Abstract
Indinavir boosted with ritonavir (IDV/r) dosing with 400/100 mg, twice daily, is preferred in Thai adults, but this dose can lead to concentrations close to the boundaries of its therapeutic window. The objectives of this analysis were to validate a population pharmacokinetic model to describe IDV/r concentrations in HIV-infected Thai patients and to investigate the impact of patient characteristics on achieving adequate IDV concentrations. IDV/r concentration data from 513 plasma samples were available. Population means and variances of pharmacokinetic parameters were estimated using a non-linear mixed effects regression model (NONMEM Version VI). Monte Carlo simulations were performed to estimate the probability of achieving IDV concentrations within its therapeutic window. IDV/r pharmacokinetics was best described by a one compartment model coupled with a single transit compartment absorption model. Body weight influenced indinavir apparent oral clearance (CL/F) and volume of distribution (Vd/F) and allometric scaling significantly reduced the interindividual variability. Final population estimates (interindividual variability in percentage) of indinavir CL/F and Vd/F were 21.3 L/h/70kg (30%) and 90.7 L/70kg (22%), respectively. Based on model simulations, the probability of achieving an IDV trough concentration > 0.1 mg/L was >99% for 600/100 mg and >98% for 400/100 mg, twice daily, in patients 40–80 kg. However, the probability of achieving IDV concentrations associated with an increased risk of drug toxicity (>10.0 mg/L), increased from 1% to 10% with 600/100 mg, compared to <1% with 400/100 mg when body weight decreased from 80 to 40 kg. The validated model developed predicts that 400/100 mg of IDV/r, twice daily, provides indinavir concentrations within the recommended therapeutic window for the majority of patients. The risk of toxic drug concentrations increases rapidly with IDV/r dose of 600/100 mg for patients <50 kg and therapeutic drug monitoring of IDV concentrations would help to reduce the risk of IDV-induced nephrotoxicity.
Keywords: HIV-1, Highly Active Antiretroviral Therapy, Indinavir, Thai patient population, therapeutic drug monitoring
Introduction
HIV protease inhibitor (PI)-based highly active antiretroviral therapy (HAART) is primarily used as a second-line regimen in resource limited settings 1. Following recent price reductions and the introduction of generic formulations, HIV protease inhibitors are slowly becoming more accessible, but options often remain limited.
Indinavir (IDV) is a potent HIV protease inhibitor that has been used widely as part of triple antiretroviral drug regimens for the treatment of HIV/AIDS 2, 3. Similar to other PIs, the concentrations of indinavir are increased when coadministered with low doses of ritonavir (RTV), a potent inhibitor of the metabolic cytochrome P450 3A4 enzyme. Indinavir boosted with ritonavir (IDV/r) allows less frequent dosing, the removal of food restrictions and provides a similar virologic response when compared to IDV alone 4, 5.
IDV/r based HAART has been one of the main second-line regimens available in Thailand. Initial studies at the recommended IDV/r dose of 800/100 mg, twice daily in Thai patients reported poor tolerability 6. The main adverse event of indinavir is nephrolithiasis, which is a result of the precipitation and crystallization of unmetabolized indinavir monohydrate in the renal tubules,; elevated indinavir plasma concentrations have been observed in patients with urological complications 7. In Thai patients receiving IDV/r 800/100 mg twice daily, an indinavir AUC > 60 mg.hr/mL was associated with an increased risk of developing nephrotoxicity 8. A therapeutic window for indinavir plasma concentrations of 0.1 to 10 mg/L is currently recommended 9. Lower IDV/r doses of 600/100 mg and 400/100 mg, twice daily, in Thai adults have been assessed and both doses provided an improved tolerability profile, while maintaining IDV concentrations within this window 10. However, at these lower doses the concentrations of IDV were near the boundaries of the therapeutic window and the interpatient variability observed suggested that a small proportion of patients may not achieve adequate levels within the wider population. Moreover, sex, body weight and concomitant RTV use can influence indinavir oral clearance (CL/F) 11, so individual patient characteristics could increase significantly the risk of suboptimal or toxic IDV concentrations at lower IDV/r doses.
In order to determine the optimal IDV/r dose for Thai patients our aims were to develop and validate a population pharmacokinetic model to describe IDV/r concentrations in HIV-infected Thai patients and to investigate the impact of patient characteristics on achieving indinavir concentrations within its therapeutic window.
Materials and Methods
Study Population
Indinavir concentration data were pooled from two pharmacokinetic studies of IDV/r in HIV-infected Thai patients. In the first study, 11 patients had intensive steady-state pharmacokinetic sampling performed at pre-dose, and 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8 and 12 hours after drug intake while receiving IDV/r 600/100 mg, twice daily, and then again while receiving 400/100 mg, twice daily 12. In a second study, 19 patients had steady-state pharmacokinetic sparse sampling performed at pre-dose, and 1, 2.5, 4 and 12 hours after drug intake 1 and 2 months after initiating IDV/r 400/100 mg, twice daily (ClinicalTrials.gov Identifiers: NCT00197639). All study procedures were identical between studies. Patients with concomitant treatments that could interfere with the pharmacokinetics of IDV/r, such as rifampin, rifapentine, fluconazole, itraconazole, St John’s Wort (Hypericum perforatum), were excluded. Drug adherence was assessed by exact pill count. This study was approved by the Ethics Committees at the Harvard School of Public Health, USA, Ministry of Public Health, Thailand, and Faculty of Associated Medical Sciences, Chiang Mai University, Thailand.
Drug Analysis
All blood samples were centrifuged, the plasma removed, aliquoted and frozen within one hour at −20°C. Indinavir and ritonavir plasma drug concentrations were measured using a validated high-performance liquid chromatography (HPLC) assay 13 at the PHPT-IRD Laboratory at the Faculty of Associated Medical Sciences, Chiang Mai University. The lower limit of assay quantification (LLOQ) was 50 ng/mL. The PHPT-IRD laboratory participates in two international external quality control (QC) programs: the AIDS Clinical Trial Group (ACTG), USA, Pharmacology Quality Control (Precision Testing) program, and ASQUALAB Quality Control program, France, and has successfully passed for both drugs.
Population Pharmacokinetic Analysis
To estimate the population means and variances of indinavir and ritonavir pharmacokinetic parameters the data were analyzed using non-linear mixed effects regression. Using the software program NONMEM (Version VI, ICON Development Solutions, Ellicott City, MD, USA), with a Fortran Compiler (Compaq Visual Fortran Version 6.6, Compaq Computer Corporation Houston, TX, USA), concentration-time data were fitted using the first-order conditional estimation method (FOCE) with interaction. Pharmacokinetic structural models were assessed using both statistical and graphical methods. The ‘goodness-of-fit’ of different models were compared using the minimal value of the objective function (OFV) provided by NONMEM (equivalent to minus twice the maximum logarithm of the likelihood of the data). An OFV decrease of 3.84 points (i.e. ΔOFV −3.84) corresponds to a statistically significant difference between hierarchical models using a Likelihood Ratio Test (p≤0.05, chi-squared distribution with one degree of freedom). Relative standard errors for parameter estimates were calculated using the COVARIANCE option in NONMEM and individual Bayesian estimates were obtained using the POSTHOC option. The software package Wings for NONMEM was utilized to run the individual models (Version 616: http://wfn.sourceforge.net/) and diagnostic graphs were generated using RfN using the R program (Version 2.11.1).
Structural Model
Single and two compartment pharmacokinetics models with linear and non-linear elimination were fitted to the data. Zero and first- order absorption models, with and without a lag time, were tested. The addition of a ‘transit’ compartment, instead of a lag time, to describe the absorption process was also assessed 14.
Statistical Model
An exponential error model was used to describe inter-individual variability (IIV) in the pharmacokinetic parameters, i.e. CL/Fi = TVCL * exp (ηi), where CL/Fi represents the oral clearance of the ith individual, TVCL is the population CL/F value and ηi is the inter-individual random effect with mean 0 and variance ω2. Covariances between the individual random effects of the pharmacokinetic parameters were assessed graphically. If correlation estimation was near 1.0, it was fixed as follows: ηVd/F = tω * ηCL/F and the proportionality term, tω, between ηVd/F and ηCL/F was estimated 15. Inter-occasion variability (IOV) in the pharmacokinetic parameters was tested using the model CL/Fi = TVCL * exp (ηI + κj), where κj is the inter-occasion random effect with mean 0 and variance π2 16. A combined additive and proportional error model was used initially to estimate residual variability.
Covariate Analysis
Individual patient characteristics that could potentially influence indinavir or ritonavir pharmacokinetic parameters were evaluated for their inclusion in the models. The patient characteristics assessed included: weight, sex, age, serum glutamic pyruvic transaminase (SGPT) and serum creatinine. Continuous covariates were modeled using a power relationship e.g. CL/Fi = TVCL * (WTi/MWT)θBW, where WT represents the weight of the ith individual, MWT is the median weight of the study population and θBW is the factor associated with body weight that influences indinavir oral clearance. Sex was the only binary covariate available and was evaluated using the following equation: CL/Fi = TVCL * θ (Sex-1), where θ is the factor associated with sex that influences indinavir oral clearance, using sex equals 1 for males and 2 for females. Covariates were tested using a stepwise forward inclusion and backward elimination model building procedure. Each covariate was introduced into the basic model one at a time and was selected if (i) its effect was biologically plausible (ii) the OFV decreased by more than 3.84 points, and (iii) it reduced the inter-individual variability of the pharmacokinetic parameter. If more than one covariate met these three criteria, the covariate that produced the largest decrease in OFV was retained and included in an intermediate model. The remaining covariates were then introduced into this intermediate model one at a time and the most significant covariate retained. This procedure was repeated until none of the remaining covariates caused the OFV to decrease by more than 3.84 points. The inclusion of covariates in the final model was determined using a backward elimination of covariates included in the intermediate model. A covariate was retained in the final model if, after its removal from the intermediate model, there was a significant difference between hierarchical models, determined by an increase in the OFV by more than 6.63 points (Likelihood Ratio Test p≤0.01, chi-squared distribution with one degree of freedom).
Model Validation
The validity of the final model was evaluated using two methods: (i) visual predictive check (VPC) validation and (ii) the bootstrap re-sampling technique. Using the final model parameters estimates, 1,000 indinavir concentration profiles were simulated for each of the subjects included in the model building (i.e. 30 patients = 30,000 concentration profiles). The 5th, 50th, and 95th percentiles of the simulated concentrations were plotted and a VPC was performed by overlying the observed concentrations. The criteria used to determine the validity of the model was based on the 90% prediction interval: if the 90% confidence interval for the proportion of observed concentration points lay outside the 90% prediction interval (i.e. below 5% or above 95%) including 10%, the model adequately described the variability observed. The bootstrap re-sampling technique was performed using the software package Wings for NONMEM. The final model was fitted to 400 replicate bootstrap datasets generated using the original dataset with replacements. The mean population pharmacokinetic parameters and their corresponding standard errors from the bootstrap analyses were calculated and compared to the estimate obtained with the original dataset.
Evaluation of the impact of patient body weight on IDV concentrations
Using the final model, the probability to achieve IDV concentrations within the therapeutic window was assessed using 1,000 Monte Carlo simulations. Several targets were assessed: (1) IDV Ctrough concentration > 0.1 mg/L, (2) 2-hour post dose concentration > 10.0 mg/L, and (3) IDV AUC > 60 mg.hr/L. The probability of achieving a target was calculated by dividing the number of simulated concentrations (or exposure) by the total number of simulated data.
Results
Indinavir/ritonavir concentration data from 513 plasma samples were included in the population PK analysis. Samples were available from 30 patients. Eleven patients, 6 men and 5 women, had intensive pharmacokinetic evaluations available at IDV/r doses of 600/100 mg and 400/100 mg; and 19 patients, 6 men and 13 women, had sparse sampling pharmacokinetic evaluations available at an IDV/r dose of 400/100 mg.
Indinavir population model
Indinavir concentrations were best described by a one compartment pharmacokinetic model with first order absorption and linear elimination. A combined additive and proportional error model was used initially to estimate residual variability, but the additive component was not significant and was removed. Inclusion of an absorption lag time did not improve the fit, but the addition of a single ‘transit’ compartment instead of a lag time significantly improved the model fit (ΔOFV −6.8). Inclusion of IIV for CL/F, Vd/F and Ka improved the fit and the addition of covariance between the IIV of CL/F and Vd/F (ηCl/F and ηV/d) further improved the model fit (ΔOFV −34.6). Inter-occasion variability (IOV) in the pharmacokinetic parameters did not improve the model. Inclusion of separate proportional error models to estimate residual variability dependent on the indinavir dose, 600 mg or 400 mg, did improve the model fit (ΔOFV −9.6).
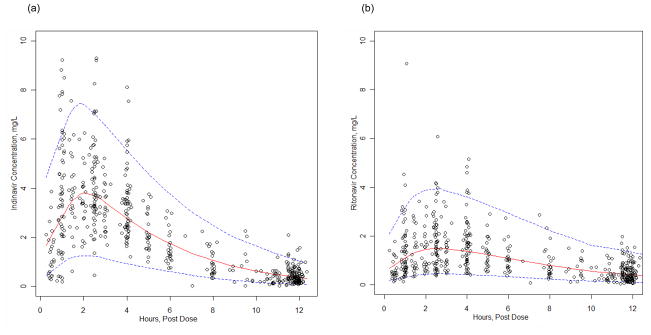
Body weight was the only covariate that significantly influenced indinavir pharmacokinetics. Vd/F and CL/F were allometrically scaled to a 70 kg adult as: Vd/F (L/h/70kg) = TVVd*(WT/70) and CL/F (L/h/70kg) = TVCL*(WT/70)0.75; this reduced the objective function by −14.7 units and the corresponding interindividual variabilities. Following this it was not possible to estimate the covariance matrix as the estimated correlation between Vd/F and Cl/F was near 1.0. Subsequently, the covariance was fixed as follows: ηVd/F = tω * ηCL/F and the proportionality term, tω, between ηVd/F and ηCL/F was estimated. A separate population model was developed for ritonavir (see below) but the influence of ritonavir concentrations on indinavir pharmacokinetics was investigated using an Emax model on indinavir CL/F, but this did not improve the model. A visual predictive check of the final model was performed and the predicted median curve provided a good fit to the observed data (Figure 1). Simulated 5th and 95th percentile curves were generated and among the observed data 5% were below the 5th percentile and 4% were above the 95th percentile. Overall, the proportion of observed concentration points outside the 90% prediction interval was 9% [95% CI, 7–12], which met the VPC criteria for model validity. The bootstrap medians were close to the final model estimates and the bootstrapped confidence intervals were narrow (Table 2).
Figure 1.
Visual predictive check for the (a) indinavir and (b) ritonavir population pharmacokinetic models (400 mg indinavir plus 100 mg ritonavir, twice daily). Dotted lines represent 95% prediction intervals (P2.5–P97.5). Observed concentrations (o) from the patients are superimposed.
Table 2.
Final indinavir and ritonavir population pharmacokinetic parameter estimates
| Final Model | Bootstrap^ | |||
|---|---|---|---|---|
| IDV PK Parameters | Estimate | RSE (%) | Median | 2.5th - 97.5th percentile |
| CL/F70 (L/h/70kg) | 21.3 | 5.5 | 21.3 | 18.9 – 23.6 |
| Vd/F70 (L70/kg) | 90.7 | 4.5 | 91.0 | 82.9 – 99.8 |
| Ktr (h−1) # | 2.32 | 9.2 | 2.37 | 1.96 – 2.91 |
| Inter-individual variability (IIV) | ||||
| IIV (CL/F) | 0.30 | 23.4 | 0.29 | 0.22 – 0.39 |
| IIV (Vd/F)* | 0.23 | - | - | - |
| IIV (Ktr) | 0.38 | 35.7 | 0.352 | 0.342 – 0.485 |
| θpV/F * | 0.58 | 10.0 | 0.58 | 0.44 – 0.73 |
| Residual Variability | ||||
| Proportional | ||||
| σ (IDV 400 mg) | 0.42 | 10.9 | 0.42 | 0.37 – 0.46 |
| σ (IDV 600 mg) | 0.29 | 24.4 | 0.29 | 0.22 – 0.39 |
| RTV PK Parameters | ||||
| CL/F (L/h) | 10.1 | 13.3 | 10.1 | 7.86 – 13.2 |
| θSEX-Female | 0.77 | 37.2 | 0.77 | 0.60 – 0.96 |
| θIDVDOSE-600mg | 0.78 | 30.8 | 0.78 | 0.62 – 0.91 |
| V/F (L) | 49.6 | 10.4 | 49.8 | 41.1 – 66.7 |
| Ktr (h-1) # | 1.45 | 13.0 | 1.48 | 1.18 – 1.90 |
| Inter-individual variability(IIV) | ||||
| IIV (CL/F) | 0.48 | 25.3 | 0.47 | 0.34 – 0.59 |
| IIV (V/F) | 0.45 | 41.5 | 0.46 | 0.23 – 0.76 |
| IIV (Ktr) | 0.61 | 44.4 | 0.55 | 0.33 – 0.84 |
| Correlation | ||||
| ηCl/F - ηV/F | 0.916 | 32.9 | 0.934 | 0.759–1.00 |
| Residual Variability | ||||
| σ (Proportional) | 0.36 | 12.7 | 0.36 | 0.32 – 0.41 |
CL/F oral clearance; V/F; apparent volume of distribution; Ka absorption rate constant
RSE%: relative standard error (standard error of estimate/estimate*100)
Ktr = Ka within a transit model
Statistics from 400 bootstrap analyses
ω2(V/F) =θpV/F × ω2(CL/F); CL/F = 21.3 (WT/70)3/4; V/F = 90.7 (WT/70)1
Evaluation of weight on IDV concentrations and exposure
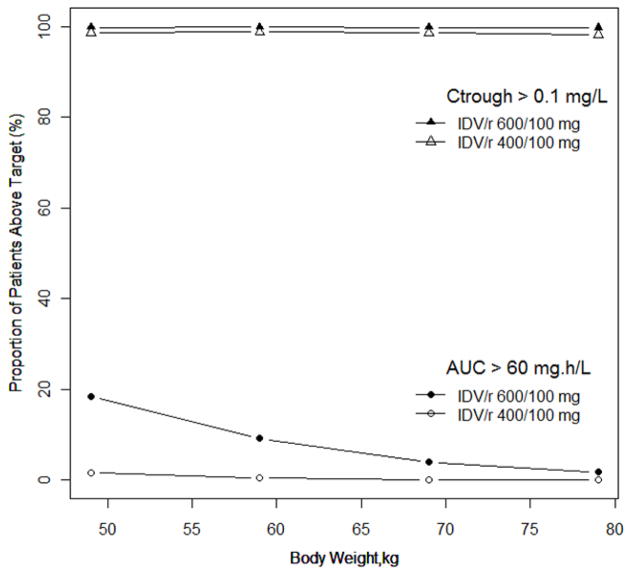
Using the final model, Monte Carlo simulation were performed for patients with body weights between 40 to 80 kg and the probabilities of achieving efficacious, Ctrough > 0.1 mg/L, or toxic exposure, AUC > 60 mg.hr/L, with 600/100 mg and 400/100 mg, twice daily, are shown in Figure 2. The probability of achieving an IDV trough concentration > 0.1 mg/L was >99% for 600/100 mg and >98% for 400/100 mg, twice daily, across all body weights. The probability of achieving an IDV AUC > 60 mg.hr/L increased from 2% to 18% with 600/100 mg compared to <1% to 2% with 400/100 mg when body weight decreased from 80 to 40 kg. The risk of having a 2-hour post drug IDV concentration >10.0 mg/L was lower than having an AUC > 60 mg.hr/L at both IDV/r doses. As the body weight decreased from 80 to 40 kg the probability of an IDV concentration >10.0 mg/L increased from 1% to 10% with 600/100 mg, while the probability was <1% with 400/100 mg across the weight range. The probability of achieving IDV concentrations within the therapeutic window (Ctrough > 0.1 and C2-hour <10.0 mg/L) for subjects weighing 40 to 80 kg was >98% for 400/100 mg and for 600/100 mg the probability was >97% for subjects between 50 to 80 kg but only 90% for subjects weighting 40–50 kg.
Figure 2.
Probabilities of achieving efficacious, Ctrough > 0.1 mg/L, or toxic exposure, AUC > 60 mg.hr/L, with IDV/r 600/100 mg and 400/100 mg, twice daily, for patients with body weights between 40 to 80 kg based on 1,000 Monte Carlo simulations.
Ritonavir population model
A separate population pharmacokinetic model was developed to describe the ritonavir concentrations for patients administered IDV/r. Ritonavir concentrations were best described by a one compartment pharmacokinetic model with a single ‘transit’ compartment. A proportional error model was used to estimate residual variability. The addition of covariance between the IIV of CL/F and Vd/F improved the model fit. Ritonavir CL/F was influenced by sex and IDV dose (i.e. 400 or 600 mg). A visual predictive check of this model showed that among the observed ritonavir data the proportion of observed concentration points outside the 90% prediction interval was 8% [95% CI, 6–11], with 4% below the 5th percentile and 4% above the 95th percentile (Figure 1). Final estimates of ritonavir CL/F and Vd/F (interindividual variability in %) were 10.1 L/h (48%) and 49.6 L (45%), respectively and the bootstrap medians were close to the final model estimates (Table 2).
Discussion
A population model to describe indinavir concentrations following administration of indinavir boosted with ritonavir in Thai HIV-infected patients was validated and model simulations predicted that IDV/r 400/100 mg, twice daily, provides indinavir concentrations within the recommended therapeutic window for over 98% of patients. Administration of IDV/r 600/100 mg, twice daily, also provides adequate indinavir concentrations for the majority of patients, but the risk of toxic exposure or concentrations increased rapidly for patients weighing less than 50 kg.
The final model estimates of CL/F and Vd/F were 21.3 L/hr/70 kg and 90.7 L/70kg, respectively, and these values are consistent with those reported in European patients 17, 18. Covariates reported to influence indinavir pharmacokinetics include concomitant ritonavir or non-nucleoside reverse transcriptase inhibitors (NNRTI) or ritonavir use, sex and body weight 11, 17. In the present analysis it was not possible to estimate the influence of concomitant ritonavir use on indinavir CL/F as all patients were receiving indinavir boosted with ritonavir at the same dose (100 mg). Given the clear pharmacological advantages of administrating indinavir with low dose ritonavir the clinical practice of prescribing indinavir without ritonavir is unlikely. Similarly, due to their common metabolic pathways, concomitant non-nucleoside reverse transcriptase inhibitors (NNRTIs) are known also to influence indinavir pharmacokinetics, but with the increasing number of antiretroviral drugs available, HAART regimens composed of NNRTIs plus boosted protease inhibitors, with the exception of etravirine, are less common.
Prior to the development of population models for indinavir there was some indication that indinavir may display sex-dependent pharmacokinetics 19 and that subgroups of female patients could be exposed to high indinavir concentrations 20. Indeed, after adjusting for concomitant ritonavir use, indinavir CL/F was reported to be 30% higher in male patients compared to female patients 11. Interestingly, no relationship between sex and indinavir pharmacokinetic variability was observed in the Thai population. A report by Bertrand et al, also reported no sex effect on indinavir pharmacokinetics in a French cohort of patients receiving IDV/r and the authors postulated that this may have been due to the low number of female subjects included in their analysis. However, an analysis including only nine women found that indinavir bioavailability was 48% higher in female patients than in males 17. It is possible that the impact of sex in cohorts of patients using indinavir with low dose ritonavir is diminished and that, in this setting, specific dosing adjustment for female patients is not necessary. Allometic scaling of indinavir Vd/F and CL/F to a 70 kg adult in the Thai population significantly improved model fit and reduced interpatient variability. While body weight has been reported to influence indinavir CL/F in other populations the impact was rather minimal compared to other reported factors such as sex. Host genetic polymorphisms could also play a role in indinavir interpatient variability. IDV is primarily metabolized by the cytochrome P450 enzyme 3A4 (CYP3A4) and is a substrate for the drug efflux transporter P-glycoprotein, coded by the ABCB1 gene. Functional variants of ABCB1 were not associated with indinavir exposure or virologic/immunological response to treatment 21, but recent evidence suggests that polymorphisms within the CYP3A gene contribute towards reduced indinavir absorption 18.
Since the presence of ritonavir significantly influences indinavir pharmacokinetics, primarily through its inhibition of CYP3A4, it seems logical to attempt to incorporate the effect of ritonavir in a model. Indeed, similar attempts have been made for other protease inhibitors boosted with low dose ritonavir, such as for lopinavir/r, where a time-independent inverse relationship between lopinavir CL/F and ritonavir AUC0–12 was utilized 22, or for atazanavir/r, where the relationship between atazanavir CL/F and ritonavir AUC0–24 was best described by a power function 23. Within this analysis, models that included a relationship between ritonavir exposure and indinavir CL/F did not improve the model significantly. Previous attempts to model the effect of ritonavir on indinavir CL/F predicted that indinavir metabolism is completely inhibited at very low ritonavir exposure and modeling ritonavir as a dichotomous variable (i.e. with or without ritonavir) was to be preferred 17.
Using the derived indinavir population PK model simulations at different IDV/r doses were performed for patients across the weight range of 40 to 80 kg. This weight range was based on data collected within a phase III, multicenter, randomised clinical trial in Thailand (PHPT-3, ClinicalTrials.gov Identifier: NCT00162682), in which the weight distribution of 716 HIV-infected Thai adults at treatment initiation was 53 (30 to 88) kg, with 38% weighing less than 50 kg and 5% less than 40 kg. After 3 years of antiretroviral treatment 95% of patients weighed between 40 to 80 kg, confirming that this weight range was representative of the HIV-infected Thai population.
Recent price reductions and the introduction of generic formulations have made lopinavir boosted with ritonavir the preferred protease inhibitor for second line regimens in many settings and, as a consequence, the use of indinavir in Asian countries is expected to decline. However, this does not mean that the use and prescription of indinavir will disappear immediately. Indeed, indinavir remains the only alternative PI to lopinavir that is available for free through the Thai National Health Care System. Patients unable to tolerate lopinavir will be switched to indinavir and many of those patients already receiving indinavir prefer not to change treatment. It is hoped that that HIV-infected patients in Thailand and other Asian countries will soon be able to access second generation PIs but, in the meantime, indinavir will continue to be a useful option for second-line treatments in this setting.
Conclusion
Due to the high interpatient variability of indinavir concentrations observed at lower doses of IDV/r concerns were raised that a small proportion of patients may not achieve adequate indinavir concentrations. The simulations performed with the derived model provided reassuring data that IDV 400/100 mg, twice daily, produces indinavir concentrations within its therapeutic window for patients between 40 to 80 kg, but highlights that he higher IDV/r dose of 600/100 mg significantly increases the risk of toxic IDV exposure, especially for patients <50kg. In such patients, therapeutic drug monitoring would be beneficial (i.e. 2 hour post dose sample <10.0 mg/L) to help to reduce the risk of IDV-induced nephrotoxicity.
Table 1.
Baseline Characteristics
| N=30 | |
|---|---|
| Sex (Male: Female) | 12:18 |
| Age (years) | 35 (23–51) |
| Weight | 53 (36–74) |
| Male (kg) | 57 (44–71) |
| Female (kg) | 52 (36–74) |
| SGPT (U/L) | 41 (10–271) |
| Creatinine (mg/dL) | 0.9 (0.4–1.2) |
Values: median, range; laboratory reference intervals: serum glutamic pyruvic transaminase (SGPT): 10–56U/L and creatinine 0.5–1.2.
Acknowledgments
Support: National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), USA.
We would like to thank all the subjects who participated and the study staff conducting the protocol at the sites. This study was supported by the National Institute of Child Health and Human Development (NICHD), National Institutes of Health, USA. Grants number: R01 HD042964 and the Institut de Recherche pour le Développement (IRD), URI 174. Indinavir and ritonavir for the antiretroviral drug assay were obtained through the NIH AIDS Research and Reference Program, Division of AIDS, NIAID, NIH. We would like to acknowledge Abbott Laboratories for providing the experimental material A86093, which was used as an internal standard in the HPLC method to measure indinavir and ritonavir.
Members of the PHPT-3 Team (PK study)
Phayao Provincial Hospital: Guttiga Halue, Anchalee Singkaew, Borwornluck (Lamduan) Changlor, Patcharaporn Krueduangkam, Ruethai Wongchai. Prapokklao Hospital: Malee Techapornroong, Supawadee Tongsakulrungruang, Suppanut (Nuttupussasorn) Khannak (Tungtongchai) Jiradsadaporn Khanmali, Renoo Wongsrisai. Chonburi Hospital: Chureeratana Bowonwatanuwong, Sirinat Toyang, Prakit Yothipitak, Somrat Matchua, Tiwacha Thimakam. Nakornping Hospital: Prattana Leenasirimakul, Orapin Khatngam, Den Roumsuk, Praneet Pinklow. PHPT-IRD174 Clinical Trial Unit, Chiang Mai: Sophie Le Coeur, Nicole Ngo-Giang-Huong, Ken McIntosh, Pra-ornsuda Sukrakanchana, Suwalai Chalermpantmetagul, Kanchana Than-in-at, Yardpiron Taworn, Pimpinun Punyati, Luc Decker, Intira Collins, Aksorn Lueanyod, Suriyan Tanasri, Sanupong Chailert, Kanjana Yoddee, Dujrudee Chinwong. Ministry of Public Health: Sombat Thanprasertsuk. Mahidol University: Suporn Koetsawang. Assistance Publique-Hopitaux de Paris, France: Michel Tod
References
- 1.World Health Organization. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access. Recommendations for a public health approach. 2010 http://www.who.int/hiv/pub/paediatric/infants2010/en/index.html. Revision. [PubMed]
- 2.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 3.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 4.Hsu A, Granneman GR, Cao G, et al. Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers. Antimicrob Agents Chemother. 1998;42:2784–2791. doi: 10.1128/aac.42.11.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnaiz JA, Mallolas J, Podzamczer D, et al. Continued indinavir versus switching to indinavir/ritonavir in HIV-infected patients with suppressed viral load. Aids. 2003;17:831–840. doi: 10.1097/00002030-200304110-00008. [DOI] [PubMed] [Google Scholar]
- 6.Boyd MA, Srasuebkul P, Khongphattanayothin M, et al. Boosted versus unboosted indinavir with zidovudine and lamivudine in nucleoside pre-treated patients: a randomized, open-label trial with 112 weeks of follow-up (HIV-NAT 005) Antivir Ther. 2006;11:223–232. [PubMed] [Google Scholar]
- 7.Dieleman JP, Gyssens IC, van der Ende ME, de Marie S, Burger DM. Urological complaints in relation to indinavir plasma concentrations in HIV-infected patients. Aids. 1999;13:473–478. doi: 10.1097/00002030-199903110-00005. [DOI] [PubMed] [Google Scholar]
- 8.Burger D, Boyd M, Duncombe C, et al. Pharmacokinetics and pharmacodynamics of indinavir with or without low-dose ritonavir in HIV-infected Thai patients. J Antimicrob Chemother. 2003;51:1231–1238. doi: 10.1093/jac/dkg198. [DOI] [PubMed] [Google Scholar]
- 9.la Porte C, Back D, Blaschke T, et al. Updated Guideline to Perform Therapeutic Drug Monitoring for Antiretroviral Agents. Reviews in Antiviral Therapy. 2006;3:4–14. [Google Scholar]
- 10.Cressey TR, Leenasirimakul P, Jourdain G, et al. Low-doses of indinavir boosted with ritonavir in HIV-infected Thai patients: pharmacokinetics, efficacy and tolerability. J Antimicrob Chemother. 2005;55:1041–1044. doi: 10.1093/jac/dki143. [DOI] [PubMed] [Google Scholar]
- 11.Csajka C, Marzolini C, Fattinger K, et al. Population pharmacokinetics of indinavir in patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 2004;48:3226–3232. doi: 10.1128/AAC.48.9.3226-3232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cressey TR, Leenasirimakul P, Sukrakanchana P, et al. A pharmacokinetic study comparing 600/100 IDV/RTV vs. 400/100 IDV/RTV as part of highly active antiretroviral therapy in HIV-infected Thai patients. XV International AIDS Conference; 11 – 16 July; Bangkok Thailand. 2004. [Google Scholar]
- 13.Droste JA, Verweij-Van Wissen CP, Burger DM. Simultaneous determination of the HIV drugs indinavir, amprenavir, saquinavir, ritonavir, lopinavir, nelfinavir, the nelfinavir hydroxymetabolite M8, and nevirapine in human plasma by reversed-phase high-performance liquid chromatography. Ther Drug Monit. 2003;25:393–399. doi: 10.1097/00007691-200306000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. Journal of pharmacokinetics and pharmacodynamics. 2007;34:711–726. doi: 10.1007/s10928-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 15.Zhou XJ, Sheiner LB, D’Aquila RT, et al. Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. The National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group Protocol 241 Investigators. Antimicrob Agents Chemother. 1999;43:121–128. doi: 10.1128/aac.43.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson MO, Sheiner LB. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm. 1993;21:735–750. doi: 10.1007/BF01113502. [DOI] [PubMed] [Google Scholar]
- 17.Kappelhoff BS, Huitema AD, Sankatsing SU, et al. Population pharmacokinetics of indinavir alone and in combination with ritonavir in HIV-1-infected patients. Br J Clin Pharmacol. 2005;60:276–286. doi: 10.1111/j.1365-2125.2005.02436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertrand J, Treluyer JM, Panhard X, et al. Influence of pharmacogenetics on indinavir disposition and short-term response in HIV patients initiating HAART. Eur J Clin Pharmacol. 2009;65:667–678. doi: 10.1007/s00228-009-0660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin JH, Chiba M, Chen IW, Nishime JA, Vastag KJ. Sex-dependent pharmacokinetics of indinavir: in vivo and in vitro evidence. Drug Metab Dispos. 1996;24:1298–1306. [PubMed] [Google Scholar]
- 20.Burger DM, Siebers MC, Hugen PW, Aarnoutse RE, Hekster YA, Koopmans PP. Pharmacokinetic variability caused by gender: do women have higher indinavir exposure than men? J Acquir Immune Defic Syndr. 2002;29:101–102. doi: 10.1097/00126334-200201010-00014. [DOI] [PubMed] [Google Scholar]
- 21.Verstuyft C, Marcellin F, Morand-Joubert L, et al. Absence of association between MDR1 genetic polymorphisms, indinavir pharmacokinetics and response to highly active antiretroviral therapy. Aids. 2005;19:2127–2131. doi: 10.1097/01.aids.0000196122.91633.04. [DOI] [PubMed] [Google Scholar]
- 22.Crommentuyn KM, Kappelhoff BS, Mulder JW, et al. Population pharmacokinetics of lopinavir in combination with ritonavir in HIV-1-infected patients. Br J Clin Pharmacol. 2005;60:378–389. doi: 10.1111/j.1365-2125.2005.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickinson L, Boffito M, Back D, et al. Population pharmacokinetics of ritonavir-boosted atazanavir in HIV-infected patients and healthy volunteers. J Antimicrob Chemother. 2009;63:1233–1243. doi: 10.1093/jac/dkp102. [DOI] [PMC free article] [PubMed] [Google Scholar]