyacrylamide–urea gels, and primer extension was quantitated by phosphoimaging with a BAS 1500 scanner (Fuji). Elongation measurements were fitted to the Michaelis–Menten equation, and the kcat and Km values were determined as previously described (Martín-Hernández et al., 1996).
Processivity
The processivity of WT BH10 and mutant enzymes was studied using the template–primer M13mp2 ssDNA/pT, in the buffer conditions used by Bebenek et al. (1995). Reactions (25 µl) containing 20 mM HEPES pH 7.8, 2 mM DTT, 10 mM MgCl2, 20 nM template–primer and a 10–15 nM active enzyme concentration were pre-incubated for 10 min at 37°C. After removing aliquots of 6 µl, extension reactions were initiated by adding an equal amount of the pre-incubation buffer containing all four dNTPs at concentrations in the range of 0.1–50 µM each, with or without DNA trap (herring sperm DNA at 2 mg/ml final concentration). After incubating the samples for 20 min at 37°C, reactions were stopped with EDTA–formamide as described above, quickly chilled on ice, and analyzed on a denaturing 6% polyacrylamide–urea gel.
Pyrophosphorolysis and PPi inhibition assays
The phosphorylated template–primer D2-47/PG5-25 (30 nM) was pre-incubated at 37°C for 10 min in the presence of WT BH10 or mutant RTs at 30–60 nM concentration, in the buffer conditions described for single nucleotide extension assays. Pyrophosphorolysis reactions were initiated by the addition of sodium PPi in the absence of dNTP to reach a final concentration of 150 µM or 1 mM, depending on the experiment. After varying incubation times, the reaction was stopped with EDTA–formamide as described above, and products were analyzed by electrophoresis in 20% polyacrylamide–urea gels. PPi inhibition was studied under the conditions described for single-nucleotide incorporation assays, using a dTTP concentration of 100 nM and varying the PPi concentration from 10 µM to 5 mM.
RT-catalyzed DNA ‘rescue’ synthesis using an AZT-terminated primer
A 20 nucleotide DNA oligonucleotide (20PG5) was labeled with [γ-32P]ATP at the 5′ end and then annealed to a 38 nucleotide DNA template oligonucleotide (D38) as described above. The phosphorylated template–primer (30 nM) was pre-incubated at 37°C for 10 min in the presence of WT BH10 or mutant RTs at 15–20 nM active enzyme concentration, in the buffer used for single-nucleotide extension assays. Reactions were initiated by adding an equal amount of the pre-incubation buffer containing a mixture of dATP, dCTP and dGTP (each at 100 µM) and 25 µM AZTTP or ddTTP, depending on the experiment. In experiments designed to assess the inhibitory effect of dATP (as the next complementary nucleotide at the 3′ end of AZT-terminated primers), the initial extension reactions were carried out in the presence of 100 µM dCTP, 100 µM dGTP and different concentrations of dATP, as indicated for each reaction. After incubating the samples for 45 min at 37°C, reactions were completed by adding dTTP to reach a final concentration of 100 µM, in the presence or absence of sodium PPi or ATP, as indicated for each assay. After incubating the samples at 37°C for up to 30 min, reactions were stopped with EDTA–formamide and products were resolved on a denaturing 20% polyacrylamide–urea gel.
Recombinant virus and drug susceptibility tests
Recombinant virus was prepared as previously described (Gutiérrez-Rivas et al., 1999). Briefly, full-length RT coding sequence DNA was amplified from plasmids carrying the different RTs using primers IN5 (5′-AATTTTCCCATTAGTCCTATTGAAACTGTACCA-3′) and IN3 (5′-TCTATTCCATCYAAAAATAGTACTTTCCTGATTCC-3′). PCR products were then co-transfected with an RT deleted HXB2-D clone into SupT1 cells (Kellam and Larder, 1994). When the HIV-1 p24 antigen concentration in the cultures surpassed 20 ng/ml, the supernatants were harvested. Progeny virus was propagated and titrated in MT-4 cells. The first 750 nucleotides of the RT coding region of the progeny virus were determined by automatic DNA sequencing to assess possible reversions or additional substitutions. The SupT1 and MT-4 cells and the deleted HXB2-D clone were obtained from the AIDS Reagent Program (Medical Research Council). HIV-1 drug susceptibility profiles were obtained after infecting 35 000 MT-4 cells with 100 50% tissue culture infective doses of virus, at a multiplicity of infection of 0.003, by exposing the HIV-1-infected cultures to various concentrations of each drug (5-fold dilutions). After MT-4 cells were allowed to proliferate for 5 days, the number of viable cells was determined by a tetrazolium-based colorimetric method (MTT method) as described elsewhere (Pauwels et al., 1988). Six replicate determinations were performed for each drug concentration.
Accession number
The DDBJ/EMBL/GenBank accession No. for the SS RT coding sequence is AF304024.
Acknowledgments
Acknowledgements
We thank Bruno Canard for critical reading of the manuscript. This work was supported by grants from Fondo de Investigación Sanitaria 98/0054-01 and -03, Comunidad Autónoma de Madrid 08.2/0014/1997, and by an institutional grant of Fundación Ramón Areces to Centro de Biología Molecular ‘Severo Ochoa’.
References
- Arion D., Kaushik,N., McCormick,S., Borkow,G. and Parniak,M.A. (1998) Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry, 37, 15908–15917. [DOI] [PubMed] [Google Scholar]
- Arion D., Sluis-Cremer,N. and Parniak,M.A. (2000) Mechanism by which phosphonoformic acid resistance mutations restore 3′-azido-3′-deoxythymidine (AZT) sensitivity to AZT-resistant HIV-1 reverse transcriptase. J. Biol. Chem., 275, 9251–9255. [DOI] [PubMed] [Google Scholar]
- Balzarini J. (1999) Suppression of resistance to drugs targeted to human immunodeficiency virus reverse transcriptase by combination therapy. Biochem. Pharmacol., 58, 1–27. [DOI] [PubMed] [Google Scholar]
- Bebenek K., Beard,W.A., Casas-Finet,J.R., Kim,H.-R., Darden,T.A., Wilson,S.H. and Kunkel,T.A. (1995) Reduced frameshift fidelity and processivity of HIV-1 reverse transcriptase mutants containing alanine substitutions in helix H of the thumb subdomain. J. Biol. Chem., 270, 19516–19523. [DOI] [PubMed] [Google Scholar]
- Boom R., Sol,C.J., Salimans,M.M., Jansen,C.L., Wertheim-van Dillen,P.M. and van der Noordaa,J. (1990) Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol., 28, 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P.L., Lisziewicz,J., Lori,F. and Hughes,S.H. (1999) Analysis of amino insertion mutations in the fingers subdomain of HIV-1 reverse transcriptase. J. Mol. Biol., 286, 995–1008. [DOI] [PubMed] [Google Scholar]
- Briones C. and Soriano,V. (1999) Different outcome in the first two patients with an HIV-1 multinucleoside drug-resistant T69SSS insertion in Spain. Antivir. Ther., 4, 125–127. [PubMed] [Google Scholar]
- Briones C., Mas,A., Gómez-Mariano,G., Altisent,C., Menéndez-Arias,L., Soriano,V. and Domingo,E. (2000) Dynamics of dominance of a dipeptide insertion in reverse transcriptase of HIV-1 from patients subjected to prolonged therapy. Virus Res., 66, 13–26. [DOI] [PubMed] [Google Scholar]
- Caliendo A.M., Savara,A., An,D., DeVore,K., Kaplan,J.C. and D’Aquila,R.T. (1996) Effects of zidovudine-selected human immunodeficiency virus type 1 reverse transcriptase amino acid substitutions on processive DNA synthesis and viral replication. J. Virol., 70, 2146–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canard B., Sarfati,S.R. and Richardson,C.C. (1998) Enhanced binding of azidothymidine-resistant human immunodeficiency virus 1 reverse transcriptase to the 3′-azido-3′-deoxythymidine 5′-monophosphate-terminated primer. J. Biol. Chem., 273, 14596–15604. [DOI] [PubMed] [Google Scholar]
- Carroll S.S., Geib,J., Olsen,D.B., Stahlhut,M., Shafer,J.A. and Kuo,L.C. (1994) Sensitivity of HIV-1 reverse transcriptase and its mutants to inhibition by azidothymidine triphosphate. Biochemistry, 33, 2113–2120. [DOI] [PubMed] [Google Scholar]
- De Antoni A., Foli,A., Lisziewicz,J. and Lori,F. (1997) Mutations in the pol gene of human immunodeficiency virus type 1 in infected patients receiving didanosine and hydroxyurea combination therapy. J. Infect. Dis., 176, 899–903. [DOI] [PubMed] [Google Scholar]
- de Jong J.J. et al. (1999) Insertion of two amino acids combined with changes in reverse transcriptase containing tyrosine-215 of HIV-1 resistant to multiple nucleoside analogs. AIDS, 13, 75–80. [DOI] [PubMed] [Google Scholar]
- Götte M., Arion,A., Parniak,M.A. and Wainberg,M.A. (2000) The M184V mutation in the reverse transcriptase of human immunodeficiency virus type 1 impairs rescue of chain-terminated DNA synthesis. J. Virol., 74, 3579–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Rivas M., Ibañez,A., Martínez,M.A., Domingo,E. and Menéndez-Arias,L. (1999) Mutational analysis of Phe160 within the ‘palm’ subdomain of human immunodeficiency virus type 1 reverse transcriptase. J. Mol. Biol., 290, 615–625. [DOI] [PubMed] [Google Scholar]
- Jonckheere H., Anné,J. and De Clercq,E. (2000) The HIV-1 reverse transcription (RT) process as target for RT inhibitors. Med. Res. Rev., 20, 129–154. [DOI] [PubMed] [Google Scholar]
- Kellam P. and Larder,B.A. (1994) Recombinant virus assay: a rapid, phenotypic assay for assessment of drug susceptibility of human immunodeficiency virus type 1 isolates. Antimicrob. Agents Chemother., 38, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellam P., Boucher,C.A.B. and Larder,B.A. (1992) Fifth mutation in human immunodeficiency virus type 1 reverse transcriptase contributes to the development of high level resistance to zidovudine. Proc. Natl Acad. Sci. USA, 89, 1934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr S.G. and Anderson,K.S. (1997) Pre-steady-state kinetic characterization of wild type and 3′-azido-3′deoxythymidine (AZT) resistant human immunodeficiency virus type 1 reverse transcriptase: implication of RNA directed DNA polymerization in the mechanism of AZT resistance. Biochemistry, 36, 14064–14070. [DOI] [PubMed] [Google Scholar]
- Krebs R., Immendorfer,U., Thrall,S.H., Wohrl,B.M. and Goody,R.S. (1997) Single-step kinetics of HIV-1 reverse transcriptase mutants responsible for virus resistance to nucleoside inhibitors zidovudine and 3TC. Biochemistry, 36, 10292–10300. [DOI] [PubMed] [Google Scholar]
- Lacey S.F., Reardon,J.E., Furfine,E.S., Kunkel,T.A., Bebenek,K., Eckert,K.A., Kemp,S.D. and Larder,B.A. (1992) Biochemical studies of the reverse transcriptase and RNase H activities from human immunodeficiency virus strains resistant to 3′-azido-3′-deoxythymidine. J. Biol. Chem., 267, 15789–15794. [PubMed] [Google Scholar]
- Larder B.A. et al. (1999) A family of insertion mutations between codons 67 and 70 of human immunodeficiency virus type 1 reverse transcriptase confer multinucleoside analog resistance Antimicrob. Agents Chemother., 43, 1961–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Hernández A.M., Domingo,E. and Menéndez-Arias,L. (1996) Human immunodeficiency virus type 1 reverse transcriptase: role of Tyr115 in deoxynucleotide binding and misinsertion fidelity of DNA synthesis. EMBO J., 15, 4434–4442. [PMC free article] [PubMed] [Google Scholar]
- Martín-Hernández A.M., Gutiérrez-Rivas,M., Domingo,E. and Menéndez-Arias,L. (1997) Mispair extension fidelity of human immunodeficiency virus type 1 reverse transcriptases with amino acid substitutions affecting Tyr115. Nucleic Acids Res., 25, 1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menéndez-Arias L. (1998) Studies on the effects of truncating α-helix E′ of p66 human immunodeficiency virus type 1 reverse transcriptase on template–primer binding and fidelity of DNA synthesis. Biochemistry, 37, 16636–16644. [DOI] [PubMed] [Google Scholar]
- Meyer P.R., Matsuura,S.E., So,A.G. and Scott,W.A. (1998) Unblocking of chain-terminated primer by HIV-1 reverse transcriptase through a nucleotide-dependent mechanism. Proc. Natl Acad. Sci. USA, 95, 13471–13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P.R., Matsuura,S.E., Mian,A.M., So,A.G. and Scott,W.A. (1999) A mechanism of AZT resistance: an increase in nucleotide-dependent primer unblocking by mutant HIV-1 reverse transcriptase. Mol. Cell, 4, 35–43. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Balzarini,J., Baba,M., Snoeck,R., Schols,D., Herdewijn,P., Desmyter,J. and De Clercq,E. (1988) Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods, 20, 309–321. [DOI] [PubMed] [Google Scholar]
- Ren J. et al. (1998) 3′-azido-3′deoxythymidine drug resistance mutations in HIV-1 reverse transcriptase can induce long range conformational changes. Proc. Natl Acad. Sci. USA, 95, 9518–9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigourd M., Lanchy,J.-M., Le Grice,S.F., Ehresmann,B., Ehresmann,C. and Marquet,R. (2000) Inhibition of the initiation of HIV-1 reverse transcription by AZT: comparison with elongation. J. Biol. Chem., 275, 26944–26951. [DOI] [PubMed] [Google Scholar]
- Ross L., Johnson,M., Graham,N., Shaefer,M. and St Clair,M. (1999) The reverse transcriptase codon 69 insertion is observed in nucleoside reverse transcriptase inhibitor-experienced HIV-1-infected individuals, including those without prior or concurrent zidovudine therapy. J. Hum. Virol., 2, 290–295. [PubMed] [Google Scholar]
- Sarafianos S.G., Das,K., Ding,J., Boyer,P.L., Hughes,S.H. and Arnold,E. (1999) Touching the heart of HIV-1 drug resistance: the fingers close down on the dNTP at the polymerase active site. Chem. Biol., 6, R137–R146. [DOI] [PubMed] [Google Scholar]
- Shirasaka T. et al. (1995) Emergence of human immunodeficiency virus type 1 variants with resistance to multiple dideoxynucleosides in patients receiving therapy with dideoxynucleosides. Proc. Natl Acad. Sci. USA, 92, 2398–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura W. et al. (1999) Identification of insertion mutations in HIV-1 reverse transcriptase causing multiple drug resistance to nucleoside analogue reverse transcriptase inhibitors. J. Hum. Virol., 2, 146–153. [PubMed] [Google Scholar]
- Tamalet C., Izopet,J., Koch,N., Fantini,J. and Yahi,N. (1998) Stable rearrangements of the β3–β4 hairpin loop of HIV-1 reverse transcriptase in plasma virus from patients receiving combination therapy. AIDS, 12, F161–F166. [DOI] [PubMed] [Google Scholar]
- Tamalet C., Yahi,N., Tourrès,C., Colson,P., Quinson,A.-M., Poizot-Martin,I., Dhiver,C. and Fantini,J. (2000) Multidrug resistance genotypes (insertions in the β3–β4 finger subdomain and MDR mutations) of HIV-1 reverse transcriptase from extremely treated patients: incidence and association with other resistance mutations. Virology, 270, 310–316. [DOI] [PubMed] [Google Scholar]
- Tong W., Lu,C.-D., Sharma,S.K., Matsuura,S., So,A.G. and Scott,W.A. (1997) Nucleotide-induced stable complex formation by HIV-1 reverse transcriptase. Biochemistry, 36, 5749–5757. [DOI] [PubMed] [Google Scholar]
- Ueno T. and Mitsuya,H. (1997) Comparative enzymatic study of HIV-1 reverse transcriptase resistant to 2′,3′-dideoxynucleotide analogs using the single-nucleotide incorporation assay. Biochemistry, 36, 1092–1099. [DOI] [PubMed] [Google Scholar]
- Ueno T., Shirasaka,T. and Mitsuya,H. (1995) Enzymatic characterization of human immunodeficiency virus type 1 reverse transcriptase resistant to multiple 2′,3′-dideoxynucleoside 5′triphosphates. J. Biol. Chem., 270, 23605–23611. [DOI] [PubMed] [Google Scholar]
- Winters M.A., Coolley,K.L., Girard,Y.A., Levee,D.J., Hamdan,H., Shafer,R.W., Katzenstein,D.A. and Merigan,T.C. (1998) A 6-basepair insert in the reverse transcriptase gene of human immunodeficiency virus type 1 confers resistance to multiple nucleoside inhibitors. J. Clin. Invest., 102, 1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]