Abstract
Extensive studies in model organisms in the last few decades have revealed that aging is subject to profound genetic influence. The conserved nutrient sensing TOR (Target Of Rapamycin) pathway is emerging as a key regulator of lifespan and healthspan in various species from yeast to mammals. The TOR signaling pathway plays a critical role in determining how an eukaryotic cell or a cellular system co-ordinates its growth, development and aging in response to constant changes in its surrounding environment? TOR integrates signals originating from changes in growth factors, nutrient availability, energy status and various physiological stresses. Each of these inputs is specialized to sense particular signal(s), and conveys it to the TOR complex which in turn relays the signal to downstream outputs to appropriately respond to the environmental changes. These outputs include mRNA translation, autophagy, transcription, metabolism, cell survival, proliferation and growth amongst a number of other cellular processes, some of which influence organismal lifespan. Here we review the contribution of the model organism Drosophila in the understanding of TOR signaling and the various biological processes it modulates that may impact on aging. Drosophila was the first organism where the nutrient dependent effects of the TOR pathway on lifespan were first uncovered. We also discuss how the nutrient-sensing TOR pathway appears to be critically important for mediating the longevity effects of dietary restriction (DR), a potent environmental method of lifespan extension by nutrient limitation. Identifying the molecular mechanisms that modulate lifespan downstream of TOR is being intensely investigated and there is hope that these are likely to serve as a potential targets for amelioration of age–related diseases and enhance healthful lifespan extension in humans.
Keywords: Dietary restriction, aging, caloric restriction, nutrients, lifespan, TOR, rapamycin, Drosophila
Introduction
TOR is an evolutionarily conserved nutrient sensing protein kinase that regulates growth and metabolism in all eukaryotic cells. As the name suggests, this kinase is a target for inhibition by rapamycin. Rapamycin was discovered as a byproduct of the soil bacterium Streptomyces hygroscopicus from the island of Rapa Nui (Vezina et al., 1975). Rapamycin was originally studied and used for its potent antifungal properties and was later shown to inhibit growth of cells and also act as an immunosuppressant. Rapamycin binds to FPR1, a peptidyl-prolyl cis-trans isomerase, which binds and regulate activities of two proteins identified as TOR1 and TOR2 in S. cerevisae (Heitman et al., 1991). These TOR proteins, encoded by two different genes, belong to the phosphatidylinositol kinase-related kinase (PIKK) family of kinases and exists in two complexes with different functions in yeast (Martin and Hall, 2005). However, in mammals there is only one gene encoding mammalian TOR (mTOR) which exists in two complexes that consist of distinct sets of protein binding partners (Dann et al., 2007; Sabatini, 2006). TOR complex I (TORC1 in yeast and mTORC1 in mammals), is rapamycin sensitive and controls temporal aspects of cellular growth mediated mostly through S6 kinase 1 (S6K1) and initiation factor 4E-binding protein 1 (4E-BP1) (Um et al., 2006; Wullschleger et al., 2006). On the other hand, TORC2 is rapamycin-insensitive and controls spatial aspects of growth within the cell and the effects are mostly mediated through protein kinase B (PKB/Akt) (Jacinto et al., 2004).
Following its initial discovery in S. cerevisae, TORs have been observed in all eukaryotes examined (Wullschleger et al., 2006). The conserved nature of the function of TOR as a nutrient sensor is reflected in the loss of function phenotypes of TOR in multiple species. In nematode, C. elegans, deletion of CeTOR leads to developmental arrest at the L3 larval stage and intestinal atrophy (Long et al., 2002). A similar phenotype was observed for mutants in daf-15, the worm ortholog of the mammalian protein Raptor, one of the TORC1 interacting partners (Jia et al., 2004). Loss of function of mTOR in mice also leads to early embryonic lethality (Hentges et al., 2001; Murakami et al., 2004). The tissue-specific removal of Raptor in skeletal muscle induces progressive muscle dystrophy, which is likely due to impaired protein synthesis (Bentzinger et al., 2008). The loss of function phenotypes of TOR pathway genes in Drosophila also show defects in growth or development and are described below. Thus, mutations in TOR show developmental or growth arrest phenotypes in different species, comparable to those observed upon nutrient deprivation, supporting the notion that it has a conserved role in coupling of availability of nutrients to growth in multiple species.
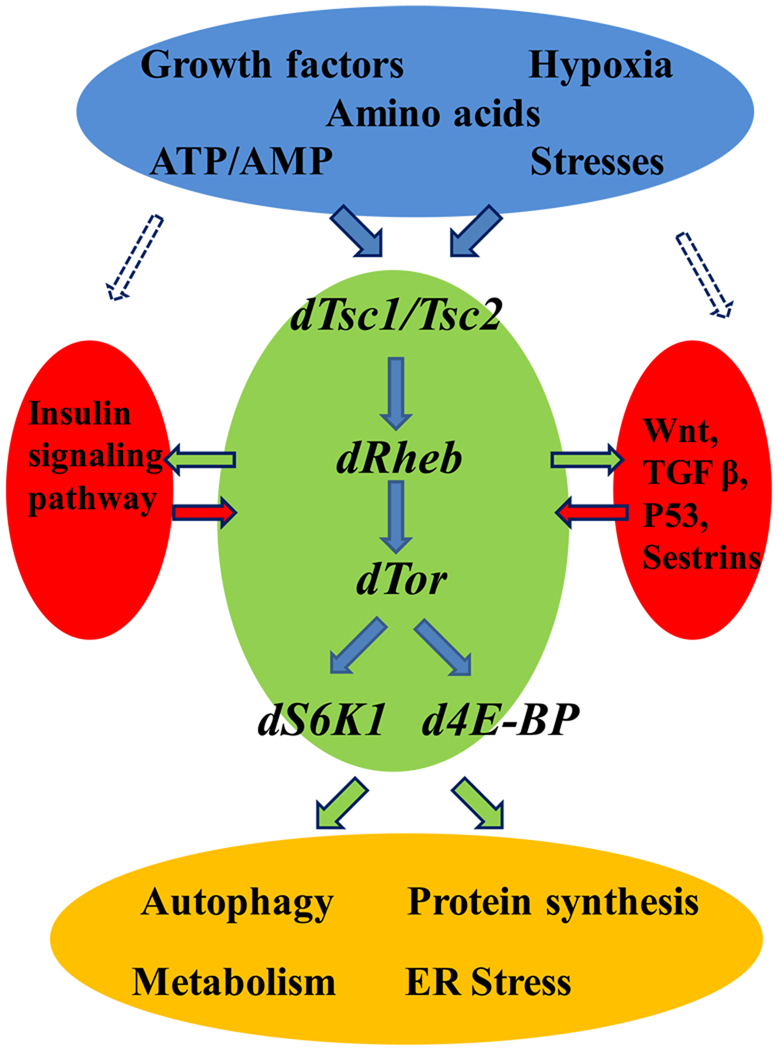
One of the exciting developments in the field has been the realization that TORC1 is involved in a large number of human diseases, including diabetes, obesity, heart disease, and cancer (Inoki and Guan, 2006). Interestingly, a common risk factor for these diseases is aging, and it has been suggested that the mechanism of the link between cellular senescence, diseases and organismal aging is via TOR (Blagosklonny, 2006; Kapahi and Zid, 2004). It was recently demonstrated that cellular aging requires mTOR and that it can be suppressed by rapamycin (Demidenko et al., 2009). Growth stimulation, on the other hand, leads to cellular senescence when the cell cycle is blocked (Demidenko and Blagosklonny, 2008). Growing evidence has directly implicated the TORC1 pathway in determining lifespan in multiple model systems. Specifically, inhibition of TORC1 (hereafter referred to as TOR), extends lifespan in yeast, worms, flies and mice (Kapahi et al., 2010). In further support of this longevity role, TOR is emerging as a robust mediator of the beneficial effects of DR, the most robust method known to increase lifespan and protect against age-related diseases by reducing nutrient intake without malnutrition in many species (Kapahi et al., 2010; Rogers and Kapahi, 2006). TOR integrates a variety of upstream signals, which aside from nutrients like amino acids also include growth factors, energy status, and various stressors (Figure 1) (Kapahi et al., 2010). According to the type of input, TOR responds by regulating a number of outputs, including protein translation, autophagy, metabolism, and stress responses (Figure1). Here, we discuss the contribution of Drosophila in understanding TOR signaling and how it impacts various biological processes with a view to discuss its relevance to aging and age-related diseases.
Figure 1. Schematic of TOR signaling network.
Various intra- and extracellular environmental cues activate the TOR signaling pathway leading to modulation of various biological processes that can modulate lifespan of flies. This figure also indicates the interaction with Insulin signaling pathway and other signalling pathways. See the main text for details.
TOR signaling pathway components in Drosophila
dS6K
The first component of the TOR pathway to be identified in Drosophila was dS6K. Two independent studies showed the existence of a single homologue for mammalian S6K and showed its sensitivity to rapamycin (Stewart et al., 1996; Watson et al., 1996). Drosophila S6K expressed in COS or NIH 3T3 cells was shown to phosphorylate mammalian 40S ribosomal protein 6 (RPS6) in a mitogen-dependent and wortmannin and rapamycin-sensitive manner, suggesting that its regulation is similar to mammalian p70S6k (Watson et al., 1996). Similarly, dS6K from S2 cells was also shown to phosphorylate mammalian 40S ribosome in a rapamycin-sensitive manner (Stewart et al., 1996).
Although most flies that are null for dS6K die during development, the surviving flies are normally proportioned but smaller than their heterozygous siblings or wild-type flies (Montagne et al., 1999). Examining cells of adult wings showed that the dS6K loss causes a reduction in cell size (by about 30%) without affecting the number of cells in the wing indicating that loss of S6K function reduces growth and body size to a lesser extent than loss of other positive components acting further upstream in the insulin cascade (Montagne et al., 1999). Using different variants of dS6K in which the conserved potential dS6K phosphorylation sites were substituted with acidic amino acids, it was shown that the kinase activity of dS6K is required for the growth phenotype (Barcelo and Stewart, 2002).
dTOR
Independent studies from two different groups identified dTOR in Drosophila and demonstrated its role in growth and development (Oldham et al., 2000; Zhang et al., 2000). In the first study, dTOR was identified in a genetic screen for recessive mutations affecting cell growth and proliferation in Drosophila compound eye. dTOR protein sequence showed about 56% identity with mTOR, with higher conservation in the kinase and FRB domains. dTOR mutants rescued the increased growth phenotype caused by loss of PTEN function. Drosophila Pten negatively regulates the growth-promoting effects of insulin signaling (Goberdhan et al., 1999). Similar to the amino acid starvation animals but not chico mutants, loss of dTOR function resulted in severe reduction of dS6 phosphorylation suggesting that insulin signaling and TOR signaling act in parallel pathways. The second study identified dTOR as one of the four members of the PIK-related family proteins. The dTOR mutants, generated by p element insertion, showed extended larval period with little or no growth suggesting that dTOR is required for normal growth of the larvae. The heterozygous mutant larvae showed increased sensitivity to rapamycin. The growth phenotype and rapamycin sensitivity of the dTOR mutant larvae was further rescued by overexpression of a constitutively active dS6K. Cells lacking dTOR exhibit reduced size proliferation in multiple tissues in the fly. The loss of dTOR function also caused a reduction in nucleolar size, aggregation of lipid vesicles and a cell type specific growth arrest. These phenotype were similar to that of the amino acid starved animals (Britton and Edgar, 1998). Interestingly, the cell cycle arrest of the dTOR mutant endoreplicative cells was rescued by overexpression of Cyclin E. In the fly, Cyclin E is required for induction of S-phase and is a G1-S phase cyclin - dimerization partner of cdc2c kinase. (Zhang et al., 2000).
dTsc1-Tsc2
Tuberous sclerosis 1 (Tsc1) and Tsc2 form a complex and was first shown to act parallel to the insulin signaling pathway to inhibit TOR signaling. The fly mutants of dTsc1 and dTsc2 were shown to modulate cell size and cell number. (Gao and Pan, 2001; Tapon et al., 2001). These mutants also antagonize the TOR-mediated response to amino acid availability (Gao et al., 2002). Loss of Tsc1 and Tsc2 resulted in a TOR-dependent increase of S6K activity(Gao et al., 2002; Radimerski et al., 2002). Although S6K is normally inactivated in animal cells in response to amino acid starvation, loss of Tsc1–Tsc2 renders cells resistant to amino acid starvation (Gao et al., 2002).
Rheb
The small GTPase RHEB (Ras homologue enriched in brain) has been shown to be a direct target of Tsc2. By using both in vivo and in vitro assays it was shown that the Tsc proteins negatively regulate Rheb through the GAP activity of Tsc2 (Zhang et al., 2003). Removal of one of the two copies of Rheb gene was sufficient to partially rescue the the lethality of Tsc1 null animals. (Zhang et al., 2003). Mutations in the Drosophila Rheb gene were also isolated as growth-inhibitors, whereas overexpression of Rheb promoted cell growth (Stocker et al., 2003). In mitotic tissues, overexpression of Rheb accelerates passage through G1–S phase without affecting rates of cell division, whereas in endoreplicating tissues, Rheb increases DNA ploidy (Saucedo et al., 2003). Mutation of Rheb suspends larval growth and prevents progression from first to second instar. Genetic and biochemical tests indicate that Rheb functions in the insulin signaling pathway downstream of Tsc1–Tsc2 and upstream of TOR (Saucedo et al., 2003; Stocker et al., 2003). Levels of Rheb mRNA are rapidly induced in response to protein starvation, and overexpression of Rheb can drive cell growth in starved animals, suggesting a role for Rheb in the nutritional control of cell growth (Saucedo et al., 2003). Furthermore, S6K was shown to be a major effector of Rheb function (Stocker et al., 2003).
d4E-BP
Drosophila contains a single 4E-BP homolog, THOR/d4E-BP, which was first described to be involved in host immune defense in Drosophila (Bernal and Kimbrell, 2000). The 4E-BPs constitute a family of low-molecular weight proteins that interact with eIF4E. Upon binding to eIF4E they prevent its interaction with the scaffold protein eIF4G and inhibit cap-dependent translation initiation (Raught et al., 2000). Translational repression by 4E-BP is relieved by the phosphorylation of a set of serine and threonine residues that causes 4E-BP dissociation from eIF4E. In flies, d4E-BP binds to deIF4E and this binding is modulated by insulin and is sensitive to both LY294002 and rapamycin. Ectopic overexpression of a strongly active form of d4E-BP causes reduction in cell size (Miron et al., 2001). Although, d4E-BP null flies are viable and showed no difference in growth and development compared to control flies, co-expression of d4E-BP with growth promoting genes (e.g. Dp110/dAkt) antagonizes their effect on growth (Miron et al., 2001). These results are consistent with d4E-BP being a downstream effector of both TOR and insulin signaling.
Other modulators of the TOR pathway
Rag GTPases (dRagA and dRagC) were recently identified as novel activators of TOR pathway in response to amino acids. Knockdown of dRagA or dRagC mRNA decreased dS6K phosphorylation upon amino acid stimulation. The Rag proteins were further shown to regulate cell size in a nutrient dependent manner. Overexpression of constitutively active dRagA Q61L increased cell size, especially upon starvation, whereas expression of dominant negative dRagA T16N decreased cell size and this effect was stronger when flies were fed. Constitutively active dRagA Q61L expression was shown to suppress starvation-induced autophagy (Kim et al., 2008).
A recent study indicates Rab1 and Arf1, two small GTPases, involved in intracellular vesicle transport to play a role in TOR activation in Drosophila S2 cells. Knockdown of both Rab1 and Arf1 mRNA caused a significant decrease of dS6K phosphorylation and the effects on dS6K phosphorylation were similar to that caused by RagA mRNA knockdown (Li et al., 2010). A protein tyrosine phosphatase 61F (Ptp61F) was also recently identified as a novel modulator of TOR activity under hypoxic conditions and suggest that its function during hypoxia contributes to the down-regulation of translation. The Ptp61F inhibited cells exhibited increased cell survival and increased TOR signaling. It was also shown that inhibition of Akt prevents both the cell survival and the increased TOR signaling seen under hypoxia and Ptp61F knockdown (Lee et al., 2008a). Recently, the B' regulatory subunit of dPP2A (dPP2A-B') was identified as a conserved component that specifically targets the PP2A complex to dephosphorylate dS6K in Drosophila (Hahn et al., 2010).
TOR is part of a complex network of signaling components. This is to be expected for a protein like TOR which is versatile and has to integrate various inputs to decide cellular fate. A key role for TOR is to match the growth rate to the availability of the resources in both intra- and extracellular environments. TOR achieves this by acting as a major hub for a complex network of signals. The various inputs and outputs from the TOR pathway have previously been described elsewhere (Kapahi et al., 2010; Polak and Hall, 2009; Wullschleger et al., 2006).
We discuss below a number of biological processes which are impacted by TOR signaling in Drosophila some of which are likely to help in improving our mechanistic understanding of how TOR signaling modulates lifespan.
Role of TOR signaling in various biological processes in Drosophila
TOR and aging
As discussed in other reviews in this issue the fruit fly Drosophila has served as an excellent model organism to study the mechanisms of organismal aging. Some of the reasons for favoring Drosophila as a model organism include the ease of manipulation, speed of discovery, availability of powerful genetic tools in an organism that has a complex body plan and exhibits diverse behaviors. The role of various genes in the TOR pathway was first identified in Drosophila demonstrating the importance of this model for aging research.
Inactivation of dTOR or activation of the upstream negative regulators dTSC1 and dTSC2 has been found to extend the mean lifespan of flies by ~30% (Kapahi et al., 2004). Consistent with these observations, rapamycin can also leads to ~10% increase in mean lifespan in Drosophila (Bjedov et al., 2010). Interestingly, upregulation of dTsc2 specifically in the fat body and muscle tissues was shown to be critical for the lifespan extension effects in Drosophila (Kapahi et al., 2004; Luong et al., 2006). A key role for fat-storing tissues in modulating lifespan was also observed in long-lived flies overexpressing dFOXO (Giannakou et al., 2004; Hwangbo et al., 2004), and in long-lived FIRKO (fat-specific insulin receptor knockout) mice (Bluher et al., 2003). While large amounts of data show that signaling through the TOR pathway can act both in parallel to but also interact with the insulin/IGF-1 pathway in flies (Kapahi and Zid, 2004; Oldham and Hafen, 2003), only few experiments have directly explored the interactions between these pathways in terms of lifespan. Rapamycin can extend lifespan of insulin/IGF-1 pathway mutants, suggesting that the two pathways may act in parallel for slowing aging (Bjedov et al., 2010). Clearly, more experiments, including genetic epistasis analysis, are needed to further clarify the interaction between the dTOR and the insulin/IGF-1 signaling pathways with regards to aging.
DR in flies, either by restriction of total food (Clancy et al., 2002; Mair et al., 2003; Rogina et al., 2002), or by reducing the amount of yeast in the food (Kapahi et al., 2004; Mair et al., 2005), robustly extends lifespan. Inhibition of TOR signaling was first proposed to extend lifespan by overlapping mechanism to DR in Drosophila. Studies in yeast and worms also support this conclusion (Kapahi et al., 2010; Katewa and Kapahi, 2010). The long lifespan of mutants overexpressing dTSC2 cannot be further extended by DR (Kapahi and Zid, 2004; Kapahi et al., 2004). Further studies have implicated a key role for d4E-BP in mediating the lifespan extension effects of DR as discussed below. However, a combination of rapamycin and caloric restriction, maximized for lifespan extension, can cause some additional longevity effects when compared to each single treatment (Bjedov et al., 2010). These experiments indicate that the effects of rapamycin, may not be identical to the mechanisms by which caloric restriction extends lifespan. However, the methods of DR utilized in the two studies were distinct suggesting that may play an important role in some forms of DR. Alternatively, it is possible that rapamycin has additional TOR-independent targets that are yet to be identified.
Role of TOR in coordination of growth regulation
TOR is a major nutrient sensing pathway which is found in both unicellular and multicellular eukaryotes (Wullschleger et al., 2006). The evolution of multi-cellularity necessitated the use of diffusible factors to co-ordinate growth (Bradley and Leevers, 2003; Neufeld, 2004; Wullschleger et al., 2006). It has been suggested that TOR is a more ancient signaling pathway that acts cell autonomously while the insulin signaling pathway evolved later to co-ordinate growth in certain multicellular organisms (Kapahi and Zid, 2004; Marygold and Leevers, 2002; Polak and Hall, 2009; Wullschleger et al., 2006). The parallel and interactive signaling of insulin/IGF and TOR signaling pathways, allows a much more robust biological sensor to assess the nutritional status of the growing organism both cell autonomously and non-autonomously and then relay this information to outputs which appropriately adjust the growth and somatic maintenance of the organism (Neufeld, 2004; Oldham and Hafen, 2003).
The co-ordination of TOR and insulin signaling pathways occurs at many levels (Shamji et al., 2003). Consistent with an interplay between the insulin and TOR/S6K pathways, lethality of loss of function of InR is was suppressed by heterozygosity of Tsc1 or Tsc2 (Gao and Pan, 2001). Furthermore, overexpressed Akt phosphorylates and inactivates Tsc2 and thereby activates S6K (Inoki et al., 2003; Manning and Cantley, 2003). Another study identified slimfast as an amino acid transporter that activates TOR signaling in fat body (Colombani et al., 2003). Animals lacking slimfast in fat body behaved as amino acid deprived animals and were smaller in size and had reduced S6 phosphorylation. Interestingly, this lack of nutrients and TOR inhibition is also communicated to other tissues by modulation of insulin signaling. The mutants showed a significant decrease in insulin signaling in other tissues. Reduced TOR signaling in fat body through a still unknown but secreted molecule, results in a retention and accumulation of dilps in the median neuro-secretory cells (mNSC). The secretion of dilps from mNSC was restored by activation of TOR in fat body or incubating the mNSC in presence of hemolymph from a fed animal suggesting a presence of a humoral factor (Geminard et al., 2009). Recent findings suggest that interaction of these two pathways is critical for cellular homeostasis. Tor/raptor signaling was shown to slow down the cell division in response to increased insulin signalling (Wu et al., 2007). Similarly, FOXO, which is a well established downstream effector of insulin signaling was shown to be critical for restricting overgrowth of eye phenotype in a Tsc1 mutant (Harvey et al., 2008).
Wnt signaling has also been demonstrated to regulate TOR activity (Inoki and Guan, 2006). Stimulation of the Wnt receptors culminates in the inhibition of glycogen synthase kinase 3 (Gsk3). When active, Gsk3 has been shown to directly phosphorylate and activate Tsc2 when primed by AMPK-dependent phosphorylation (Inoki and Guan, 2006). Therefore, Wnt mediated inactivation of Gsk3 alleviates Tsc2-driven inhibition of Rheb and results in TOR activation. Inputs from various other growth factors, like that from transforming growth factor (TGF) beta (Lamouille and Derynck, 2007), further supports the role of TOR in sensing extracellular environment to keep cellular growth in tune with it.
In a genetic screen for TOR interactors in Drosophila, clathrin-uncoating ATPase Hsc70-4, was identified as a key regulator of TOR mediated endocytosis..TOR signaling not only stimulates bulk endocytic uptake but also inhibits the targeted endocytic degradation of the amino acid importer Slimfast (Hennig et al., 2006). Drosophila Hsc70-4 (Hsc4) mutants were first shown to have an impairment in nerve-evoked neurotransmitter release in a Ca2+ dependent manner (Bronk et al., 2001).
Ecdysone is an insect hormone and is critical for pupariation and thus plays a major role in fly development. Low protein in the diet causes a delay in development by increasing the length of time taken by larvae to reach the wandering stage. Recently, it was shown that the nutrient dependent delay in development correlates negatively with the levels of dTOR mRNA. Reduce TOR signaling in prothoracic gland silences the response to prothoracicotropic hormone and reduces the activation of ecdysone production (Layalle et al., 2008). Further experiments are needed to understand the role of various growth signaling pathways including those related to ecdysone in lifespan extension by inhibition of TOR.
TOR and stress response regulation
In addition to nutrients and growth factors, appropriate control of cell growth also requires integration of information on environmental stresses. The modulation of stress pathways has been well established to contribute to lifespan extension in multiple species (Martin et al., 1996). TOR is rendered less active by high temperature, hydrogen peroxide and high salt stress in S. cerevisiae (Urban et al., 2007). This poses the question whether reduced TOR activity triggers a stress resistance response. Inhibition of the TOR signaling network and also a number of translation factor genes enhances resistance to various environmental stresses (Hansen et al., 2007; Kaeberlein et al., 2005; Pan et al., 2007; Powers et al., 2006). Evidence from both C. elegans and S. cerevisae suggest that transcription factors such as PHA-4, HIF-1, Gis1 and Msn2/4, which are all involved in mediating stress responses, are regulated by TOR signaling and mediate its effects on lifespan extension (Chen et al., 2009; Medvedik et al., 2007; Sheaffer et al., 2008).
In flies increasing Rheb-TOR-S6K signaling by overexpression of Rheb, TOR or a constitutive active form of S6K sensitize flies to oxidative stress, while expression of the Tsc2 or dominant-negative forms of TOR and S6K provides flies with resistance to oxidative stress (Patel and Tamanoi, 2006). Tissue specific analysis of stress resistance indicated that overexpression of Rheb in muscle sensitizes flies to oxidative stress and induced an age dependent loss of negative geotaxis (Patel and Tamanoi, 2006). Sestrin1 and Sestrin2, both transcriptional targets of the DNA damage sensor p53, activate AMPK, thereby inhibiting TOR pathway activity (Budanov and Karin, 2008). TOR inhibition in adult flies by rapamycin increases stress resistance to both starvation and oxidative stress (Bjedov et al., 2010). d4E-BP has been shown to play an important role in mediating the starvation and oxidative stress resistance in Drosophila (Tettweiler et al., 2005). Furthermore, starvation and oxidative stress increases the level of d4E-BP which is dependent on dFOXO (Teleman et al., 2005; Tettweiler et al., 2005). However, partial reduction of TOR, despite resulting in lifespan extension, was reported not to be sufficient to obtain significant stress resistance phenotypes (Kapahi et al., 2004; Luong et al., 2006). These results suggest that perhaps the increased stress phenotypes are not necessary for the lifespan extension effects though TOR influences both stress resistance and lifespan.
Another link of TOR with stress signaling was demonstrated in Drosophila through its interaction with sestrins. Sestrins, which are conserved proteins accumulate in cells exposed to stress. The authors propose a model whereby enhanced TOR signaling leads to increased oxidative stress and leads to increase in sestrin level. Sestrin act as feedback inhibitors of TOR by potentiating the activity of AMPK which inhibits TOR. Loss of sestrin leads to age related muscle degeneration, decline in heart function and enhanced lipid accumulation in Drosophila (Lee et al., 2010). Sestrins have been proposed to be protective through their effects on enhancing autophagy. These data provide compelling evidence for the link of ROS in age-related decline of muscle function through increased TOR activity. However, it remains to be investigated if over-expression of sestrin is sufficient to alleviate age-linked pathologies and whether it affects lifespan. In addition to the link through sestrin there are other examples in the fly where cellular stresses have been shown to modulate TOR activity.
Scylla and charybdis, two homologous genes, first identified as growth suppressors in an EP (enhancer/promoter) overexpression screen, act as negative regulators of growth. The simultaneous loss of both genes generates flies that are more susceptible to hypoxia and also show mild overgrowth phenotypes. Overactivation of either on the other hand reduces growth, which was associated with a reduction in S6K but not PKB/Akt activity. Furthermore, it was shown that scylla and charybdis are induced under hypoxic conditions and that scylla is a target of dHIF-1 (hypoxia-inducible factor-1)(Reiling and Hafen, 2004). In flies, p38 stress-activated kinase cascade has shown to be critical for activation of TOR signaling pathway in response of amino acids, insulin/IGF and certain stresses such as hydrogen peroxide and anisomycin. This stress-induced TORC1 activation could be blocked by RNAi against mitogen-activated protein kinase kinase 3 and 6 (MKK3/6) or by the overexpression of dominant negative dRags (Cully et al., 2010). Hence, TOR not only modulates stress responses but also responds to stressful situations by appropriately modifying its activity. It is likely that these responses play an important role in modulating lifespan but further experiments are required to tease apart this link between stress resistance and lifespan.
TOR and regulation of protein synthesis
The regulation of protein synthesis by TOR is one of its critical functions to keep organismal growth and development in tune with environmental conditions (Sonenberg, 2000). This regulation of protein synthesis by TOR takes place at multiple levels including modulation of S6 kinase (S6K), mRNA translation initiation and ribosomal biogenesis (Ma and Blenis, 2009; Shamji et al., 2003). S6K modulates protein synthesis by a number of mechanisms which have been previously reviewed (Kapahi et al., 2010; Ma and Blenis, 2009). Interestingly, in addition to the important role of protein synthesis in growth and development, a number of pieces of evidence from multiple species point to its importance in determining lifespan as well. Hence TOR mediated changes in protein synthesis are likely to have a major influence on its aging related phenotypes.
Drosophila was the first organism where the role of S6K in lifespan was determined. Overexpression of the dominant-negative S6K fly mutants showed extended lifespan while conversely the constitutively active form of S6K was found to exhibit significantly reduced lifespan (Kapahi and Zid, 2004). These studies prompted experiments examining the role of S6K and mRNA translation in aging in other species. Inhibition of S6K also extended lifespan in C. elegans (Hansen et al., 2007; Pan et al., 2007) and S. cerevisiae (Kaeberlein et al., 2005). In mice, female but not male animals lacking S6K1 (the mouse genome encodes 2 paralogs, S6K1 and S6K2) have been shown to display increased lifespan and slowed progression of age-related pathologies (Selman et al., 2009). These studies along with the findings that feeding rapamycin can extend lifespan in mice (Harrison et al., 2009) demonstrate a conserved role for S6K in mediating lifespan extension in vertebrate and invertebrate species which has generated much interest in targeting drugs for this pathway with the eventual goal of extending human healthspan (Kaeberlein and Kapahi, 2009; Kapahi et al., 2010; Kapahi and Vijg, 2009; Katewa and Kapahi, 2010)
TOR signaling also regulates ribosome biogenesis by multiple mechanisms which are not yet well understood (Guertin and Sabatini, 2007; Wullschleger et al., 2006). One possible mechanism is through activation of transcription initiation factor IA (TIF-IA), a conserved RNA polymerase I transcription factor. Drosophila melanogaster TIF-IA(−/−) mutants have reduced levels of rRNA synthesis and sustain a developmental arrest caused by a block in cellular growth. By using both genetic and chemical modulation of TOR, it was shown that TOR pathway not only regulates TIF-IA recruitment to rDNA but also regulates rRNA synthesis in vivo. TIF-IA overexpression was shown to maintain rRNA transcription even in the protein starved or rapamycin treated larvae (Grewal et al., 2007). Overexpression of TIF-IA also elevates levels of both 5S RNA and messenger RNAs encoding ribosomal proteins. Although it remains to be seen in flies, inhibition of various ribosomal subunits has already been shown to extend lifespan in C. elegans (Bell et al., 2009; Chen et al., 2007; Curran and Ruvkun, 2007; Hansen et al., 2007).
TOR inhibition also affects mRNA translation initiation by reducing the phosphorylation of translation-initiation factor 4 binding proteins (4E-BPs) (Hay and Sonenberg, 2004; Tee and Blenis, 2005). As a consequence, 4E-BPs bind to eIF4E, which impairs the recruitment of the 40S ribosomal subunit to the cap structure present at the 5'-end of eukaryotic mRNAs (Sonenberg and Gingras, 1998). The mRNA cap-binding protein eIF4E is a component of the eIF4E complex, which also contains the scaffold protein eIF4G and the RNA helicase eIF4A (Marintchev and Wagner, 2004). 4E-BP has been shown to play an important role in mediating the effects of DR in flies. Flies lacking d4E-BP fail to show the lifespan extension observed upon DR, while flies overexpressing an activated form of the translational repressor 4E-BP live long upon rich nutrients but fail to show lifespan extension upon DR (Zid et al., 2009). This study also demonstrated that levels of 4E-BP are increased upon DR in a FOXO independent manner. These finding support the hypothesis that DR mediates its lifespan extension effects by enhancing 4E-BP activity and inhibiting cap mediated mRNA translation (Zid et al., 2009).
Global decline in protein synthesis is known with age (Finch, 1990) and thus it is counterintuitive that a reduction in protein synthesis could extends lifespan in different species. However, evidence from multiple species supports the importance of protein synthesis in determining lifespan. Inhibition of protein synthesis by attenuating expression of various mRNA translation factors (Hansen et al., 2007; Henderson et al., 2006; Pan et al., 2007; Steffen et al., 2008; Syntichaki et al., 2007) or reducing protein in the diet (Chippindale et al., 1993; Kapahi et al., 2004; Miller et al., 2005; Orentreich et al., 1993; Richie et al., 1994) extends lifespan in multiple species. Further support for the importance of proteins synthesis in determining lifespan comes from a comparative studies done across species which showed an inverse correlation between rate of protein synthesis and longevity (Finch, 1990; Munro, 1969). Two models potentially explain the beneficial effects of inhibiting protein synthesis, though they are exclusive of each other. One possibility is that a global decrease in protein synthesis may not only improve the fidelity of protein synthesis but also a decrease of overall mRNA translation could decrease the overall burden of misfolded proteins by reducing the protein traffic intracellularly. Another possibility is that the mRNA translation of specific groups of mRNAs may be preserved or even enhanced under conditions of global inhibition of protein synthesis, and that this may slow aging. Studies from Drosophila lend support to the latter model.
The translational status of mRNA's under DR was examined in Drosophila using a genome wide translational profiling approach (Zid et al., 2009). This was achieved by examining the number of polysomes bound to each individual mRNA. Given that mRNA translation initiation is the major rate-limiting step in mRNA translation, this indicates the efficiency with which each mRNA is translated (Serikawa et al., 2003; Zong et al., 1999). Genome wide translational changes revealed an increase in the ribosomal binding of certain mRNAs, though there was an overall decrease of mRNA translation under DR in D. melanogaster (Zid et al., 2009). These mRNA's included a number of nuclear-encoded mitochondrial genes, more specifically the one that were part of the electron transport chain (ETC) complexes I and IV and mitochondrial ribosomal proteins (Zid et al., 2009). The enhanced ribosomal loading of nuclear-encoded mitochondrial genes was dependent on their simple 5’UTR structures. Interestingly, d4E-BP mediates differential translation of specific targets which is mediated by these 5’ UTR, including mitochondrial proteins (Zid et al., 2009). These results suggest a novel mode of regulation of nuclear-encoded mitochondrial genes by enhanced mRNA translation under DR. However, the precise mechanisms by which the translation machinery upregulates these genes remains to be elucidated.
TOR and regulation of autophagy
TOR was identified as a key regulator of autophagy in yeast (Noda and Ohsumi, 1998). Autophagy is a highly regulated cellular starvation response to maintain essential nutrient levels and viability. This process, sometimes also referred as macroautophagy, is described as a process in which portions of the cytoplasm, including mitochondria and other organelles, are degraded under conditions of nutrient limitation, allowing cellular macromolecules to be broken down and recycled. During autophagy, large double-membrane vesicles, called autophagosomes, are generated and degraded in lysosomes, together with their contents (Levine and Klionsky, 2004). In flies, dTOR is shown to inhibit starvation induced autophagy, as measured by increased accumulation of autolysosomes, in an Atg1 dependent manner. In cells lacking dTOR autophagy is enhanced (Scott et al., 2004). Phosphorylation and complex formation by Atg13 and Atg1 is regulated in a nutrient and dTOR dependent manner (Chang and Neufeld, 2009). Inhibition of TOR enhances autophagy and provides an attractive mechanism to explain the effects of reduced TOR activity on lifespan. Localized overexpression of the autophagic vesicle marker LC3/Atg8 using a specific promoter for expression in neurons is sufficient to extend fly lifespan (Simonsen et al., 2008). Consistent with results from other organisms, autophagy gene Atg5 is required for rapamycin to extend lifespan in flies (Bjedov et al., 2010), and fly mutants with reduced levels of autophagy components are short-lived (Juhasz et al., 2007; Toth et al., 2008). These experiments suggest that increases in autophagy could be beneficial to the organism, however the dose and tissue specificity of autophagy need to be better understood in the context of aging.
TOR and metabolism
Given the key role of TOR in regulating growth and development it is not surprising that it also regulates various aspects of metabolism including mitochondrial function, lipid metabolism and feeding behavior. These activities ensure that the ATP generating pathways are also in tune with the organism's needs.
4E-BP, the downstream target of TOR, has been shown to regulate mitochondrial function in a nutrient dependent manner. In a study to find differentially translated mRNA targets induced under DR, mitochondrial genes showed increased expression in long-lived flies subjected to DR (Zid et al., 2009). This study demonstrated for the first time that of nuclear-encoded mitochondrial genes encoding for electron transport chain (ETC) and ribosomal proteins are regulated translationally (Zid et al., 2009). The increase in translation was dependent on 4E-BP and was mediated by the simple 5'UTR structure of nuclear encoded mitochondrial genes. Interestingly, mitochondrial genes encoded by the nuclear genome in general possessed much simpler 5'UTR structure. Consistently, mitochondrial processes, including mitochondrial protein density as well as cytochrome C oxidase and complex I activity were increased in a 4E-BP-dependent fashion in response to DR (Zid et al., 2009). The role of mitochondrial function in longevity was confirmed by the observation that inhibition of complex I or complex IV throughout the whole body abrogated the lifespan extension by DR (Zid et al., 2009). Similar results were obtained independently in another study using a knockdown of mitochondrial complex V (Copeland et al., 2009). The increased translation of ETC genes as a response to DR may serve as a protective mechanism to increase mitochondrial efficiency and maintain the function of the ETC to ensure sustained ATP production, which is otherwise known to decline with age (McCarroll et al., 2004). Further work in this area is required to identify the mechanisms by which the mitochondrial function exerts protective effects to enhance longevity.
Similar to protein synthesis, lipid synthesis is also a key component for ensuing proper growth in an organism. SREBP is a conserved lipid (sterol/ fatty acid) regulated transcription factor and regulates transcription of various genes involved in lipid metabolism and cell membrane production (Dobrosotskaya et al., 2002; Seegmiller et al., 2002). In Drosophila the activity of sterol regulatory element binding protein (SREBP) is also regulated by TOR and is involved in regulation of Akt-dependent cell growth (Porstmann et al., 2008). TOR also regulates triglyceride metabolism as TOR inhibition decreases fat levels (Luong et al., 2006). This decrease in fat levels correlates with an increase in the mRNA levels of the Brummer lipase (Luong et al., 2006). However, previously it had been show that TOR mutants show increased triglyceride levels (Zhang et al., 2000) and rapamycin treatment also increases both fat stores and starvation survival and this was partially dependent on d4EBP. overexpressing the active form of d4EBP causes the animals to accumulate more fat (Teleman et al., 2005). These studies seem to apparently contradict how TOR may modulate fat metabolism. It is likely that further insight into this would be obtained by separately measuring fat synthesis and breakdown, rather than steady state levels of fat. In general there is substantial evidence that TOR modulates fat metabolism but the mechanisms of this remain poorly understood and whether this regulation is important for its effects on longevity also remain unknown.
TOR and feeding behavior
The role of TOR as a nutrient sensor is well known but how it modulates nutrient uptake behavior has been less explored. Recently two studies have described a paradigm examining for nutrient homeostasis in flies (Ribeiro and Dickson, 2010; Vargas et al., 2010). Nutrient homeostasis plays a critical role in maintaining the balance of macronutrients in the diet.
Nutrient composition plays a critical role in development, aging and metabolism (Lee et al., 2008b; Skorupa et al., 2008). Drosophila deficient in either carbohydrate or yeast in their diet show a stronger preference in choice assays for the macronutrient that they were previously deprived of (Vargas et al., 2010). Nutrient homeostasis also plays an important role in dietary switching upon mating in female flies to ensure increased egg production (Ribeiro and Dickson, 2010; Vargas et al., 2010). These studies found that enhanced S6 Kinase signaling in neurons promotes preference to a protein rich diet. These studies support the idea that the TOR pathway may also play a role in balancing of macronutrients. Non-availability of food can adversely affect the organismal growth. When food is scarce the organism increases its foraging response appropriately. In flies, this response is shown to be mediated through insulin/ neuropeptide Y signaling pathway. This response was found to be suppressed by overexpressing dS6K in dilp2 neurons (Wu et al., 2005). An interesting study has demonstrated that TOR also influences circadian behavior and mediates the link between nutrients and circadian clocks (Giebultowicz and Kapahi, 2010; Zheng and Sehgal, 2010). The significance of these findings, especially their relevance for metabolism, feeding and aging has been recently reviewed (Giebultowicz and Kapahi, 2010). Together these studies demonstrate that in addition to being a nutrient sensor TOR is also important for mediating behavioral changes that modify nutrient intake in response to environmental cues
Role of TOR pathway in models of age-related diseases
One of the promises of aging research is that agents that slow aging would also slow age-related diseases and improve healthspan. Drosophila is a great model system to examine this hypothesis. Considering that TOR regulates a number of processes that are involved in modulation of aging there is significant hope that it can also delay age related pathologies. Using Drosophila models that aim to recapitulate human age-related diseases, there are encouraging findings that indeed the benefits of inhibition of TOR may extend to a number of diseases as well. In flies, feeding rapamycin decreases the toxicity of aggregate-prone proteins such as polyglutamine-expanded huntingtin in Huntington’s disease (Berger et al., 2006; Ravikumar et al., 2004). Similar protection is observed under neurodegenerative tauopathies, including Alzheimer’s disease (Khurana et al., 2006). TOR-mediated autophagy has been shown to regulate both cell death and increased clearance of aggregated proteins in these models (Ravikumar et al., 2004; Wang et al., 2009). Rapamycin treatment, was also shown to be beneficial in protection against loss of dopaminergic neurons in a model for Parkinson's disease (Tain et al., 2009). Overexpression of 4E-BP was protective in the degenerative phenotypes observed due to loss Drosophila Parkin and PINK1, known orthologs of genes associated with Parkinson's disease in humans.
A recent study suggested that TOR activity in myocardial tissue is important for regulating cardiac stress sensitivity, and that reduction in TOR activity promotes maintenance of youthful heart function during aging. d4E-BP was shown to act autonomously in the heart and downstream of both dTOR and dFoxo to modulate cardiac aging. Overexpression of d4E-BP was sufficient to protect cardiac function against functional decline during aging, and can rescue the effects of dTOR overexpression in the heart (Wessells et al., 2009). Another study in Drosophila showed that sestrin, which inhibits TOR activity through AMPK activation is also protective against age-related loss of muscle and cardiac function (Lee et al., 2010). Reduction of dTOR activity in Drosophila also blocks the insulin resistance and metabolic syndrome phenotypes associated with increased activity of the insulin responsive transcription factor, dFOXO.
Furthermore, reduction in dTOR function also protects against age-dependent decline in cardiac function and increases longevity (Luong et al., 2006). These studies demonstrate that the beneficial effects of inhibition of TOR increases the healthspan of the flies and gives a strong rationale for exploring the effects of this pathway on healthspan in mammals.
Summary and future outlook
Drosophila is a powerful model system to explore the role of gene-environment interaction in shaping lifespan. Furthermore, it offers an excellent number of models to study age-related diseases, providing immense potential to understand the determinants of healthspan. The discovery that the TOR signaling network mediates lifespan extension caused by nutrient modulation in invertebrate and vertebrate species provides a framework to examine the genetic and molecular basis of how DR extends lifespan and protects against a number of age-related diseases.
Future studies in this area is likely to be of immense importance to understand the downstream mechanisms by which TOR and DR mediate its effects on longevity. Some of these processes include protein translation, autophagy, metabolism, and stress responses. The precise mechanism by which TOR regulates these processes and how they affect aging is not yet clear. Some of the outstanding questions in the field are to examine the tissue-specific requirements for TOR and TOR’s effectors, as well as to better understand the cross talk of TOR with other longevity pathways. Moreover, TOR is likely to utilize other effectors and upstream signals than the ones discussed here in order to modulate organismal aging, that are yet to be identified.
These findings will also provided excellent drug targets to further examine the mechanisms of extending lifespan. The findings that rapamycin was shown to extend lifespan not only in Drosophila but also in mice if given late in life has generated a lot of excitement in the field. Further analyses of this highly conserved pathway will shed light on the link between nutrients and various age-related diseases including cancer, neurodegeneration and diabetes in humans. Such knowledge might ultimately facilitate development of better therapeutic approaches for these diseases in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Long X, Spycher C, Han ZS, Rose AM, Muller F, Avruch J. TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr Biol. 2002;12:1448–1461. doi: 10.1016/s0960-9822(02)01091-6. [DOI] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Hentges KE, Sirry B, Gingeras AC, Sarbassov D, Sonenberg N, Sabatini D, Peterson AS. FRAP/mTOR is required for proliferation and patterning during embryonic development in the mouse. Proc Natl Acad Sci U S A. 2001;98:13796–13801. doi: 10.1073/pnas.241184198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–6718. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Ruegg MA. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Inoki K, Guan KL. Complexity of the TOR signaling network. Trends Cell Biol. 2006;16:206–212. doi: 10.1016/j.tcb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5:2087–2102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle. 2009;8:1888–1895. doi: 10.4161/cc.8.12.8606. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7:3355–3361. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers AN, Kapahi P. Genetic mechanisms of lifespan extension by dietary restriction. Drug Discovery Today: Disease Mechanisms. 2006;3:6–10. [Google Scholar]
- Stewart MJ, Berry CO, Zilberman F, Thomas G, Kozma SC. The Drosophila p70s6k homolog exhibits conserved regulatory elements and rapamycin sensitivity. Proc Natl Acad Sci U S A. 1996;93:10791–10796. doi: 10.1073/pnas.93.20.10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KL, Chou MM, Blenis J, Gelbart WM, Erikson RL. A Drosophila gene structurally and functionally homologous to the mammalian 70-kDa s6 kinase gene. Proc Natl Acad Sci U S A. 1996;93:13694–13698. doi: 10.1073/pnas.93.24.13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science. 1999;285:2126–2129. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- Barcelo H, Stewart MJ. Altering Drosophila S6 kinase activity is consistent with a role for S6 kinase in growth. Genesis. 2002;34:83–85. doi: 10.1002/gene.10132. [DOI] [PubMed] [Google Scholar]
- Oldham S, Montagne J, Radimerski T, Thomas G, Hafen E. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev. 2000;14:2689–2694. doi: 10.1101/gad.845700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Stallock JP, Ng JC, Reinhard C, Neufeld TP. Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 2000;14:2712–2724. doi: 10.1101/gad.835000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DC, Paricio N, Goodman EC, Mlodzik M, Wilson C. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 1999;13:3244–3258. doi: 10.1101/gad.13.24.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JS, Edgar BA. Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development. 1998;125:2149–2158. doi: 10.1242/dev.125.11.2149. [DOI] [PubMed] [Google Scholar]
- Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru B, Pan D. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- Radimerski T, Montagne J, Hemmings-Mieszczak M, Thomas G. Lethality of Drosophila lacking TSC tumor suppressor function rescued by reducing dS6K signaling. Genes Dev. 2002;16:2627–2632. doi: 10.1101/gad.239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- Bernal A, Kimbrell DA. Drosophila Thor participates in host immune defense and connects a translational regulator with innate immunity. Proc Natl Acad Sci U S A. 2000;97:6019–6024. doi: 10.1073/pnas.100391597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B, Gingras AC, Gygi SP, Imataka H, Morino S, Gradi A, Aebersold R, Sonenberg N. Serum-stimulated, rapamycin-sensitive phosphorylation sites in the eukaryotic translation initiation factor 4GI. EMBO J. 2000;19:434–444. doi: 10.1093/emboj/19.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron M, Verdu J, Lachance PE, Birnbaum MJ, Lasko PF, Sonenberg N. The translational inhibitor 4E-BP is an effector of PI(3)K/Akt signalling and cell growth in Drosophila. Nat Cell Biol. 2001;3:596–601. doi: 10.1038/35078571. [DOI] [PubMed] [Google Scholar]
- Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Kim E, Yuan H, Inoki K, Goraksha-Hicks P, Schiesher RL, Neufeld TP, Guan KL. Regulation of mTORC1 by the rab and ARF GTPases. J Biol Chem. 2010 doi: 10.1074/jbc.C110.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Feldman R, O'Farrell PH. An RNA interference screen identifies a novel regulator of target of rapamycin that mediates hypoxia suppression of translation in Drosophila S2 cells. Mol Biol Cell. 2008a;19:4051–4061. doi: 10.1091/mbc.E08-03-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn K, Miranda M, Francis VA, Vendrell J, Zorzano A, Teleman AA. PP2A regulatory subunit PP2A-B' counteracts S6K phosphorylation. Cell Metab. 2010;11:438–444. doi: 10.1016/j.cmet.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol. 2009;21:209–218. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, Bodmer R, Oldham SM. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab. 2006;4:133–142. doi: 10.1016/j.cmet.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid B. TOR pathway: linking nutrient sensing to life span. Sci Aging Knowledge Environ 2004. 2004:PE34. doi: 10.1126/sageke.2004.36.pe34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 2003;13:79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL, Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298:1745. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katewa SD, Kapahi P. Dietary restriction and aging, 2009. Aging Cell. 2010;9:105–112. doi: 10.1111/j.1474-9726.2010.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley GL, Leevers SJ. Amino acids and the humoral regulation of growth: fat bodies use slimfast. Cell. 2003;114:656–658. doi: 10.1016/s0092-8674(03)00721-9. [DOI] [PubMed] [Google Scholar]
- Neufeld TP. Genetic analysis of TOR signaling in Drosophila. Curr Top Microbiol Immunol. 2004;279:139–152. doi: 10.1007/978-3-642-18930-2_9. [DOI] [PubMed] [Google Scholar]
- Marygold SJ, Leevers SJ. Growth signaling: TSC takes its place. Curr Biol. 2002;12:R785–R787. doi: 10.1016/s0960-9822(02)01294-0. [DOI] [PubMed] [Google Scholar]
- Shamji AF, Nghiem P, Schreiber SL. Integration of growth factor and nutrient signaling: implications for cancer biology. Mol Cell. 2003;12:271–280. doi: 10.1016/j.molcel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Geminard C, Rulifson EJ, Leopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Wu MY, Cully M, Andersen D, Leevers SJ. Insulin delays the progression of Drosophila cells through G2/M by activating the dTOR/dRaptor complex. Embo J. 2007;26:371–379. doi: 10.1038/sj.emboj.7601487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Mattila J, Sofer A, Bennett FC, Ramsey MR, Ellisen LW, Puig O, Hariharan IK. FOXO-regulated transcription restricts overgrowth of Tsc mutant organs. J Cell Biol. 2008;180:691–696. doi: 10.1083/jcb.200710100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig KM, Colombani J, Neufeld TP. TOR coordinates bulk and targeted endocytosis in the Drosophila melanogaster fat body to regulate cell growth. J Cell Biol. 2006;173:963–974. doi: 10.1083/jcb.200511140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk P, Wenniger JJ, Dawson-Scully K, Guo X, Hong S, Atwood HL, Zinsmaier KE. Drosophila Hsc70-4 is critical for neurotransmitter exocytosis in vivo. Neuron. 2001;30:475–488. doi: 10.1016/s0896-6273(01)00292-6. [DOI] [PubMed] [Google Scholar]
- Layalle S, Arquier N, Leopold P. The TOR pathway couples nutrition and developmental timing in Drosophila. Dev Cell. 2008;15:568–577. doi: 10.1016/j.devcel.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, Wanke V, Anrather D, Ammerer G, Riezman H, Broach JR, De Virgilio C, Hall MN, Loewith R. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell. 2007;26:663–674. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell. 2007;6:111–119. doi: 10.1111/j.1474-9726.2006.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Thomas EL, Kapahi P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000486. doi: 10.1371/journal.pgen.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5:e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaffer KL, Updike DL, Mango SE. The Target of Rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr Biol. 2008;18:1355–1364. doi: 10.1016/j.cub.2008.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PH, Tamanoi F. Increased Rheb-TOR signaling enhances sensitivity of the whole organism to oxidative stress. J Cell Sci. 2006;119:4285–4292. doi: 10.1242/jcs.03199. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettweiler G, Miron M, Jenkins M, Sonenberg N, Lasko PF. Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 2005;19:1840–1843. doi: 10.1101/gad.1311805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman AA, Chen YW, Cohen SM. 4E-BP functions as a metabolic brake used under stress conditions but not during normal growth. Genes Dev. 2005;19:1844–1848. doi: 10.1101/gad.341505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev. 2004;18:2879–2892. doi: 10.1101/gad.322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully M, Genevet A, Warne P, Treins C, Liu T, Bastien J, Baum B, Tapon N, Leevers SJ, Downward J. A role for p38 stress-activated protein kinase in regulation of cell growth via TORC1. Mol Cell Biol. 2010;30:481–495. doi: 10.1128/MCB.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N, Hershey JWB, Mathews BM. Translational control of gene expression. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Kapahi P. Cell signaling. Aging is RSKy business. Science. 2009;326:55–56. doi: 10.1126/science.1181034. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Vijg J. Aging--lost in translation? N Engl J Med. 2009;361:2669–2670. doi: 10.1056/NEJMcibr0909815. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Grewal SS, Evans JR, Edgar BA. Drosophila TIF-IA is required for ribosome synthesis and cell growth and is regulated by the TOR pathway. J Cell Biol. 2007;179:1105–1113. doi: 10.1083/jcb.200709044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Hubbard A, Chettier R, Chen D, Miller JP, Kapahi P, Tarnopolsky M, Sahasrabuhde S, Melov S, Hughes RE. A human protein interaction network shows conservation of aging processes between human and invertebrate species. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000414. e1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Pan KZ, Palter JE, Kapahi P. Longevity determined by developmental arrest genes in Caenorhabditis elegans. Aging Cell. 2007;6:525–533. doi: 10.1111/j.1474-9726.2007.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SP, Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007;3:e56. doi: 10.1371/journal.pgen.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol. 2005;16:29–37. doi: 10.1016/j.semcdb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Gingras AC. The mRNA 5' cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- Marintchev A, Wagner G. Translation initiation: structures, mechanisms and evolution. Q Rev Biophys. 2004;37:197–284. doi: 10.1017/S0033583505004026. [DOI] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE. Longevity, Senescence, and the Genome. Chicago: University of Chicago Press; 1990. [Google Scholar]
- Henderson ST, Bonafe M, Johnson TE. daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J Gerontol A Biol Sci Med Sci. 2006;61:444–460. doi: 10.1093/gerona/61.5.444. [DOI] [PubMed] [Google Scholar]
- Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, Dang N, Johnston ED, Oakes JA, Tchao BN, Pak DN, Fields S, Kennedy BK, Kaeberlein M. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell. 2008;133:292–302. doi: 10.1016/j.cell.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syntichaki P, Troulinaki K, Tavernarakis N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature. 2007;445:922–926. doi: 10.1038/nature05603. [DOI] [PubMed] [Google Scholar]
- Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. J. Evol. Biology. 1993;6:171–193. [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. Faseb J. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Munro HN. Evolution of protein metabolism in mammals. In: Munro HN, Allison JB, editors. Mammalian Protein Metabolism. vol. 3. 1969. pp. 133–182. [Google Scholar]
- Serikawa KA, Xu XL, MacKay VL, Law GL, Zong Q, Zhao LP, Bumgarner R, Morris DR. The Transcriptome and Its Translation during Recovery from Cell Cycle Arrest in Saccharomyces cerevisiae. Mol Cell Proteomics. 2003;2:191–204. doi: 10.1074/mcp.D200002-MCP200. [DOI] [PubMed] [Google Scholar]
- Zong Q, Schummer M, Hood L, Morris DR. Messenger RNA translation state: the second dimension of high-throughput expression screening. Proc Natl Acad Sci U S A. 1999;96:10632–10636. doi: 10.1073/pnas.96.19.10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Chang YY, Neufeld TP. An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation. Mol Biol Cell. 2009;20:2004–2014. doi: 10.1091/mbc.E08-12-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Erdi B, Sass M, Neufeld TP. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev. 2007;21:3061–3066. doi: 10.1101/gad.1600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M, Vellai T. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy. 2008;4:330–338. doi: 10.4161/auto.5618. [DOI] [PubMed] [Google Scholar]
- Copeland JM, Cho J, Lo T, Jr, Hur JH, Bahadorani S, Arabyan T, Rabie J, Soh J, Walker DW. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol. 2009;19:1591–1598. doi: 10.1016/j.cub.2009.08.016. [DOI] [PubMed] [Google Scholar]
- McCarroll SA, Murphy CT, Zou S, Pletcher SD, Chin CS, Jan YN, Kenyon C, Bargmann CI, Li H. Comparing genomic expression patterns across species identifies shared transcriptional profile in aging. Nat Genet. 2004;36:197–204. doi: 10.1038/ng1291. [DOI] [PubMed] [Google Scholar]
- Dobrosotskaya IY, Seegmiller AC, Brown MS, Goldstein JL, Rawson RB. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science. 2002;296:879–883. doi: 10.1126/science.1071124. [DOI] [PubMed] [Google Scholar]
- Seegmiller AC, Dobrosotskaya I, Goldstein JL, Ho YK, Brown MS, Rawson RB. The SREBP pathway in Drosophila: regulation by palmitate, not sterols. Dev Cell. 2002;2:229–238. doi: 10.1016/s1534-5807(01)00119-8. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol. 2010;20:1000–1005. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- Vargas MA, Luo N, Yamaguchi A, Kapahi P. A Role for S6 Kinase and Serotonin in Postmating Dietary Switch and Balance of Nutrients in D. melanogaster. Curr Biol. 2010 doi: 10.1016/j.cub.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci U S A. 2008b;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Zhang Y, Xu J, Shen P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci U S A. 2005;102:13289–13294. doi: 10.1073/pnas.0501914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebultowicz J, Kapahi P. Circadian clocks and metabolism: the nutrient-sensing AKT and TOR pathways make the link. Curr Biol. 2010;20:R608–R609. doi: 10.1016/j.cub.2010.05.052. [DOI] [PubMed] [Google Scholar]
- Zheng X, Sehgal A. AKT and TOR signaling sets the circadian pacemaker. Curr Biol. 2010 doi: 10.1016/j.cub.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O'Kane CJ, Rubinsztein DC. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol. 2006;16:230–241. doi: 10.1016/j.cub.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Wang T, Lao U, Edgar BA. TOR-mediated autophagy regulates cell death in Drosophila neurodegenerative disease. J Cell Biol. 2009 doi: 10.1083/jcb.200904090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tain LS, Mortiboys H, Tao RN, Ziviani E, Bandmann O, Whitworth AJ. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat Neurosci. 2009;12:1129–1135. doi: 10.1038/nn.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells R, Fitzgerald E, Piazza N, Ocorr K, Morley S, Davies C, Lim HY, Elmen L, Hayes M, Oldham S, Bodmer R. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell. 2009;8:542–552. doi: 10.1111/j.1474-9726.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]