Abstract
Background
Atopic dermatitis (AD) is a chronic inflammatory skin disease affecting up to 20% children and 9% adults worldwide. AD patients are often sensitized against a broad variety of allergens and more than 90% of them suffer from skin superinfections with Staphylococcus aureus.
Objective
In this study, we searched for the presence of specific IgE antibodies against S. aureus and Escherichia coli antigens in AD patients.
Methods
Sera from AD patients (n=79), patients suffering only from allergic rhinoconjunctivitis (n=41) or allergic asthma (n=37) were tested for IgE reactivity to nitrocellulose-blotted S. aureus, E. coli and gut bacterial antigens. IgE-reactive bacterial antigens were affinity purified and identified by mass spectrometry.
Results
More than 30% of AD patients but not patients suffering only from allergic rhinoconjunctivitis and asthma or non-allergic persons exhibited IgE binding to several protein antigens among them DNA-binding and ribosomal proteins and flagellin. Patients with severe skin manifestations showed more frequently IgE reactivity to S. aureus compared to AD patients with mild symptoms. Positive immediate and late skin test reactions could be induced in sensitized AD patients with S. aureus extract.
Conclusion and Clinical Relevance
Specific IgE reactivities against a variety of bacterial antigens were observed in a subgroup comprising a third of AD patients and may contribute to allergic inflammation.
Keywords: Atopic dermatitis, bacterial antigens, Staphylococcus aureus, Escherichia coli, bacterial allergen
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease which affects up to 20.5% of children and between 0.2 to 8.8% of adults [1–4]. The clinical manifestations of AD vary and can range from dry skin and eczematous lesions to intense pruritus and lichenified flextures [5]. It has been reported that about 80% of AD patients exhibit elevated levels of serum IgE, and the IgE levels are often correlated with disease severity [6, 7]. As AD is associated with other atopic diseases such as asthma and allergic rhinitis, patients with AD often have specific IgE antibodies and allergic symptoms to great variety of food and inhalant allergens [8, 9].
Individuals suffering from AD show increased susceptibility to cutaneous bacterial, viral and fungal infections [10, 11]. The predominant skin infection in AD is caused by Staphylococcus aureus, which affects between 29–100% of patients [12–14]. S. aureus is present at 100–1000 fold higher density (about 105 cfu/mL) in the skin of AD patients compared to the skin of healthy individuals [15]. In contrast, only 5–8% of healthy persons harbor S. aureus which is usually concentrated in their mucosal cavities [16]. Density and frequency of S. aureus colonization is significantly correlated with the severity of eczema [14, 17]. Furthermore, treatment of S. aureus skin infections with anti-staphylococcal antibiotics significantly reduces bacterial count and clinical severity of the disease [18, 19].
Escherichia coli is not a common microflora in infected AD lesions. In a study by Brook, E. coli was isolated from secondary infected eczema lesions of 10% of AD patients, and the colonization was restricted to the leg and buttock regions [12]. This was in contrast to S. aureus which was detected in 29% of the patients in the same study, and was recovered from all body sites [12]. In another study, E. coli was isolated from the diaper area of between 0.3 – 1.1% of children with AD, which was much lower compared to S. aureus (4.2 – 10.8%) in the same study [20]. There have been no reports on the exacerbation of AD due to E. coli infection.
Beginning from the early 1980s, several groups reported that specific IgE against S. aureus proteins could be detected in the serum of AD patients [21–25]. Anti- S. aureus IgE titers were mostly observed in patients with moderate to severe AD [22, 24] but no detailed information about the IgE reactive antigens were available except that both cellular proteins and cell wall components of S. aureus may be involved [24, 26–30]. Furthermore, some of the toxins were shown to react with IgE antibodies [31–33].
In the present study, the prevalence of serum IgE binding to antigens from S. aureus and E. coli was studied in patients suffering from AD of different severity, allergic rhinoconjunctivitis or allergic asthma by IgE immunoblotting. The nature of the IgE reactive antigens was characterized by determination of their molecular weights, testing for anti-carbohydrate IgE reactivity and IgE inhibition experiments in different populations of AD patients. Additionally, effects of S. aureus and E. coli protein stimulation were evaluated by lymphoproliferations and measurements of cytokine secreted. IgE reactivity to proteins from seven most commonly occurring ileum and colon-colonizing bacteria were studied by immunoblotting. Furthermore, immune complexes consisting of IgE and bacterial antigens were affinity purified and subjected to mass spectrometry to identify IgE-reactive bacterial proteins. The allergenic activity of S. aureus antigens was investigated by skin testing in sensitized AD patients.
METHODS
Characterization of patients
Sera from 35 Austrian and 44 German patients who according to the criteria of Hanifin and Rajka [34] suffered from AD were analysed. Tables 1 and 2 summarize the demographic, clinical and serological data of these patients. For control purposes, sera from Austrian patients with allergic rhino-conjunctivitis but no AD (n=41) and allergic asthma without AD (n=37), and from 9 non-atopic individuals were included. To investigate possible associations between severity of AD, S. aureus skin superinfections and IgE reactivity profiles, AD patients from Germany were tested. Serum samples were from patients who had undergone routine clinical testing and were used in an anonymous manner, with approval from the respective local ethics committees.
Table 1.
Demographic, clinical and serological characterization of AD patients from Austria1
| Patient | Sex | Age | Symptoms | Allergies |
Total IgE (kU/L) |
SA IgE | EC IgE | SA enterotoxin RAST | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Pollen | Food | Other | ||||||||
| A1 | M | 56 | AD, RC | t, g, w | mi | 1720 | + | + | SEA (1), SEB (2), SEC (2), SED (1), SEE (2), TSST (2) | |
| A2 | M | 38 | AD, RC | t, g, w | s, n, ap, c | a, mi | >2000 | + | + | SEA (2), SEB (2), SEC (2), SED (2), SEE (2), TSST (2) |
| A3 | M | 39 | AD, RC | t, g, w | mi | 1060 | − | + | SEA (0), SEB (0), SEC (0), TSST(1) | |
| A4 | F | 61 | AD, AS | t, g | s | mi | ND | + | + | SEA (1), SEB (2), SEC (2), SED (1), SEE (1), TSST (1) |
| A5 | F | 45 | AD | t, g | mk | a, mi | ND | + | − | ND |
| A6 | F | 80 | AD, AS | t, g | a | >1000 | + | + | SEB (1), SEC (1), TSST (2) | |
| A7 | F | 44 | AD | ap, n | 165 | − | − | SEA (0), SEB (2), SEC (0), TSST (0) | ||
| A8 | F | 46 | AD | c | a, mi | 271 | − | − | SEA (0), SEB (0), SEC (0), TSST (0) | |
| A9 | F | 43 | AD, RC | t, g | ap, n | a | 802 | − | − | SEA (0), SEB (2), SEC (0), TSST (1) |
| A10 | F | 73 | AD | g | mi | >2000 | + | − | SEA (2), SEB (2), SEC (2), SED (2), SEE (2), TSST (3) | |
| A12 | M | 52 | AD, RC, AS | a, mi | >2000 | + | + | SEA (2), SEB (2), SEC (2), SED (2), SEE (2), TSST (2) | ||
| A13 | M | 67 | AD | mi | >5000 | − | + | SEA (1), SEB (2), SEC (2) | ||
| A14 | F | 29 | AD, RC | t, g, w | mi | 46 | − | − | SEA (0), SEB (0), SEC (0) | |
| A15 | F | 36 | AD, AS | t, g, w | a, mi | 580 | − | − | ND | |
| A16 | F | 38 | AD, RC | t, g, w | a | 270 | − | − | SEA (0), SEB (0), SEC (0) | |
| A17 | M | 66 | AD, RC | t, g, w | n, ap | mi | 932 | − | − | ND |
| A18 | M | 34 | AD, RC | t, g, w | >2000 | − | + | ND | ||
| A19 | F | 37 | AD, RC | t, g, w | n, ap, k | 339 | − | − | ND | |
| A20 | M | 36 | AD, RC | t, g, w | ap, ca | >2000 | − | − | SEA (2), SEB (1), SEC (1), SED (1), SEE (1), TSST (2) | |
| A21 | M | 48 | AD, RC, AS | t, g, w | n, ap, k, ca | mi | >2000 | − | + | TSST (2) |
| A22 | F | 54 | AD, RC, AS | t | ap, ca, c | 187 | − | − | SEA (0), SEB (0), SEC (0) | |
| A23 | M | 49 | AD, RC, AS | g | mi | 196 | − | − | ND | |
| A24 | F | 34 | AD, RC | t | n, ap, c | a | 189 | − | − | SEA (0), SEB (0), SEC (0) |
| A25 | F | 29 | AD, RC, AS | t, g, w | mi | 294 | − | − | ND | |
| A26 | M | 37 | AD, RC | t, g, w | 380 | − | − | ND | ||
| A27 | M | 41 | AD, RC | t, g, w | a, mi | 290 | − | − | SEA (0), SEB (0), SEC (0) | |
| A28 | M | 34 | AD, RC | t, g, w | ap | a | 309 | − | − | ND |
| A29 | M | 36 | AD, RC | t, g, w | n, ap, k | 512 | − | − | ND | |
| A30 | M | 51 | AD, RC | t, g, w | k | mi | 80 | − | − | ND |
| A31 | M | 38 | AD, RC | t | 27 | − | − | ND | ||
| A32 | F | 34 | AD, RC, AS | g | n, k, ca | 112 | − | − | ND | |
| A33 | F | 30 | AD, RC | t, g, w | a, mi | >2000 | + | + | SEA (1), SEB (2), SEC (3), SED (2), TSST (2) | |
| A34 | M | 27 | AD | t, g, w | n, ap, p | a | 205 | − | − | ND |
| A35 | F | 43 | AD, RC | t, g | n, ap, p, mk | a, mi | 19464 | + | + | ND |
| A36 | F | 31 | AD | t | ap | a | 2923 | + | + | ND |
a, animals; AD, atopic dermatitis; ap, apple; AS, asthma; c, celery; ca, carrot; EC, E. coli ; F, female; g, grass; k, kiwi; kU/L, kilo units per liter; M, male; mi, mites; mk, milk; n, nuts; ND, not determined; p, peach; RC, rhinoconjunctivitis; s, seafood; SA, S. aureus ; SE, staphylococcal enterotoxins; t, trees; TSST, toxic shock syndrome toxin; w, weeds; +, positive reaction; −, negative reaction
Table 2.
Demographic, clinical and serological characterization of AD patients from Germany2
| Patient | Sex | Age | Symptoms | SCORAD | Allergies |
Total IgE (kU/L) |
SA IgE | EC IgE | Bacterial infection |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pollen | Food | Other | |||||||||
| H1 | M | 35 | AD, AS, AR | 45 | t, g, w | so | a, f, mi, asp | 12499 | + | − | N |
| H3 | M | 32 | AD, AS, AR | 44 | t | 68.7 | − | − | N | ||
| H4 | F | 40 | AD, AR | 24 | t, g, w | a, f, mi | 1119 | − | − | N | |
| H6 | M | 72 | AD | 33 | t, g, w | a, f, mi, asp | 3668 | + | + | N | |
| H8 | F | 25 | AD, AS, AR | 42 | t, g, w | a, f, mi, asp | 1498 | − | − | N | |
| H9 | F | 47 | AD, AS, AR | 31 | t, g, w | a, mi, asp | 1020 | − | + | N | |
| H10 | M | 25 | AD, AS, AR | 31 | t, g, w | a, f, mi, asp | 2262 | − | + | Y | |
| H12 | F | 22 | AD, AS, AR | 61 | t, g, w | n, so, mk, s, wh | a, f, mi, asp | 66286 | + | + | Y |
| H13 | F | 27 | AD | 22 | mi | 36 | − | − | N | ||
| H14 | F | 29 | AD | 12 | ND | ND | ND | 38.5 | − | − | N |
| H15 | F | 29 | AD, AS, AR | 13 | ND | ND | ND | 43.9 | − | − | N |
| H16 | M | 22 | AD, AR | 16 | t, g | a, mi | 1308 | − | − | N | |
| H17 | M | 40 | AD | 28 | f | 2298 | − | − | N | ||
| H18 | F | 37 | AD, AS, AR | 51 | t, g, w | n, so | a, mi, asp | 26200 | + | + | N |
| H20 | M | 48 | AD | 30 | mi | 184 | − | − | N | ||
| H21 | F | 34 | AD | 24 | a, f, mi | 1310 | − | − | N | ||
| H23 | F | 26 | AD | 52 | w | a, f, asp | 843 | − | + | Y | |
| H24 | F | 38 | AD | 51 | t, g | a, f, mi, asp | 2661 | − | − | Y | |
| H26 | M | 20 | AD, AR | 54 | g | a, f, mi, asp | 5643 | + | − | Y | |
| H27 | M | 30 | AD, AS, AR | 20 | t, g, w | so | a, f | 1042 | − | + | N |
| H28 | M | 43 | AD, AS, AR | 60 | t, g, w | e, s, wh, n, so | a, f, mi, asp | 15119 | + | + | N |
| H29 | M | 62 | AD | 56 | t, g, w | wh, n, so | a, f, mi, asp | 11544 | + | + | N |
| H30 | M | 49 | AD, AS | 46 | t, g, w | a, f, mi, asp | 29240 | + | + | N | |
| H32 | F | 28 | AD, AS, AR | 50 | t, g, w | a, mi | 1815 | − | − | Y | |
| H34 | F | 46 | AD, AS, AR | 53 | t, g, w | a, f, mi, asp | 11403 | + | − | N | |
| H35 | M | 60 | AD, AS, AR | 41 | a, mi, asp | 12841 | + | + | N | ||
| H36 | F | 19 | AD | 48 | a, f, asp | 830 | − | − | Y | ||
| H37 | F | 23 | AD, AR | 10 | t, g | a, mi | 861 | − | − | N | |
| H38 | M | 37 | AD | 22 | ND | ND | ND | 13.8 | − | − | N |
| H39 | M | 25 | AD, AR | 31 | t, g, w | a, f, mi, asp | 3964 | + | − | N | |
| H40 | M | 32 | AD, AS | 25 | t, g, w | a, f, mi, asp | 13113 | + | + | Y | |
| H42 | F | 33 | AD, AS, AR | 61 | t, g | so | f, mi | 1216 | − | − | N |
| H43 | F | 20 | AD | 54 | ND | ND | ND | 5.9 | − | − | Y |
| H45 | F | 44 | AD | 47 | ND | ND | ND | 23.4 | − | − | N |
| H46 | M | 36 | AD, AS | 49 | t, g, w | k, s, e, mk, wh, n, so | a, f, mi, asp | 19790 | + | + | N |
| H47 | M | 44 | AD, AS, AR | 43 | t, g, w | a, f, mi, asp | 19545 | + | − | N | |
| H48 | M | 53 | AD, AS | 62 | t, g, w | a, f, mi, asp | 21428 | + | + | N | |
| H49 | M | 34 | AD, AS, AR | 56 | t, g, w | a, f, mi, asp | 33700 | + | + | N | |
| H50 | F | 18 | AD, AS, AR | 28 | mi | 31.5 | − | − | N | ||
| H51 | F | 39 | AD, AS, AR | 47 | t, g | n | a, f, mi, asp, l | 463 | − | − | N |
| H54 | F | 26 | AD, AS, AR | 8 | t | f | 68.5 | − | − | N | |
| H55 | M | 43 | AD, AR | 49 | t, g, w | a, f, mi, asp, l | 11950 | + | + | N | |
| H56 | F | 54 | AD, AS, AR | 45 | g, w | a, f, mi, asp, l | 9400 | + | + | N | |
| H57 | M | 25 | AD, AS, AR | 63 | t, g, w | a, f, mi, asp, l | 40200 | + | + | N | |
a, animals; AD, atopic dermatitis; AS, asthma; asp, Aspergillus fumigatus ; AR, allergic rhinitis; e, egg; EC, E. coli ; f, fungus; F, female; g, grass; k, kiwi; kU/L, kilo units per liter; l, latex; M, male; mi, mites; mk, milk; n, nuts; ND, not determined; s, seafood; SA, S. aureus ; so, soy; t, trees; w, weeds; wh, wheat; +, positive reaction; −, negative reaction
Preparation of bacterial total protein extracts
S. aureus (subspecies Rosenbach, ATCC 25923) and E. coli (strain Seattle 1946, ATCC 25922) were grown overnight in tryptic soy broth at 37°C. The other bacterial species (Supplementary Table 1) were cultured on trypticase soy agar plates with 5% sheep blood. All bacterial species used were well characterized type cultures, purchased from either ATCC or the German collection of microorganisms and cell cultures (DSMZ). The bacterial cells were harvested by centrifugation at 3220×g. The cells were washed twice with PBS, re-suspended in PBS and homogenized using an Ultra Turrax (IKA Labortechnik, Germany) and glass beads. The homogenates were then centrifuged at 20,000×g for 10 min and the supernatants stored at −20°C until use.
IgE immunoblotting, chemical deglycosylation, IgE-immunoblot inhibitions
Bacterial extracts (S. aureus, E. coli) were boiled for 5 min with sodium dodecyl sulfate (SDS) sample buffer containing 5% v/v β-mercaptoethanol [35]. Approximately 200 µg/cm of each bacterial extract was separated on a 12.5% preparative SDS-PAGE [35]. A protein molecular weight marker (Rainbow-Marker, Amersham, UK or PageRuler Prestained Protein Ladder Plus, Fermentas) were used as a standards. The separated proteins were then transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) by electroblotting [36]. Nitrocellulose strips were cut from the membranes and blocked twice for 10 min and once for 30 min in buffer A (50mM sodium phosphate buffer, pH 7.4, containing 0.5% w/v bovine serum albumin (BSA), 0.5% v/v Tween 20, and 0.05% w/v NaN3) at room temperature. Strips were incubated overnight with patients’ sera (diluted 1:10 in buffer A) at 4°C. The strips were then washed as described for blocking. Bound IgE was detected by incubating the strips with 125I-labeled anti-human IgE antibodies (Pharmacia, Uppsala, Sweden) diluted 1:10 in buffer A, overnight at room temperature. After repeated washes in buffer A, the strips were dried and exposed to Kodak X-OMAT S films at −70°C using intensifying screens (Kodak, Heidelberg, Germany).
For chemical deglycosylation, nitrocellulose-blotted bacterial extracts were incubated with 50 mM sodium acetate, pH 4.5 with or without 10 mM periodate for 2 h at 4°C. Strips were washed twice for 10 min and once for 30 min with buffer A, and were then incubated with patients’ sera diluted 1:10 in buffer A overnight at 4°C. Bound IgE antibodies were detected as described for immunoblotting. For inhibition experiments, patients’ sera (diluted 1:10 in buffer A) were pre-incubated with bacterial extracts overnight at 4°C (1000 µg bacterial extract per 1mL of diluted sera). The nitrocellulose-blotted bacterial extracts were then incubated with the pre-adsorbed sera overnight at 4°C, and bound IgE antibodies were detected as described for immunoblotting.
Measurement of S. aureus enterotoxin specific IgE antibodies
Serum IgE antibodies against Staphylococcus enterotoxin (SE) A, SEB, SEC, SED, SEE, and toxic shock syndrome toxin (TSST) (Toxin Technology, Florida, USA) were measured using the CAP assay (Phadia, Uppsala, Sweden) [37].
Specific lymphocyte proliferation and cytokine responses to S. aureus and E. coli protein extracts
PBMC were isolated from heparinized blood from four AD and five non-allergic individuals using Ficoll-Paque gradient centrifugation and were resuspended in Ultraculture medium (Bio Whittaker, Walkersville, MD, USA) supplemented with 2 mM glutamine, 50 mM β-mecarptoethanol and 0.1 mg/mL gentamycin. Cells were cultured in sterile 96 well plates (Nunc) at 2×105 cells/well and stimulated with S. aureus extract (50 µg/well), E. coli extract (50 µg/well), IL-2 (positive control) or medium (negative control) in triplicates at 37°C with 5% CO2. Six days after initial stimulation, 0.5 µCi of 3H-thymidine (Amersham, Buckinghamshire, UK) was added to each well, and cells were incubated for 16 h. 3H-thymidine incorporation was measured by scintillation counting (Microbeta Trilux, Perkin Elmer) and are reported as stimulation index (SI), calculated as the ratio of the mean proliferation after antigen stimulation over medium control values. Cytokine levels (IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, GM-CSF, TNF-α and IFN-γ) were measured in supernatants collected from PBMC cultures at day 6, using the Bio-Plex Pro Human Cytokine Th1/Th2 assay kit (Bio-Rad, Hercules, CA, USA) and the assay was performed according to the manufacturer’s instructions and fluorescent signals were read on a Bio-Plex system (Bio-Rad).
Skin testing
Routine skin testing was performed by intradermal injection of aliquots of 20 µL of S. aureus extract, histamine, or saline into forearms of 22 AD patients in the Russian centre. Patients had to be in remission regarding AD and pyoderma and did not use any pharmacotherapy for at least 5 days prior to skin testing. Skin reactivities were recorded after 20 min and 24 h.
Identification of IgE-reactive S. aureus and E. coli proteins by mass spectrometry
An anti-human IgE column was prepared by coupling 1.5 mg of monoclonal mouse anti-human IgE (BD Pharmingen) on a HiTrap NHS-activated HP column (GE Healthcare). The column was washed with PBS to remove unbound antibodies and loaded with serum IgE of AD patients. Then the column was washed with PBS and loaded with S. aureus or E. coli extract at a concentration of 1mg/mL. Unbound proteins were removed by washing with PBS and IgE immune complexes were eluted with 0.1M glycine pH 2.8, and neutralized immediately with 1M Tris pH 10. The eluted fraction was desalted using a PD10 column with a solution of 5mM NaH2PO4, pH 7.0. Eluted proteins were then digested overnight at 37°C with trypsin (Sigma), and subjected to mass spectrometry on Nano-LC system coupled to Bruker HCT Ultra ESI trap (Bruker Daltonics, Bremen, Germany). The peptides were fractionated on a C18 PepMap 100 column (3µm, 100Å) (LC Packings) using a 5%–80% acetonitrile solvent gradient. During elution, the mass spectra were recorded in the mass/charge range of 300 to 3000. Data were processed using DataAnalysis™ 3.4, and the peak list generated was searched against the Swiss-Prot database subset of eubacteria proteins using MASCOT (Matrix Science) search engine. The search setting included a missed cleavage site value of three, carboxymethylation of cysteine and variable oxidation of methionine, histidine and tryptophan. Protein matches with scores greater than 50 indicated relevant identity to known proteins (p<0.05).
RESULTS
A third of AD patients exhibit specific IgE reactivity to various protein antigens from S. aureus and E. coli
IgE reactivities to nitrocellulose-blotted S. aureus and E. coli antigens (Figures 1 and 2) were analysed using sera from 35 AD patients from Austria (Table 1) and 44 AD patients from Germany (Table 2). From the total of 79 patients, 39 were male, and the mean age on the patients was 39.3 years (range 18 to 80 years). The majority of patients also presented allergic manifestations other than AD, such as rhinitis and bronchial asthma, and were sensitized to multiple allergen sources. The patients displayed a wide range of total serum IgE levels (5.9 to 66286 kU/L).
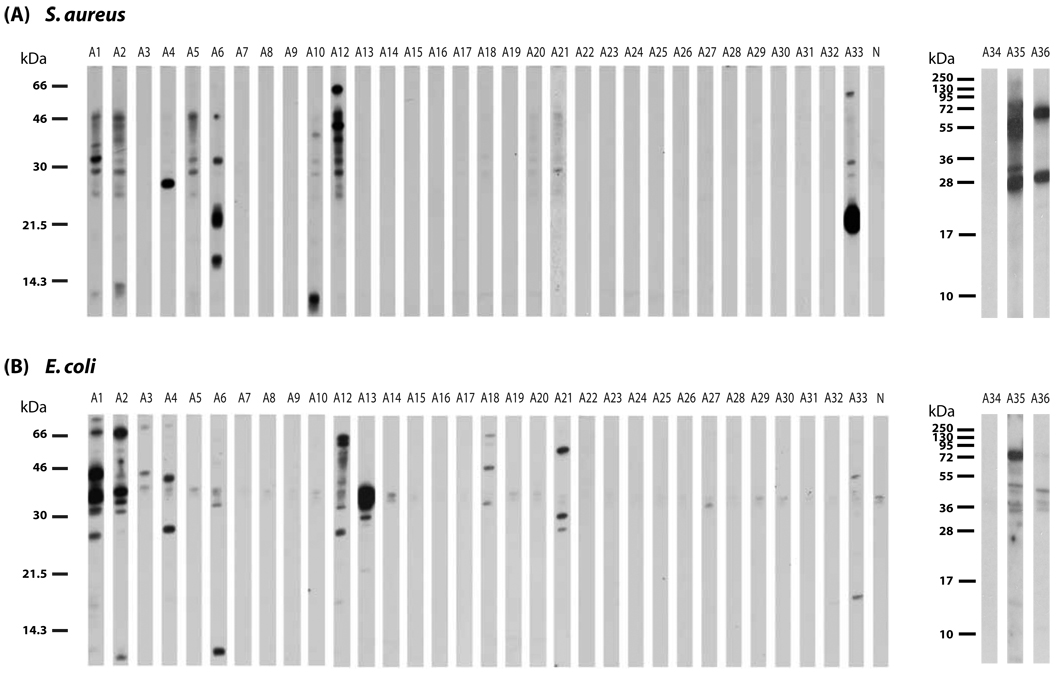
Figure 1. IgE reactivity of AD patients to nitrocellulose-blotted S. aureus and E. coli protein extracts.
Total protein extracts of S. aureus (A) and E. coli (B) were separated under reducing conditions by SDS-PAGE, blotted on to nitrocellulose membrane and probed with sera from 35 patients with AD. As a control, serum from a non-atopic individual (panel N) was used. Molecular weights (kDa) are indicated in the left margin.
Figure 2. IgE reactivity of AD patients with and without skin superinfections to nitrocellulose-blotted S. aureus and E. coli protein extracts.
Total protein extracts of S. aureus (A) and E. coli (B) were separated under reducing conditions by SDS-PAGE, blotted on to nitrocellulose membrane and probed with sera from the German AD patient populations with or without skin superinfections. As a control, serum from a non-atopic individual (panel N) was used. Molecular weights (kDa) are indicated in the left margin.
Ten of the 35 AD patients from the Austrian center showed distinct IgE reactivities to bands of different molecular weight (10–70 kDa) in extracts of S. aureus (Figure 1A). Elevated total serum IgE levels were often associated with IgE reactivity to S. aureus but were not a pre-requisite for IgE binding. In fact, not all patients with high total serum IgE (e.g., A13, A18, A20, A21) contained S. aureus-specific IgE (Table 1; Figure 1A). Similar results were obtained for the German AD patients (Table 2, Figure 2A), where some patients (e.g. H10, H17, H32) with high total IgE levels showed no S. aureus-specific IgE. When the serum from a non-atopic individual was used (panel N, Figures 1A and 2A), no IgE binding was observed.
The same sera were also probed with nitrocellulose-blotted E. coli extract (Figure 1B). Twelve of the 35 patients showed IgE reactivity to at least one band in E. coli extracts. Some of the IgE-reactive bands in E. coli extracts had similar molecular weights as those in S. aureus whereas others were different. Interestingly, certain patients showed IgE reactivity only to E. coli antigens (e.g., A13, A18, A21) but not to S. aureus antigens. Similarly in the German population, some patients (e.g., H9 and H27) had specific IgE reactivity only to E. coli, but not S. aureus proteins (Figure 2B). Again IgE reactivity was often associated with high total serum IgE but there were also patients with high total serum IgE lacking IgE reactivity to E. coli antigens (e.g., A20, H47, H1, H26). A slight anti-IgE-reactive band at approximately 34–35 kDa was observed with all sera, including the non-allergic persons (panel N) serum as well as the buffer control (data not shown). Hence it was a result of binding of the anti-IgE conjugate to this band. For control purposes, sera from 37 individuals with asthma and from 41 individuals with rhino-conjunctivitis but without allergic skin manifestations were also tested but no IgE reactivity to bacterial extracts was observed with these sera (data not shown).
Nineteen sera from the 35 AD patients were also tested for IgE reactivity to S. aureus toxins, of which 13 contained toxin-specific IgE (Table 1). Again toxin-specific IgE was mainly found in sera containing high total serum IgE levels but there were also sera (e.g., A7) which contained low total IgE levels and toxin-specific IgE (Table 1).
Periodate deglycosylation assay was used to determine if the IgE-reactive antigens were proteins or carbohydrates. Pre-treatment of nitrocellulose-blotted S. aureus antigens with periodate did not lead to a relevant reduction of IgE-binding capacity of the bacterial antigenic structures, compared to treatment with buffer alone. Periodate treatment of E. coli antigens also did not show any change in IgE binding capacity in five of the six patients tested. In one patient, periodate treatment even revealed additional IgE-reactive bands of approximately 25 kDa and 20 kDa (data not shown).
Patients with severe dermatitis symptoms show more frequently IgE reactivity to bacterial antigens than patients with mild symptoms
Based on the disease severity score of AD (SCORAD) for the German AD patients (Table 2), we classified these patients as having mild (0–24), moderate (25–50) or severe (>50) disease. Interestingly, 0%, 48% and 77% of these patients showed positive S. aureus IgE reactivity in the mild, moderate and severe groups, respectively. A similar relationship between E. coli-IgE reactivity and SCORAD was observed in this population (i.e. 10%, 48% and 77% for mild, moderate and severe AD respectively). Hence, AD patients with increased disease severity showed more frequently specific IgE reactivity to S. aureus and E. coli proteins, compared to AD patients with mild symptoms.
Next, we were interested to investigate if there was any association between S. aureus skin superinfections and IgE reactivity to bacterial antigens. For this purpose, sera from AD populations with and without skin superinfections (defined as clinically visible bacterial infection) obtained from the German center was studied (Figure 2). IgE-reactivity to S. aureus antigens were observed in 33% (3 of 9) of patients with skin superinfections, and 43% (15 of 35) of patients without skin superinfections (Figure 2A). Similarly, 44% (4 of 9) and 43% (15 of 35) patients had IgE-reactivity to E. coli proteins in this group of patients (Figure 2B). Hence, the frequency of IgE reactivity to bacterial antigens was not related to the presence of skin superinfections in AD patients.
IgE reactivity to gut bacteria
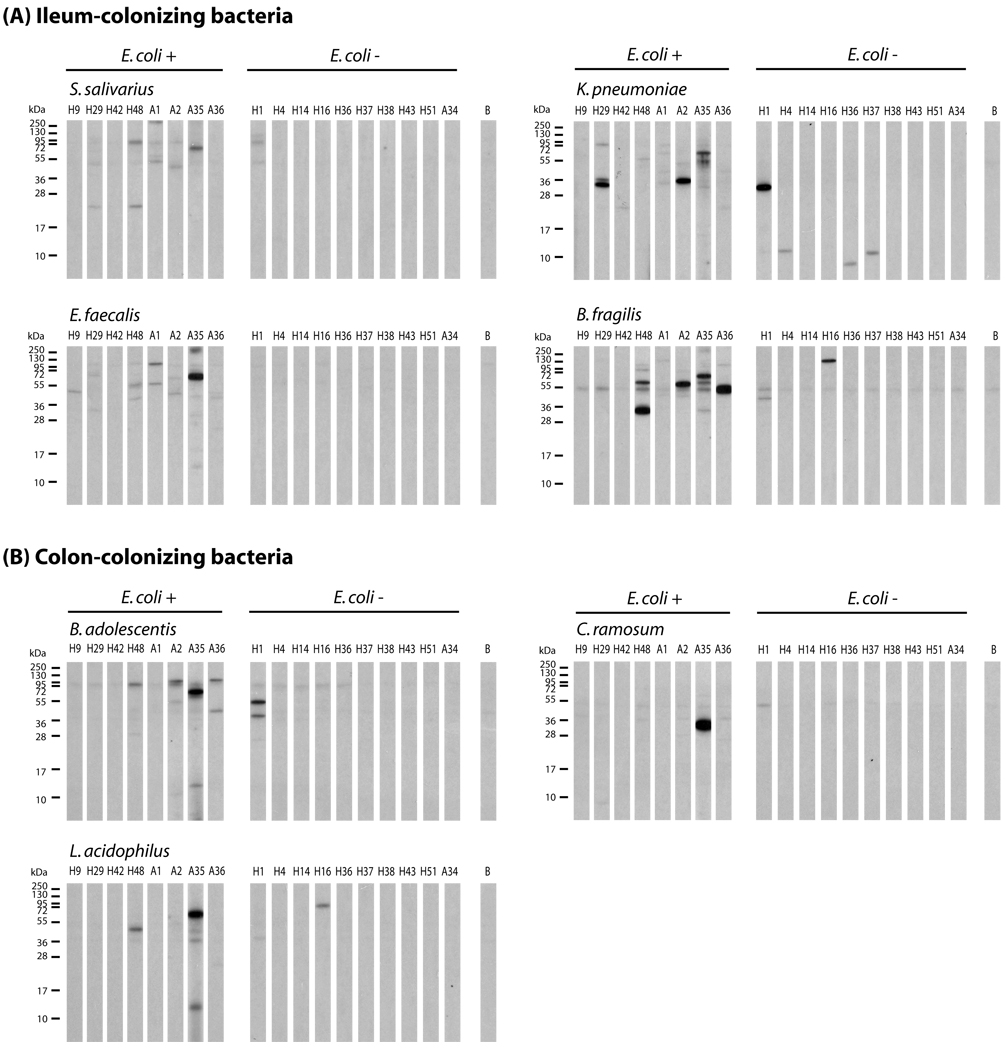
In this study, more than a third of the AD patients showed IgE-reactivity to E. coli proteins. The route of E. coli sensitization occurs most likely from the gut, and we therefore studied if AD patients also show IgE binding to antigens of other gut bacterial species. Nitrocellulose-blotted protein extracts of seven of the most commonly occurring gut bacterial species of the ileum or colon region (Supplementary Table 1) [38] were tested with remaining serum samples from AD patients with and without IgE-reactivity to E. coli proteins (Figures 3A, B). We found that both groups of AD patients showed IgE reactivity to ileum- and colon-colonizing bacteria (Figures 3A,B)
Figure 3. IgE reactivity of AD patients to nitrocellulose-blotted protein extracts from gut-colonizing bacteria.
Nitrocellulose-blotted protein extracts of S. salivarius, K. pneumoniae, E. faecalis and B. fragilis which are ileum-colonizing bacteria (A) and B. adolescentis, C. ramosum and L. acidophilus which are colon-colonizing bacteria (B) were probed with sera from patients with AD, with IgE to E. coli proteins (E. coli +) or without (E. coli −). Lane B denotes buffer control. Patient numbers are as in Tables 1 and 2. Molecular weights (kDa) are indicated in the left margin.
Low IgE cross-reactivity between S. aureus and E. coli antigens
S. aureus and E. coli extracts contained IgE-reactive bands of similar molecular weights. We therefore searched for the presence of IgE cross-reactive structures in S. aureus and E. coli in patients who displayed IgE reactivity to both extracts and for whom sufficient serum was available (Figure 4).
Figure 4. Low cross-reactivity between IgE-reactive antigens from S. aureus and E. coli.
Nitrocellulose-blotted S. aureus extracts (A) and E. coli extracts (B) were probed with sera from selected AD patients, which were pre-adsorbed with protein extracts of either S. aureus (lanes S), E. coli (lanes E), or with bovine serum albumin (BSA; lanes -). Patient numbers are as in Tables 1 and 2. Molecular weights (kDa) are indicated in the left margin.
As shown in Figure 4A, we found no (e.g., A4, A6) or limited cross-reactivity (e.g., A36, H12, H40) to nitrocellulose-blotted S. aureus extract when patient’s sera was pre-incubated with E. coli extract. Three of the four patients’ sera (A4, A21 and H49) showed no IgE cross-reactivity between S. aureus extract on nitrocellulose-blotted E. coli proteins. Sera of patient H48 showed only limited cross-reactivity, to a protein band of approximately 32 kDa (Figure 4B).
Lymphoproliferation and cytokine responses of AD patients to bacterial antigens
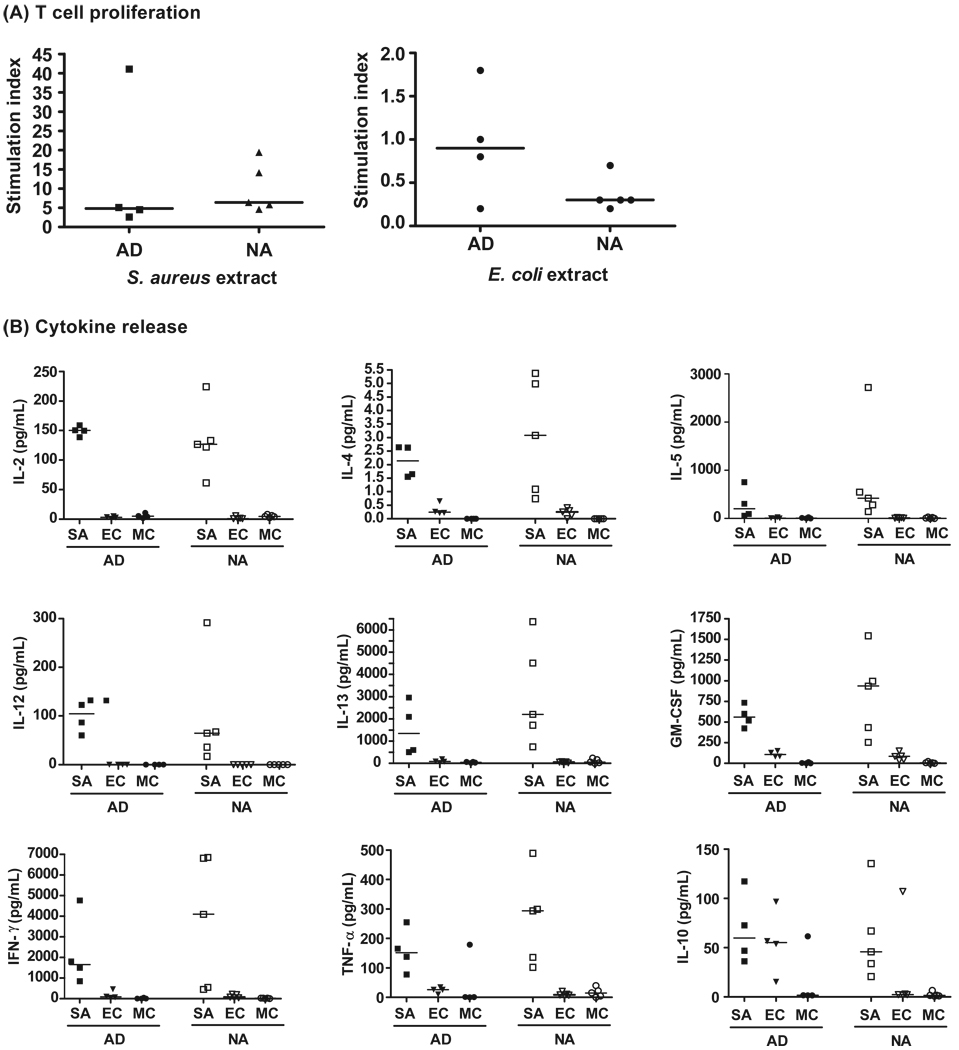
We found that S. aureus but not E. coli induced lymphoproliferative responses in AD as well as in non-allergic persons (Figure 5A). We also measured the levels of cytokines released into the cell culture supernatants after stimulation with bacterial extracts (Figure 5B). The stimulation of lymphocytes with proteins from S. aureus resulted in the release of elevated levels Th1 and Th2 cytokines, when compared to stimulation with E. coli extract or medium alone.
Figure 5. Lymphocyte proliferation and cytokine releases induced in PBMC cultures of AD patients and non-allergic individuals by stimulation with S. aureus and E. coli protein extracts.
(A) PBMCs from AD or non-atopic (NA) individuals were stimulated with S. aureus extract, E. coli extract or medium (MC). Mean lymphoproliferative responses of triplicate determinations were measured by 3H-thymidine incorporation and are displayed as stimulation indices. Horizontal lines denote the median value of each group. (B) The amounts of released cytokines are presented and horizontal lines denote the median value of each group.
S. aureus antigens can induce immediate type skin reactions in sensitized AD patients
Figure 6 shows the immediate and late skin reactions of 22 patients to S. aureus extract. All but one of the 19 patients with IgE antibodies to S. aureus proteins had both immediate and late phase inflammations upon intradermal skin testing with S. aureus extract. None of the patients who did not mount IgE against S. aureus IgE showed any skin reaction. The results show that S. aureus extracts can induce both specific immediate and late phase skin inflammation in patients with IgE antibodies against S. aureus components.
Figure 6. Immediate and late type skin reactions to S. aureus extract in AD patients.
Twenty two patients with AD, with or without IgE-reactivity to S. aureus proteins, were injected intradermally with S. aureus extract or saline buffer. Average wheal diameter was measured 20 minutes (immediate reaction) and 24 hours (late reaction) after the challenge.
Identification of IgE-reactive bacterial antigens by mass spectrometry
In order to identify IgE-reactive bacterial antigens, serum IgE from AD patients reacting with bacterial antigens was specifically immobilized on an anti-IgE column and incubated with bacterial antigens. Immune complexes consisting of IgE and bacterial antigens were then purified and subjected to MS/MS analysis. Supplementary Table 2 displays proteins from S. aureus and E. coli for which matching peptides could be identified by mass spectrometry in immune complexes obtained four reactive AD patients with a probability of >0.05. These proteins included among other proteins, DNA-binding proteins, ribosomal proteins and flagellin.
DISCUSSION
In this study, we report that a subgroup comprising more than a third of AD patients exhibits specific IgE reactivity against a variety of protein antigens of diverse molecular weights in S. aureus and E. coli. IgE binding was directed to protein bands in the molecular weight range of 20–100 kDa, with a high IgE recognition frequency to antigens in the 30–66 kDa range. The specificity of the IgE reactivity to the bacterial antigens was underlined by the fact that there was no clear relationship between total serum IgE and presence of specific IgE to antigens of S. aureus or E. coli. Elevated total serum IgE was associated with IgE reactivity to bacterial antigens, but was not a pre-requisite for this reaction. For the majority of patients, no or limited IgE cross-reactivity between antigens of S. aureus and E. coli was observed.
IgE reactivity to S. aureus has already been reported in earlier studies, but the antigens have not been characterized. We found that approximately 30% of AD patients had IgE against S. aureus antigens, whereas in earlier studies the prevalence of anti- S. aureus IgE in AD patients showed a great variation from 7– 70%. This variation might be explained by the antigen preparation (whole cells [21, 25, 27, 39], protein extracts [23, 26] or purified components [24–27, 29, 30]), choice of detection systems (RAST [21–27, 30, 39], immunoblots [30] , ELISA [29]), and also the patient population.
We investigated if the antigens of S. aureus and E. coli contain protein or carbohydrate epitopes. Based on the results of our chemical deglycosylation study, we did not detect a significant difference between IgE reactivity to periodate treated or untreated nitrocellulose-blotted antigens from S. aureus and E. coli, and hence conclude that the bacterial antigens comprise of mainly protein moieties. In fact the analysis of IgE-reactive bacterial proteins by mass spectrometry identified several of these proteins, among them DNA-binding proteins, ribosomal proteins and flagellin.
A quite unexpected finding of this study was the presence of anti-E. coli IgE reactivity. Up to 38% of the AD patients tested had specific IgE antibodies to E. coli antigens, although E. coli is not a common microflora in the infected skin lesions of AD patients. Based on the inhibition immunoblot assay, the IgE antibodies to E. coli proteins were mainly not cross-reactive to S. aureus antigens. With regards to the route of sensitization for E. coli, it is possible that it occurs via the skin, although there is little colonization. Alternatively, the gut may be a more likely entrance and sensitization point for E. coli. Based on this, IgE-reactivity to protein extracts of commonly occurring gut bacterial species of the ileum and colon regions were investigated and IgE binding to gut bacterial proteins was detected. Based on these results it may be interesting to investigate in a clinical study whether IgE antibodies against E. coli can induce food allergy-like symptoms.
In the case of S. aureus, the most probable route of sensitization is the skin route. In fact, we observed that patients suffering from severe forms of AD and with higher SCORAD values, showed more frequently IgE-reactivity to bacterial antigens compared to patients with mild AD symptoms and it is thus possible that patients with more severe skin damage experience higher loads of bacterial antigen exposure capable of boosting anti-bacterial IgE. The production of anti-microbial peptides, such as β-defensins and cathelicidins is reduced in AD patients compared to healthy persons [40, 41]. Additionally, it is known that many individuals with AD also have a defective skin barrier, and the combination of these two factors allows for the increased colonization of bacteria on the diseased skin, leading to increased penetration of S. aureus antigens through the skin [42] and elevated specific IgE production.
Specific anti- S. aureus IgE was detected exclusively in patients with AD, but not those suffering from other clinical manifestations of allergy, such as allergic asthma and rhino-conjunctivitis. This was also the case for another microorganism that was found to colonize the skin of AD patients, Malassezia sympodialis [40]. This finding could be a result of the continuous sensitizations resulting from the lack of innate immune function and disrupted skin barrier in AD patients. In contrast, patients with allergic asthma or rhino-conjunctivitis have healthy skin, with intact innate immune mechanism, and are therefore able to better defend themselves against microorganism colonization.
We found that S. aureus induced lymphoproliferative responses and the secretion of Th1 and Th2 cytokines in cultured PBMC both from AD and non-allergic persons, which is not too surprising given that both populations may have had contact with S. aureus. Interestingly, we did not observe relevant lymphoproliferative response or cytokine secretion when PBMC were stimulated with E. coli proteins, which may be explained as an effect of selective tolerance to gut bacteria. The non-specific activation of skin T- cells by S. aureus superantigens has been proposed as one pathomechanism by which S. aureus contributes to skin inflammation in AD. The presence of specific IgE reactive antigens could represent another pathomechanism in AD because they cross-link mast cells and induce specific T-cell responses. Our data demonstrate that S. aureus can induce immediate and late phase skin reactions indicating that IgE and T-cell recognition of S. aureus allergens may contribute to skin inflammation in AD. Therefore S. aureus should be considered as an allergen source for AD patients and future studies may investigate the allergenic potential of gut bacteria as well.
Our results thus provide another explanation how bacteria may contribute to the pathogenesis of AD and could open new avenues for diagnostic and therapeutic strategies. A better understanding of the individual antigens of S. aureus should provide component-resolved recombinant diagnostics, which may help to identify patients who benefit from antibiotic treatment and lead to the development of therapies targeting bacteria involved in the pathogenicity of AD.
Supplementary Material
ACKNOWLEDGEMENTS
K.R. is supported by grant F1815 of the Austrian Science Fund (FWF) and by a research grant from Biomay, Vienna, Austria. D.Y. L. is supported by NIH/NIAMS R01 AR41256.
ABBREVIATIONS
- AD
atopic dermatitis
- RAST
radioallergosorbent test
- S. aureus
Staphylococcus aureus
- E. coli
Escherichia coli
- SE
staphylococcal enterotoxin
- TSST
toxic shock syndrome toxin
- PBS
phosphate buffered saline
- IgE
immunoglobulin E
- PBMC
peripheral blood mononuclear cell
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- 1.Williams H, Robertson C, Stewart A, Ait-Khaled N, Anabwani G, Anderson R, Asher I, Beasley R, Bjorksten B, Burr M, Clayton T, Crane J, Ellwood P, Keil U, Lai C, Mallol J, Martinez F, Mitchell E, Montefort S, Pearce N, Shah J, Sibbald B, Strachan D, von Mutius E, Weiland SK. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125–138. doi: 10.1016/s0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]
- 2.Herd RM, Tidman MJ, Prescott RJ, Hunter JA. Prevalence of atopic eczema in the community: the Lothian Atopic Dermatitis study. Br J Dermatol. 1996;135:18–19. [PubMed] [Google Scholar]
- 3.Ingordo V, D'Andria G, D'Andria C. Adult-onset atopic dermatitis in a patch test population. Dermatology. 2003;206:197–203. doi: 10.1159/000068890. [DOI] [PubMed] [Google Scholar]
- 4.Beasley R, Keil U, von Mutius E, Pearce N. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225–1232. [PubMed] [Google Scholar]
- 5.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung DYM. Atopic dermatitis: the skin as a window into the pathogenesis of chronic allergic diseases. J Allergy Clin Immunol. 1995;96:302–318. doi: 10.1016/s0091-6749(95)70049-8. [DOI] [PubMed] [Google Scholar]
- 7.Jones HE, Inouye JC, McGerity JL, Lewis CW. Atopic disease and serum immunoglobulin-E. Br J Dermatol. 1975;92:17–25. doi: 10.1111/j.1365-2133.1975.tb03028.x. [DOI] [PubMed] [Google Scholar]
- 8.Ponyai G, Hidvegi B, Nemeth I, Sas A, Temesvari E, Karpati S. Contact and aeroallergens in adulthood atopic dermatitis. J Eur Acad Dermatol Venereol. 2008;22:1346–1355. doi: 10.1111/j.1468-3083.2008.02886.x. [DOI] [PubMed] [Google Scholar]
- 9.Hon KL, Leung TF, Ching G, Chow CM, Luk V, Ko WS, Ng PC. Patterns of food and aeroallergen sensitization in childhood eczema. Acta Paediatr. 2008;97:1734–1737. doi: 10.1111/j.1651-2227.2008.01034.x. [DOI] [PubMed] [Google Scholar]
- 10.Lubbe J. Secondary infections in patients with atopic dermatitis. Am J Clin Dermatol. 2003;4:641–654. doi: 10.2165/00128071-200304090-00006. [DOI] [PubMed] [Google Scholar]
- 11.Roll A, Cozzio A, Fischer B, Schmid-Grendelmeier P. Microbial colonization and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2004;4:373–378. doi: 10.1097/00130832-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Brook I. Secondary bacterial infections complicating skin lesions. J Med Microbiol. 2002;51:808–812. doi: 10.1099/0022-1317-51-10-808. [DOI] [PubMed] [Google Scholar]
- 13.David TJ, Cambridge GC. Bacterial infection and atopic eczema. Arch Dis Child. 1986;61:20–23. doi: 10.1136/adc.61.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goh CL, Wong JS, Giam YC. Skin colonization of Staphylococcus aureus in atopic dermatitis patients seen at the National Skin Centre, Singapore. Int J Dermatol. 1997;36:653–657. doi: 10.1046/j.1365-4362.1997.00290.x. [DOI] [PubMed] [Google Scholar]
- 15.Hauser C, Wuethrich B, Matter L, Wilhelm JA, Sonnabend W, Schopfer K. Staphylococcus aureus skin colonization in atopic dermatitis patients. Dermatologica. 1985;170:35–39. [PubMed] [Google Scholar]
- 16.Noble WC. Skin carriage of the Micrococcaceae. J Clin Pathol. 1969;22:249–253. doi: 10.1136/jcp.22.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson EJ, Henning CG, Magnusson J. Topical corticosteroids and Staphylococcus aureus in atopic dermatitis. J Am Acad Dermatol. 1992;27:29–34. doi: 10.1016/0190-9622(92)70151-5. [DOI] [PubMed] [Google Scholar]
- 18.Lever R, Hadley K, Downey D, Mackie R. Staphylococcal colonization in atopic dermatitis and the effect of topical mupirocin therapy. Br J Dermatol. 1988;119:189–198. doi: 10.1111/j.1365-2133.1988.tb03201.x. [DOI] [PubMed] [Google Scholar]
- 19.Niebuhr M, Mai U, Kapp A, Werfel T. Antibiotic treatment of cutaneous infections with Staphylococcus aureus in patients with atopic dermatitis: current antimicrobial resistances and susceptibilities. Exp Dermatol. 2008;17:953–957. doi: 10.1111/j.1600-0625.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 20.Keswick BH, Seymour JL, Milligan MC. Diaper area skin microflora of normal children and children with atopic dermatitis. J Clin Microbiol. 1987;25:216–221. doi: 10.1128/jcm.25.2.216-221.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abramson JS, Dahl MV, Walsh G, Blumenthal MN, Douglas SD, Quie PG. Antistaphylococcal IgE in patients with atopic dermatitis. J Am Acad Dermatol. 1982;7:105–110. doi: 10.1016/s0190-9622(82)80017-0. [DOI] [PubMed] [Google Scholar]
- 22.Yamada H, Yudate T, Orita T, Tezuka T. Serum levels of anti-Staphylococcus aureus-specific IgE in patients with atopic dermatitis. J Clin Lab Immunol. 1996;48:167–175. [PubMed] [Google Scholar]
- 23.Henocq E, Hewitt B, Guerin B. Staphylococcal and human dander IgE antibodies in superinfected atopic dermatitis. Clin Allergy. 1982;12:113–120. doi: 10.1111/j.1365-2222.1982.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 24.Motala C, Potter PC, Weinberg EG, Malherbe D, Hughes J. Anti-Staphylococcus aureus-specific IgE in atopic dermatitis. J Allergy Clin Immunol. 1986;78:583–589. doi: 10.1016/0091-6749(86)90075-8. [DOI] [PubMed] [Google Scholar]
- 25.Friedman SJ, Schroeter AL, Homburger HA. IgE antibodies to Staphylococcus aureus. Prevalence in patients with atopic dermatitis. Arch Dermatol. 1985;121:869–872. [PubMed] [Google Scholar]
- 26.Neuber K, Konig W. Effects of Staphylococcus aureus cell wall products (teichoic acid, peptidoglycan) and enterotoxin B on immunoglobulin (IgE, IgA, IgG) synthesis and CD23 expression in patients with atopic dermatitis. Immunology. 1992;75:23–28. [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh GA, Richards KL, Douglas SD, Blumenthal MN. Immunoglobulin E anti-Staphylococcus aureus antibodies in atopic patients. J Clin Microbiol. 1981;13:1046–1048. doi: 10.1128/jcm.13.6.1046-1048.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman SJ, Schroeter AL, Homburger HA. Whole organisms and purified cell walls compared as immunosorbents for the detection of IgE antibodies to Staphylococcus aureus. J Immunol Methods. 1984;66:369–375. doi: 10.1016/0022-1759(84)90350-8. [DOI] [PubMed] [Google Scholar]
- 29.Gabrielsen TO, Brandtzaeg P. Enzyme-linked immunosorbent assay (ELISA) for isotype-specific quantitation of antibodies to Staphylococcus aureus in patients with atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1985;114:61–66. doi: 10.2340/000155551146166. [DOI] [PubMed] [Google Scholar]
- 30.Hauser C, Wuethrich B, Matter L, Wilhelm JA, Schopfer K. Immune response to Staphylococcus aureus in atopic dermatitis. Dermatologica. 1985;170:114–120. doi: 10.1159/000249514. [DOI] [PubMed] [Google Scholar]
- 31.Bunikowski R, Mielke M, Skarabis H, Herz U, Bergmann RL, Wahn U, Renz H. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J Allergy Clin Immunol. 1999;103:119–124. doi: 10.1016/s0091-6749(99)70535-x. [DOI] [PubMed] [Google Scholar]
- 32.Leung DY, Harbeck R, Bina P, Reiser RF, Yang E, Norris DA, Hanifin JM, Sampson HA. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993;92:1374–1380. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong PY, Patel M, Ferdman RM, Dunaway T, Church JA. Association of staphylococcal superantigen-specific immunoglobulin e with mild and moderate atopic dermatitis. J Pediatr. 2008;153:803–806. doi: 10.1016/j.jpeds.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980 Suppl 92:44–47. [Google Scholar]
- 35.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–79. doi: 10.1111/j.1398-9995.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 38.Geiss HK. Koepereigene Flora des Menschen. Germany: Thieme Verlag KG; 1992. [Google Scholar]
- 39.Neuber K, Stephan U, Franken J, Konig W. Staphylococcus aureus modifies the cytokine-induced immunoglobulin synthesis and CD23 expression in patients with atopic dermatitis. Immunology. 1991;73:197–204. [PMC free article] [PubMed] [Google Scholar]
- 40.Casagrande BF, Fluckiger S, Linder MT, Johansson C, Scheynius A, Crameri R, Schmid-Grendelmeier P. Sensitization to the yeast Malassezia sympodialis is specific for extrinsic and intrinsic atopic eczema. J Invest Dermatol. 2006;126:2414–2421. doi: 10.1038/sj.jid.5700431. [DOI] [PubMed] [Google Scholar]
- 41.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 42.Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361:151–160. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- 43.Breuer K, Wittmann M, Kempe K, Kapp A, Mai U, Dittrich-Breiholz O, Kracht M, Mrabet-Dahbi S, Werfel T. Alpha-toxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin Exp Allergy. 2005;35:1088–1095. doi: 10.1111/j.1365-2222.2005.02295.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.