Abstract
We investigated whether the endoplasmic reticulum (ER) is a functionally connected Ca2+ store or is composed of separate subunits by monitoring movements of Ca2+ and small fluorescent probes in the ER lumen of pancreatic acinar cells, using confocal microscopy, local bleaching and uncaging. We observed rapid movements and equilibration of Ca2+ and the probes. The bulk of the ER at the base was not connected to the granules in the apical part, but diffusion into small apical ER extensions occurred. The connectivity of the ER Ca2+ store was robust, since even supramaximal acetylcholine (ACh) stimulation for 30 min did not result in functional fragmentation. ACh could elicit a uniform decrease in the ER Ca2+ concentration throughout the cell, but repetitive cytosolic Ca2+ spikes, induced by a low ACh concentration, hardly reduced the ER Ca2+ level. We conclude that the ER is a functionally continuous unit, which enables efficient Ca2+ liberation. Ca2+ released from the apical ER terminals is quickly replenished from the bulk of the rough ER at the base.
Keywords: Ca2+ movements/Ca2+ uncaging/endoplasmic reticulum/lumenal Ca2+/lumenal connectivity
Introduction
The endoplasmic reticulum (ER) is the most important dynamic Ca2+ store (Berridge and Irvine, 1989; Irvine, 1990; Berridge, 1993; Pozzan et al., 1994). The ER is a continuous tubular network structure (Villa et al., 1991; Terasaki et al., 1994), but work on astrocytes, arterial myocytes and HeLa cells suggests that it is composed of distinct heterogeneous subunits with different Ca2+ transport characteristics (Golovina and Blaustein, 1997; Montero et al., 1997a). On the other hand, there is indirect evidence from pancreatic acinar cells suggesting that the ER functions as a lumenally continuous compartment, which would allow movements of Ca2+ from the base to the apex (Mogami et al., 1997). It has also been shown that green fluorescent protein (GFP) fusion proteins can diffuse in the aqueous-phase lumen of the ER (Subramanian and Meyer, 1997; Dayel et al., 1999) and this may suggest that the free Ca2+ concentration could equilibrate throughout the cell. However, buffering by Ca2+-binding proteins could slow the movement of Ca2+ significantly and lead to the establishment of lumenal Ca2+ gradients (Subramanian and Meyer, 1997).
In order to test directly whether the ER functions dynamically as a single continuous Ca2+ storing unit or is effectively composed of separate sub-compartments, it is necessary to observe the movement of Ca2+ in the lumen of the ER under conditions of artificially imposed gradients and during stimulated Ca2+ release and re-uptake. So far such data are lacking and we have no information about the speed of Ca2+ movement in the ER lumen.
Because the pancreatic acinar cell is both structurally and functionally polarized, it is a good system in which to investigate the movement of Ca2+ between different regions within the ER. The acinar cell has an extensive rough ER network dominating its basolateral part. Electron microscope (EM) pictures show that thin ER cisternae are as densely packed as physically possible in the whole of the basolateral region surrounding the nucleus. In contrast, the apical region is densely packed with secretory (zymogen) granules (ZGs) and has almost no space for ER. Only small ER vesicular structures can occasionally be observed (Kern, 1993; Gorelick and Jamieson, 1994). This presents an interesting problem. The physiologically important cytosolic Ca2+ signals, elicited by the neurotransmitter acetylcholine (ACh) or the hormone cholecystokinin, are initiated in the apical pole and are confined to this region most of the time (Kasai et al., 1993; Thorn et al., 1993; Petersen et al., 1994). Even in the case of supramaximal agonist stimulation, when the cytosolic Ca2+ signal spreads rapidly all over the cell, the Ca2+ concentration rise is larger in the apical than in the basal part of the cell (Ito et al., 1997). If the ER is the major intracellular store from which Ca2+ is mobilized upon agonist stimulation, then the small Ca2+-releasing ER elements in the apical pole should be functionally connected to the main part of the ER in the basolateral area. Furthermore, this connection must be efficient. Ca2+ should be able to move rapidly from the major part of the store, at the base of the cell, into the termini in the granular pole, where stimulation causes opening of Ca2+ release channels in the apical ER extensions (Mogami et al., 1997).
We have used fast local photobleaching and local uncaging of substances trapped in the lumen of the ER (NP-EGTA, Mag-fluo 4 and Mag-fura 2, respectively) and studied the movements of Ca2+ and small molecular weight fluorescent indicators inside the ER. We show that the whole of the ER in the pancreatic acinar cell is functionally connected. The concentrations of Ca2+ and other small molecules appear uniform throughout the ER and suddenly established gradients, induced by local photobleaching or uncaging, equilibrated within a few seconds even over distances of up to ∼10 µm. The whole of the ER, and in particular the bulk of the rough ER in the basal part, provides a homogeneous environment with regard to the lumenal Ca2+ concentration. This may be crucial for many biological functions such as protein processing and regulation of the sensitivity of the Ca2+ release mechanism. We also show that the rapid equilibration of Ca2+ throughout the lumen of the ER enables low and physiologically relevant agonist concentrations to evoke repetitive cytosolic Ca2+ spikes with hardly any reduction in the ER Ca2+ level.
Results
Local photobleaching of Mag-fluo 4 in the lumen of the ER: the lumen of the ER in the basolateral region is continuous
The normal polarity of the acinar cells was well preserved in single cells or clusters after isolation with collagenase treatment [Figure 1A(a), B(a) and C(a)]. In order to visualize the intracellular Ca2+ stores, we loaded a low-affinity Ca2+-sensitive dye, Mag-fluo 4 (Kd = 22 µM), into the Ca2+ stores and obtained high-resolution images with a confocal microscope. As shown in Figure 1A and B, there was fluorescence from all parts of the cell except the nuclei, indicating a low level of fluorescence in the cytosol (Mogami et al., 1998). This is due to the optical properties of Mag-fluo 4, which are such that at the resting cytosolic free Ca2+ concentration there is little fluorescence. In agreement with this, washout of the cytosolic dye component only caused a small decrease in the fluorescence intensity (see below).
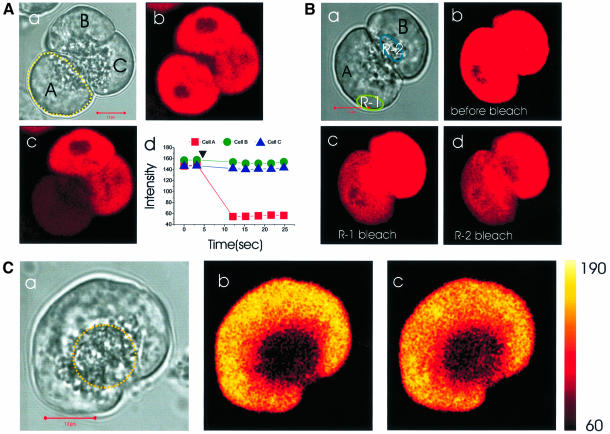
Fig. 1. The full connectivity of the whole basolateral ER Ca2+ store complex and the connection with the apical extensions is demonstrated by local photobleaching of Mag-fluo 4 in the lumen of the ER and subsequent monitoring of the movement of unbleached dye. (A) One cell [yellow dotted circle in (a)] was selectively bleached with UV laser; (b and c) show fluorescence images before and after bleaching; (d) measurement of fluorescence intensity changes in the three cells. (B) Repetitive local bleaching [R-1 in (a)] resulted in a homogeneous decrease of fluorescence intensity throughout the whole basolateral part of the cell (b and c, before and after bleaching), indicating that the whole basolaterally located Ca2+ store was fully interconnected. By contrast, local apical bleaching [R-2 in (a)] affected only the bleached part of the cell (d), suggesting that the granular Ca2+ store was not functionally connected to the basolateral ER. (C) After wide apical bleaching [yellow dotted circle in (a)], we monitored the fluorescence intensity changes in the apical and basal pole: (a) transmitted light picture; (b) fluorescence image taken immediately after apical bleaching; (c) 15 s after the bleaching. The length of the horizontal red bar in A(a), B(a) and C(a) represents 10 μm.
To test the connectivity of the lumen of the ER from different parts of the cell, we employed the local bleaching function of the confocal microscope, using a strong UV laser with near full power (351 and 360 nm, 80 mW). (It was unexpected, but helpful, that Mag-fluo 4 could be bleached by UV light. This bleaching did not affect the Ca2+ content or Ca2+ transport characteristics of the ER store—see below.) Figure 1A shows an example of the bleaching function employed. We bleached only one cell out of a triplet [cell A circled in Figure 1A(a)]. Thereafter, the fluorescence intensity of this cell decreased dramatically, but the other two cells were not affected [Figure 1A(b–d)]. Next, we bleached small areas within single cells of the doublet as shown in Figure 1B(a–d). At first, we repetitively bleached a small basolateral area (R-1). As a result, the fluorescence intensity of the whole basolateral region decreased homogeneously, whereas the apical granular pole remained almost unaffected and finally disclosed its demarcation [Figure 1B(c)] in agreement with the structure seen in the transmitted light picture [Figure 1B(a)]. In the other cell, we tried deliberately to bleach a small area (R-2) localized within the apical region containing the ZGs. Only the bleached area itself was affected strongly (although a slight decrease in the fluorescence intensity in the basolateral part is just visible), indicating that the granules are not lumenally connected with the ER in the basolateral part of the cell.
Penetration of ER elements into the apical pole of the cell
In order to investigate whether there are ER elements in the granular region, which might be connected to the bulk of the ER in the basolateral region, a wide area containing the apical pole was bleached and the fluorescence intensity changes were monitored. In the experiment shown in Figure 1C(a–c), the whole of the apical region was double bleached. The image shown in Figure 1C(b) was captured immediately after the bleaching and the one shown in Figure 1C(c), 15 s later. Following the bleaching, the fluorescence intensity in the apical pole increased, whereas that of the basolateral part decreased, suggesting that small ER elements in the apical pole can be refilled from the major portion of the ER in the basolateral region. The same result was obtained in all the 11 cells studied and also in a further six cells in which the patch–clamp whole-cell configuration was used to wash out the cytosolic dye component. Figure 1C shows that the fluorescence intensity increase in the apical pole following the bleaching is not homogeneous. However, the spatial resolution of our method is insufficient to measure the fluorescence intensity increase specifically in the tiny ER elements in the apical pole.
Rapid movement of Mag-fluo 4 in the ER lumen
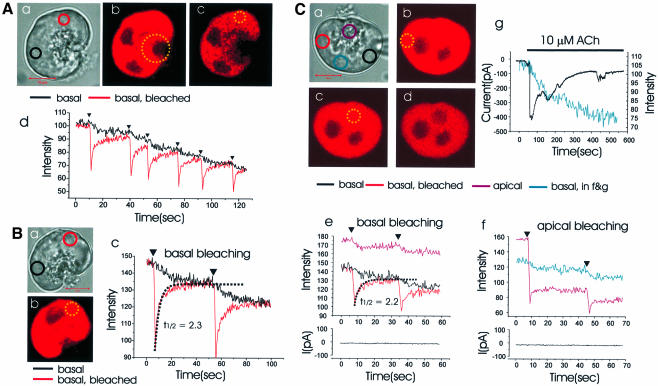
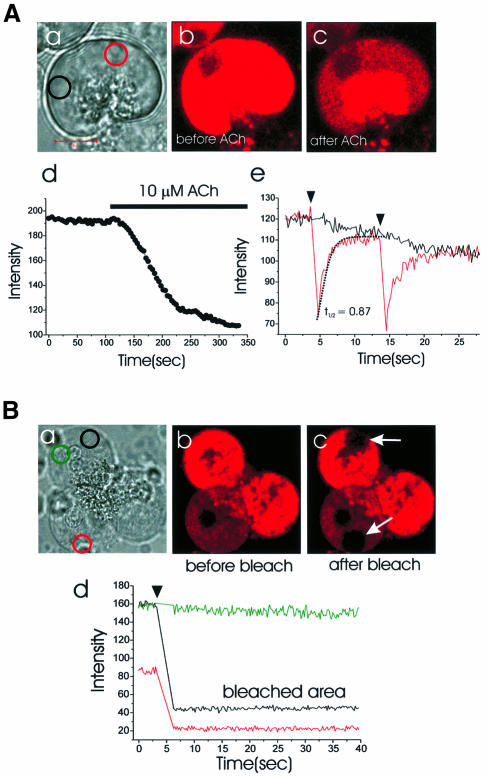
To resolve the speed of dye movement within the ER, we used the fast mode of the confocal microscope, bleached a small circular area and followed the time course of the recovery of fluorescence in that area as well as the decline in fluorescence in an area some distance away. Figure 2 illustrates experiments showing how quickly the dye moved within the ER. In single cells, we bleached a small circular area in the basolateral part [red and yellow circles in Figure 2A(a and c) and B(a and b)] and measured the fluorescence intensities of both the bleached area (red circle) and a remote area across the nucleus [black circle in Figure 2A(a) and B(a)], simultaneously. After each local bleaching event there was a sharp drop in the fluorescence intensity in the bleached field and thereafter an exponential recovery [red curve in Figure 2A(d) and B(c)]. In the remote area across the nucleus, the bleaching event resulted in a decline in fluorescence intensity (black curve). The two curves converged quickly, after each bleaching event, indicating rapid equilibration between the two sites. This could be repeated several times in the same experiment [Figure 2A(d)] and did not depend on the specific positions of the regions in the basolateral area chosen for analysis. The recovery of fluorescence intensity at the bleached site was well fitted with an exponential function, where the half recovery time was 2.3 s [Figure 2B(c)]. In the 13 cells investigated using this protocol, the average half recovery time was 1.9 ± 0.1 s (SE). After apical bleaching [Figure 2A(b)], we could detect a small decrease of the fluorescence intensity in the basolateral area [Figure 2A(c)].
Fig. 2. Time course of Mag-fluo 4 movement in the ER lumen following local bleaching. (A) (a) Transmitted light picture of a single cell with the areas of interest identified by the red and black circles. Length of red bar corresponds to 10 µm. (b and c) Fluorescence intensity images before and after wide apical bleach in region marked by large yellow dotted circle in (b). Local basal bleaching [small yellow dotted circle in (c)] was applied repeatedly and the fluorescence intensities measured both in the bleached area [red circle in (a), red curve in (d)] as well as in an unbleached basal area ∼10 µm away [black circle in (a), black curve in (d)]. (B) (a) Transmitted light picture of another cell. Local basal bleaching [red and yellow dotted circles in (a) and (b), respectively] was applied and the fluorescence intensity changes in the marked sites [colour coding corresponds to coloured circles in (a)] are shown in (c). (C) In this experiment Mag-fluo 4 was washed out of the cytosol through a patch pipette (a) (whole-cell configuration). Ten minutes after establishing whole-cell configuration, the areas represented by the dotted yellow circles (b and c) were bleached and the fluorescence intensity changes monitored in the areas represented by the marked circles in (a). (a) Transmitted light picture; (b and c) before and after basal bleaching, respectively; (c and d) before and after apical bleaching. The time course of the changes in fluorescence intensity [colour coding according to the marked sites in (a)] and the Ca2+-dependent whole-cell current during the local basal (b) and apical (c) bleaching are shown in (e) and (f), respectively. After the bleaching experiments, ACh elicited an increase in the Ca2+-dependent current (black trace) and released Ca2+ from the ER (blue trace) (g).
Short photobleaching does not disturb the Ca2+ dynamics
Since Mag-fluo 4 is a low-affinity Ca2+-sensitive dye and would be expected to have little fluorescence at the low Ca2+ concentration in the cytosol under resting conditions, we assumed that there would only be a minor interference by the dye present in the cytosol. In order to test this, the cytosolic dye component was washed out of the cytosol into a patch pipette (whole-cell configuration). During a 10 min period of dye washout, the fluorescence intensity was changed by <10%. As shown in Figure 2C, the bleaching experiments gave the same results under this condition as without the dye washout. After local basal bleaching [Figure 2C(a and b)], the recovery of fluorescence intensity in the bleached area could be fitted by a single exponential [Figure 2C(e)] and the average half recovery time was 1.8 ± 0.2 s (n = 4). Local apical bleaching [Figure 2C(c)] caused a major decrease in the fluorescence intensity in the apical part of the cell, which was only partially reversible, and also a smaller fluorescence intensity decrease in the basal part [Figure 2C(d and f)]. In these experiments it was also possible to test whether the photobleaching was associated with any change in the cytosolic Ca2+ concentration, by monitoring the Ca2+-dependent whole-cell current (Petersen, 1992). In none of these experiments (n = 4) did we observe any activation of the Ca2+-sensitive current [Figure 2C(e and f)]. However, after the bleaching, ACh could, in the same cell, generate the usual Ca2+-sensitive current and release Ca2+ from the ER [Figure 2C(g)]. This suggests that short photobleaching does not disturb the Ca2+ dynamics.
Photobleaching of Mag-fura 2: confirmation of ER lumenal connectivity
As a next step, we used Mag-fura 2 (Kd = 52 µM), which has been used to measure the Ca2+ concentration in the ER from different cell types (Hofer and Machen, 1993; Hofer and Schulz, 1996), including pancreatic acinar cells (Mogami et al., 1998; Park et al., 1999). In our experiments we used an excitation wavelength of 351 nm, which is close to the isobestic point of the dye. It was our purpose to test the lumenal connectivity within the ER without any possible interference from unwanted Ca2+ concentration changes in the cytosol or the ER. To wash out the dye from the cytosol, we used a patch pipette (whole-cell recording configuration) containing 10 mM BAPTA and 2 mM Ca2+, so that the cytosolic Ca2+ concentration was clamped at the normal resting level. As soon as the patch of plasma membrane covered by the pipette had been ruptured, the whole-cell fluorescence intensity decreased and 10 min later was reduced to ∼10% of the level observed before entering the whole-cell configuration (Mogami et al., 1998). Local bleaching experiments of the type shown in Figure 2 were then carried out, with similar results. The recovery of fluorescence intensity in a small bleached area was fast, with an average recovery half time of 0.8 ± 0.1 s (n = 10). The fluorescence intensity in an unbleached basal area, ∼10 µm away from the bleached area, decreased after each bleaching event and was equilibrated with the bleached area within ∼5 s.
Direct measurement of Ca2+ movement in the lumen of the ER: Ca2+ uncaging in the ER lumen
We loaded the caged Ca2+ compound, NP-EGTA, into the lumen of the ER together with Mag-fluo 4. Because the Ca2+ concentration in the ER is high, we used a very high dose of NP-EGTA (20.5 µM) and a long incubation time (30 min). Thereafter, to wash out the dye and the NP-EGTA in the cytosol, we established the patch–clamp whole-cell configuration. We used a pipette solution containing 10 mM BAPTA and 2 mM Ca2+ to clamp the cytosolic Ca2+ concentration at the normal resting level and thereby prevented any Ca2+-induced Ca2+ release as well as any cytosolic Ca2+ change following uncaging of Ca2+ inside the ER.
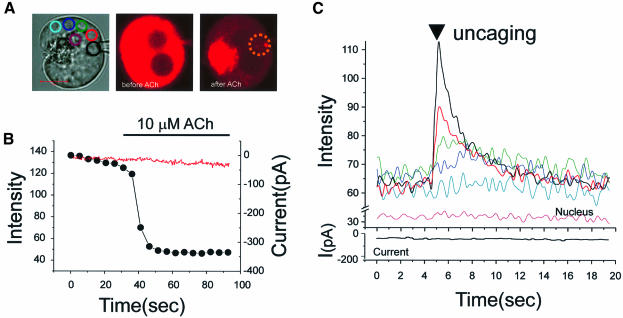
In four out of five cells, we could not detect any significant Ca2+ concentration increase after uncaging. One cell showed tiny changes. This indicates that the amount of Ca2+ released by uncaging was small in relation to the already high Ca2+ concentration in the resting ER (Mogami et al., 1998). We therefore lowered the Ca2+ concentration in the ER by stimulating with 10 µM ACh. Figure 3A shows the transmitted light image of the cell used for one of the experiments, with the regions of interest identified by coloured circles, as well as the fluorescence images of the same cell before and after ACh application. The Ca2+ concentration inside the ER in the basolateral area decreased markedly after ACh application (Figure 3B), but there was no change in the Ca2+-dependent current (red trace) because of the cytosolic Ca2+ clamp. The time course of the Ca2+ concentration change inside the ER was monitored, as shown in Figure 3B, to determine the optimal time for uncaging Ca2+. Immediately after the washout of ACh, the Ca2+ concentration inside the ER is still low and the Ca2+ release channels are closed. At this point we therefore uncaged NP-EGTA in the marked site (dotted circle in Figure 3A) and measured the Ca2+ concentration changes around the nucleus in the marked coloured circles (Figure 3A). As seen in Figure 3C, uncaging of Ca2+ elicited an instantaneous increase in the Ca2+ concentration in the targeted area (black curve). Ca2+ concentration changes, with decreasing amplitude and decreasing rate of rise with increasing distance from the site of uncaging, were observed in the other basolateral areas (red, green, dark blue and light blue curves). Thereafter the Ca2+ concentration declined in all areas, converging and attaining the same level within ∼7 s. Similar results were obtained in all six experiments carried out on separate cells. NP-EGTA has a high affinity for Ca2+ (Kd <100 nM). In mouse pancreatic acinar cells, stimulation with 10 µM ACh has previously been shown to decrease the Ca2+ concentration in the lumen of the ER (measured with low-affinity ratiometric indicators) to ∼40 µM (Mogami et al., 1998). At such free Ca2+ levels in the stores, NP-EGTA is saturated with Ca2+ and consequently cannot rebind Ca2+ following photolysis. No change in Ca2+ concentration was detected in the nucleus and the Ca2+-dependent current remained stable, due to the cytosolic Ca2+ clamp. This result (Figure 3C) indicates fast movement of Ca2+ through the lumen of the ER. Ca2+ concentration equilibration seems to occur, even over distances of ∼10 µm, within a few seconds.
Fig. 3. Rapid movement of Ca2+ in the ER lumen following local uncaging of caged Ca2+. Direct measurement of Ca2+ movement in the lumen ofthe ER. After 30 min loading with Mag-fluo 4-AM and NP-EGTA-AM, the dye and NP-EGTA were washed out of the cytosol into a patch pipette (whole-cell configuration). The cytosolic Ca2+ was clamped at the resting level by using a patch pipette containing 10 mM BAPTA and 2 mM Ca2+. Thereafter, 10 µM ACh was applied to lower the Ca2+ concentration inside the ER. (A) Transmitted light image of the cell with the patch pipette. The various regions of interest are colour coded. The fluorescence intensity images just before and after ACh application are also shown. NP-EGTA was uncaged in the lumen of the ER in the area represented by the dotted circle. (B) Time course of the ACh-evoked reduction in Ca2+ concentration inside the ER (black dots) and also the complete absence of any change in the Ca2+-dependent Cl– current (red trace). (C) Time course of the Ca2+ concentration changes in the ER, following local uncaging, in the various regions colour coded in (A). It is also seen that the Ca2+-dependent current did not change after the intralumenal uncaging.
ACh-evoked release of Ca2+ from the ER inMag-fluo 4-loaded cells
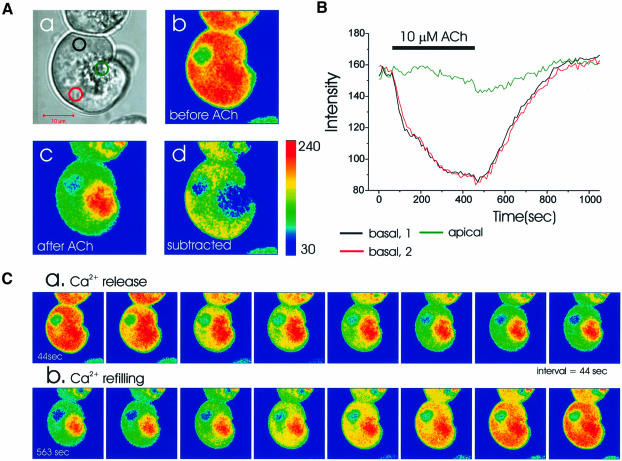
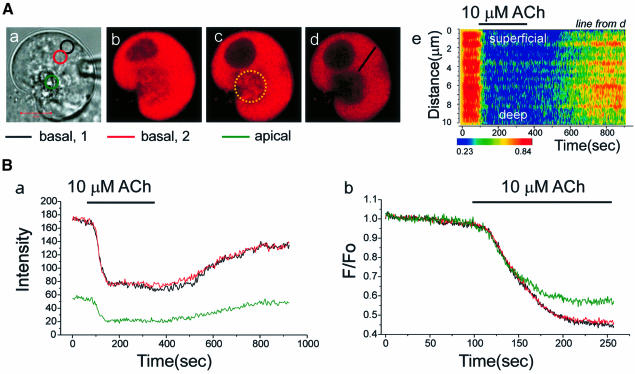
Figure 4 shows the ER Ca2+ release and refilling processes triggered by a supramaximal dose of ACh in an intact Mag-fluo 4-loaded cell. The structure of the cell, with the granule-containing pole to the right, is shown in the transmitted light picture [Figure 4A(a)]. Figure 4A(b) shows the colour-coded Ca2+-sensitive fluorescence image before the application of ACh, and in Figure 4A(c) the image taken after the maximal release of Ca2+ from the ER is shown. Subtraction [Figure 4A(d)] revealed that the main intracellular Ca2+ release site was the basolateral region, where the rough ER is densely packed (Kern, 1993; Gorelick and Jamieson, 1994). Nevertheless, the apical pole appears heterogeneously, probably due to penetration of thin ER elements into the apical pole (Figure 1).
Fig. 4. Time course of ACh-evoked Ca2+ release and the subsequent refilling of the ER. In a Mag-fluo 4-loaded cell, 10 µM ACh was applied. (A) (a) Transmitted light picture; (b and c) fluorescence intensity images before and after ACh; (d) subtracted image (b–c). (B) Time course of ACh-induced fluorescence intensity changes in the two basal as well as the apical regions marked in [A(a)]. (C) Time course of Ca2+ release following ACh stimulation and subsequent refilling after cessation of stimulation.
As shown in Figure 4B and C, Ca2+ release from the ER was slow. It took several minutes to empty the whole ER. Soon after ACh removal, Ca2+ refilling began. In the basolateral ER-rich region, all areas essentially shared the same time course of Ca2+ release and refilling (Figure 4B and C) due to the relatively rapid equilibration of Ca2+ within the lumen of the ER compared with the slow Ca2+ release. During the Ca2+ release period, focal bleaching in basal areas showed that dye equilibrium was achieved quickly, with results very similar to those shown in Figure 2 (n = 3). In the apical region, ACh initially hardly evoked any change in the Ca2+ concentration, but later there was a relatively small decrease (Figure 4B), which was spatially non-uniform (Figure 4C). This may be due to the high fluorescence intensity of the granules and the relatively small fraction of the ER extensions in the apical pole.
Since the expected ACh-elicited decrease in the Ca2+ concentration inside the ER elements in the apical pole could not be resolved (Figure 4B), most likely due to the high fluorescence of the granules, we bleached the apical pole of the cell. The remaining fluorescence would be expected to come from thin ER elements in the apical pole refilled via the connections to the main part of the basolateral ER. In this situation we could more easily observe changes in the apical pole after 10 µM ACh application (n = 5), but it was difficult to detect possible differences between the Ca2+ release rates from various parts of the ER.
The most useful experiments were those in which we dialysed the cell with a high Ca2+ buffer solution (10 mM BAPTA and 2 mM Ca2+) using the patch–clamp whole-cell configuration. In this condition, the ACh-elicited Ca2+ release was much faster (Figure 5), most likely due to the removal of the negative Ca2+ feedback on the opening of the inositol trisphosphate (IP3) receptors (Montero et al., 1997b; Mogami et al., 1998). The transmitted light picture shows the structure of the cell investigated [Figure 5A(a)]. After making the whole-cell configuration, we bleached the apical pole. The fluorescence images taken before establishing the whole-cell configuration [Figure 5A(b)], 10 min after having established this configuration [Figure 5A(c)] and after wide apical bleaching [Figure 5A(d)] are shown. The rather homogeneous Ca2+ concentration decrease throughout the basal region evoked by 10 µM ACh and the relatively homogeneous Ca2+ concentration increase following cessation of ACh stimulation are shown in Figure 5A(e). In this condition, we detected clearly Ca2+ concentration decreases both in the basal and apical poles. As seen in Figure 5B(a), there was no difference in time course between the apical pole and the basolateral part of the cell (similar results in six trials). Even in the fast scan mode (89 ms), we could not detect any time lag between the two regions [Figure 5B(b)]. This suggests that the movement of Ca2+ inside the lumen of the ER is fast relative to the rate of Ca2+ release, so that the whole of the ER is homogeneous with respect to the lumenal Ca2+ concentration at all times.
Fig. 5. Ca2+ release from the ER after ACh stimulation follows the same time course in the basal and apical regions. Mag-fluo 4-loaded cell with subsequent washout of cytosolic dye component through patch pipette in whole-cell configuration. Cytosolic Ca2+ concentration clamped at the normal resting level by BAPTA/Ca2+ mixture. (A) (a) Transmitted light picture; (b) fluorescence intensity image before cytosolic dye washout; (c) 10 min after establishment of whole-cell configuration. Thereafter wide apical bleaching of apical pole within area represented by dotted yellow circle. (d) After apical bleaching to remove fluorescence from dye in the granules. (e) Line scan image taken from the line shown in (d) illustrating the time course of the Ca2+ concentration changes in the ER at the base of the cell. (B) (a) Ca2+-dependent fluorescence intensity changes in response to ACh measured in the three areas represented by the colour-coded circles in [A(a)]. (b) Comparison of Ca2+ release time in the apical and basal parts of the cell with a fast scan mode (89 ms sampling).
Lumenal connectivity after supramaximal stimulation with ACh: local uncaging after application of ACh or ionomycin
We tested whether the ER connectivity is maintained under extreme conditions. Figure 6A shows the result from a cell loaded with Mag-fluo 4. After application of a supramaximal ACh dose (10 µM), the main decrease in the Ca2+ concentration inside stores was detected in the basolateral region [Figure 6A(b,c and d)]. Thereafter, we bleached a small area in the basal pole [Figure 6A(a, red circle)] and measured the fluorescence intensity simultaneously in the bleached as well as a remote area (black circle). As shown in Figure 6A(e), the two areas (>5 µm apart) were connected lumenally. The bleaching was carried out in eight different cells, 3–30 min after start of continuous ACh (10 µM) application. In all cells we found efficient lumenal connectivity with an average half recovery time of 1.0 ± 0.1 s (n = 8), indicating that the functional connectivity of the ER elements is robust.
Fig. 6. Supramaximal ACh stimulation fails to fragment the ER, but prolonged exposure to ionomycin in the presence of 10 mM extracellular Ca2+ abolishes lumenal diffusion of Mag-fluo 4. Local bleach experiments were carried out after application of 10 µM ACh (A) and 15 µM ionomycin (B). (A) (a) Transmitted light image of the cell investigated; (b and c) fluorescence images before and after ACh application; (d) ER Ca2+ release after 10 µM ACh; (e) a small area in the basal part [red circle in (a)] was bleached and the fluorescence intensity measured at the bleached site (red curve) and a remote site [black circle in (a) and black curve]. Half recovery time is 0.9 s. (B) Local bleaching experiment after 15 min treatment with 15 µM ionomycin in 10 mM Ca2+. We observed lumenal connectivity until 10 min after 10 µM ionomycin treatment; however, after ∼12 min,the ER appeared to be fragmented. After bleaching two local areas [marked as black and red circles in (a)], there was no recovery of fluorescence intensity (arrows). (a) Transmitted light image of thecells investigated; (b) fluorescence intensity image before bleaching; (c) fluorescence intensity image after bleaching of two local areas (arrows); (d) fluorescence intensities measured at the sites marked by the correspondingly coloured sites in (a).
Next we tried to increase artificially the cytosolic Ca2+ concentration with ionomycin in the presence of 10 mM Ca2+ in the extracellular solution. After treatment with 15 µM ionomycin, local bleaching experiments were carried out and we observed that the ER was connected normally. However, after ∼12 min of continuous exposure to ionomycin, the ER became fragmented. Figure 6B shows the result. In this case local bleaching resulted in a permanent reduction in the fluorescence intensity in the bleached area and the local bleaching had no effect on the fluorescence intensity in other parts of the cell. We conclude that the functional ER connection, in normal pancreatic acinar cells, is maintained over the whole range of physiological conditions, whereas prolonged extreme cytosolic Ca2+ loading can fragment the ER.
Short-lasting repetitive cytosolic Ca2+ spikes are only associated with small changes in the Ca2+ concentration inside the ER
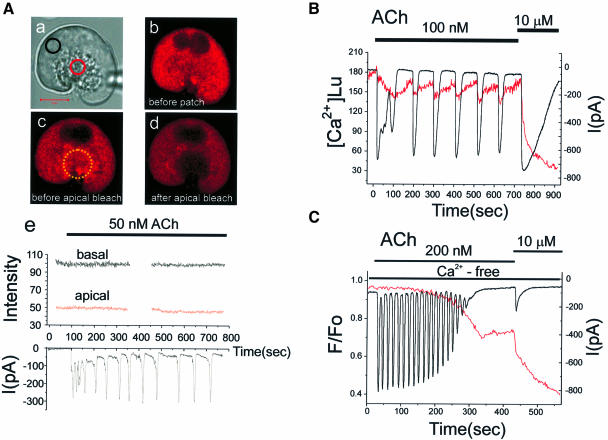
Physiological agonist concentrations elicit repetitive cytosolic Ca2+ spikes rather than a sustained increase in the Ca2+ level (Berridge and Irvine, 1989; Berridge, 1993; Petersen et al., 1994). We therefore investigated whether a low ACh concentration, evoking repetitive cytosolic Ca2+ spikes, could cause detectable decreases in the Ca2+ concentration inside the connected ER store. Figure 7A shows the result of a patch–clamp whole-cell recording experiment in which 50 nM ACh elicited repetitive cytosolic Ca2+ spikes, monitored as Ca2+-dependent current spikes (Petersen, 1992; Thorn et al., 1993). It is seen that none of these relatively short-lasting cytosolic Ca2+ spikes were associated with any detectable change in the ER Ca2+ concentration in the basal or the apical regions.
Fig. 7. Repetitive short-lasting cytosolic Ca2+ spikes evoked by a low ACh concentration are associated with no or very small changes in the Ca2+ concentration inside the ER. (A) (a) Transmitted light picture of the cell investigated, with the two regions of interest identified by the coloured circles. The fluorescence intensity images before (b) and after (c) the establishment of the patch–clamp whole-cell recording configuration as well as after the apical bleaching (d) are shown. (e) The Ca2+-sensitive fluorescence intensity from Mag-fluo 4 inside the ER before and during stimulation with 50 nM ACh in both the basal and apical regions (upper part) as well as the Ca2+-dependent whole-cell current (lower part). (B) 100 nM ACh generated more substantial Ca2+-dependent current oscillations close to the maximal activation of the current with 10 µM ACh. In this particular cell, we observed small decreases in the Ca2+ concentration of the ER, recorded using the ratiometric dye Mag-fura 2 (351 and 364 nm excitation) and calibrated as described previously (Park et al., 1999). (C) Measurement of the ER Ca2+ concentration (Mag-fluo 4) and the Ca2+-dependent current oscillations in a cell exposed to an external Ca2+-free solution. In Ca2+-free solutions, low doses of ACh (25–100 nM) failed to evoke Ca2+ oscillations and 200 nM ACh had to be used to generate repetitive cytosolic Ca2+ spikes. Individual short-lasting Ca2+-dependent current spikes were not associated with any measurable drop in the Ca2+ concentration in the ER store, but after ∼2 min of repetitive spiking the ER Ca2+ concentration began to decrease. As the ER was losing Ca2+, the spikes became smaller and finally disappeared. After the Ca2+ oscillations had ceased, there was a further decrease in the ER Ca2+ concentration during a period of non-oscillatory elevation in the cytosolic Ca2+ concentration. Finally, a supramaximal ACh (10 µM) application caused a marked further Ca2+ release from the ER.
Similar results were obtained in 16 cells investigated with Mag-fluo 4, four cells in Mag-indo 1-loaded cells and six cells in Mag-fura 2-loaded cells. We therefore increased the ACh concentration and generated more substantial cytosolic Ca2+ oscillations. In this condition, we were just able to detect, in three out of six cells, a small drop in the ER Ca2+ concentration in association with each cytosolic Ca2+ spike. Figure 7B shows the result from one of these cells, where the ER Ca2+ concentration decrease associated with a Ca2+ spike was almost fully restored before the next event. We also tested the effect of ACh on cells exposed to a Ca2+-free external solution. In this condition low doses of ACh (25–100 nM) did not generate any Ca2+ oscillations (n = 6), but 200 nM ACh elicited repetitive spikes. Figure 7C shows one of the results. There was no measurable reduction in the ER Ca2+ concentration during each cytosolic Ca2+ spike, but after ∼2 min of repetitive spiking a gradual reduction in the ER Ca2+ concentration was observed. Thereafter, the spike amplitude gradually decreased and finally the spiking stopped. Supramaximal ACh stimulation (10 µM) could nevertheless release further Ca2+ from the ER (Figure 7C).
The apical pole is dominated by non-ER elements and as we were unable to record specifically from the tiny ER elements in this region, we cannot exclude that there could be very local changes in the apical ER Ca2+ concentration during the short-lasting cytosolic spikes, which we could not resolve. Nevertheless it is clear that the amount of Ca2+ that has to be released from the ER in order to evoke a short-lasting cytosolic Ca2+ spike is insufficient to make a major impact on the Ca2+ concentration in this large continuous store.
Discussion
Our data provide direct evidence for rapid movement within the ER lumen of Ca2+ and small molecules. By localized photobleaching of Ca2+-sensitive fluorescent probes and by monitoring at high resolution the subsequent diffusion into the bleached area and away from non-bleached areas, we have characterized quantitatively the rapid movements within the ER lumen. All the basolaterally located ER elements are interconnected efficiently (Figures 1 and 2). Movements of the fluorescent Ca2+-sensitive probes occur rapidly with equilibration over distances of 10–15 µm within a few seconds (Figure 2). Most importantly, we have demonstrated that local uncaging of caged Ca2+, creating suddenly a major Ca2+ gradient inside the ER, results in rapid diffusion of Ca2+ within the lumen of the ER, followed by equilibration over distances of ∼10 µm within a few seconds (Figure 3).
By bleaching the whole of the apical granule-containing area and following the subsequent movements of non-bleached Ca2+-sensitive dye, we have shown that there is non-homogeneous refilling of the apical pole with dye from the basolateral area (Figure 1). Furthermore, ACh stimulation resulted in a reduction of the intra-store Ca2+ concentration in the apical pole with exactly the same time course as the reduction in the basal ER Ca2+ level (Figure 5). This agrees with the direct demonstration of rapid Ca2+ movement through the ER lumen (Figure 3) and illustrates the functional connection between the bulk of the ER Ca2+ reservoir at the base and the apical ER terminals.
The ER lumenal connectivity is robust (Figure 6). Previously, experiments on RBL cells have indicated that an increase in the cytosolic Ca2+ concentration can reversibly fragment the ER tubules and thereby prevent lumenal diffusion of a GFP fusion protein (Subramanian and Meyer, 1997). However, our experimental results indicate that even sustained stimulation of the acinar cells for up to 30 min with a supramaximal ACh concentration (10 µM) does not cause any reduction in the ability of Mag-fluo 4 to diffuse rapidly through the ER lumen. Only when the cytosol was flooded with Ca2+ in an unphysiological manner (ionomycin and high Ca2+ for >10 min) could we detect signs of functional ER fragmentation (Figure 6). During physiologically relevant Ca2+ signalling events there was no sign of ER fragmentation and the ER remained an efficiently connected ER store.
The major Ca2+ store that can be mobilized by agonist stimulation is the rough ER (Kern, 1993; Gorelick and Jamieson, 1994) in the basolateral region (Figures 4 and 5). The connection with apical ER extensions allows efficient cytosolic Ca2+ signalling in the strategically important apical region, without depleting any ER element of Ca2+. This may be important, since a high Ca2+ concentration inside the ER is necessary for the retention of proteins (Wileman et al., 1991). The nuclear envelope contains a mobilizable Ca2+ store (Gerasimenko et al., 1995; Stehno-Bittel et al., 1995) and is lumenally connected to the ER (Petersen et al., 1998). Ca2+ depletion of the nuclear envelope store is associated with changes in the morphology and permeability of the nuclear pore complexes (Perez-Terzic et al., 1996), but the functional connection of the whole of the ER Ca2+ store complex makes it unlikely that the nuclear envelope store could be depleted under physiological conditions.
The short-lasting cytosolic Ca2+ spikes, elicited by low (and probably physiological) levels of agonist, are due to release of Ca2+ from ER elements in the apical pole (Kasai et al., 1993; Thorn et al., 1993). Since the total ER volume in the apical pole is small (Kern, 1993; Gorelick and Jamieson, 1994), the total amount of Ca2+ held in this part of the store must also be small. The functional connection of the whole of the ER is undoubtedly essential for the repetitive Ca2+ spiking in the apical pole. As seen in Figure 3, the Ca2+ concentration in the ER lumen equilibrates over distances of >5 µm within <7 s after a local perturbation. The highest frequency of ACh-induced cytosolic Ca2+ spiking is about one spike every 10 s (Figure 7C). Hence, Ca2+ movements within the ER are rapid enough to ensure that Ca2+ availability in the apical ER lumen is preserved at all stages during oscillations. Any tendency towards Ca2+ depletion of the small ER terminals would be countered by rapid diffusion from the main bulk of the store in the basal region.
Our data also have relevance for the quantal Ca2+ liberation phenomenon: a low IP3 concentration causes only partial Ca2+ release from the ER and further Ca2+ liberation requires an increase in the messenger concentration (Muallem et al., 1989; Bootman et al., 1992). Two models have been proposed to account for this phenomenon: (i) all-or-none release from stores exhibiting heterogeneous sensitivities (Muallem et al., 1989; Bootman et al., 1992) and (ii) phasic or adaptive release from homogeneously sensitive stores (Irvine, 1990; Nunn and Taylor, 1992). Our data, demonstrating directly efficient connectivity of the whole of the ER lumen with regard to Ca2+ movements, seem incompatible with the first hypothesis. A recent study in which subcellular Ca2+ puffs evoked by IP3 were imaged in Xenopus oocytes, showed that individual sites responded repeatedly to successive increments of the IP3 concentration. It was shown that the amplitude of a second puff was little diminished even when it arose within a few hundred milliseconds of a preceding event, indicating that the local Ca2+ concentration inside the ER was not appreciably reduced (Callamaras and Parker, 2000). This could be explained by Ca2+ replenishment of local ER regions, from which release had occurred, by rapid diffusion from other parts of the ER.
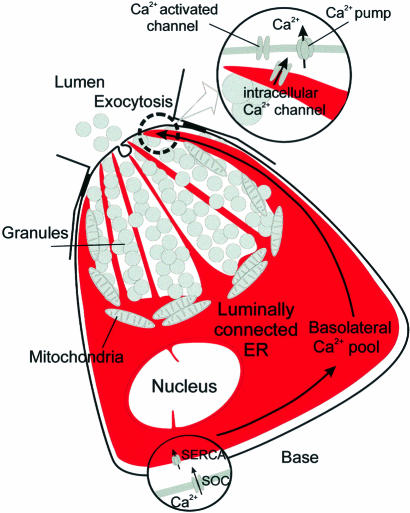
With regard to the overall pancreatic acinar cell Ca2+ homeostasis, we must differentiate between two entirely different situations. During supramaximal agonist stimulation (Figures 4 and 5), there is substantial Ca2+ depletion of the ER. In this situation there is transcellular Ca2+ transport (Figure 8). Ca2+ is taken up through store-operated Ca2+ channels (Parekh and Penner, 1997) in the basal membrane and is pumped into the basal ER by Ca2+ ATPases (Mogami et al., 1997, 1998). Ca2+ then diffuses through the lumen of the ER from the base to the apical region, where it is released into the cytosol via specific Ca2+ channels (IP3 and ryanodine receptors; Cancela et al., 2000). The route through the lumen of the ER is attractive because of the substantial buffering power of the mitochondria, which impedes Ca2+ flow through the cytosol (Tinel et al., 1999). Finally Ca2+ is pumped into the acinar lumen by plasma membrane Ca2+ ATPases concentrated in the apical membrane (Belan et al., 1996).
Fig. 8. Simplified schematic model illustrating that the whole of the basal ER is functionally connected and also that this compartment is connected to thin extensions deep into the apical granular pole. The bulk of the intracellular Ca2+ pool is located in the basal part of the cell. During agonist stimulation Ca2+ is released primarily from the thin ER extensions in the apical granular pole, where the Ca2+ release channels are located, but the apical ER elements can recruit Ca2+ from the basal store. SERCA, sarco-endoplasmic reticulum Ca2+-activated ATPase. SOC, store-operated Ca2+ channel.
In contrast, during submaximal agonist stimulation there is little ER Ca2+ depletion (Figure 7). Ca2+ spiking can continue for some time in the complete absence of Ca2+ entry from the extracellular solution. Some Ca2+ must be pumped out of the cell via the plasma membrane Ca2+ ATPases, but Ca2+ released during the spikes must also be temporarily stored in the mitochondria, helping to ensure that the Ca2+ spikes remain confined to the apical granular area (Tinel et al., 1999). In this signalling mode there can be re-uptake of the small amounts of Ca2+ lost from the apical ER terminals after each spike (Figure 7B) via Ca2+ ATPases in the ER (Petersen et al., 1993).
Materials and methods
Cell preparation
Fresh mouse pancreatic acinar cells were isolated after collagenase treatment, as described previously (Osipchuk et al., 1990) and used within 4 h. All experiments were carried out at room temperature (22–24°C). Mag-fluo 4, Mag-fura 2 and NP-EGTA were purchased from Molecular Probes and the other chemicals from Sigma Co.
Solutions
The extracellular bathing solution contained (concentration in mM): NaCl 140, KCl 4.7, MgCl2 1.13, CaCl2 1, glucose 10 and HEPES 10. pH was adjusted to 7.3 by NaOH. We used two different intracellular pipette solutions. One contained (concentration in mM): KCl 135, MgCl2 1.13, NaCl 20, HEPES 10, Na2ATP 2, EGTA 0.1. The pH was 7.2, adjusted by KOH. In the other solution we replaced 0.1 mM EGTA with 10 mM BAPTA + 2 mM Ca2+.
Imaging of Ca2+ in intracellular stores
Isolated pancreatic acinar cells were incubated with 4–6 µM Mag-fluo 4-AM and 0.01% pluronic acid for 20–30 min, or Mag-fura 2-AM and 0.01% pluronic acid for 20–25 min at 37°C. NP-EGTA (caged calcium) was loaded into pancreatic acinar cells by incubation in a solution containing 20.5 µM NP-EGTA/AM for 30 min. To remove indicators and caged probe from the cytosol we used the patch–clamp whole-cell configuration (Mogami et al., 1998). The same pipette was used to introduce Ca2+ buffer into the cell. The patch pipette contained 0.1 mM EGTA or a 10 mM BAPTA/2 mM Ca2+ mixture. Some experiments with Mag-fluo 4 were carried out on intact cells, without unloading the cytosolic Ca2+ indicator. For the image analysis, we used Zeiss confocal 510 image software as well as software developed by ourselves.
Photobleaching and uncaging experiments
We used near maximal power of UV laser (lines 351 and 364 nm, 80 mW) to induce fast bleaching of Mag-fluo 4, Mag-fura 2 or uncaging of NP-EGTA, mostly localized to small regions of the cells. Uncaging and photobleaching were combined with confocal imaging. The 488 nm laser line was used for measurements involving Mag-fluo 4 (emission filter: 505–550 or >505). The 351 nm laser line was used to excite and image the Mag-fura 2 distribution (emission filter: 505–550 or >505).
Electrophysiology
Standard patch–clamp whole-cell current recording (Hamill et al., 1981) was used. The electrophysiological recording of Ca2+-dependent current (Petersen, 1992) was made using the EPC-8 amplifier and Pulse software (HEKA). The pipette resistance was 2–3 MΩ. The detailed procedure has been described previously (Wakui et al., 1989; Thorn and Petersen, 1992).
Acknowledgments
Acknowledgements
We thank Nina Burdakova for technical assistance. This work was supported by a Medical Research Council (MRC) Programme Grant. O.H.P. is an MRC Research Professor.
References
- Belan P.V., Gerasimenko,O.V., Tepikin,A.V. and Petersen,O.H. (1996) Localization of Ca2+ extrusion sites in pancreatic acinar cells. J. Biol. Chem., 271, 7615–7619. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. (1993) Inositol trisphosphate and calcium signalling. Nature, 361, 315–325. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. and Irvine,R.F. (1989) Inositol phosphates and cell signalling. Nature, 341, 197–205. [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Berridge,M.J. and Taylor,C.W. (1992) All-or-nothing Ca2+ mobilization from the intracellular stores of histamine-stimulated HeLa cells. J. Physiol., 450, 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callamaras N. and Parker,I. (2000) Phasic characteristic of elementary Ca2+ release sites underlies quantal responses to IP3. EMBO J., 19, 3608–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela J.M., Gerasimenko,O.V., Gerasimenko,J.V., Tepikin,A.V. and Petersen,O.H. (2000) Two different but converging messenger pathways to intracellular Ca2+ release: the roles of nicotinic acid adenine dinucleotide phosphate, cyclic ADP-ribose and inositol trisphosphate. EMBO J., 19, 2549–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayel M.J., Horn,E.F.Y. and Verkman,A.S. (1999) Diffusion of green fluorescent protein in the aqueous-phase lumen of endoplasmic reticulum. Biophys. J., 76, 2843–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko O.V., Gerasimenko,J.V., Tepikin,A.V. and Petersen,O.H. (1995) ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell, 80, 439–444. [DOI] [PubMed] [Google Scholar]
- Golovina V.A. and Blaustein,M.P. (1997) Spatially and functionally distinct Ca2+ stores in sarcoplasmic and endoplasmic reticulum. Science, 275, 1643–1648. [DOI] [PubMed] [Google Scholar]
- Gorelick F.S. and Jamieson,J.D. (1994) The pancreatic acinar cells: structure–function relationships. In Johnson,L.R. (ed.), Physiology of the Gastrointestinal Tract. 3rd edn. Raven Press, New York, pp. 1353–1376. [Google Scholar]
- Hamill O.P., Marty,A., Neher,E., Sakmann,B. and Sigworth,F.J. (1981) Improved patch–clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch., 391, 85–100. [DOI] [PubMed] [Google Scholar]
- Hofer A.M. and Machen,T.E. (1993) Technique for in situ measurement of calcium in intracellular inositol 1,4,5-trisphosphate-sensitive stores using the fluorescent indicator mag-fura-2. Proc. Natl Acad. Sci. USA, 90, 2598–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer A.M. and Schulz,I. (1996) Quantification of intraluminal free [Ca2+] in the agonist-sensitive internal calcium store using compartmentalized fluorescent indicators: some considerations. Cell Calcium, 20, 235–242. [DOI] [PubMed] [Google Scholar]
- Irvine R.F. (1990) ‘Quantal’ Ca2+ release and the control of Ca2+ entry by inositol phosphates—a possible mechanism. FEBS Lett., 263, 5–9. [DOI] [PubMed] [Google Scholar]
- Ito K., Miyashita,Y. and Kasai,H. (1997) Micromolar and submicromolar Ca2+ spikes regulating distinct cellular functions in pancreatic acinar cells. EMBO J., 16, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Li,Y.X. and Miyashita,Y. (1993) Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell, 74, 669–677. [DOI] [PubMed] [Google Scholar]
- Kern H.F. (1993) Fine structure of the human exocrine pancreas. In Go,V.L.W. (ed.), The Pancreas: Biology, Pathobiology and Disease. 2nd edn. Raven Press, New York, NY, pp. 9–19. [Google Scholar]
- Mogami H., Nakano,K., Tepikin,A.V. and Petersen,O.H. (1997) Ca2+ flow via tunnels in polarized cells: recharging of apical Ca2+ stores by focal Ca 2+ entry through basal membrane patch. Cell, 88, 49–55. [DOI] [PubMed] [Google Scholar]
- Mogami H., Tepikin,A.V. and Petersen,O.H. (1998) Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J., 17, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M., Alvarez,J., Scheenen,W.J.J., Rizzuto,R., Meldolesi,J. and Pozzan,T. (1997a) Ca2+ homeostasis in the endoplasmic reticulum: coexistence of high and low [Ca2+] subcompartments in intact HeLa cells. J. Cell Biol., 139, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M., Barrero,M.J. and Alvarez,J. (1997b) [Ca2+] microdomains control agonist-induced Ca2+ release in intact HeLa cells. FASEB J., 11, 881–885. [DOI] [PubMed] [Google Scholar]
- Muallem S., Pandol,S.J. and Beeker,T.G. (1989) Hormone evoked calcium release from intracellular stores is a quantal process. J. Biol. Chem., 264, 205–212. [PubMed] [Google Scholar]
- Nunn D.L. and Taylor,C.W. (1992) Luminal Ca2+ increases the sensitivity of Ca2+ stores to inositol 1,4,5-trisphosphate. Mol. Pharmacol., 41, 115–119. [PubMed] [Google Scholar]
- Osipchuk Y.V., Wakui,M., Yule,D.I., Gallacher,D.V. and Petersen,O.H. (1990) Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl– current recording in single pancreatic acinar cells. EMBO J., 9, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. and Penner,R. (1997) Store depletion and calcium influx. Physiol. Rev., 77, 901–930. [DOI] [PubMed] [Google Scholar]
- Park M.K., Tepikin,A.V. and Petersen,O.H. (1999) The relationship between acetylcholine-evoked Ca2+-dependent current and the Ca2+ concentrations in the cytosol and the lumen of the endoplasmic reticulum in pancreatic acinar cells. Pflugers Arch., 438, 760–765. [DOI] [PubMed] [Google Scholar]
- Perez-Terzic C., Pyle,J., Jaconi,M., Stehno-Bittel,L. and Clapham,D.E. (1996) Conformational states of the nuclear pore complex induced by depletion of nuclear Ca2+ stores. Science, 273, 1875–1877. [DOI] [PubMed] [Google Scholar]
- Petersen C.C.H., Petersen,O.H. and Berridge,M.J. (1993) The role of endoplasmic reticulum calcium pumps during cytosolic calcium spiking in pancreatic acinar cells. J. Biol. Chem., 268, 22262–22264. [PubMed] [Google Scholar]
- Petersen O.H. (1992) Stimulus-secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J. Physiol., 448, 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O.H., Petersen,C.C.H. and Kasai,H. (1994) Calcium and hormone action. Annu. Rev. Physiol., 56, 297–319. [DOI] [PubMed] [Google Scholar]
- Petersen O.H., Gerasimenko,O.V., Gerasimenko,J.V., Mogami,H. and Tepikin,A.V. (1998) The calcium store in the nuclear envelope. Cell Calcium, 23, 87–90. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto,R., Volpe,P. and Meldolesi,J. (1994) Molecular and cellular physiology of intracellular Ca2+ stores. Physiol. Rev., 74, 595–636. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Perez-Terzic,C. and Clapham,D.E. (1995) Diffusion across the nuclear envelope inhibited by depletion of the nuclear Ca2+ store. Science, 270, 1835–1838. [DOI] [PubMed] [Google Scholar]
- Subramanian K. and Meyer,T. (1997) Calcium-induced restructuring of nuclear envelope and endoplasmic reticulum calcium stores. Cell, 89, 963–971. [DOI] [PubMed] [Google Scholar]
- Terasaki M., Slater,N.T., Fein,A., Schmidek,A. and Reese,T.S. (1994) Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc. Natl Acad. Sci. USA, 91, 7510–7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P. and Petersen,O.H. (1992) Activation of nonselective cation channels by physiological cholecystokinin concentrations in mouse pancreatic acinar cells. J. Gen. Physiol., 100, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P., Lawrie,A.M., Smith,P.M., Gallacher,D.V. and Petersen,O.H. (1993) Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell, 74, 661–668. [DOI] [PubMed] [Google Scholar]
- Tinel H., Cancela,J.M., Mogami,H., Gerasimenko,J.V., Gerasimenko,O.V., Tepikin,A.V. and Petersen,O.H. (1999) Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J., 18, 4999–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Podini,P., Clegg,D.O., Pozzan,T. and Meldolesi,J. (1991) Intracellular Ca2+ stores in chicken Purkinje neurons—differential distribution of the low affinity-high capacity Ca2+ binding-protein, calsequestrin, of Ca2+ ATPase and of the ER lumenal protein, BIP. J. Cell Biol., 113, 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakui M., Potter,B.V.L. and Petersen,O.H. (1989) Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature, 339, 317–320. [DOI] [PubMed] [Google Scholar]
- Wileman T., Kane,L.P., Carson,G.R. and Terhorst,C. (1991) Depletion of cellular calcium accelerates protein degradation in the endoplasmic reticulum. J. Biol. Chem., 266, 4500–4507. [PubMed] [Google Scholar]