Abstract
The CCAAT boxes of the β-like globin genes interact with three proteins: NF-Y, GATA-1 and NFE-6. We demonstrate that NFE-6 contains C/EBPγ, and address its role in globin gene regulation by erythroid overexpression of C/EBPγ, and a dominant-negative form C/EBPγΔB, in mice. Elevated levels of C/EBPγ, but not C/EBPγΔB, increase expression of the (fetal) γ-globin relative to the (adult) β-globin gene. Interestingly, fetal liver erythropoiesis is ablated when the C/EBPγ and C/EBPγΔB levels are further increased in homozygous transgenics. We suggest that targeted expression of dominant-negative leucine zipper proteins is a generally applicable approach to ablate specific tissues in mice.
Keywords: C/EBPγ/dominant-negative proteins/erythropoiesis/globin gene switching/locus control region
Introduction
Many mRNA coding genes contain functionally important CCAAT boxes in their promoter (Mantovani, 1998). Our interest in this sequence motif stems from the study of the human β-globin gene cluster. CCAAT consensus sequences are present in the minimal promoters of the human β-like globin genes (deBoer et al., 1988; Liberati et al., 1998) between a CACC box and a TATA box motif, all of which are necessary for efficient transcription (deBoer et al., 1988; Antoniou et al., 1995). The relevance of the TATA box and the CACC box has been deduced from patients with mutations in these motifs (Thein, 1993). In addition, genetic ablation studies in mice have shown that EKLF, a factor binding to the CACC box, is absolutely required for β-globin expression (Wijgerde et al., 1996).
The β-globin CCAAT box binds three major complexes in nuclear extracts from murine erythroleukaemia (MEL) cells, a tissue-culture model for adult erythropoiesis. These complexes have been designated NF-Y (previously called CP1), GATA-1 and NFE-6 (Berry et al., 1992). NFE-6 displays a similar mobility on the β-CCAAT box to a factor denoted DSFr (Delvoye et al., 1993), which was shown through expression cloning and antibody studies to contain C/EBPγ (Wall et al., 1996). C/EBPγ is a member of the CCAAT/enhancer binding protein (C/EBP) family of transcription factors, a class of bZIP proteins characterized by a basic domain followed by a leucine zipper. The leucine zipper serves as a dimerization motif that is necessary for DNA binding by the basic domain. Each member can form homodimers as well as heterodimers with C/EBP family members. In addition, they can form dimers with several other leucine zipper proteins such as jun and fos, raising the possibility that C/EBP proteins exert their biological function by switching partners during differentiation. The C/EBP family consists of six members, designated C/EBPα to C/EBPζ. The genes encoding C/EBPα, β, γ, δ and ζ have been disrupted by homologous recombination, thus elucidating some of the functions of their gene products. C/EBPα is necessary for the establishment and maintenance of energy homeostasis in neonates (Wang et al., 1995). C/EBPζ is involved in the induction of apopotosis under conditions associated with impaired function of the endoplasmic reticulum (Zinszner et al., 1998). C/EBPα and C/EBPδ have a synergistic role in terminal adipocyte differentiation (Tanaka et al., 1997). C/EBPβ has a critical role in ovarian follicle development (Pall et al., 1997; Sterneck et al., 1997) and in the immune system (Screpanti et al., 1995; Tanaka et al., 1997). C/EBPγ null mice show a high mortality rate within 48 h after birth. The analysis of lymphoid cells of these mice has demonstrated a critical role for C/EBPγ in the functional maturation of NK cells (Kaisho et al., 1999).
C/EBP proteins are expressed in the fetal liver at the onset of definitive haematopoiesis. Therefore, these proteins are likely to have a role in the differentiation and lineage commitment of the developing haematopoietic system (Scott et al., 1992; Zhang et al., 1997; Nerlov et al., 1998).
In this study, we have explored the relationship between NFE-6 and C/EBPγ, and we have addressed the role of bZIP proteins in globin gene expression and erythropoiesis. C/EBPγ was first cloned from B cells as a factor that binds to the Ig heavy chain promoter and enhancer. Unlike other C/EBP proteins, C/EBPγ is ubiquitously expressed (Roman et al., 1990). The cDNA predicts a small poly peptide of 16.4 kDa lacking the consensus N-terminus activation domain experimentally defined in C/EBPα (Friedman et al., 1989) and C/EBPβ. Thus, C/EBPγ might function as LIP, a truncated version of C/EBPβ that operates as a modulator of a series of activators (Descombes and Schibler, 1991; Cooper et al., 1995).
The CCAAT boxes of the β-like globin genes all conform to the C/EBP consensus, which is dyad symmetric, consisting of abutted half-sites bearing the CCAAT sequence (Osada et al., 1996). A role for the CCAAT boxes in globin gene expression is best demonstrated by mutations in the duplicated CCAAT box of the Aγ-globin (Berry et al., 1992; Ronchi et al., 1996). Such mutations can result in the extemporary expression of fetal γ-globin in adult life, a condition known as hereditary persistence of fetal haemoglobin (HPFH) and hence hint that factors binding to the CCAAT boxes have a role in γ-globin silencing. To investigate the potential function of C/EBPγ in globin gene expression and erythroid differentiation, we have generated transgenic mice expressing C/EBPγ under the control of β-globin regulatory sequences. We also produced mice expressing a dominant-negative version of C/EBPγ that lacks the DNA binding domain but retains the leucine zipper (C/EBPγΔB). Such a protein interferes with DNA binding and activity of bZIP transcription factors (e.g. Moitra et al., 1998). We found that moderate overexpression of C/EBPγ, but not C/EBPγΔB, results in increased γ-globin gene expression in transgenic mice carrying the human β-globin locus. Interestingly, higher overexpression of both proteins causes a block in definitive erythropoiesis leading to a severe anaemia and fetal lethality.
Results
C/EBPγ is an integral part of NFE-6
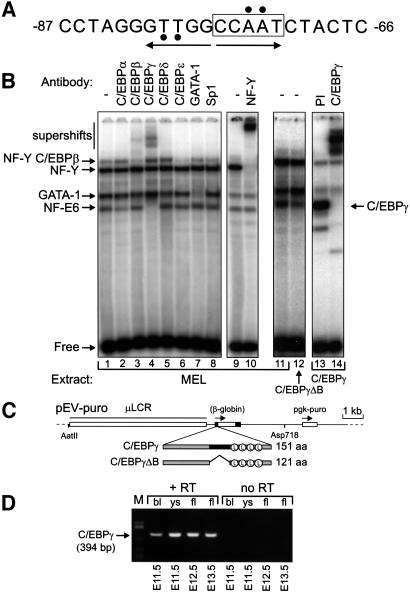
The CCAAT box of the human β-globin gene spans from position –62 to –87 of the start site (Figure 1A). At least three activities bind these sequences in MEL cell extracts. Two of these have been identified as NF-Y and GATA-1; the third was called NFE-6 (Berry et al., 1992). A complex migrating with similar mobility to NFE-6 was shown by another group to contain C/EBPγ (Wall et al., 1996). To characterize NFE-6 further we analysed its contact points on the human β-globin CCAAT box by depurination analysis (Wall et al., 1988). The contact points of NFE-6 (Figure 1A) are in agreement with the consensus binding site of C/EBP proteins (Osada et al., 1996). We therefore determined which members of this family are contained in NFE-6. For this purpose, we performed bandshifts on the β-globin CCAAT box with antibodies raised against C/EBP family members. NFE-6 is supershifted by antibodies against C/EBPγ (Figure 1B, lane 4) and not by any of the other sera used. In addition, the reduced intensity of the slowest migrating complex in lane 3 correlates with the C/EBPβ supershift. The NF-Y antibodies also remove this shift (Figure 1B, lane 10) and we conclude that this activity is composed of both NF-Y and C/EBPβ, in agreement with Wall et al. (1996).
Fig. 1. Proteins interacting with the CCAAT box of the human β-globin gene. (A) Sequence of the CCAAT box with the contact sites of NFE-6 indicated by black circles. The imperfect dyad symmetric C/EBP binding site is shown by arrows. (B) Bandshift analysis of proteins binding to the β-globin CCAAT box. An oligonucleotide probe covering the CCAAT box was incubated with 4 µg of MEL cell nuclear extract plus the indicated antibodies; PI, pre-immune serum. Lanes 12–14 contain extracts from MEL cells overexpressing the indicated EBP proteins. (C) Constructs used to express C/EBPγ and C/EBPγΔB. Restriction sites used to isolate the microinjection fragments are indicated. (D) RT–PCR analysis of C/EBPγ expression in embryonic and fetal haematopoietic tissues. Two micrograms of RNA from the indicated tissues (bl, blood; ys, yolk sac, fl, fetal liver) were used for RT–PCR (30 cycles). No RT, reverse transcriptase was omitted to exclude amplification of contaminating DNA.
To confirm further that NFE-6 contains C/EBPγ, we cloned the cDNA by RT–PCR and expressed two versions of C/EBPγ under the control of the β-globin promoter and locus control region (LCR) in MEL cells (Needham et al., 1992). The first construct, pEV-C/EBPγ, contains the 0.54 kb open reading frame of C/EBPγ. The second construct, pEV-C/EBPγΔB, is a dominant-negative version of the former, in which we deleted the DNA sequences coding for amino acids Met58–Glu89. This removes the basic DNA binding domain but leaves the leucine zipper dimerization domain intact (Figure 1C). We then analysed nuclear extracts of transfected populations in bandshifts with the human β-globin CCAAT box oligonucleotide. This shows that C/EBPγΔB causes a reduction in the intensity of the NFE-6 band (Figure 1B, lanes 11 and 12). Furthermore, C/EBPγ protein displays the same mobility as NFE-6 (Figure 1B, lanes 13 and 14). These data are in agreement with the notion that C/EBPγ is an integral part of NFE-6. Although it appears that NFE-6 is a homodimer of C/EBPγ, our results do not formally exclude the possibility that NFE-6 is a heterodimer of C/EBPγ and another polypeptide.
Overexpression of C/EBPγ in transgenic mice
It has been suggested that C/EBPγ could be involved in the regulation of the β-globin gene in MEL cells (Wall et al., 1996). However, we did not observe a change in the expression of the mouse adult globin genes upon MEL cell differentiation when C/EBPγ or C/EBPγΔB were overexpressed (data not shown). The outcome of this experiment suggests either that C/EBPγ may not be directly involved in the transcription of the globin genes or that the role of this factor is important at a stage during the differentiation of erythroid cells other than that represented by MEL cells. C/EBPγ is ubiquitously expressed in adult tissues and in most tissues of fetuses at 14 days of gestation (E14) (Cooper et al., 1995). Because we are interested in the function of C/EBPγ in erythropoiesis, we extended these studies to the erythroid organs of E11.5–E13.5 mouse fetuses through an RT–PCR analysis of C/EBPγ expression. C/EBPγ mRNA is detected in E11.5 yolk sac and E12.5/E13.5 fetal liver (Figure 1D) demonstrating that C/EBPγ is already expressed before the onset of adult erythropoiesis. Thus, C/EBPγ may have a role in erythroid cells of embryonic and fetal origin, and in globin gene switching. To investigate this, we generated transgenic mice with the pEV-C/EBPγ and pEV- C/EBPγΔB constructs (Figure 1C). These expression vectors are active in embryonic, fetal and adult erythropoiesis. Several founders were generated and bred to obtain germline transmission (Table I). Transgenic F1 pups reached adulthood with no obvious phenotype. Collectively, these mice will be referred to as EBP transgenics in this paper.
Table I. EBP transgenic mice generated for this study.
| Founder | Copy number | Remarks | γ-globin expression |
|---|---|---|---|
| PEV-C/EBPγ | |||
| 7 | (10) | no transmission | n.a. |
| 8a | 6 | homozygous alive | γ up in het. and hom. |
| 13 | 4 | transmission | n.d. |
| 14 | (42) | no transmission | n.a. |
| 19 | 26 | homozygous lethal | γ up in het. |
| 21a | 20 | homozygous lethal | γ up in het. |
| 22 | (22) | no transmission | n.a. |
| 28 | 12 | homozygous alive | n.d. |
| pEV-C/EBPγΔB | |||
| 1a | 22 | homozygous lethal | γ normal in het. |
| 2 | (16) | no transmission | n.a. |
| 10 | (20) | no transmission | n.a. |
| 12 | 6 | homozygous alive | γ normal in het. and hom. |
| 19 | 5 | homozygous alive | γ normal in het. and hom. |
| 21 | (20) | no transmission | n.a. |
| 22a | 40 | heterozygous lethal | n.d. |
| 14 | (< 1) | no transmission | n.a. |
n.a., not applicable; n.d., not determined; het., heterozygous; hom., homozygous for the EBP transgene. Expression of γ-globin refers to γ mRNA levels in E12.5 fetal liver in crosses with mice carrying the human β-globin locus (line 72; Strouboulis et al., 1992). There was no significant difference in the upregulation of γ-globin between the different C/EBPγ transgenics.
aData obtained with these lines are shown in the figures.
Expression of C/EBPγ, but not C/EBPγΔB, results in upregulation of γ-globin expression
The EBP transgenics were bred with mice harbouring the 70 kb human β-globin locus (line 72; Strouboulis et al., 1992), in order to analyse the effects of transgene-derived C/EBPγ and C/EBPγΔB on the regulation of the human β-like globin genes. This is particularly useful to study the switch from fetal (γ-) to adult (β-) globin expression, which is absent in the endogenous mouse β-globin locus.
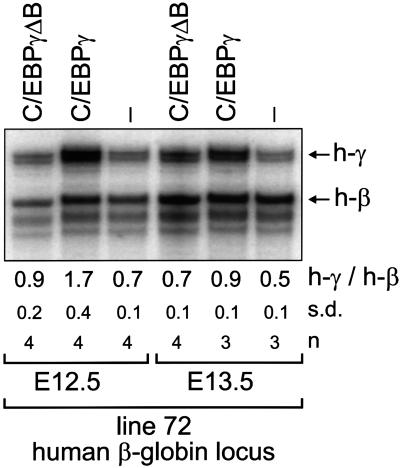
Fetal liver globin mRNA levels of compound transgenic fetuses dissected at E12.5 and E13.5 were assayed by S1 nuclease protection (Figure 2). For each transgene combination, several fetuses from independent litters were analysed. The RNA samples were also assayed for expression of the murine βH1 and εy genes to assess the contribution of primitive erythrocytes derived from the circulation; this was found to be minimal and hence the γ-globin mRNA measured is derived from fetal liver cells (data not shown).
Fig. 2. Overexpression of C/EBPγ affects globin gene switching. Mice harbouring the 70 kb human β-globin locus (line 72) were crossed with C/EBPγΔB line 1 and C/EBPγ line 21, heterozygous for the EBP transgene. RNA was isolated from E12.5 and E13.5 livers and 1 µg was used for quantitative S1 nuclease analysis of human globin gene expression (Wijgerde et al., 1996). Arrows indicate human γ-globin (h-γ) and β-globin (h-β) signals. The average of the h-γ/h-β signals with standard deviations (s.d.) and the number of fetuses analysed (n) are shown below the lanes.
Interestingly, the γ-globin gene is upregulated in the presence of the pEV-C/EBPγ transgene, but not in the presence of the pEV-C/EBPγΔB transgene (Figure 2). This is particularly evident in the E12.5 samples when we observe a 2- to 3-fold higher γ to β ratio in the presence of the pEV-C/EBPγ transgene (Figure 2). There was no significant difference in the upregulation of γ-globin expression between the different C/EBPγ transgenic lines (Table I). In adult animals, the EBP transgenes do not activate γ-globin expression, and these animals have normal numbers of erythrocytes, lymphocytes, granulocytes and platelets in their circulation (data not shown).
We conclude that overexpression of C/EBPγ in the fetal liver results in increased γ-globin expression. This prolongation is dependent on the presence of the basic DNA-binding domain in the C/EBPγ protein, suggesting that C/EBPγ acts preferentially at the γ-globin CCAAT boxes. These results are in contrast with data obtained from transfection experiments that suggested a function for C/EBPγ in transcriptional enhancement through the β-globin CCAAT box (Wall et al., 1996).
High-level expression of the EBP transgenes blocks fetal liver erythropoiesis
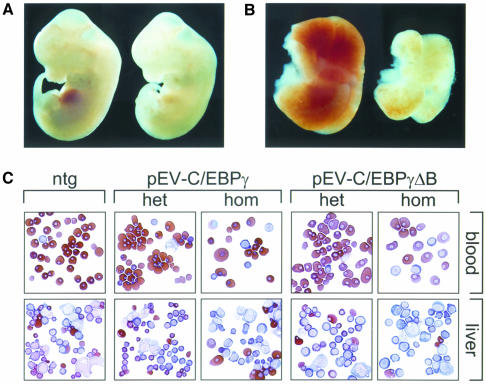
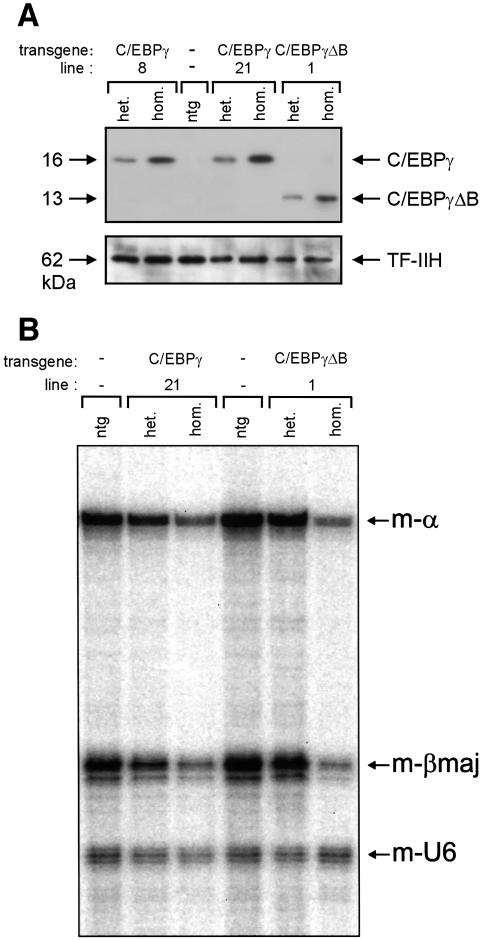
Animals heterozygous for the EBP transgenes are viable and show no morphological abnormalities in blood and fetal liver (see Figure 3). However, one of the pEV- C/EBPγΔB founder males gave rise to small litters and the pups analysed were non-transgenic. We extended the analysis of this line to E11.5–E15.5 fetuses and we found normal-sized litters up to E13.5 and re-absorbed fetuses from E14.5 onwards. At E13.5, 30% of the fetuses were severely anaemic. The affected fetuses all carried the transgene and had otherwise developed normally (Figure 3A). The fetal livers of these animals were much smaller and paler than those of non-transgenic littermates (Figure 3B). Since this phenotype could be due to high levels of transgene-derived protein, it might be reproduced in other EBP transgenic lines if the transgene could be expressed at sufficiently high levels. We therefore raised the expression levels by breeding the EBP lines to homozygosity. Western blot analysis of fetal liver nuclear extracts shows that the levels of transgenic EBP proteins are raised in homozygous transgenics, that the expression levels correlate with the transgene copy number, and that the C/EBPγ wild-type and ΔB proteins are expressed at comparable levels (Figure 4A). We found that homozygous fetuses of the high copy number lines were anaemic and died at ∼E13–E14, confirming that the phenotype observed is due to the overexpression of the EBP proteins, and not a secondary effect of transgene integration. Furthermore, there is a good correlation between transgene copy number and fetal lethality (Table I). Surprisingly, high levels of both C/EBPγ and C/EBPγΔB proteins cause anaemia and fetal lethality.
Fig. 3. Fatal anaemia caused by high level expression of the EBP transgenes. (A) Wild-type (left) and C/EBPγΔB-overexpressing line 22 (right) E12.5 littermates. Note the pallor of the transgenic fetus, which has an otherwise normal gross morphological appearance. (B) Fetal livers dissected from the fetuses shown in (A). The transgenic liver (right) is small and pale relative to the control liver (left), indicative of hampered erythropoiesis. (C) Cytospins of peripheral blood and fetal liver single cell suspensions prepared from fetuses with the indicated genotype. ntg, non-transgenic; het, heterozygous; hom, homozygous. Data shown are obtained with C/EBPγΔB line 1 and C/EBPγ line 21. The slides were stained with a combined histological and neutral benzidine stain.
Fig. 4. High levels of the EBP proteins result in reduced expression of the α- and β-globin genes. (A) Ten micrograms of E12.5 fetal liver nuclear extract were used for western blot analysis of EBP protein expression. Results are shown for non-transgenic (ntg), heterozygous (het.) and homozygous (hom.) EBP transgenics of the lines indicated. The polyclonal rabbit antiserum used was raised against the N-terminal part of C/EBPγ that is shared by the C/EBPγ and C/EBPγΔβ proteins. Note that the levels of endogenous C/EBPγ, although present in the RT–PCR analysis (Figure 1D), are too low for detection with this antiserum. A mouse monoclonal antibody recognizing the p62 subunit of TF-IIH was used as a loading control. (B) E12.5 livers were dissected from non-transgenic (ntg), heterozygous (het.) and homozygous (hom.) EBP transgenics of the lines indicated and used for RNA isolation. Approximately 1 µg of total RNA was used for quantitative S1 nuclease analysis of mouse α- (m-α) and βmajor-globin gene expression (m-βmaj); a probe detecting mouse U6 RNA (m-U6) was used as an internal loading control (Wijgerde et al., 1996).
The paleness of the fetal livers prompted us to determine by S1 nuclease analysis whether overexpression of the EBP proteins interfered with globin gene expression. We observe a general reduction in the levels of the mouse (Figure 4B) and human (data not shown) globin genes if the fetuses are homozygous for the high copy number EBP transgenes. The ratio between the α- and β-like genes is comparable to the controls, demonstrating that the block in erythroid differentiation is accompanied by reduced expression of the globin genes from both loci (Figure 4B). Thus, we conclude that the anaemia is not due to chain imbalance caused by a defect in either α- or β-globin expression, but more likely by an early block in definitive erythropoiesis resulting in fewer cells transcribing the globin genes.
Cytological analysis of fetal livers and blood
Fetal livers of mice expressing high levels of EBP proteins are pale and small (Figure 3B). We therefore analysed the morphology and distribution of the cells in the fetal livers and peripheral blood of wild-type and EBP transgenic fetuses. Cytospins of blood and E12.5 livers were prepared and stained with histological dyes and neutral benzidine (Beug et al., 1982). The pattern found in E12.5 control livers is shown in Figure 3C (ntg). A predominance of small, benzidine-positive, polychromatic and orthochromatic erythroblasts is observed, followed by fewer basophilic erythroblasts and proerythroblasts. A similar pattern is observed in the livers of heterozygous fetuses (Figure 3C, het). In contrast, liver preparations from homozygous fetuses are composed mainly of benzidine-negative blast-like cells. The absence of small, benzidine-positive cells is immediately apparent (Figure 3C, hom). Thus, it appears that the transgenic fetal livers are hypocellular and pale because erythropoiesis is impeded at a stage preceding haemoglobinized polychromatic erythroblasts. The failure of liver erythropoiesis was further confirmed by in vitro progenitor assays with pEV-C/EBPγ line 21 and pEV-C/EBPγΔB line 1 fetal livers. E12.5 livers of homozygous fetuses formed virtually no CFU-Es (>15-fold reduction) and a drastically reduced number of BFU-Es (6- to 7-fold reduction). These results indicate that the block in erythropoiesis occurs at the level of relatively early precursor represented by the BFU-E colonies. Since the colony numbers are not significantly different between wild-type and heterozygous fetal livers, we conclude that there is a critical threshold level for the dominant-negative effect of the EBP proteins. Finally, the blood shows a large number of blue nucleated cells distinct from the primitive erythrocytes (compare Figure 3C, ntg and het, with Figure 3C, hom). These abnormal cells are presumably of fetal liver origin, and are probably prematurely released in circulation in response to the severe anaemia.
We conclude that fetal liver erythropoiesis is severely impaired in homozygous EBP transgenics. This defect in erythropoiesis is independent of the presence of the DNA-binding domain of C/EBPγ, suggesting that C/EBPγ might indeed act as a modulator of the activity of other bZIP proteins that are essential for the proper execution of the erythropoietic program.
Discussion
C/EBPγ and the developmental regulation of the β-globin gene cluster
The work described in this paper was initiated to study the role of the previously described CCAAT box binding protein NFE-6 in the regulation of the human β-globin gene cluster. Several naturally occurring mutations in the promoters of the globin genes denote the involvement of factors binding to these sequences for the proper temporal and topological expression of these genes. For example, mutations in the CACC box of the human β-globin gene or ablation of EKLF, an erythroid restricted factor that binds to this motif, result in impaired expression of the β-globin gene (Thein, 1993; Wijgerde et al., 1996). Intriguingly, no patients with mutations in the β-CCAAT box are known, suggesting that the β-CCAAT box is perhaps not important for β gene expression. The relevance of the globin CCAAT boxes in vivo is best demonstrated by naturally occurring mutations clustered around the distal CCAAT box of the fetal γ-globin gene that result in an HPFH phenotype (Poncz et al., 1988). Consequently it would appear that factors binding to these sequences are causative in the proper regulation of the fetal genes and have a role in the switch from γ- to β-globin expression. Reactivation of γ-globin expression in adults is clinically important because this would be beneficial to large numbers of β-thalassaemia and sickle cell anaemia patients.
We show in this paper that NFE-6 contains C/EBPγ, and that it is most likely the same factor as the DSFr activity described by Delvoye et al. (1993). It has been postulated that C/EBPγ could play a role in the developmental upregulation of the adult β-globin gene (Wall et al., 1996). However, our data in mice overexpressing C/EBPγ do not support this model, because we observe an increase, rather than a decrease, in γ-globin expression during the switching period. If C/EBPγ were a β-promoter-specific activator, γ-globin transcription should have decreased. For instance, a robust positive correlation between the levels of the β-promoter transcription factor EKLF and β gene expression in the fetal liver has been reported, and higher β levels are accompanied by lower γ levels (Wijgerde et al., 1996). Thus, our data strongly suggest that C/EBPγ is not an activator of the β-globin promoter. We can only speculate about the mechanism by which increased C/EBPγ levels favour γ-globin expression. One obvious possibility is that it exerts this effect directly through the γ-globin CCAAT boxes. Alternatively, it could affect the developmental progression of fetal liver erythropoiesis, resulting in prolonged maintenance of a transcription factor environment favouring γ-globin expression. In this respect, it is interesting to note that C/EBP proteins are thought to be part of a developmental transcription factor cascade in haematopoiesis (Sieweke and Graf, 1998).
After the fetal liver stage, the γ-globin genes are silenced normally and we do not observe an HPFH phenotype in our EBP transgenic lines. Furthermore, the γ-globin expression levels in adult transgenic mice with the –117 Greek HPFH mutation in the distal γ-globin CCAAT box (Berry et al., 1992) remain unchanged in the presence of the EBP transgenes (data not shown). This is consistent with the notion that C/EBPγ is not an activator of the β-globin promoter, because in that case a shift in favour of β expression should occur in the presence of elevated C/EBPγ levels. However, the molecular mechanism underlying Greek HPFH remains elusive since neither point mutagenesis of the distal CCAAT box (Ronchi et al., 1996) nor manipulation of one of the factors binding to it (this study) has provided clear-cut insight into the mode of action of the –117 mutation. It appears that either more than one factor is involved, or that the –117 mutation precipitates a secondary effect like localized chromatin remodelling.
High-level expression of the EBP transgenes blocks fetal erythropoiesis
We have found that overexpression of the EBP transgenes in MEL cells does not interfere with dimethyl sulfoxide-induced MEL cell differentiation. Since MEL cells represent the proerythroblast stage of erythroid development, this suggests that the differentiation programme beyond this stage can be efficiently executed in the presence of high levels of EBP proteins. In sharp contrast, the effects of such high levels on erythropoiesis in mice are dramatic. It results in a developmental block of early erythroid progenitors in the fetal liver. C/EBP family members have distinct pivotal roles in lineage commitment in the haematopoietic system (Cooper et al., 1994; Screpanti et al., 1995; Zhang et al., 1997; Nerlov et al., 1998; Kaisho et al., 1999). These proteins are known to interact with several other factors in large multiprotein complexes that regulate the tissue-specific expression of several haematopoietic-specific genes (reviewed in Sieweke and Graf, 1998). It has been proposed that C/EBPγ is a modulator of the activity of other bZIP proteins (Cooper et al., 1995). Our data are consistent with this proposal, since we observe defective erythropoiesis in mice with high levels of C/EBPγ and the dominant-negative version C/EBPγΔB. Presumably, provided they are expressed at sufficiently high levels, both EBP proteins interfere with activator functions of bZIP proteins required for fetal liver erythropoiesis.
Tissue-specific expression of dominant-negative leucine zipper proteins
Inactivation of genes by knockout strategies is a frequently chosen approach to address the function of gene products in whole organisms. In the case of C/EBP family members, much has been learnt about the specific roles of individual family members in the development and physiology of mice (LekstromHimes and Xanthopoulos, 1998). Functional redundancy and early lethality are potential problems of gene knockouts. To some extent, these issues can be tackled by intercrossing knockout lines and by using conditional knockout alleles. However, the number of combinations that can be made is limited for practical reasons. The dominant-negative transgenic approach does not suffer from these limitations. Expression of the transgene can be directed to specific tissues and, unlike toxin genes, leaky expression in other tissues should not adversely affect these tissues because low level expression is unlikely to interfere with the functioning of the cells. Furthermore, dimerization specificity of the leucine zipper can be modulated by in vitro mutagenesis (Vinson et al., 1993), and fusion with steroid receptor ligand binding domains would give an additional level of control over the spatio-temporal activity of the dominant-negative protein (Littlewood et al., 1995).
Dominant-negative leucine zippers have been applied successfully in gene expression and differentiation studies of tissue-culture cells (i.e. Olive et al., 1997; Nerlov et al., 1998), and this approach was used to study the function of adipose tissues in mice (Moitra et al., 1998). Expression of a dominant-negative leucine zipper-containing protein under the control of the adipose-specific aP2 enhancer/promoter resulted in the complete ablation of white adipose tissue with dramatic consequences for the physiology and behaviour of the transgenic mice. In our EBP transgenics we have inactivated the erythroid system, but development of the transgenic fetuses is otherwise normal. Thus, we suggest that targeted expression of dominant-negative leucine zipper proteins might be a generally applicable approach to ablate specific cell types and tissues in order to study their role in the development, physiology and behaviour of mice.
Materials and methods
Cloning of C/EBPγ and expression constructs
Total RNA from MEL cells was isolated as described (Antoniou, 1991) and 5 µg were reverse transcribed with the γ-r primer 5′-GGCCTGGAAGGAGATCTACT-3′. One-fifth of the first strand product was subjected to 30 cycles of PCR with the γ-f primer 5′-ACGTGCCCAAATGAGCAAGC-3′. The PCR product was cloned and sequenced. To delete the DNA-binding domain, the 3′ end of C/EBPγ was amplified with the C/EBPγ reverse primer and the γ-1 primer 5′-ATGCCATGGATACACTGCAA-3′. The product was digested with NcoI and BglII, and ligated to the C/EBPγ plasmid digested with NcoI and BglII. EcoRI–BglII inserts of C/EBPγ- and C/EBPγΔB-plasmids were ligated to pEV3-puromycinr (Needham et al., 1992) to generate pEV- C/EBPγ and pEV-C/EBPγΔB.
Cell culture and protein preparation
PEV-C/EBPγ and pEV-C/EBPγΔB were linearized with ScaI and transfected into MEL cells as described (Antoniou, 1991) with the exception that puromycin (1 µg/ml) was used for the selection. RNA and nuclear extracts were prepared as described (Wall et al., 1988; Antoniou, 1991).
Production of antibodies and gel mobility shift assays
A NcoI site was introduced at the first ATG of C/EBPγ by PCR. The following primers were used: 5′-CCATGGGCAAGCTGTCGCAGCCAG-3′ and the γ-r primer. The PCR product was cloned and sequenced. The plasmid was digested with NcoI and the insert coding for the unique N-terminus of C/EBPγ was cloned into the NcoI site of pET15 and used for the production of recombinant protein in Escherichia coli BL21 (DE3). Gel-purified recombinant protein was used to immunize rabbits.
Gel mobility shift assays were performed as described by Wall et al. (1988) using the human β-globin CCAAT box oligonucleotide as probe (deBoer et al., 1988). For supershifts, appropriately diluted antibodies were added to the reactions followed by a further 20 min incubation at room temperature prior to loading of the gel.
Generation of transgenic mice and DNA analysis
PEV-C/EBPγ and pEV-C/EBPγΔB were digested with AatII and Asp718. The fragments were isolated and used for microinjection as described (Kollias et al., 1986). Transgenics were screened by Southern blotting of EcoRI–BamHI-digested DNA using the 0.54 kb EcoRI C/EBPγ fragment as probe.
RNA analysis
RNA was prepared from yolk sacs, fetal livers and adult blood as described (Strouboulis et al., 1992). RT–PCR was performed with the γ-f and the γ-r2 primer 5′-GGCTGTGCGCATGCTCAAGAAAC-3′ (394 bp product) to detect C/EBPγ expression. Globin and U6 RNA levels were analysed by S1 nuclease protection (Wijgerde et al., 1996). The S1 signals were quantitated by phosphorimager analysis.
Protein analysis
Nuclear protein was isolated from E12.5 liver (Andrews and Faller, 1991) and 10 µg were used for western blot analysis with the rabbit anti-C/EBPγ polyclonal antibodies. A mouse monoclonal antibody recognizing the 62 kDa subunit of THII-H was used as a loading control; this antibody was generously provided by Dr E.Citterio (Rotterdam).
Colony assays
Colony assays were performed essentially as previously described (Wong et al., 1986). Fetal livers were disaggregated into single cells by passage through a 100 µm mesh and plated at a density of 3 × 105 cells per ml in methyl cellulose containing 1 U/ml Epo. The appearance of CFU-E and BFU-E colonies was scored after 2 and 14 days, respectively.
Cytospin preparations and staining
E12.5 fetuses were dissected from the uterus and bled on a dish to collect fetal blood. Then, the fetal livers were dissected and rinsed several times in cold phosphate-buffered saline. A single cell suspension of the fetal livers was prepared by gentle pipetting with a yellow tip. Aliquots of blood and fetal liver cell suspensions were loaded on a cytofunnel and spun at 400 r.p.m. for 5 min on microscope slides. The preparations were left to air dry and were stained with a combined histological and neutral benzidine stain (Beug et al., 1982).
Acknowledgments
Acknowledgements
We would like to thank S.McKnight and U.Schibler for providing antibodies, and N.Gillemans for her expert technical assistance. This work was supported by the Dutch Organization for Scientific Research NWO and the EC IVth framework BMH4-98-3147.
References
- Andrews N.C. and Faller,D.V. (1991) A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res., 19, 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou M. (1991) Induction of erythroid-specific expression in murine erythroleukemia (MEL) cell lines. Methods Mol. Biol., 7, 421–434. [DOI] [PubMed] [Google Scholar]
- Antoniou M., de Boer,E., Spanopoulou,E., Imam,A. and Grosveld,F. (1995) TBP binding and the rate of transcription initiation from the human β-globin gene. Nucleic Acids Res., 23, 3473–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M., Grosveld,F. and Dillon,N. (1992) A single point mutation is the cause of the Greek form of hereditary persistence of fetal haemoglobin. Nature, 358, 499–502. [DOI] [PubMed] [Google Scholar]
- Beug H., Palmieri,S., Freudenstein,C., Zentgraf,H. and Graf,T. (1982) Hormone-dependent terminal differentiation in vitro of chicken erythroleukemia cells transformed by ts mutants of avian erythroblastosis virus. Cell, 28, 907–919. [DOI] [PubMed] [Google Scholar]
- Cooper C.L., Berrier,A.L., Roman,C. and Calame,K.L. (1994) Limited expression of C/EBP family proteins during B lymphocyte development. Negative regulator Ig/EBP predominates early and activator NF-IL-6 is induced later. J. Immunol., 153, 5049–5058. [PubMed] [Google Scholar]
- Cooper C., Henderson,A., Artandi,S., Avitahl,N. and Calame,K. (1995) Ig/EBP (C/EBPγ) is a transdominant negative inhibitor of C/EBP family transcriptional activators. Nucleic Acids Res., 23, 4371–4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deBoer E., Antoniou,M., Mignotte,V., Wall,L. and Grosveld,F. (1988) The human β-globin promoter; nuclear protein factors and erythroid specific induction of transcription. EMBO J., 7, 4203–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvoye N.L., Destroismaisons,N.M. and Wall,L.A. (1993) Activation of the β-globin promoter by the locus control region correlates with binding of a novel factor to the CAAT box in murine erythroleukemia cells but not in K562 cells. Mol. Cell. Biol., 13, 6969–6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P. and Schibler,U. (1991) A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell, 67, 569–579. [DOI] [PubMed] [Google Scholar]
- Friedman A.D., Landschulz,W.H. and McKnight,S.L. (1989) CCAAT/enhancer binding protein activates the promoter of the serum albumin gene in cultured hepatoma cells. Genes Dev., 3, 1314–1322. [DOI] [PubMed] [Google Scholar]
- Kaisho T., Tsutsui,P., Tanaka,T., Tsujimura,T., Takeda,K., Kawai,T., Yoshida,N., Nakanishi,K. and Akira,S. (1999) Impairment of natural killer cytotoxic activity and interferon γ production in CCAAT/enhancer binding protein γ-deficient mice. J. Exp. Med., 190, 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias G., Wrighton,N., Hurst,J. and Grosveldt,F. (1986) Regulated expression of human A γ-, β-, and hybrid γ β-globin genes in transgenic mice: manipulation of the developmental expression patterns. Cell, 46, 89–94. [DOI] [PubMed] [Google Scholar]
- LekstromHimes J. and Xanthopoulos,K.G. (1998) Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J. Biol. Chem., 273, 28545–28548. [DOI] [PubMed] [Google Scholar]
- Liberati C., Ronchi,A., Lievens,P., Ottolenghi,S. and Mantovani,R. (1998) NF-Y organizes the γ-globin CCAAT boxes region. J. Biol. Chem., 273, 16880–16889. [DOI] [PubMed] [Google Scholar]
- Littlewood T.D., Hancock,D.C., Danielian,P.S., Parker,M.G. and Evan,G.I. (1995) A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res., 23, 1686–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R. (1998) A survey of 178 NF-Y binding CCAAT boxes. Nucleic Acids Res., 26, 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra J. et al. (1998) Life without white fat: a transgenic mouse. Genes Dev., 12, 3168–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham M., Gooding,C., Hudson,K., Antoniou,M., Grosveld,F. and Hollis,M. (1992) LCR/MEL: a versatile system for high-level expression of heterologous proteins in erythroid cells. Nucleic Acids Res., 20, 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlov C., McNagny,K.M., Doderlein,G., Kowenzleutz,E. and Graf,T. (1998) Distinct C/EBP functions are required for eosinophil lineage commitment and maturation. Genes Dev., 12, 2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive M., Krylov,D., Echlin,D.R., Gardner,K., Taparowsky,E. and Vinson,C. (1997) A dominant negative to activation protein-1 (AP1) that abolishes DNA binding and inhibits oncogenesis. J. Biol. Chem., 272, 18586–18594. [DOI] [PubMed] [Google Scholar]
- Osada S., Yamamoto,H., Nishihara,T. and Imagawa,M. (1996) DNA binding specificity of the CCAAT/enhancer-binding protein transcription factor family. J. Biol. Chem., 271, 3891–3896. [DOI] [PubMed] [Google Scholar]
- Pall M., Hellberg,P., Brannstrom,M., Mikuni,M., Peterson,C.M., Sundfeldt,K., Norden,B., Hedin,L. and Enerback,S. (1997) The transcription factor C/EBP-β and its role in ovarian function; evidence for direct involvement in the ovulatory process. EMBO J., 16, 5273–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Henthorn,P., Stoeckert,C. and Surrey,S. (1988) Globin gene expression in hereditary persistence of fetal haemoglobin and (δβ) naught-thalassaemia. Oxf. Surv. Eukaryot. Genes, 5, 163–203. [PubMed] [Google Scholar]
- Roman C., Platero,J.S., Shuman,J. and Calame,K. (1990) Ig/EBP-1: a ubiquitously expressed immunoglobulin enhancer binding protein that is similar to C/EBP and heterodimerizes with C/EBP. Genes Dev., 4, 1404–1415. [DOI] [PubMed] [Google Scholar]
- Ronchi A., Berry,M., Raguz,S., Imam,A., Yannoutsos,N., Ottolenghi,S., Grosveld,F. and Dillon,N. (1996) Role of the duplicated CCAAT box region in γ-globin gene regulation and hereditary persistence of fetal haemoglobin. EMBO J., 15, 143–149. [PMC free article] [PubMed] [Google Scholar]
- Scott L.M., Civin,C.I., Rorth,P. and Friedman,A.D. (1992) A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood, 80, 1725–1735. [PubMed] [Google Scholar]
- Screpanti I. et al. (1995) Lymphoproliferative disorder and imbalanced T-helper response in C/EBPβ-deficient mice. EMBO J., 14, 1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieweke M.H. and Graf,T. (1998) A transcription factor party during blood cell differentiation. Curr. Opin. Genet. Dev., 8, 545–551. [DOI] [PubMed] [Google Scholar]
- Sterneck E., Tessarollo,L. and Johnson,P.F. (1997) An essential role for C/EBPβ in female reproduction. Genes Dev., 11, 2153–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouboulis J., Dillon,N. and Grosveld,F. (1992) Developmental regulation of a complete 70-kb human β-globin locus in transgenic mice. Genes Dev., 6, 1857–1864. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Yoshida,N., Kishimoto,T. and Akira,S. (1997) Defective adipocyte differentiation in mice lacking the C/EBPβ and/or C/EBPδ gene. EMBO J., 16, 7432–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein S.L. (1993) β-Thalassaemia. Baillieres Clin. Haematol., 6, 151–175. [DOI] [PubMed] [Google Scholar]
- Vinson C.R., Hai,T. and Boyd,S.M. (1993) Dimerization specificity of the leucine zipper-containing bZIP motif on DNA binding: prediction and rational design. Genes Dev., 7, 1047–1058. [DOI] [PubMed] [Google Scholar]
- Wall L., deBoer,E. and Grosveld,F. (1988) The human β-globin gene 3′ enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev., 2, 1089–1100. [DOI] [PubMed] [Google Scholar]
- Wall L., Destroismaisons,N., Delvoye,N. and Guy,L.G. (1996) CAAT/enhancer-binding proteins are involved in β-globin gene expression and are differentially expressed in murine erythroleukemia and K562 cells. J. Biol. Chem., 271, 16477–16484. [DOI] [PubMed] [Google Scholar]
- Wang N.D., Finegold,M.J., Bradley,A., Ou,C.N., Abdelsayed,S.V., Wilde,M.D., Taylor,L.R., Wilson,D.R. and Darlington,G.J. (1995) Impaired energy homeostasis in C/EBPα knockout mice. Science, 269, 1108–1112. [DOI] [PubMed] [Google Scholar]
- Wijgerde M., Gribnau,J., Trimborn,T., Nuez,B., Philipsen,S., Grosveld,F. and Fraser,P. (1996) The role of EKLF in human β-globin gene competition. Genes Dev., 10, 2894–2902. [DOI] [PubMed] [Google Scholar]
- Wong P.M.C., Chung,S.W., Chui,D.H.K. and Eaves,C.J. (1986) Properties of the earliest clonogenic hemopoietic precursors to appear in the developing murine yolk sac. Proc. Natl Acad. Sci. USA, 83, 3851–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.E., Zhang,P., Wang,N.D., Hetherington,C.J., Darlington,G.J. and Tenen,D.G. (1997) Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc. Natl Acad. Sci. USA, 94, 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H., Kuroda,M., Wang,X.Z., Batchvarova,N., Lightfoot,R.T., Remotti,H., Stevens,J.L. and Ron,D. (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev., 12, 982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]