Abstract
We previously isolated an Arabidopsis peroxisome-deficient ped2 mutant by its resistance to 2,4-dichlorophenoxybutyric acid. Here, we describe the isolation of a gene responsible for this deficiency, called the PED2 gene, by positional cloning and confirmed its identity by complementation analysis. The amino acid sequence of the predicted protein product is similar to that of human Pex14p, which is a key component of the peroxisomal protein import machinery. Therefore, we decided to call it AtPex14p. Analyses of the ped2 mutant revealed that AtPex14p controls intracellular transport of both peroxisome targeting signal (PTS)1- and PTS2-containing proteins into three different types of peroxisomes, namely glyoxysomes, leaf peroxisomes and unspecialized peroxisomes. Mutation in the PED2 gene results in reduction of enzymes in all of these functionally differentiated peroxisomes. The reduction in these enzymes induces pleiotropic defects, such as fatty acid degradation, photorespiration and the morphology of peroxisomes. These data suggest that the AtPex14p has a common role in maintaining physiological functions of each of these three kinds of plant peroxisomes by determining peroxisomal protein targeting.
Keywords: β-oxidation/peroxisome/pex14/photorespiration/protein targeting
Introduction
Peroxisomes in higher plant cells are known to differentiate into at least three different classes, namely glyoxysomes, leaf peroxisomes and unspecialized peroxisomes (Beevers, 1979). Each organelle contains a unique set of enzymes that provides special functions in various organs in higher plants. Glyoxysomes are present in cells of storage organs, such as endosperms and cotyledons during post-germinative growth of oil-seed plants, as well as in senescent organs (Nishimura et al., 1996). They contain enzymes for fatty acid β-oxidation and the glyoxylate cycle, and play a pivotal role in the conversion of lipid into sucrose. It has been suggested that fatty acids are exclusively degraded in glyoxysomes (i.e. not in mitochondria) during germination and post-germinative growth (Beevers, 1982). In contrast, leaf peroxisomes are found widely in cells of photosynthetic organs. It has been shown that some of the enzymes responsible for photorespiration are localized in leaf peroxisomes even though the entire photorespiratory process involves a combination of enzymic reactions that occur in chloroplasts, leaf peroxisomes and mitochondria (Tolbert, 1982). Other organs, such as roots and stems, contain unspecialized peroxisomes whose function is still obscure (Nishimura et al., 1996).
Glyoxysomes, leaf peroxisomes and unspecialized peroxisomes are known to be converted into one another under certain conditions (Nishimura et al., 1996). For example, glyoxysomes in etiolated cotyledons are transformed directly into leaf peroxisomes during the greening of cotyledons (Titus and Becker, 1985; Nishimura et al., 1986). During this process, glyoxysomal enzymes, such as malate synthase, are specifically degraded (Mori and Nishimura, 1989), and leaf peroxisomal enzymes, such as glycolate oxidase and hydroxypyruvate reductase, are newly synthesized and transported into the organelle as it is being transformed from a glyoxysome to a leaf peroxisome (Tsugeki et al., 1993; Hayashi et al., 1996b). Leaf peroxisomes in green cotyledons are subsequently converted to glyoxysomes when the cotyledons undergo senescence (De Bellis and Nishimura, 1991; Nishimura et al., 1993). It has been suggested that the functional transformation of plant peroxisomes is controlled by gene expression, protein translocation and protein degradation, although the detailed mechanisms underlying these processes still need to be clarified (Nishimura et al., 1996).
To identify the genes responsible for regulation of peroxisomal function in plant cells, we isolated mutants with defective peroxisomes. To screen such mutants, we used 2,4-dichlorophenoxybutyric acid (2,4-DB) as a compound for detecting Arabidopsis mutants with defects in glyoxysomal fatty acid β-oxidation (Hayashi et al., 1998). We expected that two methylene groups of the butyric side chain in 2,4-DB would be removed by the action of glyoxysomal fatty acid β-oxidation to produce a herbicide, 2,4-dichlorophenoxyacetic acid (2,4-D), in wild-type plants, whereas the mutants no longer produce a toxic level of 2,4-D from 2,4-DB because of the defect in fatty acid β-oxidation. We succeeded in identifying four mutants that were classified as carrying alleles at three independent loci. We designated these loci as ped1, ped2 and ped3, respectively, where ped stands for peroxisome defective. These mutants required sucrose for post-germinative growth, because the reduced activity of glyoxysomal fatty acid β-oxidation prevented the production of sucrose from the lipid reserves in seeds. One of these mutants, ped2, has been demonstrated previously to have a defect in the intracellular transport of 3-ketoacyl CoA thiolase, an enzyme participating in fatty acid β-oxidation, from the cytosol to glyoxysomes (Hayashi et al., 1998).
Peroxisomal enzymes are synthesized in the cytosol, and function after their post-translational transport into peroxisomes. Most of the plant peroxisomal enzymes have been shown to contain one of two peroxisome targeting signals (PTSs) within their amino acid sequences (Hayashi, 2000). One type of targeting signal (PTS1) is a unique tripeptide sequence found in the C-terminus of the proteins (Hayashi et al., 1996a; Trelease et al., 1996). The permissible combinations of tripeptide sequence for plant PTS1 are (C/A/S/P)-(K/R)-(I/L/M) (Hayashi et al., 1997). Another type of targeting signal is involved in a cleavable N-terminal presequence (Gietl et al., 1994). The N-terminal presequences contain a consensus sequence (R)-(L/Q/I)-X5-(H)-(L) (X stands for any amino acid) called PTS2 (Kato et al., 1996a, 1998). These proteins are synthesized as precursor proteins, which show a higher molecular mass due to the N-terminal presequence. The N-terminal presequence is processed to form the mature protein after its transport into peroxisomes. These peroxisomal proteins with PTS1 or PTS2 are imported into peroxisomes with oligomeric forms (Lee et al., 1997; Flynn et al., 1998; Kato et al., 1999).
Here we report the identification and analysis of the PED2 gene, and present evidence that the gene product of PED2 is a component of the protein targeting machinery involved in each of the three kinds of plant peroxisome. We discuss the pleiotropic defects in the ped2 mutant based upon the predicted function of the PED2 gene product.
Results
High-resolution mapping of the PED2 locus
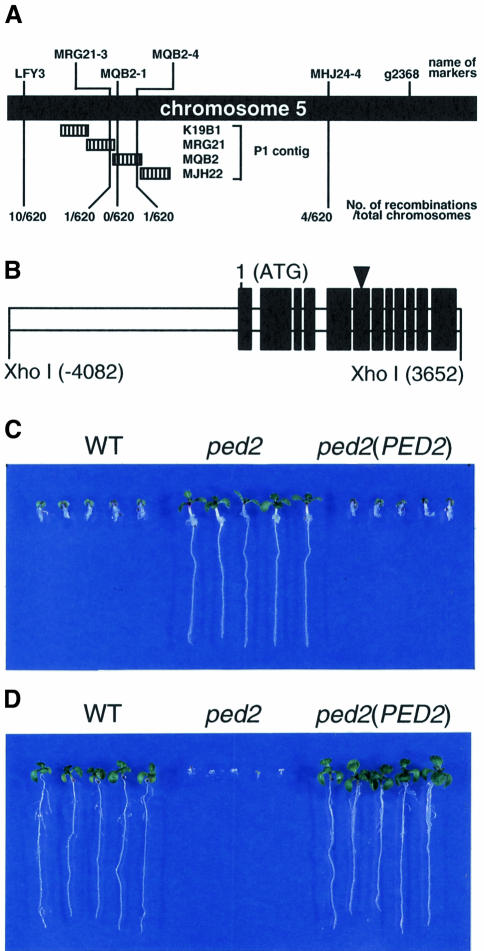
The Arabidopsis ped2 mutant, which has a Landsberg erecta ecotype background, was identified by its resistance to the presence of 2,4-DB. This mutant requires sucrose for post-germinative growth, because of its reduced activity for fatty acid β-oxidation. Our initial mapping of PED2 located it to the lower arm of chromosome 5, between two molecular markers, LFY3 and g2368 (Hayashi et al., 1998). We outcrossed the ped2 mutant (which has a Landsberg erecta ecotype background) to wild-type Arabidopsis, which has a Columbia ecotype background, and identified 310 F2 progenies that have homozygous ped2 alleles for high-resolution mapping. These progenies were subsequently scored according to their genetic background at a series of molecular markers using the cleaved amplified polymorphic sequence (CAPS) mapping procedure described by Konieczny and Ausubel (1993) (Figure 1A). The number of chromosomes that showed a Columbia background represents the number of recombinations that occurred between the PED2 locus and the position of each molecular marker, since the genomic DNA of the ped2 mutant has a Landsberg erecta background. To identify the genetic background of chromosome 5 between LFY3 and g2368, we generated four molecular markers, MRG21-3, MQB2-1, MQB2-4, MHJ24-4, based on the nucleotide sequences of P1 contigs that have been reported by the Kazusa DNA Institute, Chiba, Japan (http://www.kazusa.or.jp/kaos/). As summarized in Figure 1A, high-resolution mapping revealed that the PED2 locus is located between MRG21-3 and MQB2-4. The closest molecular marker to the PED2 locus is MQB2-1. This result strongly suggests that the PED2 gene is contained within a single P1 clone, MQB2.
Fig. 1. Positional cloning of the PED2 gene. (A) High-resolution mapping of PED2 on chromosome 5. Names and positions of the molecular markers used in this study are indicated on the top of the illustration. Hatched bars represent the regions covered by the P1 clones. We analyzed 310 F2 progeny (620 chromosomes) having homozygous ped2 alleles. The numbers of recombinations that occurred between the PED2 locus and the molecular markers are indicated at the bottom of the illustration. Mapping results with a series of molecular markers between LFY3 and MHJ24-4 are summarized schematically and indicate that the PED2 locus may be located within a single P1 clone, MQB2. (B) Schematic diagram of a 7734 bp XhoI fragment that is involved in the P1 clone, MQB2. The 12 black bars represent protein coding regions determined from the cDNA sequence. The triangle on the sixth black bar indicates the position of a nonsense mutation that occurs in the ped2 mutant. Nucleotide residue 1 corresponds to an adenine of the first methionine codon. (C) Effects of 2,4-DB on the growth of transgenic ped2 seedlings [ped2(PED2)] harboring the 7734 bp XhoI fragment shown in (B). Wild-type Arabidopsis (WT), ped2 mutant (ped2) and ped2(PED2) were grown for 10 days on growth medium containing 0.2 µg/ml 2,4-DB under constant illumination. Photographs were taken after the seedlings were removed from the media and rearranged on agar plates. (D) Effects of sucrose on the growth of ped2(PED2) seedlings. Wild-type Arabidopsis (WT), ped2 mutant (ped2) and ped2(PED2) were grown for 10 days on growth medium without sucrose under constant illumination. Photographs were taken after the seedlings were removed from the media and rearranged on agar plates.
Identification of the PED2 gene
MQB2 is reported to contain 16 predicted genes (http://www.kazusa.or.jp/kaos/). Based on the nucleotide sequences, we designed a set of oligonucleotide primers that could amplify one of the predicted genes by using the PCR. This gene is located within the 7734 bp XhoI fragment contained in MQB2 (Figure 1B). DNA fragments were amplified from genomic DNAs of wild-type Arabidopsis (ecotype Landsberg erecta) and the ped2 mutant, using this primer set, and were fully sequenced. The nucleotide sequences of the two fragments are identical except for one nucleotide substitution, from C in the wild-type plant to T in the ped2 mutant (Figure 1B, arrowhead). This result strongly indicated that the 7734 bp XhoI fragment contained the PED2 gene.
To confirm this result, the 7734 bp XhoI fragment isolated from the MQB2 clone was inserted into a plant binary vector, pBI121Δ35S, and then transformed into the ped2 mutant by Agrobacterium-mediated transformation (Bechtold et al., 1993). Seeds from individual kanamycin-resistant T2 progenies were scored for kanamycin resistance to identify the lines that are homozygous for the transgene. The homozygous T3 lines were assayed for 2,4-DB resistance and a sucrose requirement during post-germinative growth. As we have previously reported, the ped2 mutant was resistant to a toxic level of 2,4-DB, while it was sensitive to the absence of sucrose in the growth medium (Figure 1C and D, ped2). In contrast, the ped2 mutant transformed with the 7734 bp XhoI fragment became sensitive to a toxic level of 2,4-DB, whereas it was resistant to the absence of sucrose in the growth medium [Figure 1C and D, ped2(PED2)]. These phenotypes are identical to wild-type plants (Figure 1C and D, WT). These data indicated that the genomic sequence determined in this study corresponds to the PED2 gene. The nucleotide sequence data of PED2 are available in the DDBJ/EMBL/GenBank nucleotide sequence databases (AB037538).
PED2 encodes a protein similar to Pex14p
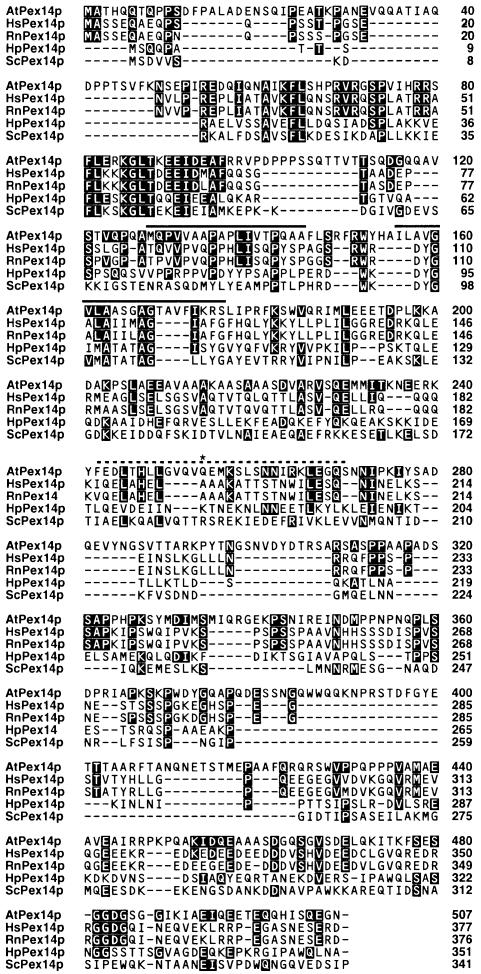
A cDNA clone of the PED2 gene was generated using RT–PCR with total RNA isolated from wild-type plants. The first methionine that appeared in this cDNA represents the start of the open reading frame, since the 5′ primer used for the PCR was designed to hybridize with the 5′ untranslated region including an in-frame stop codon. We determined the nucleotide sequence of the cDNA (DDBJ/EMBL/GenBank accession No. AB037539). Comparison of the cDNA and genomic DNA sequences showed that the PED2 gene contains 12 exons (Figure 1B). The deduced amino acid sequence of the gene product is composed of 507 amino acid residues (Figure 2). A nucleotide substitution occurred in the ped2 mutant, converting a CAA codon encoding Gln254 of the gene product to a stop codon (TAA) (Figure 2, asterisk). The amino acid sequence of the gene product shows significant similarity to mammalian and fungal Pex14p, one of the peroxisomal membrane proteins involved in the peroxisomal protein targeting machinery (Albertini et al., 1997; Brocard et al., 1997; Komori et al., 1997; Shimizu et al., 1999; Will et al., 1999), and is most similar to that of human Pex14p (Figure 2). Therefore we decided to call it AtPex14p. AtPex14p contains at lease two hydrophobic segments and a coiled-coil region (Figure 2). Although two yeast Pex14p are known to contain the class II SH3 ligand consensus sequence (Albertini et al., 1997), AtPex14p does not contain such a motif. In addition, there is no obvious PTS.
Fig. 2. Alignment of amino acid sequences for the PED2 gene product with mammalian and yeast Pex14p. Deduced amino acid sequence of the PED2 gene product (AtPex14p) was compared with Pex14p identified from human (HsPex14p), rat (RnPex14p), H.polymorpha (HpPex14p) and Saccharomyces cerevisiae (ScPex14p). Identical amino acid residues between AtPex14p and other Pex14p are highlighted. Amino acid sequence of AtPex14p is 29.6% identical to that of human Pex14p. The asterisk on Gln254 represents the position of a nonsense mutation (CAA to TAA) in the ped2 gene. Two putative membrane spanning domains are indicated by a line. A dashed line represents a putative coiled-coil region.
Subcellular localization of AtPex14p
To analyze the subcellular localization of AtPex14p, we prepared an antiserum raised against a fusion protein containing a partial amino acid sequence of AtPex14p (Met1–Pro100). This antiserum recognized a 75 kDa protein in wild-type plant (Figure 3, WT), while no cross-reactive band was detected in the ped2 mutant (Figure 3, ped2). These data indicate that AtPex14p is the 75 kDa protein.
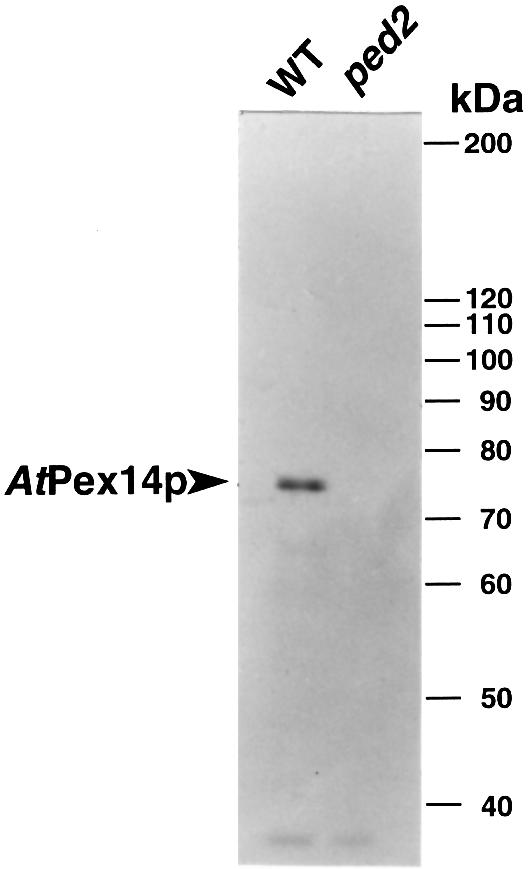
Fig. 3. Immunodetection of AtPex14p in etiolated cotyledons of wild-type Arabidopsis and ped2 mutant. Extracts were prepared from 5-day-old etiolated cotyledons of wild-type Arabidopsis (WT) and ped2 mutant (ped2). For each sample, 10 µg of total protein were subjected to SDS–PAGE. Immunoblot analysis was performed using the antibody raised against AtPex14p. Markers are shown on the right with molecular masses in kDa.
To investigate the subcellular localization of Pex14p in plant cells, homogenates prepared from pumpkin etiolated cotyledons were subjected to sucrose density gradient centrifugation. Fractions thus obtained were analyzed using an immunoblotting technique with the antibody raised against AtPex14p (Figure 4A). The 75 kDa protein was detected in fractions 23–25, whose densities were 1.25 g/cm3. These fractions also contained other glyoxysomal marker enzymes, such as isocitrate lyase and catalase, while they did not contain a mitochondrial marker enzyme (cytochrome c oxidase) activity (Figure 4A and B).
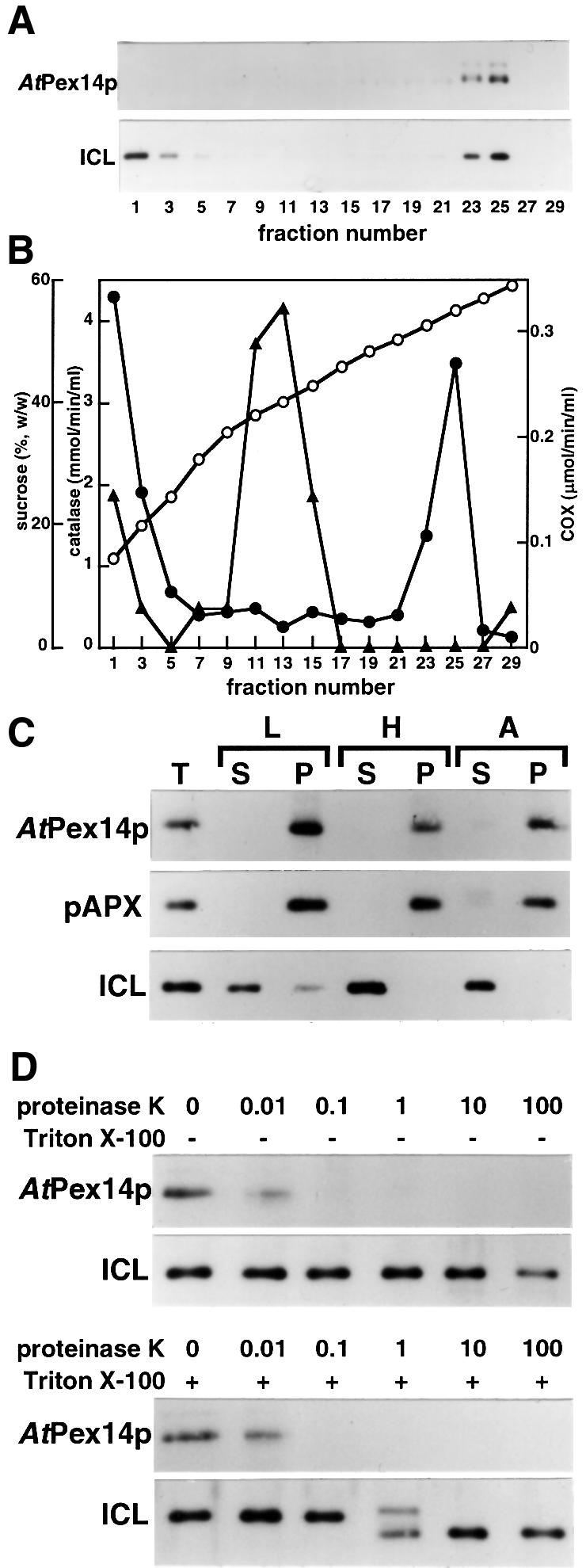
Fig. 4. Subcellular localization of Pex14p in pumpkin etiolated cotyledons. (A) Subcellular fractionation of etiolated pumpkin cotyledons was performed by 30–60% sucrose density gradient centrifugation. Fraction number 1 represents the top fraction of the gradient. Pex14p and isocitrate lyase in each fraction were detected by immunoblot analyses using antibody raised against AtPex14p (AtPex14p) and isocitrate lyase (ICL). (B) Sucrose concentration (open circles), activities of catalase (filled circles) and cytochrome c oxidase (COX; filled triangles) in the same fractions as used in (A) were also measured. (C) Intact glyoxysomes were resuspended in either low salt buffer (L), high salt buffer (H) or alkaline solution (A). Each buffer consists of 10 mM HEPES–KOH pH 7.2, 500 mM KCl with 10 mM HEPES–KOH pH 7.2 and 0.1 M Na2CO3, respectively. These samples were then centrifuged and separated into soluble (S) and insoluble (P) fractions. T represents total proteins of the intact glyoxysomes. Pex14p (AtPex14p), peroxisomal ascorbate peroxidase (pAPX) and isocitrate lyase (ICL) were detected by immunoblot analysis. (D) The intact glyoxysomes were treated with various concentrations of proteinase K in the absence (–) or presence (+) of Triton X-100. The concentration of proteinase K is indicated in µg/ml on the top of each lane. Pex14p (AtPex14p) and isocitrate lyase (ICL) were detected by immunoblot analysis.
Figure 4C represents the result of the extensive subfractionation studies performed by the treatment of intact glyoxysomes with various solutions. Pex14p and ascorbate peroxidase, a marker enzyme for peroxisomal membranes (Yamaguchi et al., 1995), were found in the insoluble fraction even after treatment with alkaline solution. However, isocitrate lyase, a marker enzyme for the glyoxysomal matrix, was dissolved completely both in high-salt buffer and alkaline solution. In addition, Pex14p in intact glyoxysomes was sensitive to the digestion of proteinase K both in the absence and presence of Triton X-100, whereas isocitrate lyase was degraded only in the presence of Triton X-100 (Figure 4D). Overall results suggest that the 75 kDa protein is a peroxisomal membrane-associated protein, and that at least a part of the polypeptide is located in the cytosol.
Intracellular transport of PTS1-containing proteins in the ped2 mutant
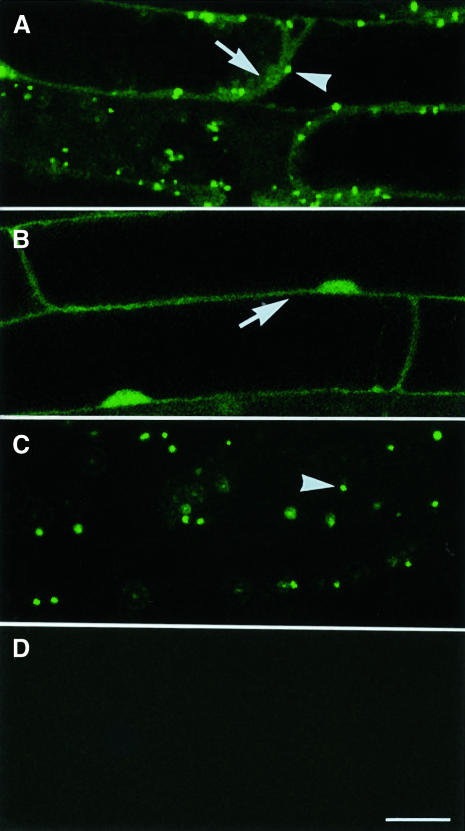
To analyze the intracellular transport of PTS1-containing proteins in the ped2 mutant, we generated plants expressing a jellyfish green fluorescent protein (GFP)–PTS1 fusion protein (GFP–PTS1) in a ped2 background, as described previously (Mano et al., 1999). GFP–PTS1 consisted of GFP fused to a dodecapeptide containing serine-lysine-leucine at the C-terminal end. These plants were created by outcrossing the ped2 mutant with trans genic Arabidopsis expressing GFP–PTS1. Additional control plants were created by outcrossing the ped2 mutant with transgenic Arabidopsis expressing only GFP. When GFP–PTS1 is expressed in cells of the F3 progeny that are homozygous for the ped2 allele, green fluorescence was observed both in the periphery of the cells (Figure 5A, arrow) and in small spots distributed diffusely throughout the periphery (Figure 5A, arrowhead). The fluorescence detected in the periphery indicated that a part of the GFP–PTS1 remains in the cytosol, since GFP without PTS1 showed a similar fluorescent pattern in the ped2 mutant (Figure 5B, arrow) and in wild-type plants (data not shown). In Figure 5A and B, the dark space surrounded by the cytosol corresponds to a central vacuole. The fluorescent spots distributed in the cytosol indicate that a significant amount of GFP–PTS1 was recognized correctly and transported into the peroxisomes in the cells of the ped2 mutant. In contrast, only punctate fluorescence was observed when GFP–PTS1 was expressed in wild-type plants (Figure 5C). These data indicate that the ability for intracellular transport of PTS1-containing proteins is reduced in the ped2 mutant.
Fig. 5. Subcellular localization of GFP–PTS1 fusion protein in ped2 mutant. Seedlings were grown under continuous illumination for 10 days. Images of the green fluorescence derived from GFP in root cells were taken by a confocal laser microscope as single optical sections. (A) Subcellular localization of GFP–PTS1 expressed in the cells of a ped2 mutant. (B) Subcellular localization of GFP expressed in the cells of a ped2 mutant. (C) Subcellular localization of GFP–PTS1 expressed in the cells of a wild-type plant. (D) No fluorescence was observed in non-transformed cell of a ped2 mutant. Arrowheads indicate fluorescence detected in peroxisomes, whereas the arrows indicate fluorescence detected in the cytosol. Bar in (D), 20 µm. Magnifications of (A)–(D) are the same.
Intracellular transport of PTS2-containing proteins in the ped2 mutant
To analyze intracellular transport of PTS2-containing proteins in the ped2 mutant, two PTS2-containing proteins, 3-ketoacyl CoA thiolase (Figure 6A, ped2) and malate dehydrogenase (Figure 6B, ped2), were analyzed in 3- and 5-day-old etiolated cotyledons and 7-day-old green cotyledons by using an immunoblotting technique. As shown in Figure 6A, 3-day-old etiolated cotyledons contained two types of 3-ketoacyl CoA thiolase. One of these corresponds to the mature form of 3-ketoacyl CoA thiolase (45 kDa) (Figure 6A, arrowhead), whereas the other was an additional protein with a higher molecular mass (48 kDa) (Figure 6A, arrow). We have demonstrated previously that the larger protein corresponded to the precursor form of 3-ketoacyl CoA thiolase that accumulated in the cytosol (Kato et al., 1996a; Hayashi et al., 1998). In addition, 3-day-old etiolated cotyledons contained the mature form of malate dehydrogenase (33 kDa) (Figure 6B, arrowhead) and the precursor form of the enzyme (37 kDa) (Figure 6B, arrow). In contrast, the wild-type plants did not contain detectable amounts of the precursor proteins during any stages of post-germinative growth (Figure 6A and B, WT). The precursor proteins detected in 3-day-old etiolated cotyledons of the ped2 mutant rapidly disappeared, whereas the amounts of the mature proteins remained at similar levels during subsequent seedling growth. 3-Ketoacyl CoA thiolase and malate dehydrogenase are known to be actively synthesized in cells of etiolated cotyledons but not in the green cotyledons (Kato et al., 1996a, 1998). Accumulation of the precursors for PTS2-containing proteins in the ped2 mutant occurred only during the period of active protein synthesis. These data indicate that the ped2 mutant has reduced activity for the intracellular transport of PTS2-containing proteins, and is not able to import all of the PTS2-containing proteins when these proteins are actively synthesized.
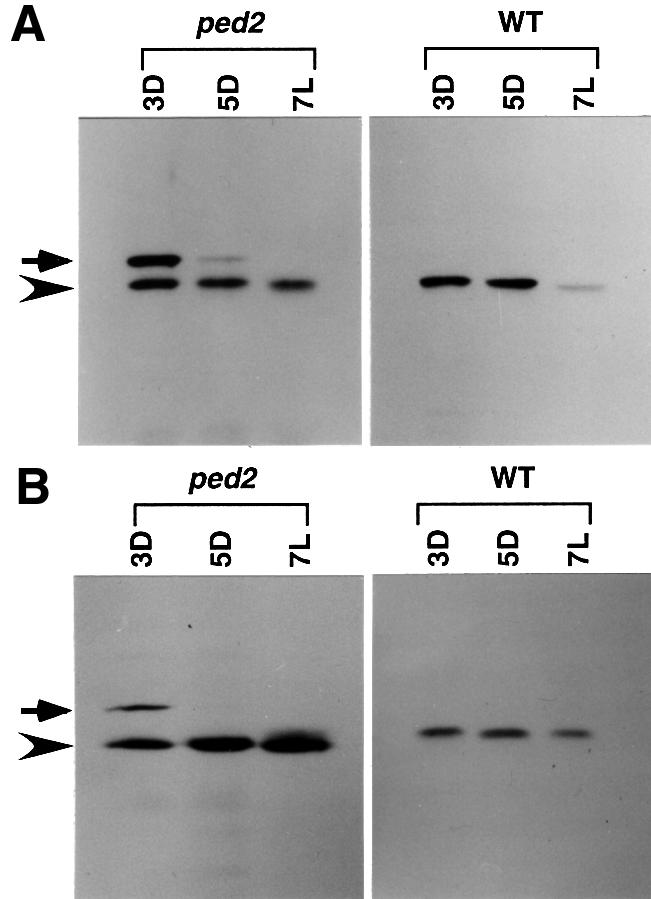
Fig. 6. Immunodetection of thiolase and malate dehydrogenase in cotyledonary cells of ped2 mutants. Seedlings of the ped2 mutant (ped2) and wild-type plant (WT) were grown in continuous darkness for 3 days (3D), 5 days (5D) or under continuous illumination for 7 days (7L). Ten micrograms of total protein prepared from the cotyledons were subjected to immunoblotting using an antibody raised against 3-ketoacyl CoA thiolase (A) and malate dehydrogenase (B). Arrowheads indicate the positions of the mature proteins, whereas the arrows indicate the positions of the precursors.
Morphology of glyoxysomes, leaf peroxisomes and unspecialized peroxisomes in the ped2 mutant
Figure 7 shows an immunoelectron microscopic analysis of various peroxisomes in wild-type plants and the ped2 mutant. As mentioned above, there are three types of plant peroxisomes: glyoxysomes, leaf peroxisomes and non-specialized peroxisomes. In wild-type plants, these peroxisomes have similar morphologies (Nishimura et al., 1996). As shown in Figure 7A, glyoxysomes found in the 5-day-old etiolated cotyledons of wild-type plants are ∼0.5 µm in diameter and have a round or oval shape containing a uniform matrix. The glyoxysomes contain enzymes for fatty acid β-oxidation. When cells were immunogold labeled using antibodies raised against one of these enzymes, 3-ketoacyl CoA thiolase, the gold particles were exclusively localized on the glyoxysomes (Figure 7A). In contrast, the peroxisomes in the ped2 mutant showed an abnormal morphology (Figure 7B–E). Glyoxysomes found in the etiolated cotyledons of the ped2 mutant were shrunken and not round (Figure 7B). Therefore, they looked very different from the glyoxysomes of wild-type plants. A small but significant number of gold particles were detected when the glyoxysomes of the ped2 mutant were stained with antibodies raised against 3-ketoacyl CoA thiolase. In contrast, the number of gold particles detected in the cytosol was not significant, in spite of the fact that the precursor of 3-ketoacyl CoA thiolase is accumulated in the cytosol. This may be because the concentration of the precursor that accumulated in the cytosol was not sufficient to be clearly detected. A similar abnormal morphology was detected in leaf peroxisomes found in cells of green cotyledons (Figure 7C) and leaves (Figure 7D), as well as in unspecialized peroxisomes found in cells of root (Figure 7E). Since the leaf peroxisomes contain photorespiration enzymes, such as hydroxypyruvate reductase, the leaf peroxisomes were stained using antibodies raised against hydroxypyruvate reductase (Figure 7C and D). However, fewer gold particles were detected in the leaf peroxisomes of the ped2 mutant than in those of the wild-type plant (data not shown). A similar phenomenon was observed when unspecialized peroxisomes in root cells were stained with antibodies raised against catalase (Figure 7E). These data indicate that all three kinds of peroxisome in the ped2 mutant have abnormal morphologies, and contain fewer enzymes than do the peroxisomes of wild-type plants.
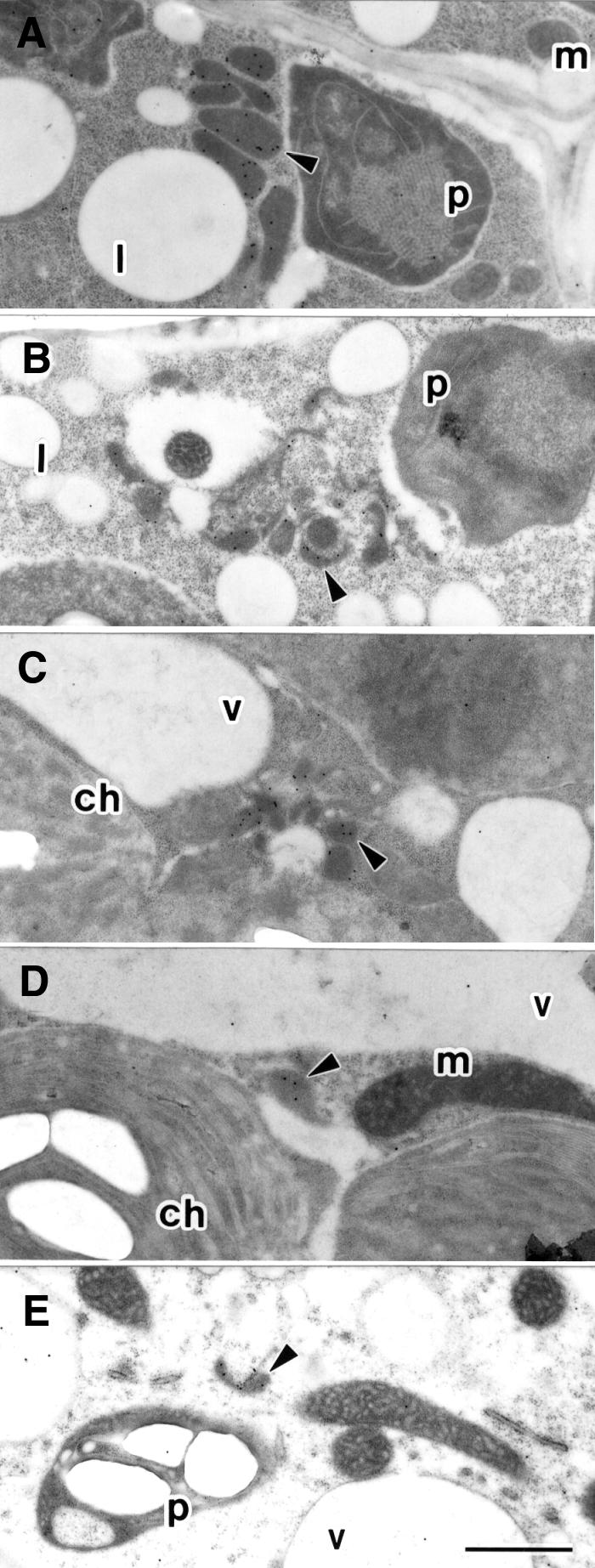
Fig. 7. Electron microscopic analysis of peroxisomes in the cells of the ped2 mutant. (A) Etiolated cotyledon of wild-type Arabidopsis, grown for 5 days in darkness, was stained with 3-ketoacyl CoA thiolase-specific antibody. (B) Etiolated cotyledon of ped2 mutant, grown for 5 days in darkness, was stained with 3-ketoacyl CoA thiolase-specific antibody. (C) Green cotyledon of ped2 mutant, grown for 7 days under continuous illumination, was stained with hydroxypyruvate reductase-specific antibody. (D) Leaf of ped2 mutant, grown for 14 days under continuous illumination, was stained with hydroxypyruvate reductase-specific antibody. (E) Root of ped2 mutant, grown for 14 days under continuous illumination, was stained with catalase-specific antibody. Arrowhead, peroxisome; m, mitochondrion; l, lipid body; p, plastid; ch, chloroplast; v, vacuole. Bar in (E), 1 µm. Magnification of (A)–(E) is the same.
Reduced activity of photorespiration in ped2 mutant
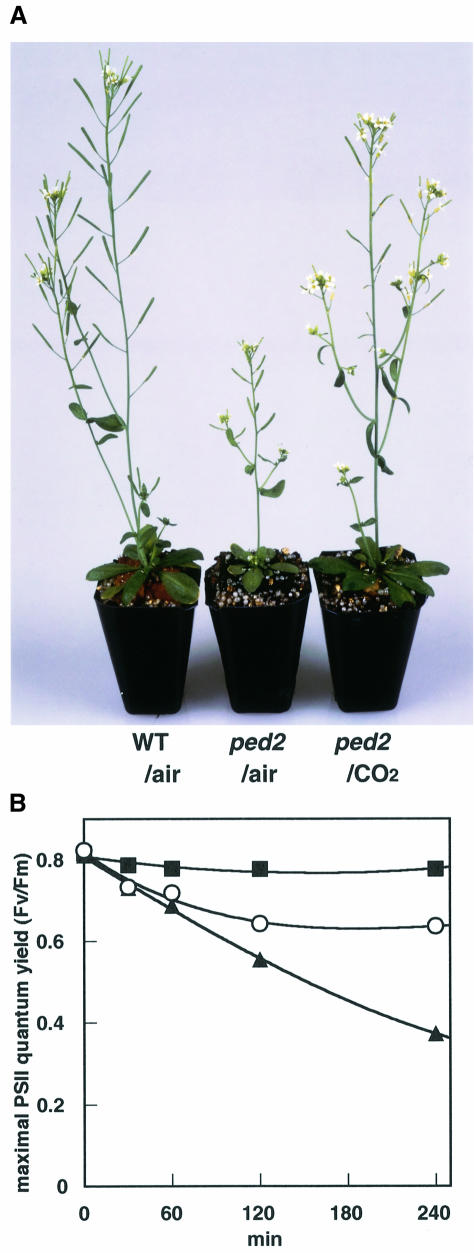
When ped2 mutants were grown in a normal atmosphere (36 Pa CO2), they always had yellow-green leaves and showed a dwarf phenotype (Figure 8A, ped2/air) compared with wild-type plants (Figure 8A, WT/air). No such phenotypes were observed in transgenic ped2 mutants transformed with the wild-type PED2 gene (data not shown). These phenotypes were recovered when the ped2 mutant was grown under high CO2 conditions (1000 Pa CO2) (Figure 8A, ped2/CO2). A similar phenomenon was observed in mutants with alterations in the photorespiratory pathway (Somerville and Ogren, 1982). Since some enzymes involved in the photorespiratory pathway are PTS1-containing proteins that are located in leaf peroxisomes, we assumed that these effects are induced by the reduced activity of photorespiration in the ped2 mutant. We tested this hypothesis by measuring the maximal quantum yield of photosystem II, which can be estimated from the ratio of the variable fluorescence of dark-adapted chlorophyll a to the maximum fluorescence (Fv/Fm) (Krause, 1988; Franklin et al., 1992; Öquist et al., 1992). We compared the Fv/Fm of dark-adapted leaves of the ped2 mutant with that of wild-type plants. We also compared it with the Fv/Fm of the stm mutant, which lacks one of the enzymes for photorespiration, a mitochondrial serine transhydroxymethylase (Somerville and Ogren, 1981).
Fig. 8. Reduced activity of photorespiration in ped2 mutant. (A) Effect of CO2 on the growth of ped2 mutant. Wild-type Arabidopsis (WT/air) and ped2 mutant (ped2/air) were grown for 8 weeks in a normal atmosphere (36 Pa CO2) under constant illumination (100 µE/m2/s). The ped2 mutant was also grown for 8 weeks in an atmosphere containing 1000 Pa CO2 (ped2/CO2) under constant illumination (50 µE/m2/s). (B) Effect of strong irradiation on maximal quantum yield of photosystem II (Fv/Fm). Wild-type Arabidopsis (filled squares), ped2 mutant (open circles) and the stm mutant (filled triangles) were grown for 3 weeks in an atmosphere containing 1000 Pa CO2 under low light (50 µE/m2/s). These plants were illuminated with strong light (450 µE/m2/s) in a normal atmosphere (36 Pa CO2). Maximum quantum yield of photosystem II (Fv/Fm) of the leaves at each defined time was measured after these plants were kept for 30 min in darkness. Each point represents the average Fv/Fm measured in six leaves of independent plants. The standard error of each point is <0.01.
To reduce the effect of photoinhibition, plants were grown for 3 weeks in an atmosphere containing high CO2 (1000 Pa) under low light (50 µE/m2/s). Under these conditions, the ped2 mutant, the wild-type plant and the stm mutant showed normal growth, and had similar Fv/Fm values of ∼0.8 (Figure 8B, 0 min). These plants were then transferred to a normal atmosphere (36 Pa CO2), where they were illuminated with a strong light (450 µE/m2/s). Under these conditions, ribulose bisphosphate carboxylase/oxygenase (RuBisCO) has an oxygenase activity in addition to its carboxylase activity, which is necessary for CO2 fixation in photosynthesis. Phosphoglycolate, a byproduct of the oxygenase reaction, is metabolized by the photorespiration enzymes, and finally returned to the Calvin–Benson cycle. Therefore, the Fv/Fm of the wild-type plants would not be expected to change after the strong illumination, since photorespiration operates properly and photoinhibition is not induced under such conditions. This is what was observed (Figure 8B, filled squares). In contrast, the Fv/Fm ratios of the ped2 and stm mutants decreased after the transfer to the same conditions (Figure 8B, open circles and filled triangles). These mutants were unable to maintain sufficient activity of the Calvin–Benson cycle under these conditions, because they could not metabolize phosphoglycolate due to the defect in photorespiration. The reduced activity of the Calvin– Benson cycle induced an excessive supply of energy from the light reaction of photosynthesis, and caused photoinhibition under high light irradiation. The imbalance between the light reaction and the Calvin–Benson cycle might have been responsible for the observed reduction of Fv/Fm. After 120 min of illumination, Fv/Fm of the ped2 mutant reached a plateau (Fv/Fm = 0.64), while that of the stm mutant kept decreasing. These results suggest that the activity for photorespiration in the ped2 mutant is partially inhibited, but is stronger than that in the stm mutant.
Discussion
The chemically induced ped2 strain is one of a series of Arabidopsis thaliana mutants that has defects in glyoxysomal fatty acid β-oxidation. Positional cloning and subsequent analyses revealed that the PED2 gene encodes AtPex14p, a 75 kDa peroxisomal membrane protein, whose amino acid sequence is most similar to human Pex14p. At present, there is no other gene that encodes a protein similar to AtPex14p in Arabidopsis genome database. Recently, Lopez-Huertas et al. (1999) reported that antibody raised against human Pex14p recognizes a 66 kDa protein in the sunflower glyoxysomal membrane.
Recent analyses of yeast mutants allowed the identification of >20 PEX genes and their products, peroxins (Subramani, 1998). Among these peroxins, it has been suggested that Pex14p forms a protein complex in the peroxisomal membrane with other peroxins, such as Pex13p and Pex17p (Albertini et al., 1997; Huhse et al., 1998). This protein complex is sometimes called a docking protein complex, since it may control intracellular transport of both PTS1- and PTS2-containing proteins (Subramani, 1998). Pex14p binds directly to both PTS1 receptor (Pex5p) and PTS2 receptor (Pex7p) (Albertini et al., 1997). Thus, Pex14p is believed to be a point of convergence of the PTS1-dependent and PTS2-dependent peroxisomal protein import pathway in mammalian and fungal cells. The present data strongly suggest that the AtPex14p is a key component of the peroxisomal protein import machinery that determines the intracellular transport of both PTS1-containing and PTS2-containing proteins in plant cells. PTS1 receptors have been identified from several plants (Brickner et al., 1998; Kragler et al., 1998; Wimmer et al., 1998), and a PTS2 receptor has been identified from Arabidopsis (Schumann et al., 1999). Whether AtPex14p binds directly to both PTS1 and PTS2 receptors remains to be tested.
In higher plant cells, all enzymes involved in fatty acid β-oxidation and the glyoxylate cycle except cytosolic aconitase are localized predominantly in glyoxysomes (Hayashi, 2000). Among these enzymes, short-chain acyl CoA oxidase, multifunctional enzyme, malate synthase and isocitrate lyase are PTS1-containing proteins, whereas long-chain acyl CoA oxidase, 3-ketoacyl CoA thiolase, malate dehydrogenase and citrate synthase are PTS2-containing proteins (Hayashi, 2000). The defect in the PED2 gene resulted in reduced amounts of enzymes in glyoxysomes, which reduced the ability of glyoxysomes to carry out fatty acid β-oxidation. The loss of glyoxysomal matrix proteins also caused a defect in the morphology of the glyoxysomes. The PEX14 disruption mutant of Hansenula polymorpha was also shown to lack intact peroxisomes but it contained several small membranous vesicles (Komori et al., 1997).
Fatty acid β-oxidation is an important physiological function of glyoxysomes during germination and the early stage of post-germinative growth, since it metabolizes lipid reserves in the seeds to produce sucrose, which is necessary for seedling growth (Beevers, 1982). However, this activity decreases rapidly along with the functional transformation of glyoxysomes into leaf peroxisomes that occurs during subsequent seedling growth. Since the ped2 mutant has been identified by its defect in fatty acid β-oxidation, we had assumed that the defect was limited to glyoxysomes. However, electron microscopic analyses indicated that the ped2 mutant had morphological defects not only in glyoxysomes but also in leaf peroxisomes and unspecialized peroxisomes. In addition, we found that the ped2 mutant had defects in the physiological function of leaf peroxisomes, i.e. in photorespiration. Measurements of maximal quantum yield of photosystem II suggested that the activity of photorespiration in the ped2 mutant is lower than that in wild-type plants but higher than that in the stm mutant. Leaf peroxisomes contain enzymes for photorespiration such as hydroxypyruvate reductase and glycolate oxidase (Tolbert, 1982). These enzymes contain PTS1 at their C-termini (Tsugeki et al., 1993; Hayashi et al., 1996b; Mano et al., 1997, 1999). Since the ped2 mutant has a weakened ability to import PTS1-containing proteins, leaf peroxisomes in the mutant contain reduced amounts of these enzymes. Although photorespiration consists of many enzymic reactions located not only in leaf peroxisomes, but also in chloroplasts and mitochondria (Tolbert, 1982), reduced amounts of leaf peroxisomal enzymes diminish the overall activity of photorespiration in the ped2 mutant. The partial inhibition of photorespiration in the ped2 mutant is in good agreement with the observation that the ped2 mutant has a reduced ability to import PTS1- and PTS2-containing proteins.
Functional differentiation is a remarkable feature of peroxisomes in higher plant cells (Nishimura et al., 1996). In order to perform different physiological functions, glyoxysomes, leaf peroxisomes and unspecialized peroxisomes contain different sets of enzymes (Beevers, 1979; Tolbert, 1982). However, the present data suggest that each of the plant peroxisomes imports these enzymes using the same protein import machinery that involves AtPex14p, and that the pleiotropic phenotype of the ped2 mutant is caused by a defect in a single gene product, AtPex14p.
Materials and methods
Plant materials and growth conditions
Identification of the ped2 mutant of Arabidopsis thaliana has been described previously (Hayashi et al., 1998). Progenies that had been back-crossed twice were used in this study. Arabidopsis thaliana ecotype Landsberg erecta was used as the wild-type plant. Seeds of the stm mutant were kindly provided by the Arabidopsis Biological Resource Center, Ohio State University. Plants were grown under a 16 h light (100 µE/m2/s)/8 h dark cycle at 22°C.
Fine mapping of the PED2 gene
The ped2 mutant was outcrossed to the wild-type plant [ecotype Columbia (Col-0)]. F2 progeny, obtained by self-fertilization of the F1 plants, were germinated on growth medium without sucrose (Hayashi et al., 1998). Three hundred and ten seedlings that could not expand green cotyledons and leaves on the growth medium were recovered after transferring these seedlings to medium containing sucrose. The genomic DNA of these F2 plants was individually isolated. Recombinations that occurred between the PED2 locus and the molecular markers were scored by using the CAPS mapping procedure (Konieczny and Ausubel, 1993). The molecular marker, LFY3, has been described previously (Konieczny and Ausubel, 1993). The nucleotide sequences of the primers and the enzymes for other molecular markers are as follows. MRG21-3: GAGCATCGAAATGCGTCACG and GTCTTCTTTGATCCGATTAGACCG, RsaI; MQB2-1: TGACTTGCTGTCTGAGGTTCC and TCACTGATTCCACCGATTCC, RsaI; MQB2-4: CGCCTTGATTGTTGCTTCTACC and CGTGTCAAGGCCAATAGTCC, HinfI; MHJ24-4: TGGTCCATATTCCTGAAGACG and CGCTCTTCACAATGATCTGC, NcoI.
DNA sequencing analyses
DNA and RNA extraction, sequence determination and routine molecular biological techniques were performed by standard techniques (Sambrook et al., 1989). For identification of the PED2 gene, the DNA fragments were amplified by the PCR using 100 ng of genomic DNA isolated from wild-type plants and the ped2 mutant as templates, a 5′ primer (CTTCCAAGGTTAGTGAGCTGC) and a 3′ primer (GGCTCTTCACTCATGCTTCC). A PED2 cDNA clone was generated by RT–PCR with total RNA isolated from 7-day-old cotyledons of wild-type plants using a 5′ primer (CTTCCAAGGTTAGTGAGCTGC) and a 3′ primer (GTTTTTAGTTCCCTTCCTGGC). Analysis of the nucleotide sequences of those DNA fragments was performed according to a previous report (Hayashi et al., 1998).
Construction of clone for complementation studies and plant transformation
The P1 clone, MQB2, which contains the PED2 gene was kindly sent from the Kazusa DNA Institute, Chiba, Japan. XhoI fragments obtained after complete digestion of MQB2 DNA were subcloned into the XhoI site of Bluescript KS+. The XhoI fragment (7734 bp) that had been inserted in the vector was recovered, and then ligated to the XhoI site of pBI121Δ35S. A plant binary vector, pBI121Δ35S, was constructed from pBI121 (Clontech, Palo Alto, CA) by replacing a HindIII–SacI fragment with a polylinker consisting of HindIII, XhoI, SmaI, SpeI and SacI sites. The vector containing the XhoI fragment was designated as pBI-PED2. pBI-PED2 was introduced into the ped2 mutant by the vacuum infiltration method (Bechtold et al., 1993) using Agrobacterium tumefaciens (strain C58C1RifR) harboring pBI-PED2. T3 transformants harboring homozygous transgenes were analyzed by plating on either growth medium containing 0.2 µg/ml 2,4-DB or growth medium without sucrose.
Immunoblotting
A DNA fragment encoding from Met1 to Pro100 of AtPex14p was amplified from the PED2 cDNA by PCR using a 5′ primer (GGGAGCTCGCTGCTATGGCGACT) and a 3′ primer (CCCTCGAGTTAAGGAACACGGCGGAAAGCTT). The amplified DNA was inserted into the pET32 vector (Novagen, Madison, WI). A fusion protein was synthesized in Escherichia coli cells, and used for the production of rabbit antibody raised against AtPex14p according to the method reported previously (Hayashi et al., 1999). We also used antibodies raised against pumpkin 3-ketoacyl CoA thiolase (Kato et al., 1996b), pumpkin malate dehydrogenase (Kato et al., 1998), caster bean isocitrate lyase (Maeshima et al., 1988) and pumpkin ascorbate peroxidase (Yamaguchi et al., 1995). Immunoblot analyses were performed according to protocols described previously (Hayashi et al., 1998).
Subcellular fractionation of etiolated pumpkin cotyledons and analyses of the intact glyoxysomes
Subcellular fractionation of 5-day-old pumpkin etiolated cotyledons (2 g, fresh weight) was performed using 30–60% (w/w) sucrose density gradient centrifugation according to the method previously reported (Hayashi et al., 1999). After the centrifugation, fractions of 0.5 ml were collected, and used for immunoblot analyses and enzyme assays.
Isolation of intact glyoxysomes from 5-day-old pumpkin etiolated cotyledons (100 g, fresh weight) by Percoll density gradient centrifugation has also been reported previously (Yamaguchi et al., 1995). The intact glyoxysomes (250 µg total protein) were resuspended in 200 µl of either low salt buffer, high salt buffer or alkaline solution. Each solution consists of 10 mM HEPES–KOH pH 7.2, 500 mM KCl with 10 mM HEPES–KOH pH 7.2 and 0.1 M Na2CO3 pH 11, respectively. After centrifugation at 100 000 g for 30 min, these samples were separated into supernatant and pellet. The pellets were resuspended in 200 µl of 100 mM HEPES–KOH pH 7.2. In some experiments, the intact glyoxysomes (250 µg total protein) were incubated in buffer containing 10 mM HEPES–KOH pH 7.2, 1 mM EDTA and 0.3 M mannitol with an appropriate concentration of proteinase K for 30 min at 4°C in the presence or absence of 5% Triton X-100. The reactions were terminated by the addition of 5 mM phenylmethylsulfonyl fluoride.
Detection of fluorescence in ped2 mutants expressing GFP–PTS1 and GFP
To produce ped2 mutants expressing GFP–PTS1 and GFP, ped2 mutants were outcrossed to transgenic Arabidopsis expressing GFP–PTS1 or GFP. Generation of these kanamycin-resistant transgenic plants was reported previously (Mano et al., 1999). Homozygous F3 plants (ped2/ped2, GFP–PTS1/GFP–PTS1 and ped2/ped2, GFP/GFP) were identified by determining the sucrose requirement and the kanamycin resistance during post-germinative growth. The homozygous F3 plants and transgenic wild-type plant expressing GFP were grown for 10 days on growth medium under constant illumination. Roots of these plants were mounted under coverslips with phosphate-buffered saline. The specimens were examined using a TCS NT laser-scanning confocal microscope under the same condition with ×40 objective lens and an FITC filter set (Leica Japan, Tokyo, Japan).
Immunoelectron microscopic analysis
Five-day-old etiolated cotyledons, 7-day-old green cotyledons, 14-day-old leaves and 14-day-old roots were harvested from plants that were grown for the appropriate number of days in darkness or under constant illumination. Preparation of the ultrathin sections, and the immunoelectron microscopic analyses were performed according to the protocol described previously (Hayashi et al., 1998).
Measurements of maximum quantum yield of photosystem II (Fv/Fm)
To reduce the effect of photoinhibition, plants were grown for 4 weeks in an atmosphere containing 1000 Pa CO2 under low light (50 µE/m2/s). These plants were then illuminated with strong light (450 µE/m2/s) for 0, 30, 60, 120 and 240 min in a normal atmosphere (36 Pa CO2). After the end of each illumination period, plants were kept for 30 min in the dark. The ratio of variable fluorescence to maximum fluorescence (Fv/Fm) was automatically calculated from the result of the modulated chlorophyll fluorescence emission from the upper surface of dark-adapted leaves, which was measured using a pulse amplitude modulation fluorometer (Mini-PAM; H.Walz, Effeltrich, Germany).
Acknowledgments
Acknowledgements
This work was supported in part by a grant from the Research for the Future Program of the Japanese Society for the Promotion of Science (JSPS-RFTF96L00407), and by grants-in-aid for scientific research from the Ministry of Education, Science and Culture of Japan (12440231 to M.N. and 12640625 to M.H.) and by a grant from the Nissan Science Foundation (Tokyo, Japan) to M.H.
References
- Albertini M., Rehling,P., Erdmann,R., Girzalsky,W., Kiel,J.A.K.W., Veenhuis,M. and Kunau,W.H. (1997) Pex14p, a peroxisomal membrane protein binding both receptors of the two PTS-dependent import pathways. Cell, 89, 83–92. [DOI] [PubMed] [Google Scholar]
- Bechtold N., Ellis,J. and Pelletier,G. (1993) In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci., 316, 1194–1199. [Google Scholar]
- Beevers H. (1979) Microbodies in higher plants. Annu. Rev. Plant Physiol., 30, 159–193. [Google Scholar]
- Beevers H. (1982) Glyoxysomes in higher plants. Ann. N Y Acad. Sci., 386, 243–251. [Google Scholar]
- Brickner D.G., Brickner,J.H. and Olsen,J.J. (1998) Sequence analysis of a cDNA encoding Pex5p, a peroxisomal targeting signal type 1 receptor from Arabidopsis. Plant Physiol., 118, 330. [Google Scholar]
- Brocard C., Lametschwandtner,G., Koudelka,R. and Hartig,A. (1997) Pex14p is a member of the protein linkage map of Pex5p. EMBO J., 16, 5491–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis L. and Nishimura,M. (1991) Development of enzymes of the glyoxylate cycle during senescence of pumpkin cotyledons. Plant Cell Physiol., 32, 555–561. [Google Scholar]
- Flynn C.R., Mullen,R.T. and Trelease,R.N. (1998) Mutational analyses of a type 2 peroxisomal targeting signal that is capable of directing oligomeric protein import into tobacco BY-2 glyoxysomes. Plant J., 16, 709–720. [DOI] [PubMed] [Google Scholar]
- Franklin L.A., Levavasseur,G., Osmond,C.B., Henley,W.J. and Ramus,J. (1992) Two components of onset and recovery during photoinhibition of Ulva rotundata. Planta, 187, 399–408. [DOI] [PubMed] [Google Scholar]
- Gietl C., Faber,K.N., Vanderklei,I.J. and Veenhuis,M. (1994) Mutational analysis of the N-terminal topogenic signal of watermelon glyoxysomal malate dehydrogenase using the heterologous host Hansenula polymorpha. Proc. Natl Acad. Sci. USA, 91, 3151–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., De Bellis,L., Ciurli,A., Kondo,M., Hayashi,M. and Nishimura,M. (1999) A novel acyl-CoA oxidase that can oxidize short-chain acyl-CoA in plant peroxisomes. J. Biol. Chem., 274, 12715–12721. [DOI] [PubMed] [Google Scholar]
- Hayashi M. (2000) Plant peroxisomes: molecular basis of the regulation of their functions. J. Plant Res., 113, 103–109. [Google Scholar]
- Hayashi M., Aoki,M., Kato,A., Kondo,M. and Nishimura,M. (1996a) Transport of chimeric proteins that contain a carboxy-terminal targeting signal into plant microbodies. Plant J., 10, 225–234. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Tsugeki,R., Kondo,M., Mori,H. and Nishimura,M. (1996b) Pumpkin hydroxypyruvate reductases with and without a putative C-terminal signal for targeting to microbodies may be produced by alternative splicing. Plant Mol. Biol., 30, 183–189. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Aoki,M., Kondo,M. and Nishimura,M. (1997) Changes in targeting efficiencies of proteins to plant microbodies caused by amino acid substitutions in the carboxy-terminal tripeptide. Plant Cell Physiol., 38, 759–768. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Toriyama,K., Kondo,M. and Nishimura,M. (1998) 2,4-di chlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell, 10, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhse B., Rehling,P., Albertini,M., Blank,L., Meller,K. and Kunau,W.H. (1998) Pex17p of Saccharomyces cerevisiae is a novel peroxin and component of the peroxisomal protein translocation machinery. J. Cell Biol., 140, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Hayashi,M., Kondo,M. and Nishimura,M. (1996a) Targeting and processing of a chimeric protein with the N-terminal presequence of the precursor to glyoxysomal citrate synthase. Plant Cell, 8, 1601–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Hayashi,M., Takeuchi,Y. and Nishimura,M. (1996b) cDNA cloning and expression of a gene for 3-ketoacyl-CoA thiolase in pumpkin cotyledons. Plant Mol. Biol., 31, 843–852. [DOI] [PubMed] [Google Scholar]
- Kato A., Takeda-Yoshikawa,Y., Hayashi,M., Kondo,M., Hara-Nishimura,I. and Nishimura,M. (1998) Glyoxysomal malate dehydrogenase in pumpkin: cloning of a cDNA and functional analysis of its presequence. Plant Cell Physiol., 39, 186–195. [DOI] [PubMed] [Google Scholar]
- Kato A., Hayashi,M. and Nishimura,M. (1999) Oligomeric proteins containing N-terminal targeting signals are imported into peroxisomes in transgenic Arabidopsis. Plant Cell Physiol., 40, 586–591. [DOI] [PubMed] [Google Scholar]
- Komori M., Rasmussen,S.W., Kiel,J.A.K.W., Baerends,R.J.S., Cregg,J.M., Van der Klei,I.J. and Veenhuis,M. (1997) The Hansenula polymorpha PEX14 gene encodes a novel peroxisomal membrane protein essential for peroxisome biogenesis. EMBO J., 16, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A. and Ausubel,F.M. (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J., 4, 403–410. [DOI] [PubMed] [Google Scholar]
- Kragler F., Lametschwandtner,G., Christmann,J., Hartig,A. and Harada,J.J. (1998) Identification and analysis of the plant peroxisomal targeting signal 1 receptor NtPEX5. Proc. Natl Acad. Sci. USA, 95, 13336–13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause G.H. (1988) Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant., 74, 566–574. [Google Scholar]
- Lee M.S., Mullen,R.T. and Trelease,R.N. (1997) Oilseed isocitrate lyases lacking their essential type 1 peroxisomal targeting signal are piggybacked to glyoxysomes. Plant Cell, 9, 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Huertas E., Oh,J.S. and Baker,A. (1999) Antibodies against Pex14p block ATP-independent binding of matrix proteins to peroxisomes in vitro. FEBS Lett., 459, 227–229. [DOI] [PubMed] [Google Scholar]
- Maeshima M., Yokoi,H. and Asahi,T. (1988) Evidence for no proteolytic processing during transport of isocitrate lyase into glyoxysomes in castor bean endosperm. Plant Cell Physiol., 29, 381–384. [Google Scholar]
- Mano S., Hayashi,M., Kondo,M. and Nishimura,M. (1997) Hydroxy pyruvate reductase with a carboxy-terminal targeting signal to microbodies is expressed in Arabidopsis. Plant Cell Physiol., 38, 449–455. [DOI] [PubMed] [Google Scholar]
- Mano S., Hayashi,M. and Nishimura,M. (1999) Light regulates alternative splicing of hydroxypyruvate reductase in pumpkin. Plant J., 17, 309–320. [DOI] [PubMed] [Google Scholar]
- Mori H. and Nishimura,M. (1989) Glyoxysomal malate synthase is specifically degraded in microbodies during greening of pumpkin cotyledons. FEBS Lett., 244, 163–166. [Google Scholar]
- Nishimura M., Yamaguchi,J., Mori,H., Akazawa,T. and Yokota,S. (1986) Immunocytochemical analysis shows that glyoxysomes are directly transformed to leaf peroxisomes during greening of pumpkin cotyledons. Plant Physiol., 80, 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Takeuchi,Y., De Bellis,L. and Hara-Nishimura,I. (1993) Leaf peroxisomes are directly transformed to glyoxysomes during senescence of pumpkin cotyledons. Protoplasma, 175, 131–137. [Google Scholar]
- Nishimura M., Hayashi,M., Kato,A., Yamaguchi,K. and Mano,S. (1996) Functional transformation of microbodies in higher plant cells. Cell Struct. Funct., 21, 387–393. [DOI] [PubMed] [Google Scholar]
- Öquist G., Chow,W.S. and Andersson,J.M. (1992) Photoinhibition of photosynthesis represents a mechanism for the long term regulation of photosystem II. Planta, 186, 450–460. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Schumann U., Gietl,C. and Schmid,M. (1999) Sequence analysis of a cDNA encoding Pex7p, a peroxisomal targeting signal 2 receptor from Arabidopsis. Plant Physiol., 120, 339.10409053 [Google Scholar]
- Shimizu N. et al. (1999) The peroxin Pex14p—cDNA cloning by functional complementation on a Chinese hamster ovary cell mutant, characterization and functional analysis. J. Biol. Chem., 274, 12593–12604. [DOI] [PubMed] [Google Scholar]
- Somerville C.R. and Ogren,W.L. (1981) Photorespiration-deficient mutants of Arabidopsis thaliana lacking mitochondrial serine transhydroxymethylase activity. Plant Physiol., 67, 666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C.R. and Ogren,W.L. (1982) Genetic modification of photorespiration. Trends Biochem. Sci., 7, 171–174. [Google Scholar]
- Subramani S. (1998) Components involved in peroxisome import, bio genesis, proliferation, turnover and movement. Physiol. Rev., 78, 171–188. [DOI] [PubMed] [Google Scholar]
- Titus D.E. and Becker,W.M. (1985) Investigation of the glyoxysome– peroxisome transition in germinating cucumber cotyledons using double-label immunoelectron microscopy. J. Cell Biol., 101, 1288–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N.E. (1982) Leaf peroxisomes. Ann. N Y Acad. Sci., 386, 254–268. [Google Scholar]
- Trelease R.N., Lee,M.S., Banjoko,A. and Bunkelmann,J. (1996) C-terminal polypeptides are necessary and sufficient for in vivo targeting of transiently-expressed proteins to peroxisomes in suspension-cultured plant cells. Protoplasma, 195, 156–167. [Google Scholar]
- Tsugeki R., Hara-Nishimura,I., Mori,H. and Nishimura,M. (1993) Cloning and sequencing of cDNA for glycolate oxidase from pumpkin cotyledons and Northern blot analysis. Plant Cell Physiol., 34, 51–57. [PubMed] [Google Scholar]
- Will G.K., Soukupova,M., Hong,X., Erdmann,K.S., Kiel,J., Dodt,G., Kunau,W.H. and Erdmann,R. (1999) Identification and characterization of the human orthologue of yeast pex14p. Mol. Cell. Biol., 19, 2265–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer C., Schmid,M., Veenhuis,M. and Gietl,C. (1998) The plant PTS1 receptor: similarities and differences to its human and yeast counterparts. Plant J., 16, 453–464. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Mori,H. and Nishimura,M. (1995) A novel isoenzyme of ascorbate peroxidase localized on glyoxysomal and leaf peroxisomal membranes in pumpkin. Plant Cell Physiol., 36, 1157–1162. [DOI] [PubMed] [Google Scholar]