Abstract
Id proteins are thought to be negative regulators of cell differentiation and positive regulators of cell proliferation. Mammary glands of Id2–/– female mice reveal severely impaired lobulo-alveolar development during pregnancy. Id2–/– mammary epithelia show no precocious maturation, but instead exhibit intrinsic defects in both cell proliferation and cell survival, implying that the role of Id2 in pregnant mammary epithelia is mainly stimulation of cell proliferation and support of cell viability. Expression studies of genes required for mammary gland development suggest Id2 to be a downstream or parallel factor of these genes. A decrease in the DNA binding activity of Stat5 was also observed in Id2–/– mammary glands at 7 days post-coitus. Our results indicate an indispensable role of Id2 in pregnant mammary glands.
Keywords: apoptosis/cell proliferation/HLH inhibitor/Id2/mammary gland
Introduction
The mammary gland is a unique organ, terminal differentiation of which is achieved during pregnancy under the influence of various hormones, including estrogen, progesterone and prolactin, and epithelial–mesenchymal interaction (Neville and Daniel, 1987). Dramatic changes in the mammary epithelia during pregnancy, characterized by expansion of the lobulo-alveolar compartment and maturation of epithelial cells for milk secretion, provides a good experimental model system to study the mechanisms that regulate cell proliferation and differentiation in vivo (Neville and Daniel, 1987).
Mammary gland development consists of multiple steps; establishment of the anlage during embryogenesis, ductal elongation and branching in puberty and lobulo-alveolar expansion in pregnancy (Neville and Daniel, 1987). Recent studies using gene targeting experiments have revealed some of the genetic components involved in each step of mammary gland development in mice (reviewed in Hennighausen and Robinson, 1998). Parathyroid hormone-related peptide is required to establish the mammary gland anlage during development (Wysolmerski et al., 1998). The estrogen receptor is essential for ductal elongation (Korach et al., 1996) and genes required for lobulo-alveolar development include those encoding prolactin, prolactin receptor, Stat5a, progesterone receptor, SRC-1, cyclin D1, C/EBPβ and A-myb (Fantl et al., 1995, 1999; Lydon et al., 1995; Sicinski et al., 1995; Horseman et al., 1997; Humphreys et al., 1997; Liu et al., 1997; Ormandy et al., 1997; Toscani et al., 1997; Brisken et al., 1998, 1999; X.Liu et al., 1998; Robinson et al., 1998; Seagroves et al., 1998; Teglund et al., 1998; Xu et al., 1998). Although each of these genes has been shown to play an essential role in mammary gland development, their relationships are still largely unknown.
Transcription factors bearing a basic helix–loop–helix (bHLH) motif, exemplified by MyoD, are crucial for various cell differentiation processes during development in multicellular organisms (Weintraub et al., 1991; Massari et al., 2000). These factors are divided into two classes according to their expression patterns; ubiquitous bHLH factors (also known as E proteins) such as the E2A gene products E12 and E47, and tissue-specific bHLH factors such as MyoD and neurogenin (Weintraub et al., 1991; Massari and Murre, 2000). Id proteins, inhibitors of DNA binding/differentiation, which are characterized by their lack of the basic DNA-binding domain but retention of the HLH dimerization motif, inhibit the functions of these bHLH transcription factors in a dominant-negative manner by suppressing their heterodimerization partners through the HLH domains (Benezra et al., 1990; Norton et al., 1998). They have also been shown to stimulate the G1/S transition in the cell cycle in several cell culture systems, implying their roles in cell proliferation (reviewed in Norton et al., 1998). Thus, Id proteins are considered to be positive regulators of cell proliferation as well as negative regulators of cell differentiation. Four related proteins have been identified in mammals so far (Benezra et al., 1990; Christy et al., 1991; Sun et al., 1991; Riechmann et al., 1994) and several lines of emerging evidence have demonstrated their involvement in various cell differentiation processes and cellular functions in vivo. We previously reported that mice deficient in Id2 are devoid of lymph nodes and Peyer’s patches due to lack of a cell population essential for the generation of peripheral lymphoid organs (Yokota et al., 1999). In addition, Id2 null mutant mice display disturbed differentiation of natural killer cells (Yokota et al., 1999). In mice lacking Id3, the immune response and B-cell proliferation are impaired (Pan et al., 1999). Moreover, Id1 and Id3 double knockout mice exhibit precocious maturation of neurons with elevated expression of cyclin-dependent kinase inhibitors and vascular malformation in the central nervous system (Lyden et al., 1999).
Here we report that Id2 is indispensable for mammary gland development during pregnancy. The observed defect is intrinsic to mammary epithelial cells. Our results suggest that the function of Id2 in pregnant mammary glands is to stimulate cell cycle progression and to support cell survival.
Results
During the course of colony expansion of Id2-deficient mice we noticed that pups born to Id2–/– mothers died within 2 days after birth. The deaths were presumably due to dehydration, as examination revealed the absence of milk in their stomachs. This phenomenon was specific to homozygosity of the mothers and was not related to the genotype of the pups. Since Id2–/– mothers showed normal nursing behavior, a lactation defect was suspected. In cross-fostering experiments between Id2+/– and Id2–/– mothers none of 24 pups survived with Id2–/– mothers, while 34 out of 36 pups nursed by Id2+/– mothers did, irrespective of the genotype of the fostered pups (genotype ratios +/+:+/–:–/– were 0:22:14 and 1:11:12 for pups fostered by Id2+/– and Id2–/– mothers, respectively). The results confirmed that Id2–/– mothers have a lactation defect.
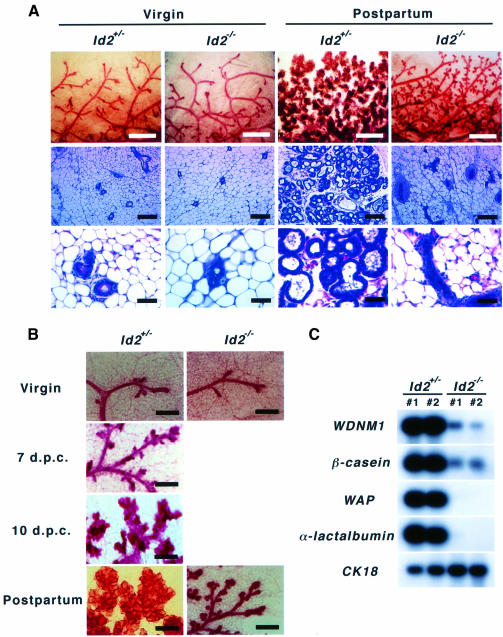
Whole mount analyses of the mammary glands demonstrated that Id2–/– virgin female mice have normal ductal trees, similar to those of heterozygous mice, indicating that normal development occurs before pregnancy (Figure 1A). Examination on the day of delivery, however, revealed that the glands of Id2–/– females were poor in lobulo-alveolar tissues and remained immature, comparable with those of Id2+/– mice at ∼7 days post-coitus (d.p.c.), although side branching of mammary ducts was observed (Figure 1A, upper, and B). This failure of lobulo-alveolar expansion in Id2 null mammary glands was also confirmed by histological analyses of sectioned specimens (Figure 1A, middle and lower). To evaluate the maturation status of the Id2–/– mammary glands, gene expression of milk proteins was investigated by northern blotting. Milk protein genes such as WDNM1, β-casein, α-lactalbumin and WAP start to be expressed sequentially in the mammary glands during pregnancy (Robinson et al., 1995). In contrast to the abundant expression of all of these genes in Id2+/– mammary glands, only the early markers, WDNM1 and β-casein, were weakly detected in Id2–/– mammary glands on the day of delivery (Figure 1C), indicating poor differentiation. These results demonstrated that Id2 is dispensable prior to pregnancy but essential during pregnancy for mammary gland development.
Fig. 1. Impaired mammary gland development in Id2–/– mice during pregnancy. (A) (Upper) Whole mount analyses of mammary glands by carmine alum staining (lower magnification, scale bars represent 500 µm). (Middle and lower) Histological analyses of mammary glands by hematoxylin and eosin staining. The middle and lower panels are lower and higher magnification, respectively (scale bars represent 400 and 100 µm, respectively). Virgin glands of 8-week-old females (left) and glands on the day of delivery (right). Genotypes are indicated at the top of the panels. (B) Whole mount analyses of mammary glands (higher magnification). The glands of Id2+/– (left) (virgin, 7 and 10 d.p.c. and 1 day post-partum) and Id2–/– (right) (virgin and 1 day post-partum) mice are shown. Scale bars represent 50 µm. (C) Expression of milk protein genes. Total RNA extracted from mammary glands on the day of delivery was analyzed by northern hybridization. WDNM1 and β-casein were used as early markers of milk genes. As markers for later stages, α-lactalbumin and WAP were used. CK18 is a marker for epithelial cells. Probes used are indicated on the left. Two examples are shown for each genotype group.
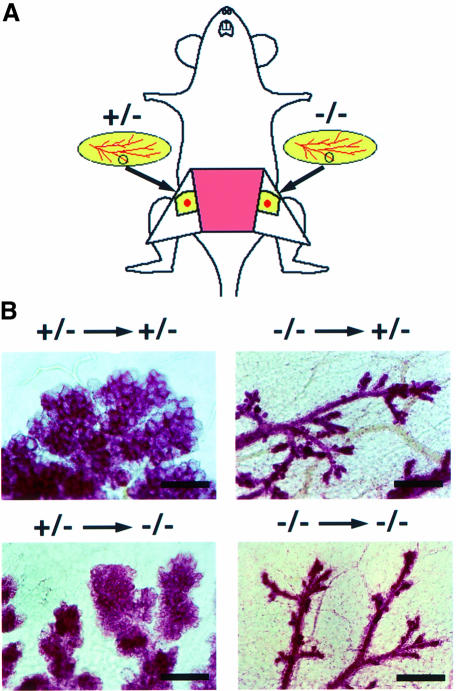
Maturation of mammary glands during pregnancy requires complex hormonal stimulation. Epithelial– mesenchymal interaction is important for mammary gland development not only during generation of the glands but also in pregnancy (Neville and Daniel, 1987). To determine which component is responsible for the lactation defect, we performed transplantation experiments (Figure 2). A cleared fat pad was prepared by completely removing the area containing endogenous mammary glands at 3 weeks of age. As shown in Figure 2A, small pieces of mammary glands were then implanted into a cleared fat pad and allowed to develop into secondary mammary glands in the host. Using this technique, the epithelium contained in the transplanted mammary gland can grow and penetrate the host fat pad and become associated with the stroma of the host (Hoshino et al., 1976; Robinson and Hennighausen, 1997), although a contribution of stromal cells of donor origin is not completely excluded. These mice with chimeric mammary glands were mated and mammary tissues were analyzed on the day of delivery. Id2+/– glands showed lobulo-alveolar development not only in heterozygous but also in homozygous recipients, similar to the glands of normal pregnant mice (Figure 2B). These results indicate that normal hormonal function is preserved in Id2–/– females and suggested that Id2–/– stroma seem to be able to interact normally with donor type epithelial cells. On the other hand, transplanted Id2–/– epithelia failed to develop lobulo-alveolar tissues in both heterozygous and homozygous mice (Figure 2B), suggesting that Id2–/– mammary epithelial cells cannot respond to the normal environment of the host in the presence of hormones and mammary stroma. Taken together, the transplantation experiments suggest that the basis of the lactation defect of Id2 null mutants is intrinsic to the mammary epithelial cells.
Fig. 2. Intrinsic defect in Id2–/– mammary epithelial cells. (A) Schematic presentation of transplantations. Tissues prepared from Id2+/– and Id2–/– donors were transplanted into right and left clearedfat pads, respectively, of a recipient. (B) Whole mount analyses of engrafted mammary glands. Transplanted mammary glands were stained with carmine alum on the day of delivery. Directions of transplantation are indicated on the top of each panel. Scale bars represent 50 µm.
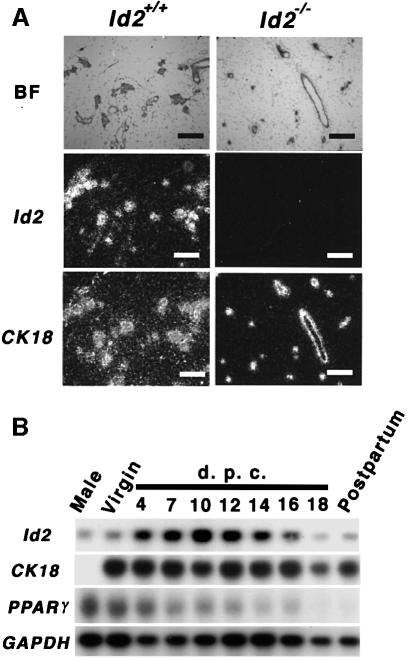
To support this notion we analyzed the spatio-temporal expression pattern of Id2. In situ hybridization revealed expression of Id2 in cells that are positive for cytokeratin 18 (CK18) (Figure 3A), indicating that Id2 is expressed in ductal and glandular epithelia of mouse mammary glands. Moreover, northern blot analyses revealed that Id2 expression is up-regulated during pregnancy and reaches its maximal level at ∼10 d.p.c. (Figure 3B). The stage that Id2 null mammary glands show an attenuation of gland development corresponds almost to the peak of Id2 expression (Figure 1A and B). These results confirmed that mammary epithelial cells are responsible for the lactation defect.
Fig. 3. Id2 expression in mammary glands. (A) In situ hybridization analyses of mammary glands at 7 d.p.c. Serial sections were hybridized to a CK18 antisense probe. (Left) Results for Id2+/+ mammary glands (Right) Results for Id2–/– mammary glands. Probes used are indicated on the left. Scale bars represent 400 µm. BF, bright field. (B) Northern blot analysis of the time course of Id2 expression in mammary glands. CK18 and PPARγ were used as markers of epithelial cells and adipocytes, respectively. As a loading control for RNA in each lane, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used. Probes used are indicated on the left.
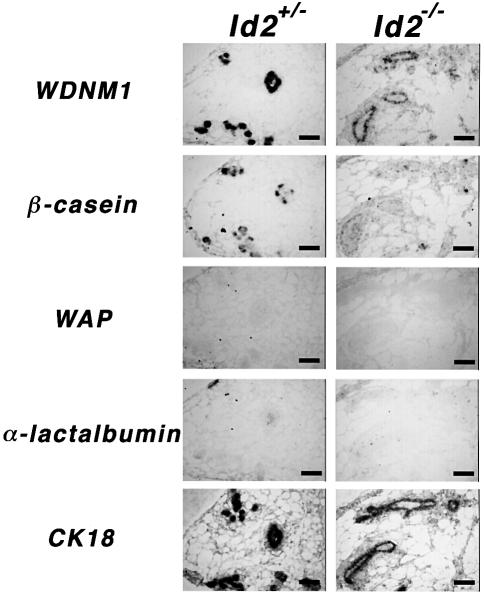
Id proteins have been shown to inhibit cell differentiation in many cell culture systems (reviewed in Norton et al., 1998), raising the possibility that the loss of Id2 leads to premature differentiation of epithelial cells and exit from the cell cycle. Incomplete differentiation of Id2–/– mammary glands at parturition, as shown in Figure 1A and B, may be the result of precocious maturation of epithelial cells during the early phase of pregnancy. We performed in situ hybridization analyses of mammary glands at 7 d.p.c. with probes for milk protein genes such as WDNM1, β-casein, WAP and α-lactalbumin. Id2–/– glands expressed WDNM1 and β-casein transcripts but the expression levels were much lower than those in Id2+/– glands. Neither WAP nor α-lactalbumin was detected in heterozygous and homozygous glands (Figure 4). These observations were compatible with northern blot analyses of mammary glands on the day of delivery, as shown in Figure 1C, and demonstrate no sign of precocious maturation in Id2 null mammary cells. Similar results were obtained in specimens at 10 d.p.c. (data not shown). Thus, it is unlikely that the defective alveolar development in Id2–/– glands is due to premature differentiation of epithelial cells.
Fig. 4. No precocious maturation in Id2–/– mammary glands. Mammary glands of Id2+/– (left) and Id2–/– (right) mice at 7 d.p.c. were analyzed by in situ hybridization. Probes used were the genes encoding milk proteins, WDNM1, β-casein, WAP and α-lactalbumin, as indicated on the left. CK18 was used as a marker of epithelial cells. Scale bars represent 200 µm.
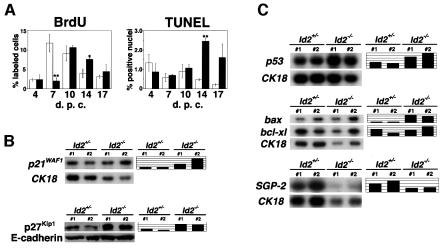
Next, bromodeoxyuridine (BrdU) incorporation and terminal deoxynucleotidyltransferase-mediated dUTP end-labeling (TUNEL) assays were performed to determine whether the decreased size of Id2 null glands was the result of attenuated cell proliferation or an increased rate of cell elimination by apoptosis (Figure 5A). Several reports have indicated that Id2 is a stimulator of the G1/S transition and able to enhance cell proliferation (Barone et al., 1994; Hara et al., 1994; Iavarone et al., 1994; Lasorella et al., 1996). The percentages of BrdU-positive epithelial cells in Id2+/– and Id2–/– mice were 11.8 ± 2.3 and 2.0 ± 0.8% at 7 d.p.c., respectively, demonstrating a marked contrast in the number of proliferating epithelial cells (Figure 5A). In contrast, no significant differences were detected at 4, 10 and 17 d.p.c. and more BrdU-positive epithelial cells were observed in Id2–/– mammary glands at 14 d.p.c. (4.0 ± 1.1 and 7.6 ± 0.4% in Id2+/– and Id2–/– epithelial cells, respectively) (Figure 5A). On the other hand, the TUNEL assay detected no significant differences at 4, 7 and 10 d.p.c. between Id2+/– and Id2–/– mammary epithelia, but the percentage of apoptotic cells increased in Id2–/– mammary epithelia at 14 d.p.c., as shown in Figure 5A (0.5 ± 0.04 and 2.5 ± 0.2% in Id2+/– and Id2–/– epithelial cells, respectively). This tendency was also observed at 17 d.p.c., although it is not statistically significant due to a variation among Id2–/– mammary epithelia. Thus, the defective mammopoiesis in the mutant mice seems to be derived from a combinatorial effect of a proliferation defect and apoptosis, which are observed in the early and late phases of pregnancy, respectively.
Fig. 5. Defective proliferation and enhanced apoptosis of pregnant Id2–/– mammary epithelial cells. (A) BrdU labeling index (left) and TUNEL assay (right). In these analyses >4000 nuclei of epithelial cells were examined in each of the samples taken at different days of pregnancy as indicated, and the percentages of BrdU-labeled or TUNEL-positive cells were determined. Columns represent the mean percentages of Id2+/– (white) and Id2–/– (black) mammary epithelia. Standard errors of the mean (n = 3) are shown. Student’s t-test was used for statistical analysis. Single and double asterisks indicate P values of <0.03 and <0.01, respectively. (B) Up-regulation of p21WAF1 and p27Kip1 in Id2–/– mammary glands. Total RNA or protein was extracted from mammary glands at 7 d.p.c. (Upper) RNase protection assays for p21WAF1 and CK18 in Id2+/– and Id2–/– mammary glands. Relative expression levels of p21WAF1 to CK18 are indicated on the right. (Lower) Western blot analysis of p27Kip1 and E-cadherin. Relative expression levels of p27Kip1 to E-cadherin are indicated on the right. The relative expression level of heterozygous mouse no. 2 is designated 100%. Genotypes are indicated at the top of each panel. (C) Examination of expression levels of several apoptosis-related genes in Id2 null mammary glands. Total RNA was prepared from 14 d.p.c. mammary glands. (Upper) p53 and CK18 expression determined by northern analysis. (Middle) RNase protection assays of bax, bcl-x and CK18. (Lower) Northern blot analysis of SGP-2 and CK18. Relative expression levels of p53, bax and bcl-xl compared with CK18 are shown on the right of each panel. The expression level of heterozygous mouse no. 2 is designated 100%. Genotypes are indicated at the top of each panel.
To investigate the molecular event underlying the proliferation defect in Id2–/– mammary epithelia in the early phase of pregnancy, expression levels of cell cycle regulators were next analyzed. Cyclin-dependent kinase inhibitors play important roles in cell cycle regulation (Sherr and Roberts, 1999) and Id proteins have been reported to be involved in the regulation of p16INK4a and p27Kip1 protein expression (Lyden et al., 1999) and p21WAF1 mRNA transcription (Prabhu et al., 1997). We therefore focused on their expression levels in mammary glands in early pregnancy. As shown in Figure 5B, p21WAF1 mRNA in Id2–/– glands was expressed at 2- to 5-fold higher levels than that in Id2+/– glands. p27Kip1 protein, which is regulated by translational controls (Hengst and Reed, 1996; Millard et al., 1997) and post-translational mechanisms (Pagano et al., 1995; Sheaff et al., 1997), was up-regulated in Id2 null mammary tissues, when compared with that of heterozygous glands (3- to 6-fold, as demonstrated in Figure 5B). Expression of p16INK4a was not detected even by RT–PCR analysis (data not shown), indicating that its level is substantially low in pregnant mammary glands. Thus, the proliferation defect of Id2 null mammary epithelial cells is accompanied by up-regulation of p21WAF1 and p27Kip1.
To explore the molecular basis of the enhanced apoptosis of Id2 null mammary cells in the late phase of pregnancy, p53 expression in 14 d.p.c. mammary glands was first analyzed by northern analysis (Figure 5C). Its expression level in Id2–/– mammary glands was elevated 2- to 3-fold (Figure 5C), suggesting that the p53-dependent pathway is involved in the cell death of Id2–/– mammary epithelia. Examination of the expression level of bax, which is a pro-apoptotic gene belonging to the Bcl-2 family and transactivated by p53 (Oltvai et al., 1993; Miyashita and Reed, 1995), revealed that there was a 7- to 8-fold higher level in Id2–/– mammary glands than in Id2+/– glands (Figure 5C). In contrast, no remarkable difference was observed in bcl-xl and bcl-2 mRNA expression (Figure 5C and data not shown), anti-apoptotic members of the Bcl-2 family (Vaux et al., 1988; Boise et al., 1993; Gonzalez-Garcia et al., 1994). bcl-xs mRNA was not detected, even after extended exposure (data not shown). These observations suggested that Id2 plays a role in late pregnant mammary cells as a cell survival factor by inhibiting the p53–Bax pathway. Elevated expression of p53 and bax is reminiscent of the situation that occurs during involution. Involution is a step by which secretory mammary epithelia are removed by massive apoptosis and lactation is terminated (Neville and Daniel, 1987). During involution the gene encoding sulfated glycoprotein-2 (SGP-2) (Buttyan et al., 1989) is strongly induced, in addition to p53 and bax (Strange et al., 1992; Heermeier et al., 1996; Metcalfe et al., 1999). As shown in Figure 5C, however, SGP-2 was not induced in Id2–/– mammary glands, excluding the possibility of an involution-like mechanism.
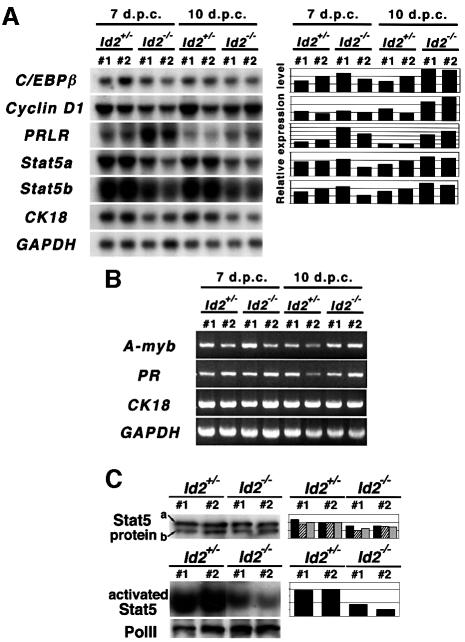
Recent studies have revealed genes that are essential for mammary gland development during pregnancy. Examples include cyclin D1, A-myb, progesterone receptor, prolactin receptor, Stat5a, Stat5b and C/EBPβ (Fantl et al., 1995, 1999; Lydon et al., 1995; Robinson et al., 1995; Sicinski et al., 1995; Humphreys et al., 1997; Liu et al., 1997; Ormandy et al., 1997; Toscani et al., 1997; Brisken et al., 1998, 1999; X.Liu et al., 1998; Seagroves et al., 1998; Teglund et al., 1998). To examine the relationship of Id2 to these genes, northern blot and RT–PCR analyses were performed in mammary glands at 7 and 10 d.p.c. (Figure 6). Northern blotting indicated no reduction in gene expression of C/EBPβ, cyclin D1, prolactin receptor, Stat5a or Stat5b at 7 and 10 d.p.c. Rather, up-regulation was observed for C/EBPβ and cyclin D1 at 10 d.p.c. (>2-fold compared with the control). Expression of prolactin receptor at 7 and 10 d.p.c. was also higher than that of the control, 2- and 4-fold, respectively (Figure 6A). Additionally, RT–PCR analyses demonstrated similar levels of expression of the A-myb and progesterone receptor genes (Figure 6B). These results revealed that Id2 is not an upstream factor of these genes in terms of transcriptional regulation.
Fig. 6. Id2 is a downstream or parallel factor of genes indispensable for lobulo-alveolar expansion. Expression of genes and proteins, indicated on the left, was analyzed in Id2+/– and Id2–/– mammary glands at the indicated d.p.c. (A) Northern analyses for C/EBPβ, prolactin receptor (PRLR), Stat5a and Stat5b expression. CK18 is an epithelial cell marker and GAPDH was used to quantitate total amount of RNA. The expression levels of C/EBPβ, PRLR, Stat5a and Stat5b relative to CK18 expression are shown on the right. The expression level of each gene in the 10 d.p.c. mammary glands of heterozygote no. 2 is designated 100%. (B) RT–PCR analyses for A-myb and progesterone receptor (PR) expression. CK18 was used as an epithelial cell marker. As an internal control, GAPDH was included. (C) Down-regulation of the DNA binding activity of Stat5. (Upper) Protein levels of Stat5 in whole cell extracts of 7 d.p.c. mammary glands were analyzed by immunoblotting. The samples used in Figure 5B were subjected to this analysis. Positions of proteins Stat5a and Stat5b are indicated by a and b, respectively. Relative expression levels of Stat5a (black columns), Stat5b (hatched columns) and the sum of Stat5a and Stat5b (dotted columns) to E-cadherin are indicated on the right. (Lower) The DNA binding activity of Stat5 in nuclear extracts of 7 d.p.c. mammary glands was analyzed by EMSA with a Stat5 recognition sequence. The protein levels of RNA polymerase II, analyzed by western blotting, serves as a loading control of nuclear extract. Quantitated and normalized DNA binding activity is shown on the right. In all panels two examples are shown for each genotype group.
Among the molecules analyzed for their gene expression, Stat5 is an intracellular transducer of the signal induced by prolactin (Wakao et al., 1994) and its activity as a transcription factor depends on phosphorylation of tyrosine residues (Hennighausen et al., 1997). We therefore tried to determine the actual activity of Stat5 (Figure 6C). For this the electromobility shift assay (EMSA) was applied, because preliminary experiments indicated that EMSA is more sensitive than detection of phosphotyrosine residues of activated Stat5 by immunoprecipitation–western blot analysis (data not shown). The amounts of Stat5a and Stat5b proteins in whole cell extracts were slightly decreased in Id2–/– mammary glands (Figure 6C, upper). The total DNA-binding activity of Stat5 in nuclear extracts of Id2–/– mammary glands, however, exhibited a further decrease to ∼40% of that of the control (Figure 6C, lower). These results suggest that decreased activity of Stat5, at least in part, participates in the lactation defect in Id2-deficient mice. Moreover, this raises the interesting possibility that Id2 plays a role in the Stat5 signaling pathway.
Discussion
We have shown that Id2-deficient female mice exhibit a severe lactation defect. Transplantation experiments suggest that the defect is intrinsic to mammary epithelial cells. This was confirmed by expression analyses of Id2 during pregnancy. Impaired cell proliferation and enhanced apoptosis of Id2-deficient mammary epithelia are evident in the early and late phases of pregnancy, respectively. Id2-deficient mammary epithelia remain poorly differentiated. Analyses of the expression levels of genes required for mammary gland development suggest that Id2 possibly acts downstream or parallel to these factors in mammary epithelia during pregnancy. In addition, the functional activity of Stat5 is decreased in Id2–/– mammary glands, implying the possible participation of Id2 in the Stat5 signaling pathway. Our results demonstrate that Id2 is involved in cell cycle progression and cell survival in pregnant mammary glands, enabling the expansion of epithelial cells during pregnancy.
Id proteins have been proposed to possess two functions; inhibition of cell differentiation and stimulation of cell proliferation (reviewed in Norton et al., 1998), both of which are mutually related. The former activity is executed by suppression of bHLH factors through interaction with the HLH regions, which has been considered to be a major role of Id proteins, particularly during development (Benezra et al., 1990; Norton et al., 1998). For the latter function an antagonistic activity of Id2 against the retinoblastoma protein and release of the suppression of p21WAF1 transcription by bHLH factors have been reported (Sorrentino et al., 1990; Iavarone et al., 1994; Prabhu et al., 1997), although the detailed mechanisms are still largely unclear. The former function allows us to speculate that a deficiency of Id2 would lead to accelerated or premature differentiation of mammary glands. In fact, precocious maturation of neuroepithelia is observed in Id1–/–Id3–/– mice, in association with up-regulation of expression of neuronal bHLH factors and other markers of neuronal differentiation (Lyden et al., 1999). Conversely, an overexpression study of Id1 in a mammary gland-derived cell line shows disturbed differentiation (Desprez et al., 1995). In this context we speculated that premature differentiation might be a cause of the lactation defect in Id2 mutant mice and thus that a bHLH factor(s) could participate in mammary gland development during pregnancy. However, no signs of precocious maturation were observed by in situ analyses of genes encoding milk proteins. We have also failed so far to identify any tissue-specific bHLH transcription factors that may be potentially involved in mammary gland development in pregnancy (S.Boku and Y.Yokota, unpublished data). This would suggest that the function of Id2 in pregnant mammary glands appears to be to regulate cell differentiation by controlling the activity of a potential bHLH factor, which in turn promotes differentiation of mammary glands. However, we cannot exclude the possibility of the involvement of a bHLH factor that suppresses differentiation of mammary glands. By inhibiting the activity of such a bHLH factor, Id2 may promote cell differentiation of mammary glands during normal mammary gland development.
BrdU incorporation experiments indicated that Id2 null mammary glands exhibit a defect in cell proliferation at 7 d.p.c., during the early phase of pregnancy. This time point corresponds to the induction of Id2 expression in wild-type mammary glands as shown by northern analysis. Id2–/– mammary glands remained attenuated at this stage, as demonstrated morphologically by whole mount staining and biochemically by the expression of milk protein genes. Proliferation of mammary epithelial cells during pregnancy reaches its maximum in the early phase, coinciding with high concentrations of serum hormones such as prolactin and progesterone (Virgo and Bellward, 1974; Talamantes et al., 1984; Neville and Daniel, 1987). Thus, the defect identified in Id2 null mutant mice suggests that the function of Id2 in the early phase of pregnancy is to stimulate cell cycle progression of mammary epithelial cells.
The high levels of p21WAF1 and p27Kip1 expression are compatible with the observed cell cycle arrest in Id2 null mammary glands. These observations suggest that activation of cyclin-dependent kinase inhibitors is responsible for the attenuated cell proliferation in Id2 null mammary epithelia during early pregnancy. Overexpression of the E2A gene in cultured cells enhances p21WAF1 transcription resulting in cell cycle arrest, which can be prevented by Id1 (Peverali et al., 1994; Prabhu et al., 1997). A similar effect is also induced by MyoD (Sorrentino et al., 1990). As the activity of bHLH factors such as the E2A gene products and MyoD is negatively regulated by Id proteins (Benezra et al., 1990), it is natural to think that loss of Id2 results in functional up-regulation of bHLH factors and therefore up-regulation of p21WAF1 transcription. However, although E2A and other E protein genes are actually expressed in mammary glands during pregnancy at constant levels (S.Mori and Y.Yokota, unpublished observation), a tissue-specific bHLH factor is less likely to be present in mammary glands, as discussed above. Homo- or heterodimers consisting of E proteins, which are usually formed in lymphoid cells (Bain et al., 1993; Benezra, 1994; Shen and Kadesch, 1995), may be involved in activation of p21WAF1 transcription. Alternatively, further investigations may identify a bHLH factor in pregnant mammary glands. p21WAF1 transcription is also known to be activated by p53 (el-Deiry et al., 1993) and Stat5 (Matsumura et al., 1997). In Id2 null mammary glands at 7 d.p.c. p53 expression is not elevated (S.Mori and Y.Yokota, unpublished observation) and the DNA-binding activity of Stat5 is decreased, making it unlikely that these factors are involved. The protein level of p27Kip1, on the other hand, is regulated by several mechanisms, such as degradation by the ubiquitin–proteasome pathway through phosphorylation of p27Kip1 by cyclin E–cyclin-dependent kinase 2 complexes (Pagano et al., 1995; Sheaff et al., 1997). The fact that developing neuroepithelia of Id1/Id3 compound null mutant embryos show a similar enhancement of p27Kip1 (Lyden et al., 1999) implies a common mechanism in altering the p27Kip1 level by Id proteins, although the detailed mechanism underlying the up-regulation of p27Kip1 is unclear at present.
In the late phase of pregnancy Id2–/– mammary epithelia exhibit significant BrdU incorporation and enhanced apoptosis. These suggest that the absence of Id2 does not affect the proliferation of mammary cells but that Id2 additionally plays a role as a survival factor in vivo, at least for late pregnant mammary epithelia. Considering that augmented apoptosis is observed on overexpression of Id proteins (Florio et al., 1998; Norton and Atherton, 1998), an appropriate level of Id2 protein may be important for cell growth and survival. Interestingly, cyclin D1, which is essential for development of mammary glands during pregnancy (Sicinski et al., 1995; Fantl et al., 1999), has been reported to be required for the viability of retinal cells (Ma et al., 1998). These observations imply that Id2 and cyclin D1 share similar functions in the regulation of cell proliferation and cell death. We have so far failed, however, to detect a physical interaction between Id2 and cyclin D1 using the yeast two-hybrid system (M.Tanji and Y.Yokota, unpublished observation).
In 7 d.p.c. Id2 null mammary glands, expression of the early maturation marker genes WDNM1 and β-casein is hardly detectable and apoptosis of epithelial cells is not significant. These data do not support the notion that differentiated Id2–/– mammary epithelia are removed by apoptosis in the early phase of pregnancy. In the late phase of pregnancy, on the other hand, WAP and α-lactalbumin expression commence in the glandular structure (Robinson et al., 1995), which fails to develop in Id2-deficient mammary glands. The loss of the alveolar component seems to be responsible for the impaired expression of these late milk proteins. Alternatively, differentiated cells expressing WAP and α-lactalbumin may be eliminated by apoptosis in Id2 null mammary glands.
Northern blot and RT–PCR analyses demonstrated that expression of genes involved in lobulo-alveolar development was not significantly reduced in Id2–/– mammary glands and, instead, genes such as prolactin receptor were up-regulated, indicating that expression of these genes is not dependent on the activity of Id2. It is tempting to think that signals elicited by hormonal stimuli converge on Id2 to promote cell cycle progression of mammary epithelial cells during early pregnancy. Alternatively, Id2 may act in parallel with molecules involved in lobulo-alveolar development of pregnant mammary glands. Among the genes analyzed, prolactin receptor was observed as being up-regulated at both 7 and 10 d.p.c. in Id2–/– mammary glands. This may reflect the immature state of Id2 null mammary glands, since prolactin receptor mRNA expression is gradually down-regulated as pregnancy proceeds in wild-type mice (S.Mori and Y.Yokota, unpublished observation).
In fact, the DNA-binding activity of Stat5 is decreased in Id2 null mammary glands, as demonstrated by EMSA, despite the increased prolactin receptor expression revealed by northern analyses and the normal hormonal environment as demonstrated by transplantation experiments. The mammary phenotype of Stat5a–/– mice (Liu et al., 1997; X.Liu et al., 1998; Teglund et al., 1998) is less severe than that of Id2–/– mice, suggesting that decreased activity of Stat5 is not a major cause of the lactation defect of Id2-deficient mice. Since we have failed so far to detect a direct association between Id2 and Stat5 by mammalian two-hybrid assays (S.Mori and Y.Yokota, unpublished observation), an indirect interaction might be involved in Stat5 signaling via molecules such as SOCS/CIS (Yoshimura et al., 1995; Starr et al., 1997) and PIAS (Chung et al., 1997; B.Liu et al., 1998), which regulate Stat5 activity at several steps (Starr and Hilton, 1999).
Among the genes required for lobulo-alveolar development, of note is cyclin D1, a cell cycle regulator, as discussed above. In ∼30–50% of breast cancer cases cyclin D1 is overexpressed and ∼15–20% of cases demonstrate amplification of the cyclin D1 gene locus (Lammie et al., 1991; Bartkova et al., 1994; Gillett et al., 1994; reviewed in Barnes and Gillett, 1998). In addition, transgenic mice expressing the cyclin D1 gene under control of the MMTV LTR develop mammary adenoma and hyperplasia (Wang et al., 1994). Id2 has been shown to be overexpressed in pancreatic cancers (Kleeff et al., 1998; Maruyama et al., 1999). Id2, which has the ability to stimulate cell cycle progression, may also be involved in the development of breast cancer. Preliminary overexpression experiments of Id2 using HC11, a mammary epithelial cell line, have failed so far to show anchorage-independent growth or tumorigenic potential when injected into nude mice (S.Mori and Y.Yokota, unpublished observation), as in the case of cyclin D1 (Han et al., 1995, 1996). We are now examining a role of Id2 in human breast cancer and establishing transgenic mouse lines that target Id2 expression in the mammary gland. These investigations would clarify a potential role of Id2 in mammary carcinogenesis, combined with studies using crosses of Id2–/– mice with mouse models for breast cancer, such as MMTV–cyclin D1 mice.
Materials and methods
Mice
Id2-deficient mice of mixed genetic background (129/Sv × NMRI) were used in most of the experiments (Yokota et al., 1999). For transplantation experiments mice with the 129/Sv background were used. No strain-dependent variation has been identified in mammary glands.
Tissue preparations and histology
Inguinal mammary glands no. 4 were used for all experiments. For histological analyses mice were anesthetized with diethyl ether and perfused with phosphate-buffered saline (PBS) containing 4% paraformaldehyde (PFA). Mammary glands were dissected out and post-fixed with PBS containing 4% PFA and 0.2% Tween-20 on ice overnight. They were embedded in paraffin according to standard procedures. Sections of 8 µm were used for each assay. Whole mount staining of mammary glands was performed as previously described (Horseman et al., 1997).
Transplantation experiments
Transplantations were carried out as described (DeOme et al., 1959; Hoshino et al., 1976). Briefly, fat pads containing inguinal mammary glands no. 4 on both sides were removed from 3-week-old female recipient mice. Complete removal of the mammary glands was confirmed by whole mount staining of dissected mammary glands no. 4. Two or three pieces of mammary tissue isolated from inguinal glands (approximately <2 mm in diameter) of 6-week-old mice were inserted into the cleared fat pads of recipient mice. Tissues prepared from Id2+/– and Id2–/– donors were transplanted into the right and left cleared fat pads, respectively, of the recipient. The transplanted mammary tissue formed chimeric secondary mammary glands mainly consisting of a donor-derived epithelial component and host-derived stroma (Hoshino et al., 1976; Robinson and Hennighausen, 1997). However, a minor contribution of donor-derived stroma and fatty tissues is not completely excluded. Six weeks after the operation, the recipients were mated and the grafted fat pads were harvested on the day of delivery for whole mount staining.
Cell proliferation and TUNEL assays
In vivo incorporation of BrdU was used for cell proliferation assays. Two hours before killing, BrdU (120 µg/g body wt) was injected i.p. into mice on the indicated days of pregnancy. After embedding the mammary glands in paraffin and sectioning, incorporated BrdU was detected with anti-BrdU antibody conjugated to horseradish peroxidase (Boehringer Mannheim) according to the manufacturer’s instructions.
Apoptotic cells were determined by the TUNEL method with an In Situ Cell Death Detection Kit (Boehringer Mannheim), combined with their apoptotic morphology.
Samples were counterstained with hematoxylin. Serially sectioned samples were examined by these analyses.
Northern blot, RNase protection and RT–PCR analyses
The northern blot and RNase protection assays were carried out using standard protocols as previously described (Chanda, 1995; Narumi et al., 2000). Probes for northern analyses were labeled with [32P]dCTP by the random priming method and those for RNase protection assays were prepared by in vitro transcription with [32P]UTP (Chanda, 1995). The probes used in this study were derived from the following plasmids: Id2, pRcCMV-Id2; PPARγ, pCMXmPPARg; cytokeratin 18, pBluecBSE; cyclin D1, pcBZ05.4; C/EBPβ, pMSV/EBPb; Stat5a, pME18S-Stat5a; Stat5b, pME18S-Stat5b; PRLR, pBS-mPRLR; p21WAF1, pBSmp21; p53, pp53-176; bax, pSFFVneo-HA-mBAX; bcl-xL, pSFFVneo-mBclXL; bcl-2, pSKMBBR; SGP-2, p1321. Plasmids carrying cDNAs for WDNM1, β-casein, α-lactalbumin, WAP and GAPDH were obtained by RT–PCR using primers designed according to the reported sequences in DDBJ/EMBL/GenBank. The expression levels of various molecular markers were quantitated with a BAS5000 Image Analyzer (Fuji Film).
RT–PCR was carried out using a standard protocol. Primers were designed according to the reported sequences in DDBJ/EMBL/GenBank.
Western blot and EMSA
Western blot analyses and EMSA were performed essentially as described previously (Narumi et al., 2000). Whole cell lysate prepared using NP-40 lysis buffer (Nakayama et al., 1996) was used for analyses of p27, E-cadherin and Stat5a/5b expression. E-cadherin was used as a marker of lumenal epithelial cells of mammary glands (Radice et al., 1997) and an internal control for whole cell lysates. RNA polymerase II was used as an internal control for nuclear extracts. The probe used in EMSA for detection of activated Stat5 was derived from the bovine β-casein promoter/enhancer region (Wakao et al., 1994). The specificity of shifted bands was confirmed by a supershift assay with anti-Stat5a/Stat5b specific antibodies (data not shown). Antibodies used in this study were anti-p27 rabbit antisera (15606E; Pharmingen), anti-E-cadherin rat monoclonal antibody (ECCD2; TaKaRa), anti-Stat5a rabbit antisera (L-20; Santa Cruz Biotechnology), anti-Stat5 mouse monoclonal antibody (G-2; Santa Cruz Biotechnology) and anti-RNA polymerase II rabbit antisera (C-21; Santa Cruz Biotechnology). Enhanced chemiluminescence reagents (Dupont-NEN Life Science) were used for visualization. Quantitation for EMSA and western analyses was done with a BAS5000 system (Fuji Film) and a Fluor-S MultiImager (Bio-Rad), respectively.
In situ hybridization
In situ hybridization was conducted as described (Mori et al., 1999). In the case of digoxygenin (DIG)-labeled RNA probes, alkaline phosphatase-conjugated anti-DIG antibody (Boehringer Mannheim) was used for detection. To enhance sensitivity, polyvinylalcohol was added to the color reaction (Yokota et al., 1999). The sense probe of CK18 was used as a negative control (data not shown).
Acknowledgments
Acknowledgements
We thank T.Neuman, K.Umesono, M.Nozaki, H.Matsushime, S.L.Mcknight, A.Miyajima, M.Sasaki, O.Niwa, I.Matsumura, G.Nunez, Y.Tsujimoto and R.Strange for providing us with materials. We thank P. Gruss for support during the initial stages of this study, including generation of mutant mice. This work was supported in part by grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan (07CE2005, 06277102, 10670119 and 06NP1101). S.M. is supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
References
- Bain G., Gruenwald,S. and Murre,C. (1993) E2A and E2-2 are subunits of B-cell-specific E2-box DNA-binding proteins. Mol. Cell. Biol., 13, 3522–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D.M. and Gillett,C.E. (1998) Cyclin D1 in breast cancer. Breast Cancer Res. Treat., 52, 1–15. [DOI] [PubMed] [Google Scholar]
- Barone M.V., Pepperkok,R., Peverali,F.A. and Philipson,L. (1994) Id proteins control growth induction in mammalian cells. Proc. Natl Acad. Sci. USA, 91, 4985–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J., Lukas,J., Muller,H., Lutzhoft,D, Strauss,M. and Bartek,J. (1994) Cyclin D1 protein expression and function in human breast cancer. Int. J. Cancer, 57, 353–361. [DOI] [PubMed] [Google Scholar]
- Benezra R. (1994) An intermolecular disulfide bond stabilizes E2A homodimers and is required for DNA binding at physiological temperatures. Cell, 79, 1057–1067. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis,R.L., Lockshon,D., Turner,D.L. and Weintraub,H. (1990) The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell, 61, 49–59. [DOI] [PubMed] [Google Scholar]
- Boise L.H., Gonzalez-Garcia,M., Postema,C.E., Ding,L., Lindsten,T., Turka,L.A., Mao,X., Nunez,G. and Thomson,C.B. (1993) bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell, 74, 597–608. [DOI] [PubMed] [Google Scholar]
- Brisken C., Park,S., Vass,T., Lydon,J.P., O’Malley,B.W. and Weinberg,R.A. (1998) A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl Acad. Sci. USA, 95, 5076–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C., Kaur,S., Chavarria,T.E., Binart,N., Sutherland,R.L., Weinberg,R.A., Kelly,P.A. and Ormandy,C.J. (1999) Prolactin controls mammary gland development via direct and indirect mechanisms. Dev. Biol., 210, 96–106. [DOI] [PubMed] [Google Scholar]
- Buttyan R., Olsson,C.A., Pintar,J., Chang,C., Bandyk,M., Ng,P.Y., and Sawczuk,I.S. (1989) Induction of the TRPM-2 gene in cells undergoing programmed death. Mol. Cell. Biol., 9, 3473–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda V.B. (1995) Current Protocols in Molecular Biology. Wiley, New York, NY. [Google Scholar]
- Christy B.A., Sanders,L.K., Lau,L.F., Copeland,N.G., Jenkins,N.A. and Nathans,D. (1991) An Id-related helix-loop-helix protein encoded by a growth factor-inducible gene. Proc. Natl Acad. Sci. USA, 88, 1815–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C.D., Liao,J., Liu,B., Rao,X., Jay,P., Berta,P. and Shuai,K. (1997) Specific inhibition of Stat3 signal transduction by PIAS3. Science, 278, 1803–1805. [DOI] [PubMed] [Google Scholar]
- DeOme K.B., Faulkin,L.J.,Jr, Bern,H.A. and Blair,P.B. (1959) Development of mammary tumours from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res., 19, 515–520. [PubMed] [Google Scholar]
- Desprez P.Y., Hara,E., Bissell,M.J. and Campisi,J. (1995) Suppression of mammary epithelial cell differentiation by the helix-loop-helix protein Id-1. Mol. Cell. Biol., 15, 3398–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W.S et al. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell, 75, 817–825. [DOI] [PubMed] [Google Scholar]
- Fantl V., Stamp,G., Andrews,A., Rosewell,I. and Dickson,C. (1995) Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev., 9, 2364–2372. [DOI] [PubMed] [Google Scholar]
- Fantl V., Edwards,P.A., Steel,J.H., Vonderhaar,B.K. and Dickson,C. (1999) Impaired mammary gland development in Cyl-1–/– mice during pregnancy and lactation is epithelial cell autonomous. Dev. Biol., 212, 1–11. [DOI] [PubMed] [Google Scholar]
- Florio M., Hernandez,M.-C., Yang,H., Shu,H.-K., Cleveland,J.L. and Israel,M.A. (1998) Id2 promotes apoptosis by a novel mechanism independent of dimerization to basic helix-loop-helix factors. Mol. Cell. Biol., 18, 5435–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett C., Fantl,V., Smith,R., Fisher,C., Bartek,J., Dickson,C., Barnes,D. and Peters,G. (1994) Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res., 54, 1812–1817. [PubMed] [Google Scholar]
- Gonzalez-Garcia M., Perez-Ballestero,R., Ding,L., Duan,L., Boise,L.H., Thomson,C.B. and Nunez,G. (1994) bcl-xL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria. Development, 120, 3033–3042. [DOI] [PubMed] [Google Scholar]
- Han E.K., Sgambato,A., Jiang,W., Zhang,Y.J., Santella,R.M., Doki,Y., Cacace,A.M., Schieren,I. and Weinstein,I.B. (1995) Stable overexpression of cyclin D1 in a human mammary epithelial cell line prolongs the S-phase and inhibits growth. Oncogene, 10, 953–961. [PubMed] [Google Scholar]
- Han E.K., Begemann,M., Sgambato,A., Soh,J.W., Doki,Y., Xing,W.Q., Liu,W. and Weinstein,I.B. (1996) Increased expression of cyclin D1 in a murine mammary epithelial cell line induces p27kip1, inhibits growth, and enhances apoptosis. Cell Growth Differ., 7, 699–710. [PubMed] [Google Scholar]
- Hara E., Yamaguchi,T., Nojima,H., Ide,T., Campisi,J., Okayama,H. and Oda,K. (1994) Id-related genes encoding helix-loop-helix proteins are required for G1 progression and are repressed in senescent human fibroblasts. J. Biol. Chem., 269, 2139–2145. [PubMed] [Google Scholar]
- Heermeier K., Benedict,M., Li,M., Furth,P., Nunez,G. and Hennighausen,L. (1996) Bax and Bcl-xs are induced at the onset of apoptosis in involuting mammary epithelial cells. Mech. Dev., 56, 197–207. [DOI] [PubMed] [Google Scholar]
- Hengst L. and Reed,S.I. (1996) Translational control of p27Kip1 accumulation during the cell cycle. Science, 271, 1861–1864. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. and Robinson,G.W. (1998) Think globally, act locally: the making of a mouse mammary gland. Genes Dev., 12, 449–455. [DOI] [PubMed] [Google Scholar]
- Hennighausen L., Robinson,G.W., Wagner,K.-U. and Liu,X. (1997) Prolactin signaling in mammary gland development. J. Biol. Chem., 272, 7567–7569. [DOI] [PubMed] [Google Scholar]
- Horseman N.D., Zhao,W., Montecino-Rodriguez,E., Tanaka,M., Nakashima,K., Engle,S.J., Smith,F., Markoff,E. and Dorshkind,K. (1997) Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J., 16, 6926–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K., Martin,F. and Machino,M. (1976) Early histogenesis of transplanted mouse mammary glands. III. Parenchymal–stromal interactions. Dev. Growth Differ., 18, 79–94. [DOI] [PubMed] [Google Scholar]
- Humphreys R.C., Lydon,J., O’Malley,B.W. and Rosen,J.M. (1997) Mammary gland development is mediated by both stromal and epithelial progesterone receptors. Mol. Endocrinol., 11, 801–811. [DOI] [PubMed] [Google Scholar]
- Iavarone A., Garg,P., Lasorella,A., Hsu,J. and Israel,M.A. (1994) The helix-loop-helix protein Id-2 enhances cell proliferation and binds to the retinoblastoma protein. Genes Dev., 8, 1270–1284. [DOI] [PubMed] [Google Scholar]
- Kleeff J., Ishiwata,T., Friess,H., Buchler,M.W., Israel,M.A. and Korc,M. (1998) The helix-loop-helix protein Id2 is overexpressed in human pancreatic cancer. Cancer Res., 58, 3769–3772. [PubMed] [Google Scholar]
- Korach K.S. et al. (1996) Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog. Horm. Res., 51, 159–188. [PubMed] [Google Scholar]
- Lammie G.A., Fantl,V., Smith,R., Schuuring,E., Brookes,S., Michalides,R., Dickson,C., Arnold,A. and Peters,G. (1991) D11S287, a putative oncogene on chromosome 11q13, is amplified and expressed in squamous cell and mammary carcinomas and linked to BCL-1. Oncogene, 6, 439–444. [PubMed] [Google Scholar]
- Lasorella A., Iavarone,A. and Israel,M.A. (1996) Id2 specifically alters regulation of the cell cycle by tumor suppressor proteins. Mol. Cell. Biol., 16, 2570–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Liao,J., Rao,X., Kushner,S.A., Chung,C.D., Chang,D.D. and Shuai,K. (1998) Inhibition of Stat1-mediated gene activation by PIAS1. Proc. Natl Acad. Sci. USA, 95, 10626–10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Robinson,G.W., Wagner,K.-U., Garrett,L., Wynshaw-Boris,A. and Hennighausen,L. (1997) Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev., 11, 179–186. [DOI] [PubMed] [Google Scholar]
- Liu X., Gallego,M.I., Smith,G.H., Robinson,G.W. and Hennighausen,L. (1998) Functional release of Stat5a-null mammary tissue through the activation of compensating signals including Stat5b. Cell Growth Differ., 9, 795–803. [PubMed] [Google Scholar]
- Lyden D. et al. (1999) Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature, 401, 670–677. [DOI] [PubMed] [Google Scholar]
- Lydon J.P., DeMayo,F.J., Funk,C.R., Mani,S.K., Hughes,A.R., Montgomery,C.A.,Jr, Shyamala,G., Conneely,O.M. and O’Mally,B.W. (1995) Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev., 9, 2266–2278. [DOI] [PubMed] [Google Scholar]
- Ma C., Papermaster,D. and Cepko,C.L. (1998) A unique pattern of photoreceptor degeneration in cyclin D1 mutant mice. Proc. Natl Acad. Sci. USA, 95, 9938–9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H., Kleeff,J., Wildi,S., Friess,H., Buchler,M.W., Israel,M.A. and Korc,M. (1999) Id-1 and Id-2 are overexpressed in pancreatic cancer and in dysplastic lesions in chronic pancreatitis. Am. J. Pathol., 155, 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari M.E. and Murre,C. (2000) Helix-loop-helix proteins: regulators of transcription in eukaryotic organisms. Mol. Cell. Biol., 20, 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura I. et al. (1997) Thrombopoietin-induced differentiation of a human megakaryoblastic leukemia cell line, CMK, involves transcriptional activation of p21WAF1/Cip1 by STAT5. Mol. Cell. Biol., 17, 2933–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe A.D. et al. (1999) Developmental regulation of Bcl-2 family protein expression in the involuting mammary gland. J. Cell Sci., 112, 1771–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard S.S., Yan,J.S., Nguyen,H., Pagano,M., Kiyokawa,H. and Koff,A. (1997) Enhanced ribosomal association of p27Kip1 mRNA is a mechanism contributing to accumulation during growth arrest. J. Biol. Chem., 272, 7093–7098. [DOI] [PubMed] [Google Scholar]
- Miyashita T. and Reed,J.C. (1995) Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell, 80, 293–299. [DOI] [PubMed] [Google Scholar]
- Mori S., Sugawara,S., Kikuchi,T., Tanji,M., Narumi,O., Stoykova,A., Nishikawa,S.I. and Yokota,Y. (1999) The leukemic oncogene tal-2 is expressed in the developing mouse brain. Brain Res. Mol. Brain Res., 64, 199–210. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Ishida,N., Shirane,M., Inomata,A., Inoue,T., Shishido,N., Horii,I., Loh,D.Y. and Nakayama,K.I. (1996) Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell, 85, 707–720. [DOI] [PubMed] [Google Scholar]
- Narumi O., Mori,S., Boku,S., Tsuji,Y., Hashimoto,N., Nishikawa,S.I. and Yokota,Y. (2000) OUT, a novel basic helix-loop-helix transcriptional factor with an Id-like inhibitory activity. J. Biol. Chem., 275, 3510–3521. [DOI] [PubMed] [Google Scholar]
- Neville M.C. and Daniel,C.W. (1987) The Mammary Gland: Development, Regulation, and Function. Plenum Press, New York, NY. [Google Scholar]
- Norton J.D. and Atherton,G.T. (1998) Coupling of cell growth control and apoptosis functions of Id proteins. Mol. Cell. Biol., 18, 2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J.D., Deed,R.W., Craggs,G. and Sablitzky,F. (1998) Id helix-loop-helix proteins in cell growth and differentiation. Trends Cell Biol., 8, 58–65. [PubMed] [Google Scholar]
- Oltvai Z.N., Milliman,C.L. and Korsmeyer,S.J. (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell, 74, 609–619. [DOI] [PubMed] [Google Scholar]
- Ormandy C.J. et al. (1997) Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev., 11, 167–178. [DOI] [PubMed] [Google Scholar]
- Pagano M., Tam,S.W., Theodoras,A.M., Beer-Romero,P., Del-Sal,G., Chau,V., Yew,P.R., Draetta,G.F. and Rolfe,M (1995) Role of ubiquitin–proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science, 269, 682–685. [DOI] [PubMed] [Google Scholar]
- Pan L., Sato,S., Frederick,J.P., Sun,X.H. and Zhuang,Y. (1999) Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol. Cell. Biol., 19, 5969–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peverali F.A., Ramqvist,T., Saffrich,R., Pepperkok,R., Barone,M.V. and Philipson,L. (1994) Regulation of G1 progression by E2A and Id helix–loop–helix proteins. EMBO J., 13, 4291–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu S., Ignatova,A., Park,S.T. and Sun,X.H. (1997) Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol. Cell. Biol., 17, 5888–5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice G.L., Ferreira-Cornwell,M.C., Robinson,S.D., Rayburn,H., Chodosh,L.A., Takeichi,M. and Hynes,R.O. (1997) Precocious mammary gland development in P-cadherin-deficient mice. J. Cell Biol., 139, 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann V., van Cruechten,I. and Sabilitzky,F. (1994) The expression pattern of Id4, a novel dominant negative helix–loop–helix protein, is distinct from Id1, Id2 and Id3. Nucleic Acids Res., 22, 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G.W. and Hennighausen,L. (1997) Inhibins and activins regulate mammary epithelial cell differentiation through mesenchymal–epithelial interactions. Development, 124, 2701–2708. [DOI] [PubMed] [Google Scholar]
- Robinson G.W., McKnight,R.A., Smith,G.H. and Hennighausen,L. (1995) Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development, 121, 2079–2090. [DOI] [PubMed] [Google Scholar]
- Robinson G.W., Johnson,P.F., Hennighausen,L. and Sterneck,E. (1998) The C/EBPβ transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev., 12, 1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves T.N., Krnacik,S., Raught,B., Gay,J., Burgess-Beusse,B., Darlington,G.J. and Rosen,J.M. (1998) C/EBPβ, but not C/EBPα, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev., 12, 1917–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaff R.J., Groudine,M., Gordon,M., Roberts,J.M. and Clurman,B.E. (1997) Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev., 11, 1464–1478. [DOI] [PubMed] [Google Scholar]
- Shen C.P. and Kadesch,T. (1995) B-cell-specific DNA binding by an E47 homodimer. Mol. Cell. Biol., 15, 4518–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C.J. and Roberts,J.M. (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev., 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Donaher,J.L., Parker,S.B., Li,T., Fazeli,A., Gardner,H., Haslam,S.Z., Bronson,R.T., Elledge,S.J. and Weinberg,R.A. (1995) Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell, 82, 621–630. [DOI] [PubMed] [Google Scholar]
- Sorrentino V., Pepperkok,R., Davis,R.L., Ansorge,W. and Philipson,L. (1990) Cell proliferation inhibited by MyoD1 independently of myogenic differentiation. Nature, 345, 813–815. [DOI] [PubMed] [Google Scholar]
- Starr R and Hilton,D.J. (1999) Negative regulation of the JAK/STAT pathway. BioEssays, 21, 47–52. [DOI] [PubMed] [Google Scholar]
- Starr R. et al. (1997) A family of cytokine-inducible inhibitors of signaling. Nature, 387, 917–921. [DOI] [PubMed] [Google Scholar]
- Strange R., Li,F., Saurer,S.,Burkhardt,A. and Friis,R.R. (1992) Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development, 115, 49–58. [DOI] [PubMed] [Google Scholar]
- Sun X.-H., Copeland,N.G., Jenkins,N.A. and Baltimore,D. (1991) Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol. Cell. Biol., 11, 5603–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamantes F., Soares,M.J., Colosi,P., Haro,L. and Ogren,L. (1984) In Mena,F. and Valverde-R.,C.M. (eds), The Biochemistry and Physiology of Mouse Placental Lactogen. Academic Press, New York, NY, pp. 31–41. [Google Scholar]
- Teglund S. et al. (1998) Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell, 93, 841–850. [DOI] [PubMed] [Google Scholar]
- Toscani A., Mettus,R.V., Coupland,R., Simpkins,H., Litvin,J., Orth,J., Hatton,K.S. and Reddy,E.P. (1997) Arrest of spermatogenesis and defective breast development in mice lacking A-myb. Nature, 386, 713–717. [DOI] [PubMed] [Google Scholar]
- Vaux D.L., Cory,S. and Adams,J.M. (1988) Bcl-2 gene promotes haematopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature, 335, 440–442. [DOI] [PubMed] [Google Scholar]
- Virgo B.B. and Bellward,G.D. (1974) Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology, 95, 1486–1490. [DOI] [PubMed] [Google Scholar]
- Wakao H., Gouilleux,F. and Groner,B. (1994) Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J., 13, 2182–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.C., Cardiff,R.D., Zukerberg,L., Lees,E., Arnold,A. and Schmidt,E.V. (1994) Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature, 369, 669–671. [DOI] [PubMed] [Google Scholar]
- Weintraub H. et al. (1991) The myoD gene family: nodal point during specification of the muscle cell lineage. Science, 251, 761–766. [DOI] [PubMed] [Google Scholar]
- Wysolmerski J.J., Philbrick,W.M., Dunbar,M.E., Lanske,B., Kronenberg,H. and Broadus,A.E. (1998) Rescue of the parathyroid hormone-related protein knockout mouse demonstrates that parathyroid hormone-related protein is essential for mammary gland development. Development, 125, 1285–1294. [DOI] [PubMed] [Google Scholar]
- Xu J., Qiu,Y., DeMayo,F.J., Tsai,S.Y., Tsai,M.-J. and O’Mally,B.W. (1998) Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science, 279, 1922–1925. [DOI] [PubMed] [Google Scholar]
- Yokota Y., Mansouri,A., Mori,S., Sugawara,S., Adachi,S., Nishikawa,S.I. and Gruss,P. (1999) Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature, 397, 702–706. [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Ohkubo,T., Kiguchi,T., Jenkins,N.A., Gilbert,D.J., Copeland,N.G., Hara,T. and Miyajima,A. (1995) A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin-3 and erythropoietin receptors. EMBO J., 14, 2816–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]