Abstract
Importance
Mesenchymal stem cells have the ability to differentiate into osteoblasts, chondrocytes and adipocytes. Along with differentiation, MSCs can modulate inflammation, home to damaged tissues and secret bioactive molecules. These properties can be enhanced through genetic-modification that would combine the best of both cell and gene therapy fields to treat monogenic and multigenic diseases.
Areas covered
A review of the findings demonstrating the immunomodulation, homing and paracrine activities of MSCs followed by a summary of the current research utilizing MSCs as a vector for gene therapy, focusing on skeletal disorders, but also cardiovascular disease, ischemic damage and cancer.
What the reader will gain
MSCs are a possible therapeutic for many diseases, especially those related to the musculoskeletal system, as a standalone treatment, or in combination with factors that enhance the abilities of these cells to migrate, survive or promote healing through anti-inflammatory and immunomodulatory effects, differentiation, angiogenesis, delivery of cytolytic or anabolic agents.
Take home message
Genetically-modified MSCs are a promising area of research that would be improved by focusing on the biology of MSCs that could lead to identification of the natural and engrafting MSC-niche and a consensus on how to isolate and expand MSCs for therapeutic purposes.
Keywords: mesenchymal stem cell, gene therapy, genetic modifications, genetic engineering, immunomodulatory, paracrine effects, skeletal disorders
1. Introduction
The discovery of adult stem cells incited expectations for novel therapies to multiple diseases and disorders. Multipotent stromal cells, or mesenchymal stem cells (MSC), are adult stem cells that can differentiate into cells of the mesoderm-lineage, including osteoblasts, chondrocytes and adipocytes [1, 2]. The field of regenerative medicine developed to utilize the intrinsic multipotentiality of these cells to overcome failed, faulty or ineffective repair processes. The potential usefulness of MSCs in gene therapy was quickly recognized during their characterization. Along with differentiation, MSCs have been found to be both hypoimmunogenic and immunomodulatory, can home to damaged tissues and depend on secretion of bioactive molecules to initiate healing in repair processes. These characteristics suggest that genetically-modified MSCs could combine the best of both cell and gene therapy fields to treat diverse diseases from cancer to cardiovascular or skeletal diseases.
Despite successes in animal models, the use of gene therapy in humans has remained unfulfilled. The few clinical trials involving delivery of genes to humans have seen only limited success with severe drawbacks, which highlight the challenges that this emerging field is still facing. Delivery of genes is achieved using one of several types of viral vectors and even these modified vectors can lead to toxicity and inflammatory responses. In one clinical study aimed to evaluate safety and efficacy of gene therapy in subjects with enzyme ornithine transcarboxylase deficiency, a patient died of a severe immune response likely caused by the viral vector used as a delivery mechanism [3]. Work is ongoing to alter viral vectors to make them safe. Other non-viral techniques are also being pursued, such as the use of liposomes or nanoparticles to help cell uptake of naked or unmodified plasmid DNA. Targeting the DNA for genomic incorporation also remains an issue. In clinical studies using bone marrow (BM) cells to deliver interleukin-2 to X-linked severe combined immunodeficiency patients in Europe, despite promising results, some patients developed leukemia-like symptoms leading to death likely due to the insertion of the IL-2 gene near an oncogene [4]. Based on these fatalities, in April of 2003 the FDA banned gene therapy trials using retroviral vectors in blood stem cells. Current research is focusing on targeting gene insertion into “safe harbor” areas of the genome specifically in MSCs [5]. Other complications in the use of gene therapy include lack of tissue specific targeting, control over timing and expression level of the additive gene as well as the elicitation of an undesired immune response. All these issues could be addressed by using improved gene delivery vector systems with an immune-modulating, engraftable, highly plastic, yet naturally occurring, injury-seeking stem cell such as MSCs.
MSCs are rare, non-hematopoietic progenitor cells first isolated from BM mainly based on their ability to adhere to plastic culture dishes. Freidenstein et al., in characterizing these cells found them to be clonogenic in culture and multipotent for stromal precursors [6]. Though mainly found in BM and adipose tissue, examples of isolation from peripheral blood, umbilical cord blood, synovial membranes, deciduous teeth, amniotic fluid and perivascular regions exist, leaving open the question of the natural niche for MSCs [7]. Pericytes are cells that surround blood vessels throughout the body and recent work has compared the phenotype of pericytes and MSCs and found that MSCs express pericyte markers [8]. It is possible then that all MSCs are pericytes and the natural niche is perivascular, which would allow MSCs easy access to cytokines and chemokines and a means to migrate to injured tissue, though whether these are truly the same cell is still up for debate. The osteo-, chondro- and adipo-genic ability of MSCs has since been established and examples of differentiation into cells such as, cardiomyocytes, neurons and hepatocytes have been described [2, 9–11]. The ability of MSC to differentiate into non-mesodermal lineages remains controversial and could be attributable to a lack of homogeneity within isolated MSC populations, or due to disparate isolation and expansion techniques. In fact, a specific, true multipotent MSC population has yet to be clearly defined. Currently the International Society for Cell Therapy defines human multipotent mesenchymal stromal cells as plastic adhering cultured cells that express CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79alpha or CD19 and HLA-DR surface molecules, while maintaining the ability to differentiate into osteoblasts, adipocytes and chondrocytes in vitro [12]. These requirements are not completely specific and do not extend to rodent and other animal sources of MSCs, in which a significant portion of the basic research is being performed, thus further identification of markers is required.
MSCs have several favorable qualities that signify their usefulness as a source of therapeutic treatments for multiple disorders. Besides the ease of isolation and expansion in culture and their capacity to differentiate into multiple lineages, MSCs: have key interactions with the immune system; have been shown to migrate to sites of injury; generate strong paracrine effects with surface and shed molecules.
Transplanted MSCs have been shown to home to sites of damaged tissue. Bone fractures, infarcted heart muscle, rat ischemic brain and renal injury are all sites of localization of transplanted MSCs in animal models [13–16]. The fact that MSCs can be isolated from peripheral blood and can be found in increased numbers under stress of total body irradiation or hypoxia implies that MSCs are part of the innate healing response involving a natural trafficking signal that can be utilized by transplanted MSCs [17, 18]. MSCs express an assortment of chemokine receptors that allow for their migration in response to the chemokine-attractive gradients generated by the inflamed injured site. The functional chemokine receptors expressed by MSCs include CCR1, CCR7, CCR9, CXCR3, CXCR4, CXCR5 and CX3CR1 [19]. The CXCR4 receptor and its single, specific chemokine, SDF1/CXCL12 has an important role in the regulation of hematopoietic stem cells (HSC) and other stem cell trafficking, along with a part in controlling the metastasis of several types of cancer [20, 21]. This signaling axis has been shown to regulate MSC localization to damaged heart tissue in a rat model of myocardial infarction and to fractured mouse tibias [13, 15]. Both in vitro and in vivo experiments with MSCs overexpressing CXCR4 have shown increased migration, localization and healing in the case of transplantation into mice suffering coronary occlusion-reperfusion injury [22, 23]. SDF1 expression is upregulated in rodent hearts following myocardial infarction and this led to an increase in the number of MSCs recruited to the injured tissue [24, 25]. However, Ip et al. showed that inhibition of CXCR4 in MSC had no effect on MSC migration to ischemic myocardium, but instead was dependent on integrin β1 [26]. Similarly, MSC homing to bone was improved with ectopic expression of integrin α4 and ts association with integrin β1 in mice [27]. The chemokine receptor CCR7 has been associated with MSC localization to CCL2- expressing skin wound sites [28]. The chemokine CCL2 (MCP-1) is also produced at sites of inflammation and reported to be critical for recruiting MSCs expressing its receptor CCR2 [29]. The innate ability of MSCs to target sites of injury and damage and the determination of the mechanisms involved will have important implications for the therapeutic use of MSCs.
There are an increasing number of studies showing MSC-dependent improvement of damaged tissues without a significant contribution of engrafted and differentiated cells. The mechanism for this is still under investigation, but likely depends on paracrine-mediated effects of MSCs. In response to injury, MSCs in culture secrete a large number of cytokines, chemokines and other trophic factors [30, 31]. Improvement or protective effects of MSCs despite low levels of engraftment within hepatotoxin damaged liver and mouse myocardial infarction model led the authors to test MSC conditioned media in their models, which reproduced the results of intact MSC transplant [32, 33]. The paracrine-mediated effects of MSC can be seen in another of MSCs beneficial properties, immunomodulation.
MSCs were first isolated from BM, a key site of hematopoiesis in which MSCs are now thought to play a role. HSCs require stromal supporting cells for proper differentiation and for the maintenance of the quiescent state within the endosteal niche of the BM and both supporting functions can be carried out by the MSC-progeny, osteoblasts [34]. MSCs reside and engraft after transplant within this endosteal niche suggesting a direct interplay between hematopoietic and skeletal homeostasis [13, 35]. This has been the basis for the use of MSCs in combination with HSC transplantation in the hopes of enhancing engraftment and proliferation of donor HSC which is under investigation in several clinical trials [36]. As a possible consequenc e of this tight interaction with immune cell progenitors MSCs are non-immunogenic. This is believed to be due to low or complete lack of MHC I, II and costimulatory molecules, meaning they can avoid activation of and clearance by the immune system [37].
MSCs also directly modulate the immune responses of several immune cells. MSCs inhibit the proliferation of lymphocytes, antigen presenting cells and natural killer cells, though the mechanism is still up for debate [38]. In in vitro experiments, MSCs block activation of T cells in response to a host of immunogenic stimuli by release of paracrine factors, though Krampera et al. have shown a requirement for direct MSC:T cell interaction to modulate the T cell reaction [39–41]. A diverse set of soluble factors have been proposed for this function, including TGFβ, IGF, VEGF, HGF, IL-2, -10 and PGE2 possibly regulated by toll-like receptors [38, 41]. Indoleamine 2, 3-dioxygenase is another soluble factor released by MSCs in response to IFNγ that depletes T cell tryptophan levels leading to inhibition of T cell activation, proliferation and apoptosis [42]. While MSCs lack the full array of costimulatory machinery to activate T cells, low levels of MHC-I are expressed on MSCs and IFNγ stimulation increases MHC II molecules on the surface of MSCs. The absence of costimulatory machinery has been associated with T cell anergy, whereby the cells become unresponsive and unable to respond to further challenges which could also explain the ability of MSCs to suppress the immune response [40]. Another mechanism involves MSCs both inhibiting the maturation and inducing the reversion of mature dendritic cells to immature cells, leading to a lack of mature antigen presenting cells and thus activated T cells [43]. Furthermore, MSCs can decrease the release of the pro-inflammatory cytokines TNFα and IFNγ and IL-12 produced by dendritic cells while increasing the anti-inflammatory molecule IL-10 [44–46]. We have reported a similar effect in in vivo studies with transplanted MSCs in a tibia fracture model in mice which lead to a decrease in the pro-inflammatory cytokines TNFα, IL-1β and IL-6 in circulation and at the fracture site [13]. Less clear is the effect on B cells, where high volumes of MSC inhibited proliferation and differentiation through paracrine factors in response to a B cell activating cytokine cocktail, while MSC contact with B cells leads to increased antibody production and proliferation [47, 48]. Lastly, natural killer cell proliferation and activity are also impeded in the presence of MSC, though this may require a combination of cell-cell contact and soluble factors [44, 49, 50].
The importance of these effects was established in in vivo animal models of acute lung injury. MSCs transplanted into mice following bleomycin-induced lung injury attenuated tissue damage and inhibited the infiltration of neutrophils despite the low level of cell engraftment within the lung. Instead, plasma levels of inflammatory cytokines were reduced while lung cells produced growth factors and chemokines which were able to stimulate MSC proliferation and migration towards injured tissue [51]. Further limiting the damage from inflammation in MSC-transplanted mice could be the ability of MSCs to regulate the oxidative state of the local environment. It has been proposed that MSC secrete antioxidant molecules such as glutathione and disulfide cysteine, to maintain redox homeostasis, as seen when conditioned media from MSCs were able to rescue oxidized cells and in an endotoxin model of lung injury [52]. Thus, while the mechanism remains unresolved, the immunomodulating properties of MSCs, which seem to depend on paracrine mediated effects, are now an important factor in driving the interest of these cells for therapeutic research.
The initial focus for therapeutic use of MSC was on regeneration of musculoskeletal system diseases. As the new paradigm for MSC function emerges, where the more important role is as an initiator of healing through modulation of the initial inflammatory response and regulation of the environmental niche through paracrine delivery of bioactive molecules that stimulate healing, the value of developing cytokine and growth factor gene-modified MSCs, increases. Indeed, several preclinical studies of genetically-altered MSCs have demonstrated the enhancement of the MSC effects. This review will now summarize the current use of gene-modified MSCs in animal models, focusing on bone related disorders.
2. Skeletal Disorders
MSCs seem tailor made to fulfill the need for novel therapies for the repair and replacement of bone, cartilage, ligaments and tendons. In our recent studies, we have reported that MSC-transplant improves fracture repair by improving the callus biomechanical properties and promoting cartilage formation [13]. While these studies clearly indicate that MSCs have beneficial effects in the healing process, the use of MSCs is far from the silver bullet first envisioned, due to our incomplete understanding of differentiation cues and regulatory mechanisms that trigger the regenerative response. Preclinical studies on the effect of MSCs in fracture repair have shown promise, but also suggest that genetically manipulated MSCs may enhance the use of MSCs in skeletal disorders.
2.1. Bone Regeneration and Repair
By far the most effort into genetically-modified MSCs has been with the BMP family of growth factors. BMPs are members of the TGFβ superfamily of growth factors which play a major role in the formation of bone and cartilage along with other nonosteoblastic developmental functions [53, 54]. BMP2 was discovered as a powerful bone stimulating factor and along with other family members, such as BMP-4 and -7, have been examined as a therapy for bone defects in several animal models [55]. BMP2 has been studied in several clinical trials for tibial non-union fractures and has been approved for use in spinal fusion and non-unions [56]. However, high concentrations of BMPs are required for effectiveness which is still not always successful [57]. Using MSCs from BMP2-LacZ reporter mice we have been able to demonstrate that transplanted MSCs improve fracture healing while engrafting within the fracture site including a specific endosteal niche where they express BMP2 [13]. We have also found that BMP2 expression within this niche is not driven by the osteoblastic differentiation of MSCs. BMP2 has been reported to be necessary for the initiation of the fracture repair process [58]. Taken together, these data indicate that MSCs engraft within specific fracture repairing niches where they induce BMP2 which acts as a critical initiator of the regenerative process. This suggests that combining the bone trophic effects of BMP2 with a system to regulate the localization, temporal and levels of expression from MSCs could be a potential target for BMP2 based therapies in non-unions.
Early experiments using BMP2 and BM stromal cells formed intramuscular ectopic bone following implantation of adenoviral transduced cells within a femoral defect in rats [59]. The ability of BMP2-MSC to induce ectopic bone formation in immunocompromised mice has also been shown with lentiviral vectors and the non-viral nucleofection technique [60, 61]. Human MSCs, soaked into a collagen sponge, delivered BMP2 into radial fractures of immune incompetent mice and completely bridged the gap with bone and cartilage [62]. In a rat articular fracture model, MSCs expressing adenovirus-BMP2 (AdBMP2) induced healing of both bone and cartilage in as little as 14 days [63]. MSCs with AdBMP2 have also been shown to heal cranio- and maxillo-facial critical size defects in swine [64, 65]. Transplant of AdBMP2-MSC into the spinal processes of rabbits induced new bone formation and fusion with similar results in rats using adeno- or lentiviral vectors without the need for mechanical fixation [66–69]. BMP2-MSCs also increased bone mineral density and content in a mouse model of osteoporosis through osteoblastic differentiation of both the donor cells and a stimulated effect of the endogenous progenitors [70]. Taken together these results demonstrate the enhancement of bone growth and repair when BMP2 overexpression is combined with cells that can also respond to BMP2 signaling.
BMP2 is not the only family member with bone inducing properties. BMP4-MSCs transplanted into the femoral BM cavity increased both trabecular and total bone density, while the similar muscle-derived stem cells transduced with BMP4 have demonstrated improved osteogenesis in immune competent mice [71]. BMP7 in combination with MSCs has not been reported yet, but in periosteal cells and dermal fibroblasts, BMP7 was able to increase intramuscular bone growth in rabbit and rat calvarial defects [72, 73].
Other bone-related factors may also be effective in promoting bone growth and healing. RunX2 is a key transcription factor in osteogenesis and can help direct cells towards osteogenic and chondrogenic differentiation. In AdRunX2 MSCs, more bone was formed following subcutaneous injection or transplantation into a calvarial defect in immunecompetent mice compared to MSCs alone [74, 75]. Another transcription factor, sonic hedgehog, in adipose-derived stem cells, commonly believed to be MSCs, also increased bone formation in a cranial defect in rabbits, though no mechanism was pursued [76]. Basic fibroblast growth factor (bFGF) is a powerful osteogenic proliferation growth factor that when transfected into MSC and transplanted into radial segmental defects in rabbits induced greater bone and capillary formation, though it’s possible that the proliferation response triggered by bFGF may ultimately inhibit the differentiation signals in progenitor cells [77, 78]. The Wnt signaling pathway has also been described as influencing osteogenesis [79]. Wnt4-MSCs loaded on scaffolds were able to increase mineralized bone and complete bridging of both a periodontal and calvarial defect [80]. In an attempt to surpass telomere shortening-induced senescence of MSCs, adenoviral telomerase was used to extend the lifespan of the cells in culture and increase ectopic bone formation and the number of osteogenic cells after subcutaneous injection into mice [81]. LIM mineralization protein (LMP) is a novel regulator of osteogenesis, possibly through the regulation of osteogenic genes such as BMP2, osterix and RunX2. LMP can be spliced into 3 variants and adenovirus-encoding LMP1 has been used in buffy coat-derived marrow cells to achieve new bone growth and spinal fusion in rabbits, while LMP3 in the same cells, has induced greater ectopic bone formation than AdBMP2 in skeletal muscle [82, 83]. Recent work in our laboratory has shown the positive effects of transplanted AdIGF-I-MSC in mouse tibial fracture healing [84]. Delivery of MSCs and over expressing IGF-I at the fracture site induced an increase in callus size and biomechanical properties over MSCs alone, or a no cell control, due to additional bone formation [84].
In normal fracture healing, a complex milieu of growth factors and cells act in a specific spatially and temporally regulated fashion. Current research is focused on combination therapy with BMPs and other important osteotrophic factors to increase bone regeneration. RunX2 may complement BMP2 signaling, as seen in vitro when AdRunX2 cells treated with recombinant BMP2 displayed enhanced expression of osteogenic markers [85]. When RunX2 and BMP2 were combined into an immortalized mesenchymal cell line and injected into mice, bone and cartilage ossicles with a marrow cavity were formed to a greater extent compared to AdBMP2 cells alone [86]. The angiogenic factor VEGF can also enhance BMP family bone formation. When AdBMP4 and AdVEGF were co-transduced into muscle derived stem cells and transplanted into mice, only in the presence of both factors was their increased angiogenesis, cell survival and bone healing [87]. More recently, BMP2 and VEGF were combined in MSCs using AAV vectors and demonstrated enhanced bone formation, bone density and biomechanical properties following systemic administration in a mouse tibial defect model [88]. These results allude to the power of combinatorial gene modifications and expand the possibilities of factors to be considered for use with MSCs.
Investigation has also begun on temporal control of growth factors in genetically-modified MSCs. Use of regulatable vectors will allow superior control of individual factors to mimic the intrinsic healing process and utilization of specific features of factors in combination gene therapy, while limiting the likelihood of excess bone formation or tumorigenesis from an unregulated cell. By using the “tet-off” system where expression of target genes can be exogenously regulated by tetracycline treatment, Hasharoni et al. demonstrated that when AdBMP2 was expressed by MSCs transplanted into the muscle surrounding the lumbar spine of mice it induced a considerable amount of new bone leading to spine fusion [67]. The “tet-on” system has also been used with BMP4 in muscle derived stem cells, a cell type related to MSCs with a history of osteogenic potential, to demonstrate ectopic bone formation, though in a critical-sized calvarial defect regulation was unable to halt excess bone growth [89–91]. The “tet-off” system with RunX2 in skeletal myoblasts demonstrates the repression in the presence, recoverability in the absence and dose-dependence of tetracycline on the formation of ectopic bone [92].
2.2. Cartilage Repair
Cartilage damage is slow to heal due to limitation of progenitor cells and appropriate anabolic signals. MSCs have the potential to differentiate into chondrocytes and have been shown to slow the progression of osteoarthritis and initiate partial cartilage repair [7]. The combination of cell transplant and gene therapy could greatly aid the cartilage repair process by directing the differentiation and chondrogenic response of the cells. Initial gene therapy targeting cartilage damage employed transplantation of chondrocytes transduced with known chondrogenic stimulators IGF-I, BMP7, or FGF into rabbit and horse models of arthritis [93–96]. In vitro, overexpression of IGF-I alone in MSCs could not induce chondrogenic differentiation, but in concert with TGFβ1, or BMP2, induced greater chondrogenic tissue than either alone [97, 98]. This is in support of in vivo experiments where MSCs were transfected or transduced withTGFβ1 to form ectopic cartilage in pigs, or filling full-thickness articular cartilage defects in rabbits and rats [99–101]. AdBMP2-induced cartilage repair was compared in adipose, BM and periosteal MSCs of rat cartilage lesions. BM and periosteal cells showed enhanced cartilage repair compared to adipose MSCs, but all cell types required BMP2 to initiate chondrogenesis [102]. Another member of the TGFβ superfamily, GDF5/CDMP1 with MSCs, accelerated cartilage formation in rabbit full thickness cartilage defects [103]. Similar to bone repair, transcription factors can be used to regulate a host of important chondrogenic pathways. Sox9, which has been shown to regulate a number of chondrogenic genes including collagen II, was transfected into MSCs and transplanted subcutaneously into mice, where a collagen II-positive thick mass of tissue formed over 4 weeks compared to untransfected cells [104]. The BMP2- and FGFR3-regulated transcription factor Brachyury was used to force chondrogenic differentiation and ectopic cartilage formation of a MSC-like cell line in subcutaneously injected mice [105]. More recently Sprouty1 has been postulated as a critical regulatory switch of MSC cell lineage allocation from adipose to osteoblast progenitors [106]. These studies clearly indicate the need to identify signaling pathways to direct stem cells to pre-determined lineages.
2.3. Ligament and Tendon Repair
The repair of ligaments and tendons using genetically-modified MSCs is a relatively new field of study. MSCs can be forced to differentiate into tenocytes in culture and autologous cells have been used in equine systems to repair partially damaged digital flexor tendons [107]. Hoffmann et al. combined BMP2 signaling and Smad8 inhibition of osteogenesis in an MSC-like cell line to form ectopic tenocytes and fill partially injured tendons in rats [108]. AdIGF-I-MSCs were also used in equine damaged digital flexor tendons to induce healing of lesions, though there was no difference between empty MSCs, suggesting some other factor may be more prominently involved [109]. Modified MSCs can also aid in the repair of tendons indirectly. Membrane type I matrix metalloproteinase (MT1-MMP), one of the MMPs involved in cartilage proteolysis and supporting its mineralization, was transduced into MSCs in an attempt to improve the tendon adhesion to the bone interface by increasing fibrocartilage formation [110]. BMP12 can induce MSCs to differentiate into tenocytes and ligaments in vitro and in skeletal muscle cells was able to improve healing progression and biomechanical properties of the injured rat tendon over controls [111–113]. In a tendon allograft used to repair an ACL njury in rabbits, PDGF-B transduced MSCs and MSCs alone were able to increase cell recruitment to the ligament but only PDGF-B-MSCs increased angiogenesis, thus the direct contribution of MSCs versus PDGF to this process remains to be determined [114]. A better understanding of the signaling molecules and pathways that regulate tenocyte and ligamentogenesis will build upon these early findings by using genetically modified MSCs.
2.4. Monogenic Bone Diseases
Hypophosphatasia is a disease characterized by reduced mineralization of the bone due to inactivating mutations in tissue non-specific alkaline phosphatase (ALP) from osteoblasts and chondrocytes. Transplanted MSC-differentiation into osteoblasts could be an effective treatment for delivery of a population of cells able to produce normal ALP enzyme. Whole BM transplants have been used to treat infants suffering from hypophosphatasia in combination with cultured osteoblast cells, however the use of ex vivo expanded MSCs six months after initial BM transplant yielded dramatic improvements likely due to long term engraftment of these progenitor-supplying cells [115–117]. In all cases, despite varying degrees of improvement, ALP levels remained low, which could be addressed by the use of ALP-genetically-modified MSCs to deliver greater expression of the enzyme. Osteogenesis imperfecta (OI) is a group of at least nine genetic disorders characterized by bone disease leading to incomplete bone lengthening and increased risk of fracture with different degrees of severity. In most affected patients, it is caused by the lack, or abnormal synthesis, of type I collagen. The main treatment is with anti-resorptive bisphosphonates to increase bone density, but this fails to address the underlying cause. MSCs may be useful for the treatment of patients with OI because they can differentiate into osteoblasts and supply normal collagen I. MSCs have already been evaluated in clinical case studies. Children with a severe type of OI underwent BM transplant with additional MSC transplantation and exhibited increased bone density and lengthening [118]. Allogenic MSCs were also transplanted in utero to a female fetus, which at 9 months of age had normal growth rates and detectable donor MSCs [119]. To test the feasibility of collagen gene targeting, MSCs from a patient suffering from OI were transduced with a viral vector intended to target and disrupt the mutant collagen. MSCs with successfully targeted deletion of mutant collagen processed and formed collagen and collagen fibrils similar to wild type cells. In vivo, these cells were able to form ectopic bone in mice [120]. This strategy would allow the use of autologous ex vivo expanded MSC to treat OI, hypophosphatasia and possibly other bone monogenic diseases.
3. Cardiovascular and Ischemic Disease
In the last decade, there has been an escalation of studies involving MSCs and cardiovascular disease. Specifically in myocardial infarctions, the plasticity, homing and inflammatory modulation of MSCs are all important properties that come into play following the hypoxia-induced damage to cardiomyocytes due to occluded arteries. The myocardium lacks the ability to efficiently replace these cells, and thus the goal of any treatment is the replacement of cardiomyocytes. Several studies have shown the ability of MSCs to transdifferentiate into cardiomyocytes in vitro and in animal models of myocardial infarction, though there is also evidence that the number of stem cells that engraft and differentiate into cardiomyocytes is extremely low [121]. Still, clinical trials for the use of MSCs to treat acute myocardial infarctionare under way with promising results [122]. The use of genetically-modified MSCs in these animal models attempts to improve the homing, survival and paracrine-mediated effects of MSCs. Systemically infused CXCR4-overexpressing MSCs are more abundant in and around the infarcted area in rat hearts and led to improved function compared to MSCs alone [23, 123, 124]. Similar results were seen with the chemokine receptor CCR1 [125]. In rodent models of myocardial infarction, besides increased homing to the injury, CXCR4- and SDF1-modified MSCs expressed increased amounts of angiogenic and growth factors, improved endothelial differentiation and angiogenesis leading to improved cardiac function [123, 126].
Most other studies have aimed to improve MSC survival following transplantation. A major cause of cell death in the infarcted heart is due to the low oxygen content. To enhance the ability of MSCs to tolerate the hypoxic conditions, MSCs were modified with the hypoxic-regulated, anti-apoptotic proteins heme oxygenase-1 or angiogenin, which is also a protective and angiogenic molecule, resulting in increased cell survival, signs of neoangiogenesis and improved heart function [127–129]. TNFα receptor is a mediator of inflammation in ischemic heart but may have survival effects for MSCs as seen with TNFα receptor overexpressing-MSCs in a rat model of acute myocardial infarction and supported by the loss of cardioprotective effects in mice transplanted with TNFα receptor-2 knockout MSCs [130, 131]. Other anti-apoptotic molecules such as Bcl2, survivin, adrenomedullin, and erythropoietin have all induced increased survival of transplanted cells within the infarcted area and increased angiogenesis as measured by capillary density [132–135]. IGF-I is a potent proliferative and differentiative factor for MSCs and when expressed by MSCs in the context of a coronary artery occlusion, induced expression of anti-apoptotic factors such as Bcl.xL and the homing factor SDF1 which in turn led to an increase in the number stem cells around the blockage [136]. Transplant of MSCs modified to express VEGF under hypoxic conditions increased MSC cell survival, differentiation into cardiomyocytes, induce angiogenesis and improve overall heart function [137]. HGF transduced into MSCs had similar effects to VEGF-MSCs, while SDF1-VEGF-MSCs were superior to either single transduced MSCs in augmenting survival of MSCs, shrinking infarct sizes and increasing vascular density, possibly dependent on the Akt pathway to moderate the effects [126, 138]. The direct effects of Akt1 expressed in transplanted MSC have also been evaluated. This prosurvival protein in MSCs dose-dependently increased myocardial volume and heart function in a heart ischemia model despite low engraftment and differentiation of MSCs [139]. Akt1 actions were dependent on an increase in the secreted frizzled related protein 2 and its subsequent paracrine activation of Wnt signaling in endogenous cells that then normalized cell metabolism and environment [140, 141]. The angiogenic factor angiopoietin-1 alone or in collaboration with Akt1 in MSCs also improve vascular density and heart function [142, 143]. Lastly, Song et al. were able to increase the survival rate of MSCs transplanted into infarcted heart by transducing cells with integrin-linked inase which amplified expression of anti-apoptotic genes and proliferative signaling to improve heart function, presumably due to increased adhesion of the cells within the damaged tissue [144].
Along with ischemic heart, other organs are also sensitive to MSC treatment in response to the hypoxic conditions following ischemia/reperfusion. MSC transplant into rodent models of renal, limb, lung and brain ischemia has shown beneficial effects. Much of the genetic modifications used with MSCs in these models have focused on protecting the cells from the hypoxic conditions along with stimulating neoangiogenesis. In rodent models of hindlimb ischemia, transplanted MSCs modified to express VEGF or angiopoietin-1, increased vessel density as early as 14 days post injury/transplant and demonstrated enhanced MSC survival within the damaged tissue [145, 146]. Interestingly, VEGF was transfected into MSCs using polymeric nanoparticles that avoid potential safety concerns of the generally used viral delivery systems [145]. Angiopoietin-1-MSCs were studied in a lung ischemia model which limited damage and scarring by inhibiting the recruitment of inflammatory cells [147]. Likewise, the anti-inflammatory cytokine IL-10, delivered to ischemic lung tissue in rats by genetically engineered MSCs, inhibited inflammatory cell infiltration, decreased damage to lung tissue and increased lung function over MSCs alone [148]. MSCs have also been used to prevent lung ischemia by preventing pulmonary hypertension. Prostacyclin synthase and eNOS expressing-MSCs transplanted into rats with monocrotalin-induced pulmonary hypertension, with the goal of expanding the lung arteries, eased the strain on the heart and extended the survival time of the animals [149, 150]. MSCs can protect against acute kidney failure which have lead to more recent experiments to enhance this effect [151]. Kallikrein is a renoprotective molecule due its anti-inflammatory, -apoptotic and -dilation activities. When delivered to kidneys of rats in a renal ischemia/reperfusion injury model by modified MSCs caused a decrease in blood urea nitrogen and creatine levels and the number of apoptotic kidney cells and inflammatory cells within the kidney [152]. While these studies demonstrate the various and numerous potential factors for the enhancement of MSC-dependent treatment of acute myocardial infarctions and other ischemic injuries, it seems likely the best approach will be a combination of molecules that target the cells appropriately and regulate both the paracrine stimulation of surrounding cells and the differentiation of the transplanted MSCs.
4. Cancer
Targeting solid tumors with anti-tumor gene therapy has been hindered by systemic toxicity, low efficiency of delivery and nominal temporal expression. Cell-based targeting with MSCs has recently been utilized to overcome these issues. MSCs are capable of preferentially homing to sites of primary and metastatic tumor growth and delivering anti-tumor agents to highly specific niches surrounding the tumors. The two main categories for gene targeting are cytotoxic, or pro-apoptotic genes, and immunostimulatory genes. Receptors for the pro-apoptotic gene TNF-related apoptosis inducing ligand are expressed on many types of tumors and when soluble forms of the ligand are expressed in MSCs, and properly localized in xenograft mouse models of human cervical and breast cancers and gliomas, have caused the decrease in proliferation and tumor size and increased apoptosis and survival time [153–157]. Inducible nitric oxide synthase has also been shown to be potentially powerful anti-tumor therapy that when delivered to a fibrosarcoma model in mice by genetically-modified MSCs reduced tumor growth [158]. Replication competent viruses that target cancer cells are another means to eliminate tumors. Once the virus has replicated within the cancer cells the cell is lysed to release the virus. Due to toxicity to normal cells, targeting and delivery are especially important. MSCs carrying engineered adenoviruses have been used to target ovarian, breast, lung and brain tumors leading to decreased tumor burdens and increased survival times [159–163]. MSC-based gene therapy can also increase the usefulness of current anti-cancer therapies. A combination of genetically-modified MSCs to deliver cytosine deaminase and treatment with the less toxic prodrug 5-fluorocytosine, which is converted to 5-fluorouracil by cytosine deaminase within the MSC-containing tumor niche, could replace systemic delivery of the highly toxic 5FU as demonstrated in mouse models for colon, prostate and skin cancers [164–166]. MSCs delivering the suicide gene herpes simplex virus thymidine kinase, in combination with ganciclovir treatment to produce tumor-localized toxic metabolites to surrounding cells, has shown promising results in rodent xenograft models of human pancreatic carcinomas and gliomas [167, 168].
The second class of therapeutic transgenes is immunomodulatory targets. MSCs engineered to express IL-2, -7, -12 and -18 have decreased tumor size in rodent xenograft models of primary, established and metastatic tumors [169–175]. MSCs bearing other immune cytokines such as IFN-α and –β and CX3CL1/fractalkine increase tumor cell apoptosis and animal survival times in prostate, lung, pancreatic and skin cancers through activation of innate immune activity such as natural killer cells, or adaptive immune response through increased activation of T cells [176–180]. Other emerging transgenes for tumor therapy combined with MSCs are anti-angiogenic factors such as soluble VEGF receptor, a urokinase-type plasminogen pathway antagonist, amino terminal fragment, and the HGF pathway antagonist NK4 which inhibited tumor growth and increased survival time in xenograft mouse models of lung tumors, lymphoma and bone metastasized prostate cancer [181–183].
While initial animal studies are promising, there are still issues to be addressed. Some evidence exists that the location of the injection may affect the efficacy of the genetically-modified MSCs, thus further studies are required to determine whether systemic versus local injection is optimal [184]. More directly, the properties that make MSCs attractive as a therapeutic vector may in fact facilitate tumor growth [184, 185]. The immunosuppressive effects of MSCs could disable the intrinsic antitumor immune response as when a subcutaneous injection of melanoma cells in mice only formed tumors when co-injected with MSCs, presumably due to modulation of the immune system [186]. In this model MSCs were localized to the stromal periphery of the tumor suggesting that MSCs may also affect the tumor niche by adding structural support for tumor proliferation by differentiation into fibroblasts [187]. Tumor support and growth could also be supplemented by release of trophic factors from MSCs. Several examples of the angiogenic effects of MSCs have been presented above and VEGF from MSCs aided in the growth of Burkitt’s lymphoma cells, while secretion of chemokines could promote tumor metastasis [183, 188]. Lastly, the unlimited potential for proliferation could also lead to transformation of MSCs into malignant tissue. Long term passaging may lead to chromosomal abnormalities, though current data suggests that human MSCs are not susceptible to transformation [189, 190]. Thus, while the role of MSCs in tumor proliferation remains unresolved, these concerns need to be addressed before MSC-based anti-tumor gene therapy becomes a realistic possibility.
5. Conclusion
These preclinical animal studies of genetically-modified MSCs reveal the possibilities for clinical application with the potential for wider applications. Exciting new research into repair of the nervous system with MSCs engineered to express brain-derived neurotrophic factor or other neural growth factors could have implications for the regeneration of neurons following stroke, spinal injury or genetic neurological disorders, while recapitulation of pancreatic cells using pancreatic and duodenal homeobox-1-MSCs could restore insulin producing islet cells to diabetic patients [191–198]. Other MSC-based therapies that do not involve genetic engineering, especially the use of MSCs to improve BM and solid organ transplants, are further along, as evidenced by the number of clinical trials using MSCs compared to genetically-engineered MSCs [199]. This is due to the fundamental concerns facing the use of both MSCs and the gene therapy. For MSCs, identifying a standardized tissue and donor source, isolation and expansion procedures, definition of markers to improve homogeneity of MSCs, dosing and administration site will affect the safety and efficacy of cell therapy. The safety concerns facing gene therapy are much more daunting. The transfer of genetic material is still most efficiently conducted using the viral vectors: adenovirus, adeno-associated virus, retroviruses or lentiviruses. Each has advantages, but the concerns of an immune response outweigh the risk in all but the most dire of cases. Refinement and design of novel, non-viral delivery methods for naked or plasmid DNA, such as the use of lipids or nanoparticles, will reduce the likelihood of an immune response and limit incorporation of DNA into the genome [145, 200]. Use of regulatable gene systems will allow precise control over temporal expression patterns to eliminate the issues of over- or under-dosing during the repair process. There remains much work to be done, but by utilizing the inherent properties of MSCs and improved gene delivery, we are entering an exciting new era where the use of genetic engineering therapies will be safe, efficient and efficacious.
6. Expert Opinion
The increasing interest and research employing MSCs to treat a myriad of diseases justifies the early expectations of these cells. There are now nearly 150 clinical trials targeting skeletal and cardiac problems to transplants and graft versus host disease, diabetes and multiple sclerosis, all utilizing unmodified MSCs [199]. These studies will provide critical information on the safety profile and efficacy of MSCs. We envision that future MSC-based therapies will employ genetically programmed cells to enhance the natural effects of MSCs. By utilizing cell therapy techniques, gene therapy will be propelled into a new era. The characteristics and properties described throughout this review, in our opinion, make MSCs the most promising and viable vector for the delivery of genetic therapies. They have none of the ethical concerns of embryonic stem cells, are expandable and are more efficient in accepting non-viral gene transfer, such as lipid or nanoparticle-based methods. These combined with their apparent natural healing properties, such as the ability to modulate the immune system, home to injured tissue, regulate angiogenesis and possibly incorporate into surrounding tissue following differentiation, advocate for the continued work in understanding MSC biology. Discoveries of MSC-pathways and cell-cell interactions may lead to MSC-based targeted gene therapy.
While solving the mechanistic questions surrounding MSCs would surely aid in harnessing their unique capabilities, some of the more pressing issues facing the use of MSCs is the handling of these cells. A lack of a standardized protocol for culturing and expanding MSCs has led to three main concerns. First, it seems apparent now that long-term culture increases heterogeneity of the cell population. An increasingly diverse population of cells makes it hard to clearly define the functional population and the mechanisms responsible for any detectable effect. This could also explain the loss of efficient MSC engraftment along with differences between results from individual laboratories and clinical trials that have led to controversy. Thus, the identification of markers is a critical need to truly define MSCs and to identify and track the progeny of these cells, similar to the highly organized and comprehendible lineage of HSC. Secondly, long-term culturing can also lead to down regulation of functional surface proteins, such as CXCR4 and long-term ex vivo expanded cells show reduced migration and homing potential in vivo [201, 202]. This has caused problems when attempting to transfer results from the bench to the bedside as cell behavior can be drastically different depending on the methods employed by various laboratories. Finally, along with concerns of serum-dependent growth for transplant into humans, cultured MSCs can acquire mutations and become transformative as in the case with the formations of sarcomas from transplanted MSCs in mice [203]. Several laboratories have endeavored to optimize the growth conditions for various sources of MSCs, but ultimately it will require a consensus among the leaders of the field and a commitment across all laboratories before consistent and reproducible results will yield a complete understanding of MSC biology.
Along with handling these cells, it is important to consider the source used to isolate and expand MSCs. There are reports describing the isolation of MSCs from peripheral blood and many different tissues. Most effort on MSCs has been with BM derived cells, but increasingly from adipose tissue and umbilical cord blood. Mostly cells from divergent sources are more similar than different, yet inherently tend to favor the tissue type from which they were harvested and can lead to transplanted cells engrafting in these niches instead of their intended target. This implies that the microenvironment where MSCs reside, whether it is perivascular, in the BM or some yet to be defined area, has a substantial effect on the homing, differentiative and regenerative capabilities of MSCs even after isolation. The surrounding cells and extracellular milieu also seems to influence the surface proteins and epitopes which would be used as identifying markers [204]. Defining the interaction of MSCs within its natural environment will be crucial to understanding the molecular cues that maintain MSC stemness, influence migration and drive proliferation versus differentiation decisions and thus our ability to fully harness the potential of MSCs for cell based therapies. Identifying the natural niche could also lead to a better understanding of the surface proteome of MSCs and possibly to the discovery of a common MSC progenitor and its markers for identification and progeny-tracking. Embryonic studies indicate that MSCs, along with other adult stem cell lines, derive from epiblast stem cell progeny primordial germ cells (PGC) [205] and PGC-like cells have been found in the adult bone marrow [206]. Further studies are required to confirm that these are truly postnatal MSC progenitors, to determine the origin niche and whether the embryonic niche is the same as the natural adult niche, but this is a promising area of research for the identification of a MSC niche. However, until then, it is our opinion that the therapeutic application of MSCs should be based on their source-niche to benefit from the influence of this microenvironment on the differentiation fate of MSCs.
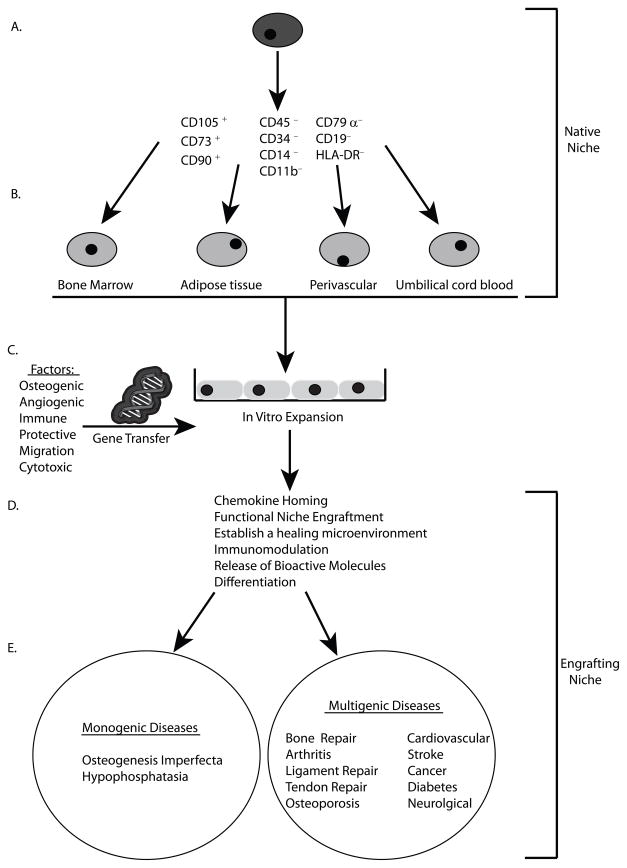
To summarize, we propose that the identification of the natural niche and engrafting niche for MSCs after transplant is the key for unlocking the untapped potential of MSC-based therapeutics (Fig. 1). By identifying the natural niche, we gain a better understanding of the basic biology that influences fate decisions of the common progenitor (Fig. 1A) and the prospective differences in MSCs from various adult niches. This knowledge will lead to better targeting of transplanted MSCs to distinct engraftment niches (Fig. 1B). This would also allow for identification of markers and the eventual conformity in ex vivo culturing procedures for MSCs (Fig. 1C). A better understanding of MSC biology would also lead to better choices in genes for enhancing the intrinsic MSC characteristics that make them valuable as a therapeutic option (Fig. 1C and D). Applying what we have learned will result in favorable conditions to employ genetically-modified MSCs for monogenic as well as multigenic diseases (Fig. 1E).
Figure 1.
MSCs from niche to bedside. A) Homogenous, pure MSCs would be isolated from their natural niche to be utilized for therapeutic purposes. B) These MSC progenitors give rise to tissue-specific heterogeneous populations of MSCs from bone marrow, adipose tissue perivascular regions and umbilical cord blood that can also be used as a therapeutic treatment. C) Ex vivo expanded cells could then be expanded and modified by gene transfer with appropriate genes to enhance D) the chemokine homing, functional niche engraftment, establishment of a healing microenvironment, modulation of the immune system, release of bioactive paracrine factors and differentiation into functional tissue E) for use in treatment of monogenic diseases, such as OI or hypophosphatasia, or more complex multigenic diseases.
Article Highlights.
Introduction: Multipotent stromal cells can be used to treat a wide range of diseases by harnessing the best of both cell and gene therapy characteristics.
Skeletal disorders: MSCs can deliver immunomodulatory, anti-inflammatory, bone stimulating and angiogenic factors to initiate healing, promote differentiation of self and intrinsic cells to repair and form new bone.
Cardiovascular and Ischemia: MSCs can home to sites of vascular injury to initiate healing, supply angiogenic and anti-inflammatory effects.
Cancer: MSCs can home to sites of cancerous growth to deliver cytolytic or apoptotic-inducing factors.
Conclusion: MSCs have a great potential for clinical applications though hurdles remain before the clinical use of MSCs-based gene therapy is implemented.
Expert Opinion: A greater understanding of MSC biology, identification of the natural and engrafting niche for MSCs and a consensus for how to culture and expand MSCs will lead to quicker implementation of MSC-based therapies.
Footnotes
Declaration of interest: The author’s work reported in this manuscript has been supported by a National Institutes of Health (NIH) grant RO1DK070929
Bibliography
- 1.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991 Sep;9(5):641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr 2;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003 Sep-Oct;80(1–2):148–58. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003 Jan 16;348(3):255–6. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 5.Benabdallah BF, Allard E, Yao S, et al. Targeted gene addition to human mesenchymal stromal cells as a cell-based plasma-soluble protein delivery platform. Cytotherapy. May;12(3):394–9. doi: 10.3109/14653240903583803. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970 Oct;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 7.Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol. 2009 Feb;218(2):237–45. doi: 10.1002/jcp.21592. [DOI] [PubMed] [Google Scholar]
- 8.Crisan M, Chen CW, Corselli M, et al. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009 Sep;1176:118–23. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 9.Dezawa M, Kanno H, Hoshino M, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004 Jun;113(12):1701–10. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luk JM, Wang PP, Lee CK, et al. Hepatic potential of bone marrow stromal cells: development of in vitro co-culture and intra-portal transplantation models. J Immunol Methods. 2005 Oct 20;305(1):39–47. doi: 10.1016/j.jim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Tokcaer-Keskin Z, Akar AR, Ayaloglu-Butun F, et al. Timing of induction of cardiomyocyte differentiation for in vitro cultured mesenchymal stem cells: a perspective for emergencies. Can J Physiol Pharmacol. 2009 Feb;87(2):143–50. doi: 10.1139/Y08-111. [DOI] [PubMed] [Google Scholar]
- 12.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 13.Granero-Molto F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009 Aug;27(8):1887–98. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004 Jul;15(7):1794–804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 15.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003 Aug 19;108(7):863–8. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Chen J, Wang L, et al. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001 Jun 26;56(12):1666–72. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- 17.Francois S, Bensidhoum M, Mouiseddine M, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006 Apr;24(4):1020–9. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 18.Rochefort GY, Delorme B, Lopez A, et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006 Oct;24(10):2202–8. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- 19.Granero-Molto F, Weis JA, Longobardi L, et al. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther. 2008 Mar;8(3):255–68. doi: 10.1517/14712598.8.3.255. [DOI] [PubMed] [Google Scholar]
- 20.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005 Sep 15;106(6):1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006 Sep;25(3):357–71. doi: 10.1007/s10555-006-9003-5. [DOI] [PubMed] [Google Scholar]
- 22.Cho HH, Kyoung KM, Seo MJ, et al. Overexpression of CXCR4 increases migration and proliferation of human adipose tissue stromal cells. Stem Cells Dev. 2006 Dec;15(6):853–64. doi: 10.1089/scd.2006.15.853. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Z, Ou L, Zhou X, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008 Mar;16(3):571–9. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 24.Hiasa K, Ishibashi M, Ohtani K, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004 May 25;109(20):2454–61. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 25.Abbott JD, Huang Y, Liu D, et al. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004 Nov 23;110(21):3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 26.Ip JE, Wu Y, Huang J, et al. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007 Aug;18(8):2873–82. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007 Dec;21(14):3917–27. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008 Feb 15;180(4):2581–7. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 29.Dwyer RM, Potter-Beirne SM, Harrington KA, et al. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007 Sep 1;13(17):5020–7. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 30.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006 Aug 1;98(5):1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 31.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007 Nov;25(11):2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 32.Parekkadan B, van Poll D, Suganuma K, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2(9):e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen BK, Maltais S, Perrault LP, et al. Improved Function and Myocardial Repair of Infarcted Heart by Intracoronary Injection of Mesenchymal Stem Cell-Derived Growth Factors. J Cardiovasc Transl Res. Feb 26; doi: 10.1007/s12265-010-9171-0. [DOI] [PubMed] [Google Scholar]
- 34.Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009 May;24(5):759–64. doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valtieri M, Sorrentino A. The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol. 2008 Nov;217(2):296–300. doi: 10.1002/jcp.21521. [DOI] [PubMed] [Google Scholar]
- 36.Tolar J, Le Blanc K, Keating A, et al. Hitting the Right Spot with Mesenchymal Stromal Cells (MSCs) Stem Cells. Jul 1; doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003 Feb 15;75(3):389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 38.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008 Sep;8(9):726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 39.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003 May 1;101(9):3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 40.Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003 Jan;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 41.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007 Nov 15;110(10):3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 42.Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004 Jun 15;103(12):4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004 Jun;13(3):263–71. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 44.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005 Feb 15;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 45.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005 May 15;105(10):4120–6. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 46.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005 Mar 1;105(5):2214–9. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 47.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006 Jan 1;107(1):367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 48.Rasmusson I, Le Blanc K, Sundberg B, et al. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007 Apr;65(4):336–43. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 49.Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006 Feb 15;107(4):1484–90. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 50.Sotiropoulou PA, Perez SA, Gritzapis AD, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006 Jan;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 51.Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005 Aug;33(2):145–52. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iyer SS, Co C, Rojas M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med. 2009 Mar;51(1):5–16. [PubMed] [Google Scholar]
- 53.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996 Jul 1;10(13):1580–94. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 54.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004 Dec;22(4):233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 55.Oreffo RO. Growth factors for skeletal reconstruction and fracture repair. Curr Opin Investig Drugs. 2004 Apr;5(4):419–23. [PubMed] [Google Scholar]
- 56.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007 Dec;31(6):729–34. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002 Dec;84-A(12):2123–34. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Tsuji K, Bandyopadhyay A, Harfe BD, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006 Dec;38(12):1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 59.Lieberman JR, Le LQ, Wu L, et al. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998 May;16(3):330–9. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- 60.Aslan H, Zilberman Y, Arbeli V, et al. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006 Apr;12(4):877–89. doi: 10.1089/ten.2006.12.877. [DOI] [PubMed] [Google Scholar]
- 61.Sugiyama O, An DS, Kung SP, et al. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther. 2005 Mar;11(3):390–8. doi: 10.1016/j.ymthe.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 62.Turgeman G, Pittman DD, Muller R, et al. Engineered human mesenchymal stem cells: a novel platform for skeletal cell mediated gene therapy. J Gene Med. 2001 May-Jun;3(3):240–51. doi: 10.1002/1521-2254(200105/06)3:3<240::AID-JGM181>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 63.Zachos T, Diggs A, Weisbrode S, et al. Mesenchymal stem cell-mediated gene delivery of bone morphogenetic protein-2 in an articular fracture model. Mol Ther. 2007 Aug;15(8):1543–50. doi: 10.1038/sj.mt.6300192. [DOI] [PubMed] [Google Scholar]
- 64.Chang SC, Wei FC, Chuang H, et al. Ex vivo gene therapy in autologous critical-size craniofacial bone regeneration. Plast Reconstr Surg. 2003 Dec;112(7):1841–50. doi: 10.1097/01.PRS.0000091168.73462.1A. [DOI] [PubMed] [Google Scholar]
- 65.Chang SC, Chuang HL, Chen YR, et al. Ex vivo gene therapy in autologous bone marrow stromal stem cells for tissue-engineered maxillofacial bone regeneration. Gene Ther. 2003 Nov;10(24):2013–9. doi: 10.1038/sj.gt.3302106. [DOI] [PubMed] [Google Scholar]
- 66.Sheyn D, Ruthemann M, Mizrahi O, et al. Genetically Modified Mesenchymal Stem Cells Induce Mechanically Stable Posterior Spine Fusion. Tissue Eng Part A. Jul 10; doi: 10.1089/ten.tea.2009.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hasharoni A, Zilberman Y, Turgeman G, et al. Murine spinal fusion induced by engineered mesenchymal stem cells that conditionally express bone morphogenetic protein-2. J Neurosurg Spine. 2005 Jul;3(1):47–52. doi: 10.3171/spi.2005.3.1.0047. [DOI] [PubMed] [Google Scholar]
- 68.Miyazaki M, Sugiyama O, Tow B, et al. The effects of lentiviral gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J Spinal Disord Tech. 2008 Jul;21(5):372–9. doi: 10.1097/BSD.0b013e31814cf51d. [DOI] [PubMed] [Google Scholar]
- 69.Riew KD, Wright NM, Cheng S, et al. Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model. Calcif Tissue Int. 1998 Oct;63(4):357–60. doi: 10.1007/s002239900540. [DOI] [PubMed] [Google Scholar]
- 70.Kumar S, Mahendra G, Nagy TR, et al. Osteogenic differentiation of recombinant adeno-associated virus 2-transduced murine mesenchymal stem cells and development of an immunocompetent mouse model for ex vivo osteoporosis gene therapy. Hum Gene Ther. 2004 Dec;15(12):1197–206. doi: 10.1089/hum.2004.15.1197. [DOI] [PubMed] [Google Scholar]
- 71.Zhang XS, Linkhart TA, Chen ST, et al. Local ex vivo gene therapy with bone marrow stromal cells expressing human BMP4 promotes endosteal bone formation in mice. J Gene Med. 2004 Jan;6(1):4–15. doi: 10.1002/jgm.477. [DOI] [PubMed] [Google Scholar]
- 72.Breitbart AS, Grande DA, Mason JM, et al. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg. 1999 May;42(5):488–95. [PubMed] [Google Scholar]
- 73.Krebsbach PH, Gu K, Franceschi RT, et al. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000 May 20;11(8):1201–10. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- 74.Zheng H, Guo Z, Ma Q, et al. Cbfa1/osf2 transduced bone marrow stromal cells facilitate bone formation in vitro and in vivo. Calcif Tissue Int. 2004 Feb;74(2):194–203. doi: 10.1007/s00223-003-0004-x. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Z, Wang Z, Ge C, et al. Healing cranial defects with AdRunx2-transduced marrow stromal cells. J Dent Res. 2007 Dec;86(12):1207–11. doi: 10.1177/154405910708601213. [DOI] [PubMed] [Google Scholar]
- 76.Edwards PC, Ruggiero S, Fantasia J, et al. Sonic hedgehog gene-enhanced tissue engineering for bone regeneration. Gene Ther. 2005 Jan;12(1):75–86. doi: 10.1038/sj.gt.3302386. [DOI] [PubMed] [Google Scholar]
- 77.Guo X, Zheng Q, Kulbatski I, et al. Bone regeneration with active angiogenesis by basic fibroblast growth factor gene transfected mesenchymal stem cells seeded on porous beta-TCP ceramic scaffolds. Biomed Mater. 2006 Sep;1(3):93–9. doi: 10.1088/1748-6041/1/3/001. [DOI] [PubMed] [Google Scholar]
- 78.Terkeltaub RA, Johnson K, Rohnow D, et al. Bone morphogenetic proteins and bFGF exert opposing regulatory effects on PTHrP expression and inorganic pyrophosphate elaboration in immortalized murine endochondral hypertrophic chondrocytes (MCT cells) J Bone Miner Res. 1998 Jun;13(6):931–41. doi: 10.1359/jbmr.1998.13.6.931. [DOI] [PubMed] [Google Scholar]
- 79.Milat F, Ng KW. Is Wnt signalling the final common pathway leading to bone formation? Mol Cell Endocrinol. 2009 Oct 30;310(1–2):52–62. doi: 10.1016/j.mce.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 80.Chang J, Sonoyama W, Wang Z, et al. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007 Oct 19;282(42):30938–48. doi: 10.1074/jbc.M702391200. [DOI] [PubMed] [Google Scholar]
- 81.Gronthos S, Chen S, Wang CY, et al. Telomerase accelerates osteogenesis of bone marrow stromal stem cells by upregulation of CBFA1, osterix, and osteocalcin. J Bone Miner Res. 2003 Apr;18(4):716–22. doi: 10.1359/jbmr.2003.18.4.716. [DOI] [PubMed] [Google Scholar]
- 82.Pola E, Gao W, Zhou Y, et al. Efficient bone formation by gene transfer of human LIM mineralization protein-3. Gene Ther. 2004 Apr;11(8):683–93. doi: 10.1038/sj.gt.3302207. [DOI] [PubMed] [Google Scholar]
- 83.Viggeswarapu M, Boden SD, Liu Y, et al. Adenoviral delivery of LIM mineralization protein-1 induces new-bone formation in vitro and in vivo. J Bone Joint Surg Am. 2001 Mar;83-A(3):364–76. doi: 10.2106/00004623-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Granero-Molto F, Myers TJ, Weis JA, Yan Y, Li T, Longobardi L, Miga MI, Spagnoli A. Mesenchymal Stem Cells Expressing IGF-I Improve Fracture Repair: The Seed and the Soil for Tissue Regeneration. 92nd Annual Meeting of The Endocrine Society; 2010; San Diego. 2010. [Google Scholar]
- 85.Phimphilai M, Zhao Z, Boules H, et al. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006 Apr;21(4):637–46. doi: 10.1359/JBMR.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang S, Wei D, Wang D, et al. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J Bone Miner Res. 2003 Apr;18(4):705–15. doi: 10.1359/jbmr.2003.18.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peng H, Wright V, Usas A, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002 Sep;110(6):751–9. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar S, Wan C, Ramaswamy G, et al. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. May;18(5):1026–34. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee JY, Musgrave D, Pelinkovic D, et al. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am. 2001 Jul;83-A(7):1032–9. doi: 10.2106/00004623-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 90.Peng H, Usas A, Gearhart B, et al. Development of a self-inactivating tet-on retroviral vector expressing bone morphogenetic protein 4 to achieve regulated bone formation. Mol Ther. 2004 Jun;9(6):885–94. doi: 10.1016/j.ymthe.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 91.Peng H, Usas A, Olshanski A, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005 Nov;20(11):2017–27. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 92.Gersbach CA, Le Doux JM, Guldberg RE, et al. Inducible regulation of Runx2-stimulated osteogenesis. Gene Ther. 2006 Jun;13(11):873–82. doi: 10.1038/sj.gt.3302725. [DOI] [PubMed] [Google Scholar]
- 93.Goodrich LR, Hidaka C, Robbins PD, et al. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. 2007 May;89(5):672–85. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- 94.Hidaka C, Quitoriano M, Warren RF, et al. Enhanced matrix synthesis and in vitro formation of cartilage-like tissue by genetically modified chondrocytes expressing BMP-7. J Orthop Res. 2001 Sep;19(5):751–8. doi: 10.1016/S0736-0266(01)00019-5. [DOI] [PubMed] [Google Scholar]
- 95.Yokoo N, Saito T, Uesugi M, et al. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005 Jan;52(1):164–70. doi: 10.1002/art.20739. [DOI] [PubMed] [Google Scholar]
- 96.Cucchiarini M, Madry H, Ma C, et al. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol Ther. 2005 Aug;12(2):229–38. doi: 10.1016/j.ymthe.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 97.Longobardi L, O’Rear L, Aakula S, et al. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006 Apr;21(4):626–36. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 98.Steinert AF, Palmer GD, Pilapil C, et al. Enhanced in vitro chondrogenesis of primary mesenchymal stem cells by combined gene transfer. Tissue Eng Part A. 2009 May;15(5):1127–39. doi: 10.1089/ten.tea.2007.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo CA, Liu XG, Huo JZ, et al. Novel gene-modified-tissue engineering of cartilage using stable transforming growth factor-beta1-transfected mesenchymal stem cells grown on chitosan scaffolds. J Biosci Bioeng. 2007 Jun;103(6):547–56. doi: 10.1263/jbb.103.547. [DOI] [PubMed] [Google Scholar]
- 100.Pagnotto MR, Wang Z, Karpie JC, et al. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007 May;14(10):804–13. doi: 10.1038/sj.gt.3302938. [DOI] [PubMed] [Google Scholar]
- 101.Xia W, Jin YQ, Kretlow JD, et al. Adenoviral transduction of hTGF-beta1 enhances the chondrogenesis of bone marrow derived stromal cells. Biotechnol Lett. 2009 May;31(5):639–46. doi: 10.1007/s10529-009-9930-7. [DOI] [PubMed] [Google Scholar]
- 102.Park J, Gelse K, Frank S, et al. Transgene-activated mesenchymal cells for articular cartilage repair: a comparison of primary bone marrow-, perichondrium/periosteum- and fat-derived cells. J Gene Med. 2006 Jan;8(1):112–25. doi: 10.1002/jgm.826. [DOI] [PubMed] [Google Scholar]
- 103.Katayama R, Wakitani S, Tsumaki N, et al. Repair of articular cartilage defects in rabbits using CDMP1 gene-transfected autologous mesenchymal cells derived from bone marrow. Rheumatology (Oxford) 2004 Aug;43(8):980–5. doi: 10.1093/rheumatology/keh240. [DOI] [PubMed] [Google Scholar]
- 104.Tsuchiya H, Kitoh H, Sugiura F, et al. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2003 Feb 7;301(2):338–43. doi: 10.1016/s0006-291x(02)03026-7. [DOI] [PubMed] [Google Scholar]
- 105.Hoffmann A, Czichos S, Kaps C, et al. The T-box transcription factor Brachyury mediates cartilage development in mesenchymal stem cell line C3H10T1/2. J Cell Sci. 2002 Feb 15;115(Pt 4):769–81. doi: 10.1242/jcs.115.4.769. [DOI] [PubMed] [Google Scholar]
- 106.Urs S, Venkatesh D, Tang Y, et al. Sprouty1 is a critical regulatory switch of mesenchymal stem cell lineage allocation. FASEB J. Sep;24(9):3264–73. doi: 10.1096/fj.10-155127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pacini S, Spinabella S, Trombi L, et al. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 2007 Dec;13(12):2949–55. doi: 10.1089/ten.2007.0108. [DOI] [PubMed] [Google Scholar]
- 108.Hoffmann A, Pelled G, Turgeman G, et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006 Apr;116(4):940–52. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schnabel LV, Lynch ME, van der Meulen MC, et al. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthop Res. 2009 Oct;27(10):1392–8. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- 110.Gulotta LV, Kovacevic D, Montgomery S, et al. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Am J Sports Med. Jul;38(7):1429–37. doi: 10.1177/0363546510361235. [DOI] [PubMed] [Google Scholar]
- 111.Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 2005 Oct;100(4):418–22. doi: 10.1263/jbb.100.418. [DOI] [PubMed] [Google Scholar]
- 112.Majewski M, Betz O, Ochsner PE, et al. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene Ther. 2008 Aug;15(16):1139–46. doi: 10.1038/gt.2008.48. [DOI] [PubMed] [Google Scholar]
- 113.Haddad-Weber M, Prager P, Kunz M, et al. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy. Jul;12(4):505–13. doi: 10.3109/14653241003709652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li F, Jia H, Yu C. ACL reconstruction in a rabbit model using irradiated Achilles allograft seeded with mesenchymal stem cells or PDGF-B gene-transfected mesenchymal stem cells. Knee Surg Sports Traumatol Arthrosc. 2007 Oct;15(10):1219–27. doi: 10.1007/s00167-007-0385-x. [DOI] [PubMed] [Google Scholar]
- 115.Whyte MP, Kurtzberg J, McAlister WH, et al. Marrow cell transplantation for infantile hypophosphatasia. J Bone Miner Res. 2003 Apr;18(4):624–36. doi: 10.1359/jbmr.2003.18.4.624. [DOI] [PubMed] [Google Scholar]
- 116.Cahill RA, Jones OY, Klemperer M, et al. Replacement of recipient stromal/mesenchymal cells after bone marrow transplantation using bone fragments and cultured osteoblast-like cells. Biol Blood Marrow Transplant. 2004 Oct;10(10):709–17. doi: 10.1016/j.bbmt.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 117.Cahill RA, Wenkert D, Perlman SA, et al. Infantile hypophosphatasia: transplantation therapy trial using bone fragments and cultured osteoblasts. J Clin Endocrinol Metab. 2007 Aug;92(8):2923–30. doi: 10.1210/jc.2006-2131. [DOI] [PubMed] [Google Scholar]
- 118.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002 Jun 25;99(13):8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Le Blanc K, Gotherstrom C, Ringden O, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005 Jun 15;79(11):1607–14. doi: 10.1097/01.tp.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- 120.Chamberlain JR, Schwarze U, Wang PR, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004 Feb 20;303(5661):1198–201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- 121.Song H, Song BW, Cha MJ, et al. Modification of mesenchymal stem cells for cardiac regeneration. Expert Opin Biol Ther. Mar;10(3):309–19. doi: 10.1517/14712590903455997. [DOI] [PubMed] [Google Scholar]
- 122.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006 Sep 21;355(12):1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 123.Huang W, Zhang D, Millard RW, et al. Gene manipulated peritoneal cell patch repairs infarcted myocardium. J Mol Cell Cardiol. Apr;48(4):702–12. doi: 10.1016/j.yjmcc.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang D, Fan GC, Zhou X, et al. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008 Feb;44(2):281–92. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang J, Zhang Z, Guo J, et al. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res. Jun 11;106(11):1753–62. doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tang J, Wang J, Zheng F, et al. Combination of chemokine and angiogenic factor genes and mesenchymal stem cells could enhance angiogenesis and improve cardiac function after acute myocardial infarction in rats. Mol Cell Biochem. Jun;339(1–2):107–18. doi: 10.1007/s11010-009-0374-0. [DOI] [PubMed] [Google Scholar]
- 127.Liu XH, Bai CG, Xu ZY, et al. Therapeutic potential of angiogenin modified mesenchymal stem cells: angiogenin improves mesenchymal stem cells survival under hypoxia and enhances vasculogenesis in myocardial infarction. Microvasc Res. 2008 May;76(1):23–30. doi: 10.1016/j.mvr.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 128.Tang YL, Tang Y, Zhang YC, et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005 Oct 4;46(7):1339–50. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 129.Tsubokawa T, Yagi K, Nakanishi C, et al. Impact of anti-apoptotic and anti-oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am J Physiol Heart Circ Physiol. May;298(5):H1320–9. doi: 10.1152/ajpheart.01330.2008. [DOI] [PubMed] [Google Scholar]
- 130.Kelly ML, Wang M, Crisostomo PR, et al. TNF receptor 2, NOT tnf receptor 1, enhances mesenchymal stem cell-mediated cardiac protection following acute ischemia. Shock. Jun;33(6):602–7. doi: 10.1097/SHK.0b013e3181cc0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bao C, Guo J, Zheng M, et al. Enhancement of the survival of engrafted mesenchymal stem cells in the ischemic heart by TNFR gene transfection. Biochem Cell Biol. Aug;88(4):629–34. doi: 10.1139/O10-018. [DOI] [PubMed] [Google Scholar]
- 132.Jo J, Nagaya N, Miyahara Y, et al. Transplantation of genetically engineered mesenchymal stem cells improves cardiac function in rats with myocardial infarction: benefit of a novel nonviral vector, cationized dextran. Tissue Eng. 2007 Feb;13(2):313–22. doi: 10.1089/ten.2006.0133. [DOI] [PubMed] [Google Scholar]
- 133.Li W, Ma N, Ong LL, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007 Aug;25(8):2118–27. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 134.Copland IB, Jolicoeur EM, Gillis MA, et al. Coupling erythropoietin secretion to mesenchymal stromal cells enhances their regenerative properties. Cardiovasc Res. 2008 Aug 1;79(3):405–15. doi: 10.1093/cvr/cvn090. [DOI] [PubMed] [Google Scholar]
- 135.Fan L, Lin C, Zhuo S, et al. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail. 2009 Nov;11(11):1023–30. doi: 10.1093/eurjhf/hfp135. [DOI] [PubMed] [Google Scholar]
- 136.Haider H, Jiang S, Idris NM, et al. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008 Nov 21;103(11):1300–8. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 137.Pons J, Huang Y, Takagawa J, et al. Combining angiogenic gene and stem cell therapies for myocardial infarction. J Gene Med. 2009 Sep;11(9):743–53. doi: 10.1002/jgm.1362. [DOI] [PubMed] [Google Scholar]
- 138.Deuse T, Peter C, Fedak PW, et al. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation. 2009 Sep 15;120(11 Suppl):S247–54. doi: 10.1161/CIRCULATIONAHA.108.843680. [DOI] [PubMed] [Google Scholar]
- 139.Noiseux N, Gnecchi M, Lopez-Ilasaca M, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006 Dec;14(6):840–50. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 140.Gnecchi M, He H, Melo LG, et al. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009 Apr;27(4):971–9. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007 Jan 30;104(5):1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shujia J, Haider HK, Idris NM, et al. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res. 2008 Feb 1;77(3):525–33. doi: 10.1093/cvr/cvm077. [DOI] [PubMed] [Google Scholar]
- 143.Sun L, Cui M, Wang Z, et al. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun. 2007 Jun 8;357(3):779–84. doi: 10.1016/j.bbrc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 144.Song SW, Chang W, Song BW, et al. Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells. 2009 Jun;27(6):1358–65. doi: 10.1002/stem.47. [DOI] [PubMed] [Google Scholar]
- 145.Yang F, Cho SW, Son SM, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A. Feb 23;107(8):3317–22. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Piao W, Wang H, Inoue M, et al. Transplantation of sendai viral angiopoietin-1-modified mesenchymal stem cells for ischemic limb disease. Angiogenesis. May 11; doi: 10.1007/s10456-010-9169-x. [DOI] [PubMed] [Google Scholar]
- 147.Xu J, Qu J, Cao L, et al. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008 Mar;214(4):472–81. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 148.Manning E, Pham S, Li S, et al. Interleukin-10 delivery via mesenchymal stem cells: a novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum Gene Ther. Jun;21(6):713–27. doi: 10.1089/hum.2009.147. [DOI] [PubMed] [Google Scholar]
- 149.Takemiya K, Kai H, Yasukawa H, et al. Mesenchymal stem cell-based prostacyclin synthase gene therapy for pulmonary hypertension rats. Basic Res Cardiol. May;105(3):409–17. doi: 10.1007/s00395-009-0065-8. [DOI] [PubMed] [Google Scholar]
- 150.Kanki-Horimoto S, Horimoto H, Mieno S, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006 Jul 4;114(1 Suppl):I181–5. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- 151.Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005 Jul;289(1):F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 152.Hagiwara M, Shen B, Chao L, et al. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther. 2008 Aug;19(8):807–19. doi: 10.1089/hum.2008.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grisendi G, Bussolari R, Cafarelli L, et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. May 1;70(9):3718–29. doi: 10.1158/0008-5472.CAN-09-1865. [DOI] [PubMed] [Google Scholar]
- 154.Loebinger MR, Eddaoudi A, Davies D, et al. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009 May 15;69(10):4134–42. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Menon LG, Kelly K, Yang HW, et al. Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells. 2009 Sep;27(9):2320–30. doi: 10.1002/stem.136. [DOI] [PubMed] [Google Scholar]
- 156.Sasportas LS, Kasmieh R, Wakimoto H, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009 Mar 24;106(12):4822–7. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim SM, Lim JY, Park SI, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008 Dec 1;68(23):9614–23. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 158.Xiang J, Tang J, Song C, et al. Mesenchymal stem cells as a gene therapy carrier for treatment of fibrosarcoma. Cytotherapy. 2009;11(5):516–26. doi: 10.1080/14653240902960429. [DOI] [PubMed] [Google Scholar]
- 159.Komarova S, Kawakami Y, Stoff-Khalili MA, et al. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006 Mar;5(3):755–66. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 160.Hakkarainen T, Sarkioja M, Lehenkari P, et al. Human mesenchymal stem cells lack tumor tropism but enhance the antitumor activity of oncolytic adenoviruses in orthotopic lung and breast tumors. Hum Gene Ther. 2007 Jul;18(7):627–41. doi: 10.1089/hum.2007.034. [DOI] [PubMed] [Google Scholar]
- 161.Dembinski JL, Spaeth EL, Fueyo J, et al. Reduction of nontarget infection and systemic toxicity by targeted delivery of conditionally replicating viruses transported in mesenchymal stem cells. Cancer Gene Ther. Apr;17(4):289–97. doi: 10.1038/cgt.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sonabend AM, Ulasov IV, Tyler MA, et al. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008 Mar;26(3):831–41. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 163.Yong RL, Shinojima N, Fueyo J, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009 Dec 1;69(23):8932–40. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kucerova L, Matuskova M, Pastorakova A, et al. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J Gene Med. 2008 Oct;10(10):1071–82. doi: 10.1002/jgm.1239. [DOI] [PubMed] [Google Scholar]
- 165.Kucerova L, Altanerova V, Matuskova M, et al. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007 Jul 1;67(13):6304–13. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 166.Cavarretta IT, Altanerova V, Matuskova M, et al. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol Ther. Jan;18(1):223–31. doi: 10.1038/mt.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Uchibori R, Okada T, Ito T, et al. Retroviral vector-producing mesenchymal stem cells for targeted suicide cancer gene therapy. J Gene Med. 2009 May;11(5):373–81. doi: 10.1002/jgm.1313. [DOI] [PubMed] [Google Scholar]
- 168.Zischek C, Niess H, Ischenko I, et al. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann Surg. 2009 Nov;250(5):747–53. doi: 10.1097/SLA.0b013e3181bd62d0. [DOI] [PubMed] [Google Scholar]
- 169.Xu G, Jiang XD, Xu Y, et al. Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats. Cell Biol Int. 2009 Apr;33(4):466–74. doi: 10.1016/j.cellbi.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 170.Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004 Jul;11(14):1155–64. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 171.Gunnarsson S, Bexell D, Svensson A, et al. Intratumoral IL-7 delivery by mesenchymal stromal cells potentiates IFNgamma-transduced tumor cell immunotherapy of experimental glioma. J Neuroimmunol. Jan 25;218(1–2):140–4. doi: 10.1016/j.jneuroim.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 172.Chen X, Lin X, Zhao J, et al. A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Mol Ther. 2008 Apr;16(4):749–56. doi: 10.1038/mt.2008.3. [DOI] [PubMed] [Google Scholar]
- 173.Gao P, Ding Q, Wu Z, et al. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett. Apr 28;290(2):157–66. doi: 10.1016/j.canlet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 174.Hong X, Miller C, Savant-Bhonsale S, et al. Antitumor treatment using interleukin- 12-secreting marrow stromal cells in an invasive glioma model. Neurosurgery. 2009 Jun;64(6):1139–46. doi: 10.1227/01.NEU.0000345646.85472.EA. discussion 46–7. [DOI] [PubMed] [Google Scholar]
- 175.Elzaouk L, Moelling K, Pavlovic J. Anti-tumor activity of mesenchymal stem cells producing IL-12 in a mouse melanoma model. Exp Dermatol. 2006 Nov;15(11):865–74. doi: 10.1111/j.1600-0625.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 176.Kidd S, Caldwell L, Dietrich M, et al. Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. Sep;12(5):615–25. doi: 10.3109/14653241003631815. [DOI] [PubMed] [Google Scholar]
- 177.Sato H, Kuwashima N, Sakaida T, et al. Epidermal growth factor receptor-transfected bone marrow stromal cells exhibit enhanced migratory response and therapeutic potential against murine brain tumors. Cancer Gene Ther. 2005 Sep;12(9):757–68. doi: 10.1038/sj.cgt.7700827. [DOI] [PubMed] [Google Scholar]
- 178.Ren C, Kumar S, Chanda D, et al. Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Ther. 2008 Nov;15(21):1446–53. doi: 10.1038/gt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xin H, Kanehira M, Mizuguchi H, et al. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007 Jul;25(7):1618–26. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- 180.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002 Jul 1;62(13):3603–8. [PubMed] [Google Scholar]
- 181.Fritz V, Noel D, Bouquet C, et al. Antitumoral activity and osteogenic potential of mesenchymal stem cells expressing the urokinase-type plasminogen antagonist amino-terminal fragment in a murine model of osteolytic tumor. Stem Cells. 2008 Nov;26(11):2981–90. doi: 10.1634/stemcells.2008-0139. [DOI] [PubMed] [Google Scholar]
- 182.Kanehira M, Xin H, Hoshino K, et al. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007 Nov;14(11):894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- 183.Kyriakou CA, Yong KL, Benjamin R, et al. Human mesenchymal stem cells (hMSCs) expressing truncated soluble vascular endothelial growth factor receptor (tsFlk-1) following lentiviral-mediated gene transfer inhibit growth of Burkitt’s lymphoma in a murine model. J Gene Med. 2006 Mar;8(3):253–64. doi: 10.1002/jgm.840. [DOI] [PubMed] [Google Scholar]
- 184.Bexell D, Scheding S, Bengzon J. Toward brain tumor gene therapy using multipotent mesenchymal stromal cell vectors. Mol Ther. Jun;18(6):1067–75. doi: 10.1038/mt.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Loebinger MR, Janes SM. Stem cells as vectors for antitumour therapy. Thorax. Apr;65(4):362–9. doi: 10.1136/thx.2009.128025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003 Nov 15;102(10):3837–44. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 187.Spaeth EL, Dembinski JL, Sasser AK, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4(4):e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007 Oct 4;449(7162):557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 189.Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005 Apr 15;65(8):3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 190.Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007 Oct 1;67(19):9142–9. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 191.Kurozumi K, Nakamura K, Tamiya T, et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004 Feb;9(2):189–97. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 192.Ikeda N, Nonoguchi N, Zhao MZ, et al. Bone marrow stromal cells that enhanced fibroblast growth factor-2 secretion by herpes simplex virus vector improve neurological outcome after transient focal cerebral ischemia in rats. Stroke. 2005 Dec;36(12):2725–30. doi: 10.1161/01.STR.0000190006.88896.d3. [DOI] [PubMed] [Google Scholar]
- 193.Zhao MZ, Nonoguchi N, Ikeda N, et al. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab. 2006 Sep;26(9):1176–88. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]
- 194.Glavaski-Joksimovic A, Virag T, Chang QA, et al. Reversal of dopaminergic degeneration in a parkinsonian rat following micrografting of human bone marrow-derived neural progenitors. Cell Transplant. 2009;18(7):801–14. doi: 10.3727/096368909X470801. [DOI] [PubMed] [Google Scholar]
- 195.Ronsyn MW, Daans J, Spaepen G, et al. Plasmid-based genetic modification of human bone marrow-derived stromal cells: analysis of cell survival and transgene expression after transplantation in rat spinal cord. BMC Biotechnol. 2007;7:90. doi: 10.1186/1472-6750-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Karnieli O, Izhar-Prato Y, Bulvik S, et al. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007 Nov;25(11):2837–44. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- 197.Liu H, Honmou O, Harada K, et al. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006 Oct;129(Pt 10):2734–45. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu AM, Lu G, Tsang KS, et al. Umbilical cord-derived mesenchymal stem cells with forced expression of hepatocyte growth factor enhance remyelination and functional recovery in a rat intracerebral hemorrhage model. Neurosurgery. Aug;67(2):357–65. doi: 10.1227/01.NEU.0000371983.06278.B3. discussion 65–6. [DOI] [PubMed] [Google Scholar]
- 199.trials c. [cited August 2010; Available from: www.clinicaltrials.gov.
- 200.Madeira C, Mendes RD, Ribeiro SC, et al. Nonviral gene delivery to mesenchymal stem cells using cationic liposomes for gene and cell therapy. J Biomed Biotechnol. 2010:735349. doi: 10.1155/2010/735349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Honczarenko M, Le Y, Swierkowski M, et al. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006 Apr;24(4):1030–41. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 202.Son BR, Marquez-Curtis LA, Kucia M, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006 May;24(5):1254–64. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 203.Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007 Feb;25(2):371–9. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 204.Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. Feb;222(2):268–77. doi: 10.1002/jcp.21940. [DOI] [PubMed] [Google Scholar]
- 205.De Miguel MP, Arnalich Montiel F, Lopez Iglesias P, et al. Epiblast-derived stem cells in embryonic and adult tissues. Int J Dev Biol. 2009;53(8–10):1529–40. doi: 10.1387/ijdb.072413md. [DOI] [PubMed] [Google Scholar]
- 206.Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006 May;20(5):857–69. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]