Summary
Background
Aphasia affects 1/3 of stroke patients with improvements noted only in some of them. The goal of this exploratory study was to provide preliminary evidence regarding safety and efficacy of fMRI-guided excitatory repetitive transcranial magnetic stimulation (rTMS) applied to the residual left-hemispheric Broca’s area for chronic aphasia treatment.
Material/Methods
We enrolled 8 patients with moderate or severe aphasia >1 year after LMCA stroke. Linguistic battery was administered pre-/post-rTMS; a semantic decision/tone decision (SDTD) fMRI task was used to localize left-hemispheric Broca’s area. RTMS protocol consisted of 10 daily treatments of 200 seconds each using an excitatory stimulation protocol called intermittent theta burst stimulation (iTBS). Coil placement was targeted individually to the left Broca’s.
Results
6/8 patients showed significant pre-/post-rTMS improvements in semantic fluency (p=0.028); they were able to generate more appropriate words when prompted with a semantic category. Pre-/post-rTMS fMRI maps showed increases in left fronto-temporo-parietal language networks with a significant left-hemispheric shift in the left frontal (p=0.025), left temporo-parietal (p=0.038) regions and global language LI (p=0.018). Patients tended to report subjective improvement on Communicative Activities Log (mini-CAL; p=0.075). None of the subjects reported ill effects of rTMS.
Conclusions
FMRI-guided, excitatory rTMS applied to the affected Broca’s area improved language skills in patients with chronic post-stroke aphasia; these improvements correlated with increased language lateralization to the left hemisphere. This rTMS protocol appears to be safe and should be further tested in blinded studies assessing its short- and long-term safety/efficacy for post-stroke aphasia rehabilitation.
Keywords: aphasia, language, fMRI, rTMS, rehabilitation, stroke
Background
Aphasia, impaired communication ability that usually occurs in patients with left middle cerebral artery (LMCA) stroke, is associated with high mortality, significant motor impairment, and severe limitations in social participation [1–5]. While 1/3 of new strokes have aphasia as one of its symptoms, the progress of aphasia rehabilitation has not matched the great strides in developing acute treatment strategies that, in some patients, decreased or eliminated the stroke-related deficits. Further, the development of post-stroke aphasia treatments lags behind the development of therapies that alleviate motor deficits in patients with history of stroke such as constraint-induced motor therapy [6,7], mental practice [8], electrical stimulation of the affected extremity [9] or neurostimulation [10,11]. The current aphasia therapy approaches are based largely on compensatory strategies or repetitive training of lost functions rather than function restoration. While these therapies may lead to improvements in some patients, many continue to be aphasic despite interventions [12]. Further, while language recovery >1 year after stroke is thought to be less likely, studies showed that improvements even in patients with chronic aphasia are possible when they are provided with an intervention [13,14]. These patients need additional restorative therapies that will enable them to return to society as productive members. The question raised in this study is whether some form of repetitive transcranial magnetic stimulation (rTMS) may be one of those interventions [15]. TMS is a noninvasive method of stimulating neurons by inducing weak electric currents by electromagnetic induction. When applied in repetitive paradigms, synaptic plasticity can be altered to transiently increase or decrease localized cortical activities. Changes in the neuronal networks induced by TMS can persist well beyond the actual period of stimulation with improved language functions observed in some excitatory rTMS studies [16]. But, the safety and efficacy of the excitatory rTMS applied directly to the stroke/peri-stroke areas is unclear. Further, early reports of rTMS-related seizures have led to the development of safety rules regarding stimulation frequency, intensity, train duration and intertrain interval [17].
Neuroimaging studies have revealed that post-stroke reorganization of language functions may be related to increased cortical involvement of the non-dominant [18,19] or dominant cortical areas [20–22]. Unfortunately, these discoveries have thus far not contributed to the development of specific aphasia treatments. Therefore, the primary goals of this study were to determine whether excitatory repetitive transcranial magnetic stimulation with fMRI guidance (neuronavigated rTMS; nerTMS) applied directly to the language area identified by fMRI is safe and whether it might facilitate rehabilitation from post-stroke aphasia and improve patient outcomes. We hypothesized that nerTMS will have positive impact on language skills evaluated with aphasia battery.
Material and Methods
Subjects
This exploratory study was approved by the Institutional Review Board and all subjects provided written informed consent. The inclusion criteria were: 1. LMCA distribution stroke ≥12 months prior to study participation, 2. moderate aphasia at time of enrollment, 3. no contraindication(s) to fMRI at 4T, 4. no history of seizures. All subjects were right-handed prior to stroke, as determined by an Edinburgh Handedness Inventory (EHI) score ≥50.
Functional MRI task and scanning procedures
FMRI procedures at 4T Varian Unity INOVA Whole Body MRI/MRS scanner have been previously described in detail [20,23,24]. Briefly, participants were oriented to the equipment and engaged in explicit practice of the tasks; they were allowed to enter the scanner only after expressing complete understanding of all procedures. All fMRIs were performed following the same protocol. First, an alignment scan was performed for head position adjustments so that the AC-PC reference line was as close as possible to the vertical axis of the scanner. A high resolution, T1-weighted 3-D MDEFT (Modified Driven Equilibrium Fourier Transform) anatomical image was obtained using the following parameters: TR/TE=13.1/6 ms, FOV=25.6×19.2×19.2 cm, flip angle=22°, and voxel dimensions=1×1×1 mm. Finally, T2*-weighted functional images were obtained using the following parameters: TR/TE=3000/30 ms, FOV=25.6×25.6 cm, matrix=64×64 pixels, number of slices=30, slice thickness=4 mm, and flip angle=75°.
For language localization we used a highly reliable semantic decision/tone decision (SDTD) task [20,25–28]. Briefly, subjects performed two different alternating conditions, the control condition (tones recognition) and the active condition (semantic recognition/association), starting with the control condition. In the tone decision task, subjects heard brief sequences of four to seven low- (500 Hz) and high-pitch (750 Hz) tones every 3.75 seconds and responded with a non-dominant hand button press for any sequence containing two high-pitched tones. In the semantic task, subjects heard spoken English nouns designating animals every 3.75 seconds and responded with a non-dominant hand button press to stimuli that meet two criteria: “lives in the United States” and “is commonly used by humans” (e.g., “horse”). The contrast between the tone discrimination task and the semantic task results in an activation pattern that is inherent only to semantic, word-form, and phoneme processing, all of which are part of the language system. Each subject performed this task twice during each scanning session and the runs were concatenated for processing.
Functional MRI data post-processing and analyses
The fMRI image post-processing was performed using software developed in the Imaging Research Center at the Cincinnati Children’s Hospital Medical Center in the IDL software environment (IDL 7.0; Research Systems Inc., Boulder, CO) [27,28]. As previously, Hamming-filtering preceded data reconstruction and the geometric distortion correction using the multi-echo reference method. Data were then co-registered to further reduce the effects of motion artifact using a previously developed pyramid co-registration algorithm. A general linear model (GLM) was used to process the data with a set of cosine basis functions used to minimize artifacts due to signal drift from aliased respiratory and cardiac effects. Finally, z-score maps were computed on a pixel-by-pixel basis and transformed into Talairach space for composite mapping. Data in native space were utilized to localize left-hemispheric language centers for subsequent rTMS administration.
To parameterize the fMRI data, we used previously generated regions of interest (ROIs) from 49 healthy controls, to calculate the laterality indices (LI) in the frontal and temporo-parietal brain regions (see Figure 1 in Szaflarski et. al., 2008) [28]. These primary ROIs include the lateral inferior and middle frontal region that corresponds to Broca’s area and the lateral/posterior temporal and parietal region that corresponds to Wernicke’s area; these ROIs were superimposed on the corresponding right hemispheric homologues (right hemispheric ROIs mirrored the left hemispheric ones). We also combined both ROIs into one “global language ROI.” Next, to avoid biases induced by arbitrary thresholding schemes, we determined the mean value of the t-statistics for all voxels within each ROIs; the number of voxels above the mean for each ROI was entered in the following formula to calculate the lateralization index: LI = (NL−NR)/(NL+NR), where NL and NR represent the number of voxels (left and right, respectively) above the mean t for each ROI [24]. This calculation method produces LI ranging from 1 (left lateralized) to −1 (right lateralized).
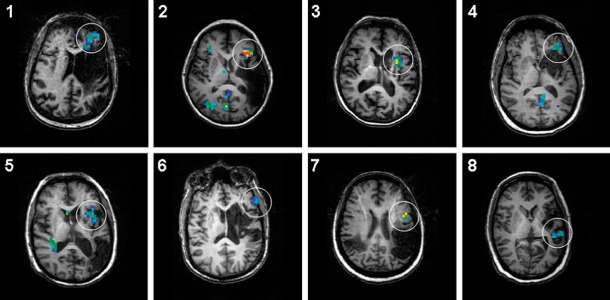
Figure 1.
Individual areas of fMRI activation that correspond to the nerTMS target (white circle) superimposed on individual T1-weighted anatomical images to illustrate the extent of the ischemic strokes and the location of the BOLD signal changes. Subjects 1, 3, 4 and 6 presented with anomic aphasias (1 and 3 had also dysarthria and 6 had conduction aphasia); other subjects presented with non-fluent aphasias, all Broca’s type. All images are in radiological convention (left on the picture corresponds to right in the brain).
Neuropsychological testing of aphasia
All subjects completed a previously described aphasia testing protocol [14]. A battery of neuropsychological measures was obtained on the day of fMRI or within 1 week of initiating the nerTMS and again, within 1 week of completing the nerTMS protocol on the same day as the follow-up fMRI. The battery included 1) Boston Naming Test (BNT), 2) Controlled Oral Word Association Test (COWAT), 3) Semantic Fluency Test (SFT), 4) Complex Ideation subtest from the Boston Diagnostic Aphasia Examination (BDAE) and 5) Peabody Picture Vocabulary Test IV (PPVT IV). For COWAT, SFT, and PPVT IV, different forms of the tests were administered pre-/post-nerTMS. All subjects also completed Communicative Abilities Log (mini-CAL), a subjective, self-administered measure of communicative abilities [14].
Neuronavigated excitatory repetitive transcranial magnetic stimulation (nerTMS)
Single-pulse TMS was performed to establish resting motor threshold (RMT) and active motor threshold (AMT) with a Magstim 200® stimulator connected through a Bistim® module to a 70 mm figure-8 coil (Magstim Co., Wales, UK) [29]. Surface electromyography (EMG) leads were placed over the first dorsal interosseous (FDI) muscle of both hands. Subjects were seated comfortably, with both arms fully supported on a pillow. Full muscle relaxation was maintained through visual and online EMG monitoring. The coil was then placed over the primary motor cortex in the right and left hemisphere at the optimal site for obtaining a MEP in the FDI. After the RMT and AMT were determined, iTBS was performed using Magstim Rapid2® (Magstim Co., Wales, UK) with intensity set at 80% of AMT obtained from the right hemisphere. Motor threshold values from the left hemisphere were too high due to the LMCA stroke; therefore, these values were not used to set the rTMS intensity. The figure-8 coil was positioned tangentially to the skull, with the handle parallel to the sagittal axis pointing occipitally. ITBS consisted of bursts of three pulses at 50 Hz given every 200 milliseconds in two second trains, repeated every 10 seconds over 200 seconds for a total of 600 pulses [30]. BrainSight™2 (Rogue Research Inc., Montreal, Canada) was used for neuronavigation to guide rTMS stimulation to the fMRI-identified residual left hemispheric Broca’s area of each individual patient. This technology also enabled reliable three-dimensionally precise reapplication of rTMS throughout the study. Subjects received one session of iTBS each day for 10 consecutive days from Monday through Friday.
Data analyses
Data were initially characterized using descriptive statistics (means and standard deviations or frequencies and percentages as appropriate). Next, the pre-/post-rTMS aphasia testing and fMRI lateralization indices were compared using the Wilcoxon signed-rank test. All data management and analyses were performed using SPSS version 18.0 (SPSS, Chicago, IL, USA).
Results
Eight subjects fulfilled the inclusion criteria and completed all study procedures (Table 1); additional 3 subjects signed the informed consent but were later excluded due to metallic artifact (2) and history of a single seizure at the time of stroke (1). All subjects were ≥1 year since the aphasia-producing stroke and were not engaged in any specific aphasia rehabilitation. Aphasia testing showed trends towards improvement with all performed tests except for COWAT (Table 1); improvements in semantic fluency were significant (p=0.028) and mini-CAL showed trend towards post-rTMS communication improvements (p=0.075). No subjects reported any seizures or serious adverse events.
Table 1.
Demographic and performance data for study subjects (N=8).
| Pre-rTMS | Post-rTMS | P value | ||
|---|---|---|---|---|
| Age | 54.4 (±12.7) | |||
| Gender (% male) | 50 | |||
| Years since stroke | 5.3 (±3.6) | |||
| EHI | 90.8 (±13) | |||
| Aphasia testing | ||||
| SFT | 20.75 (±10) | 25.62 (±13.3) | 0.028 | |
| COWAT | 12.13 (±6.4) | 11.25 (±6) | 0.833 | |
| BNT | 42.88 (±13.3) | 44.25 (±12) | 0.482 | |
| BDAE CompId | 8.75 (±2.9) | 9.62 (±2.3) | 0.272 | |
| PPVT-IV | 198.50 (±17.2) | 202.00 (±12.2) | 0.249 | |
| CAL | 55.30 (±6.3) | 63.30 (±8.9) | 0.075 | |
| FMRI LI | ||||
| Broca’s | 0.14 (±0.27) | 0.30 (±0.14) | 0.025 | |
| Wernicke’s | −0.15 (±0.33) | 0.14 (±0.12) | 0.036 | |
| Global | 0.10 (±0.2) | 0.27 (±0.9) | 0.018 | |
| Semantic Decision Performance | 60.10 (±16.3) | 60.80 (±14.9) | 0.500 | |
| Tone Decision Performance | 59.50 (±19.5) | 59.50 (±21.7) | 0.753 | |
EHI – Edinburgh Handedness Inventory; SFT – Semantic Fluency Test; COWAT – Controlled Oral Word Association Test; BNT – Boston Naming Test; PPVT – Peabody Picture Vocabulary Test; CAL – Communicative Abilities Log; BDAE CompId – Complex Ideation subtest from the Boston Diagnostic Aphasia Examination (BDAE); LI – lateralization index; Data reported as mean (± standard deviation); significance level based on the results of Wilcoxon matched-pair signed rank test.
MR images of individual strokes are presented in Figure 1. On these images, clearly noted are individual activations that served as a target for nerTMS (enclosed in circles). There does not appear to be any overlap between the areas of activation and the location of the stroke. Types of individual aphasias, based on clinical impression are provided in the legend. Group pre- and post-rTMS fMRI results are shown in Figure 2. Pre-rTMS scans showed areas of increased activation for the tone decision baseline in the right hemisphere and low-level activations predominantly in the left hemisphere. Post-rTMS group images showed clear overall increase in the BOLD signal changes in response to the intervention. Further, pre-/post-rTMS contrasts showed significant overall increases in the BOLD signal with apparent shifts to the left hemisphere (p=0.025 for Broca’s; p=0.036 for Wernicke’s; p=0.018 for the global ROI). While the pre- and post-rTMS locations of the BOLD signal changes are similar and include language areas typical for this task (Table 2), post-rTMS images showed shifts in activations predominantly to the left hemispheric head regions (Figure 3).
Figure 2.
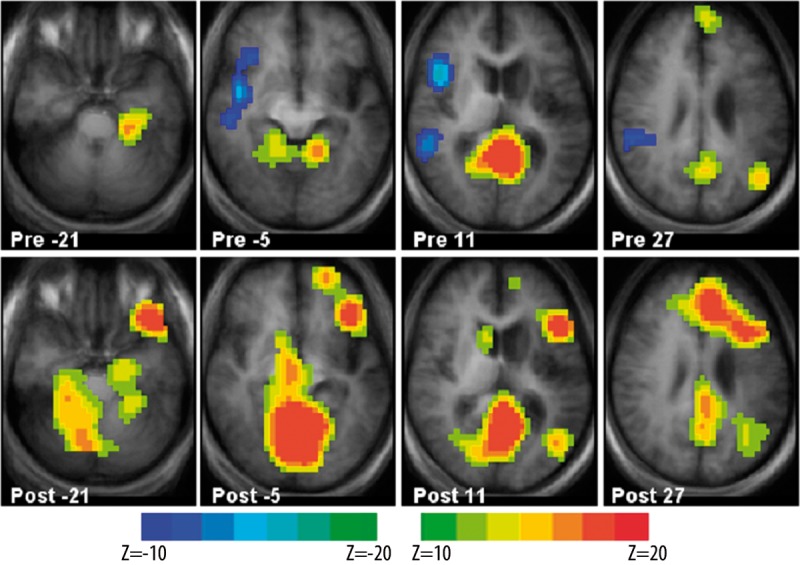
Group z-score maps for the pre-rTMS (upper row) and post-rTMS (lower row) semantic decision and tone decision fMRI task. Brain regions showing BOLD signal increases for the tone decision > semantic decision contrast are shown in cyan/blue; regions showing BOLD signal increases for the semantic decision > tone decision are shown in yellow/red. BOLD signal changes are significant at an uncorrected p <0.05. Each z-score map is presented in radiological convention, with left on the picture corresponding to the right hemisphere, and are superimposed on an average T1-weighted image generated from all subjects (dilatation of the left lateral ventricle and area of encephalomalacia in the left MCA distribution is clearly seen). Talairach coordinates of the selected slices for each panel range from z=−21 mm (left) to z=+27 mm (right).
Table 2.
Locations of activation (blood oxygenation level dependent (BOLD) signal) differences between pre- and post-rTMS intervention fMRI scans as shown in Figure 2. Talairach coordinates of the centroids (X, Y, Z) are given with the corresponding Brodmann’s areas (BA) and anatomical location of the activation differences.
| Region | BA | X, Y, Z |
|---|---|---|
| Areas with more post- than pre-rTMS BOLD signal | ||
| Left gryus frontalis inferior | 47 | −40, 29, −15 |
| Left gryus frontalis inferior | 44, 45 | −43, 18, 12 |
| Left gyrus frontalis superior | 10 | −14, 59, 8 |
| Left gyrus frontalis medialis/superior and anterior cingulate gyrus | 8, 9, 32 | −11, 31, 33 |
| Left hippocampus and parahippocampal gyrus | −24, −16, −22 | |
| Left nucleus caudalis and globus pallidus | −16, −2, 2 | |
| Left lobulus parietalis inferior, gyrus supramarginalis, | 39, 40 | −43, −46, 22 |
| Midline/bilateral lingular gyrus and posterior/retrosplenial cingulate gyrus | 18, 19, 23, 29, 30, 31 | 0, −56, 5 |
| Right gyrus precentralis | 4 | 26, −17, 44 |
| Right nucleus caudalis and globus pallidus | 8, 9, 8 | |
| Right hippocampus and parahippocampal gyrus | 22, −34, −22 | |
| Areas with more pre- than post-rTMS BOLD signal | ||
| Right inferior frontal gyrus and insula | 45, 47 | 43, 3, −1 |
| Right gyrus temporalis superior and lobulus parietalis inferior | 22, 40 | 48, −31, 15 |
Figure 3.
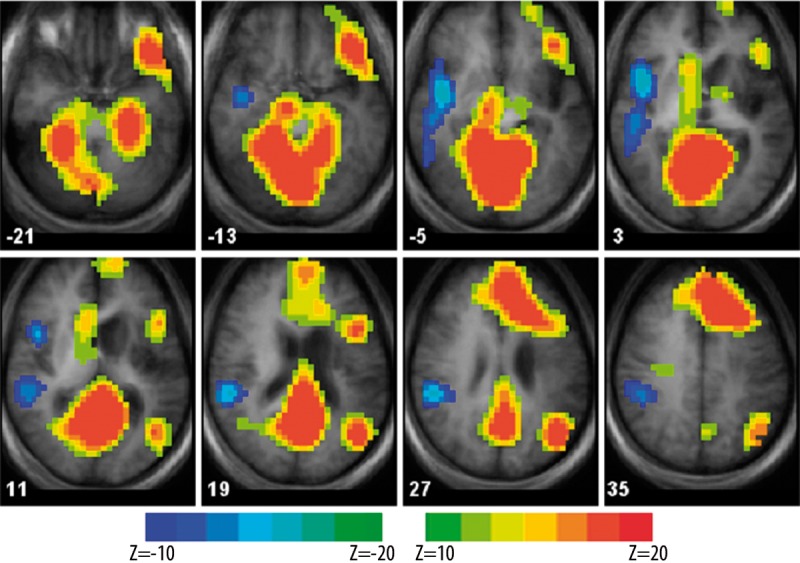
Pre-/post-rTMS group differences for the SDTD fMRI task shown as z-score maps. Brain regions showing post- > pre-rTMS BOLD signal are shown in yellow/red; regions showing pre- > post-rTMS BOLD signal are shown in cyan/blue. BOLD signal changes are significant at an uncorrected p <0.05. Each z-score map is presented in radiological convention, with left on the picture corresponding to the right hemisphere, and is superimposed on an average T1-weighted image generated from all subjects (dilatation of the left lateral ventricle and area of encephalomalacia in the left MCA distribution is clearly seen). Talairach coordinates of the selected slices range from z=−21 mm (top left) to z=+35 mm (bottom right). Locations of the centroids and the corresponding Brodmann’s areas are provided in Table 2.
Discussion
In this exploratory study we evaluated the safety and efficacy of the neuronavigated excitatory rTMS (nerTMS) applied to the identified by fMRI frontal language areas as a tool for post-stroke aphasia rehabilitation. Despite a relatively small sample size (n=8), we were able to show significant improvements in language function after 2 weeks of stimulation that were associated with significant shifts of fMRI signal to the affected hemisphere; patients also tended to report subjective improvements in language skills after therapy completion (p=0.075). These results are very encouraging and support further testing of nerTMS as a post-stroke aphasia rehabilitation tool.
In general, rTMS involves repeated brain stimulation at regular frequencies; it is a noninvasive method of exciting or inhibiting neurons through repeated induction of weak electric currents in the brain via rapidly changing magnetic fields. If used with appropriate intensity, brain activity can be modulated using rTMS without discomfort or ill effects [31]. The cortical plasticity induced by rTMS and its therapeutic effects are thought to be primarily related to synaptic plasticity; short- and long-term modifications in neural communication mediated by post-synaptic NMDA receptors in which rTMS releases Mg++ blockade allowing Ca++ entering the post-synaptic cell and inducing long-term potentiation [16]. The rTMS stimulation area is usually very focal [32] and the changes induced by rTMS can persist well beyond the duration of the stimulation [30,33]. We speculate that the efficacy of the nerTMS observed in this study is related to the excitatory effect on the peri-stroke area and to the induction of long-term potentiation in this area. Further, we are encouraged by the fact that nerT-MS induced changes in the entire semantic decision network [26] (Figure 2) and not only in the left frontal (stimulated) area. This finding further supports the notion that the effect of nerTMS on a single area, if associated with improvement(s), may correspond to both local and remote changes in the relevant network, and, therefore, improvements in all language-related skills. While a further discussion about the localization of the pre-/post-rTMS BOLD signal changes is beyond the scope of this manuscript, of importance is that the post-rTMS increases are seen in brain regions typically active during this fMRI task – left inferior frontal and left temporo-parietal regions [25,26]. Certainly, the left frontal BOLD signal increase may be related to the direct stimulation of that area in the course of therapy; this increase is in agreement with the improvements on an expressive language measure (SFT) and subjective verbal output reported by the patients (CAL) [14]. The BOLD signal increase in the left temporo-parietal region (the area that is typically responsible for lexical retrieval and word meaning) was observed even though this region was not directly stimulated with rTMS, and is also consistent with the observed improvements on the semantic fluency test (SFT).
In healthy controls, inhibitory rTMS may decrease motor and cognitive performance in the stimulated area [16]. In patients with post-stroke aphasia caused by LMCA stroke, inhibitory rTMS applied to the unaffected, non-dominant right-hemispheric language homologues improved the left-hemispheric language functions [34]. RTMS-evoked inhibition of redundant language circuits in the non-dominant hemisphere may release the damaged, dominant language networks allowing them to participate in post-stroke recovery. These findings support the hypothesis that the residual left-hemispheric network(s) may be more important or effective for post-stroke language processing when not negatively influenced by the redundant non-dominant (less effective) circuitry.
In contrast to the inhibitory rTMS, excitatory rTMS has been shown to facilitate language processing via increased short- and long-term cortical excitability; rTMS applied to the left-hemispheric speech area facilitated naming in healthy controls [35] or patients with Alzheimer’s disease [36] or Primary Progressive Aphasia [37]. In general, as a treatment, rTMS has been shown in numerous pilot studies to be efficacious in various neurological and psychiatric conditions [38] and is now FDA-cleared for the treatment of depression. The findings from the above studies combined with our findings suggest that the application of nerTMS to the damaged by stroke residual language circuits in the dominant-hemisphere may indeed lead to improved language skill as observed in our preliminary study.
Our original hypothesis that stimulating the left hemispheric language areas with nerTMS would produce aphasia improvements is further supported by the finding of increased post-nerTMS BOLD signal activation (when compared to the pre-rTMS fMRI) in the dominant-hemisphere language circuits and decreased involvement of the non-dominant for language hemisphere after the course of therapy as observed with fMRI. Post-stroke neuroimaging studies that have evaluated recovery from aphasia in adults with unilateral lesions show evidence of cortical reorganization and migration of language functions to the non-dominant hemisphere after a dominant hemisphere insult [20,23,39]. Interestingly, one study that showed such a redistribution pattern using PET and fMRI language tasks also found negative association between increased non-dominant inferior frontal gyrus activation and recovery after an ischemic stroke [40]. The best recovery was observed in patients with peri-infarct activation on the fMRI and PET studies. Winhuisen et al. (2007) applied inhibitory rTMS to the non-dominant language circuits and found improved post-stroke aphasia recovery in patients with preserved left-hemispheric language centers; recovery was independent of the recruitment of the non-dominant homologues [19]. A recent study showed that the fMRI activation may be dependent on the phase of post-stroke recovery with early right-hemispheric upregulation of the fMRI signal changes correlating with language recovery and late consolidation of the activation in the left-hemispheric language centers [41]. This and other studies support the presence of preexisting language pathways in both, the dominant and non-dominant hemispheres and suggest that in health the circuitry in the non-dominant hemisphere may be inhibited by the active circuitry in the dominant hemisphere; when the preferred pathway is interrupted (as in a stroke), the non-dominant circuitry is uninhibited, hence activated. These studies suggest that increased reliance on language circuits in the dominant hemisphere supports higher levels of recovery from aphasia after stroke [20,23]. As hypothesized, in this study nerTMS applied to the identified by fMRI language areas facilitated the increased reliance on these areas for post-stroke aphasia recovery.
Early reports [42] of possible seizures associated with TMS led over 10 years ago to the development of safety rules regarding stimulation frequency, intensity, train duration and intertrain interval [17,43]. Multiple subsequent studies of rTMS have demonstrated this to be a safe technique for use in studies of stroke [44] and even in epilepsy [45]. In healthy adult studies, TBS has been well tolerated. However, there is one report of a seizure in a healthy subject caused by continuous TBS stimulation with intensity set at approximately 100% of the resting motor threshold, a threshold far exceeding our protocol [46]. Our results also demonstrate the safety of the nerTMS protocol; no adverse reactions were observed in our pilot group of stroke patients despite 10 daily 200 seconds long nerTMS (iTBS) sessions; subjects reported no discomfort and the subjective assessment of pre-/post-rTMS language skills (mini-CAL) indicated the patients felt the treatments they received were beneficial. Therefore we are confident in proposing nerTMS as a safe and potentially beneficial intervention in patients with chronic aphasia after stroke.
The shortcomings of this study include small sample size, lack of sham treatment, un-blinded application of rTMS and only short-term follow-up. Nevertheless, we were able to show improved language skills and shifts of fMRI activations to the left hemisphere in response to the neuronavigated rTMS. Future studies should focus on addressing these limitations.
Conclusions
In this study, we provide preliminary evidence that fMRI-guided, excitatory rTMS applied to the affected Broca’s area improves language skills in patients with chronic post-stroke aphasia. We also show that these linguistic gains are associated with stronger language lateralization to the dominant left hemisphere. Finally, in addition to the objective gains, patients tend to report improvements in their language skills after the intervention. Therefore, the used in this study rTMS protocol appears to be safe and should be further tested in blinded studies assessing its short- and long-term safety and efficacy for post-stroke aphasia rehabilitation.
Footnotes
This study was presented in part at the 2010 Human Brain Mapping meeting in Barcelona, Spain
Source of support: Support for this study was provided by grants from University Research Council at the University of Cincinnati and, in part, by the National Institute of Neurological Disorders and Stroke (NINDS; NIH R01 NS04828); both to JPS
References
- 1.Laska A, Hellblom A, Murray V, et al. Aphasia in acute stroke and rehabilitation outcome. J Int Med. 2001;249:413–22. doi: 10.1046/j.1365-2796.2001.00812.x. [DOI] [PubMed] [Google Scholar]
- 2.Pohjasvaara T, Erkinjuntti T, Ylikoski R, et al. Clinical determinants of poststroke dementia. Stroke. 1998;29(1):75–81. doi: 10.1161/01.str.29.1.75. [DOI] [PubMed] [Google Scholar]
- 3.Tatemichi TK, Desmond DW, Paik M, et al. Clinical determinants of dementia related to stroke. Ann Neurol. 1993;33(6):568–75. doi: 10.1002/ana.410330603. [DOI] [PubMed] [Google Scholar]
- 4.Tatemichi TK, Desmond DW, Stern Y, et al. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57(2):202–7. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry. 1986;49(1):11–16. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page SJ, Levine P, Leonard A, et al. Modified Constraint-Induced Therapy in Chronic Stroke: Results of a Single-Blinded Randomized Controlled Trial. Phys Ther. 2008;88(3):333–40. doi: 10.2522/ptj.20060029. [DOI] [PubMed] [Google Scholar]
- 7.Szaflarski JP, Page SJ, Kissela BM, et al. Cortical reorganization following modified constraint-induced movement therapy: a study of 4 patients with chronic stroke. Arch Phys Med Rehabil. 2006;87(8):1052–58. doi: 10.1016/j.apmr.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Page SJ, Szaflarski JP, Eliassen JC, et al. LCortical plasticity following motor skill learning during mental practice in stroke. Neurorehabil Neural Repair. 2009;23(4):382–88. doi: 10.1177/1545968308326427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page SJ, Harnish SM, Lamy M, et al. Affected arm use and cortical change in stroke patients exhibiting minimal hand movement. Neurorehabil Neural Repair. 2010;24(2):195–203. doi: 10.1177/1545968309360501. [DOI] [PubMed] [Google Scholar]
- 10.Khedr EM, Abdel-Fadeil MR, Farghali A, Qaid M. Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol. 2009;16(12):1323–30. doi: 10.1111/j.1468-1331.2009.02746.x. [DOI] [PubMed] [Google Scholar]
- 11.Lindenberg R, Renga V, Zhu LL, et al. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176–84. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazar RM, Minzer B, Antoniello D, et al. Improvement in aphasia scores after stroke is well predicted by initial severity. Stroke. 2010;41(7):1485–88. doi: 10.1161/STROKEAHA.109.577338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. J Speech Lang Hear Res. 1998;41(1):172–87. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- 14.Szaflarski JP, Ball A, Grether S, et al. Constraint-induced aphasia therapy stimulates language recovery in patients with chronic aphasia after ischemic stroke. Med Sci Monit. 2008;14(5):CR243–50. [PMC free article] [PubMed] [Google Scholar]
- 15.Kakuda W, Abo M, Kaito N, et al. Functional MRI-based therapeutic rTMS strategy for aphasic stroke patients: a case series pilot study. Int J Neurosci. 2010;120(1):60–66. doi: 10.3109/00207450903445628. [DOI] [PubMed] [Google Scholar]
- 16.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3(2):95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tillema JM, Byars AW, Jacola LM, et al. Cortical reorganization of language functioning following perinatal left MCA stroke. Brain Lang. 2008;105(2):99–111. doi: 10.1016/j.bandl.2007.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winhuisen L, Thiel A, Schumacher B, et al. The right inferior frontal gyrus and poststroke aphasia: a follow-up investigation. Stroke. 2007;38(4):1286–92. doi: 10.1161/01.STR.0000259632.04324.6c. [DOI] [PubMed] [Google Scholar]
- 20.Eaton KP, Szaflarski JP, Altaye M, et al. Reliability of fMRI for studies of language in post-stroke aphasia subjects. Neuroimage. 2008;41(2):311–22. doi: 10.1016/j.neuroimage.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raja Beharelle A, Dick AS, Josse G, et al. Left hemisphere regions are critical for language in the face of early left focal brain injury. Brain. 2010;133(Pt 6):1707–16. doi: 10.1093/brain/awq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saur D, Ronneberger O, Kummerer D, et al. Early functional magnetic resonance imaging activations predict language outcome after stroke. Brain. 2010;133(Pt 4):1252–64. doi: 10.1093/brain/awq021. [DOI] [PubMed] [Google Scholar]
- 23.Szaflarski J, Eaton K, Ball A, et al. Post-stroke aphasia recovery assessed with functional magnetic resonance imaging and a picture identification task. J Stroke Cerebrovasc Dis. doi: 10.1016/j.jstrokecerebrovasdis.2010.02.003. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27(3):202–12. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karunanayaka P, Kim KK, Holland SK, Szaflarski JP. The effects of left or right hemispheric epilepsy on language networks investigated with semantic decision fMRI task and independent component analysis. Epilepsy Behav. doi: 10.1016/j.yebeh.2010.12.029. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim K, Karunanayaka P, Privitra M, et al. Semantic association investigated with fMRI and independent component analysis. Epilepsy Behav. doi: 10.1016/j.yebeh.2010.11.010. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szaflarski JP, Binder JR, Possing ET, et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59(2):238–44. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- 28.Szaflarski JP, Holland SK, Jacola LM, et al. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008;12(1):74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills KR, Nithi KA. Corticomotor threshold is reduced in early sporadic amyotrophic lateral sclerosis. Muscle Nerve. 1997;20(9):1137–41. doi: 10.1002/(sici)1097-4598(199709)20:9<1137::aid-mus7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Huang YZ, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 31.Epstein CM, Lah JJ, Meador K, et al. Optimum stimulus parameters for lateralized suppression of speech with magnetic brain stimulation. Neurology. 1996;47(6):1590–93. doi: 10.1212/wnl.47.6.1590. [DOI] [PubMed] [Google Scholar]
- 32.Siebner HR, Bergmann TO, Bestmann S, et al. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2(2):58–80. doi: 10.1016/j.brs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Pascual-Leone A, Tormos JM, Keenan J, et al. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15(4):333–43. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Naeser MA, Martin PI, Nicholas M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang. 2005;93(1):95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Cappa SF, Sandrini M, Rossini PM, et al. The role of the left frontal lobe in action naming: rTMS evidence. Neurology. 2002;59(5):720–23. doi: 10.1212/wnl.59.5.720. [DOI] [PubMed] [Google Scholar]
- 36.Cotelli M, Manenti R, Cappa SF, et al. Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Arch Neurol. 2006;63(11):1602–4. doi: 10.1001/archneur.63.11.1602. [DOI] [PubMed] [Google Scholar]
- 37.Finocchiaro C, Maimone M, Brighina F, et al. A case study of Primary Progressive Aphasia: improvement on verbs after rTMS treatment. Neurocase. 2006;12(6):317–21. doi: 10.1080/13554790601126203. [DOI] [PubMed] [Google Scholar]
- 38.Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8(7):559–67. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- 39.Weiller C, Isensee C, Rijntjes M, et al. Recovery from Wernicke’s aphasia: a positron emission tomographic study. Ann Neurol. 1995;37(6):723–32. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- 40.Rosen HJ, Petersen SE, Linenweber MR, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–94. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- 41.Saur D, Lange R, Baumgaertner A, et al. Dynamics of language reorganization after stroke. Brain. 2006;129(Pt 6):1371–84. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 42.Homberg V, Netz J. Generalised seizures induced by transcranial magnetic stimulation of motor cortex. Lancet. 1989;2(8673):1223. doi: 10.1016/s0140-6736(89)91835-7. [DOI] [PubMed] [Google Scholar]
- 43.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 44.Malcolm MP, Triggs WJ, Light KE, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint-induced therapy: an exploratory randomized controlled trial. Am J Phys Med Rehabil. 2007;86(9):707–15. doi: 10.1097/PHM.0b013e31813e0de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae EH, Schrader LM, Machii K, et al. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behav. 2007;10(4):521–28. doi: 10.1016/j.yebeh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Oberman LM, Pascual-Leone A. Report of Seizure Induced by Continuous Theta Burst Stimulation. Brain Stimulat. 2009;2(4):246–47. doi: 10.1016/j.brs.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]