Among the 26% of Americans that showed a 12-month prevalence for a DSM-IV psychiatric disorder in 2005, most suffered from anxiety disorders (18.1%), followed by mood disorders at 9.5% (1). Therefore, anxiety disorders affect nearly one fifth of the population directly in their lifetime, albeit with substantial variation in range of affliction. Despite the current availability of numerous pharmaceutical anxiolytic treatments, most exert only a temporary relief on acute symptomatology, whilst few traditional anxiolytic agents promote long-term alleviation of core symptoms such as the cognitive aspects of anxiety disorders. While a considerable amount of work has explored the role of GABA and GABA modulators in anxiety, more recently, pharmaceutical “anti-depressant” treatments (ADT) such as SSRI’s have been demonstrated as somewhat successful in treating anxiety disorders. Despite their indisputable contributions to psychiatric therapeutics, ADT’s have considerable limitations clinically, such as the prolonged latency (3–5 weeks of chronic administration) until core symptom relief is observed. Thus, there is an urgent need to define the neural systems that mediate anxiety and understand its pathophysiology, which would allow us to explore new treatment avenues.
Recent work from the Akil laboratory has investigated a potential role for fibroblast growth factor 2 (FGF2) in the hippocampus in anxiety and mood disorders. FGF2, a potent central nervous system growth factor and glial mitogen, has been shown to play fundamental roles in growth of the cerebral cortex and hippocampus and genesis of excitatory neurons in these regions during development (2, 3). Patients with affective disorders including major depression and post-traumatic stress disorder have decreased hippocampal volume (4). Decreases in levels of FGF2 have been observed postmortem in humans that suffered from major depressive disorder (MDD), and, in rodents, increases in FGF2 have been reported in response to ADT treatment (5).
In addition to serving a developmental role in building up hippocampal and cortical circuitry, FGF2 levels vary in response to acute changes in CNS homeostasis in adulthood. For example, FGF2 is increased in the hippocampus of animals that have undergone acute stressors, whereas repeated stress and/or chronic corticosterone treatment are associated with decreased levels of hippocampal FGF2 (6). However, alterations in FGF2 levels are not confined to “emotional” perturbations. Hypoxia upregulates both FGF1 and FGF2 in several regions (7) and the knockout of Fgf2 or Fgfr1 (one of the FGF receptors that is stimulated by FGF2) abolishes endogenous compensatory neurogenic responses that are induced after hypoxic or hypoxic/ischemic insults (8). Indeed, FGF2 is a potent neurogenic and trophic factor that improves the outcome of many types of injury.
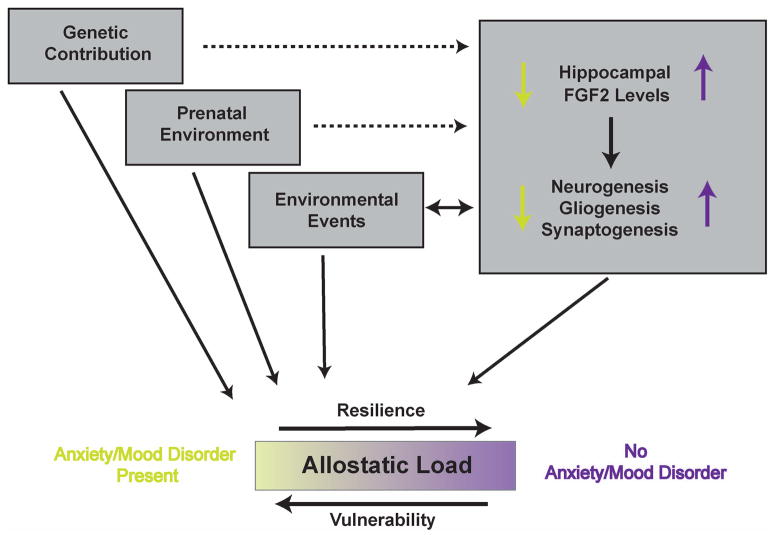
Despite an abundance of correlative evidence for a relationship between FGF2 and depression/anxiety behavior, a causal relationship has not been suggested until recently, when the Akil group showed that chronic peripheral administration of FGF2 in rats showing high anxiety has anxiolytic and potentially antidepressant-like effects (9). In “shRNA silencing of endogenous FGF2 in rat hippocampus increases anxiety behavior” (10), this group goes further by establishing a role for endogenous FGF2 in anxiety behavior by using RNA interference (RNAi) to knockdown FGF2 specifically in the hippocampus. In this paper, they first establish that levels of hippocampal FGF2 gene expression are positively correlated to levels of exhibited anxiety behavior on the elevated plus maze. Following this, they administered a lentivirus expressing short hairpin RNA (shRNA) targeting FGF2 bilaterally into the hippocampus and showed a significant knockdown of FGF2 limited to the DG and extending somewhat to the CA3. Silencing FGF2 in the hippocampus leads to significant increases in anxiety behavior on the elevated plus maze. Taken together, these data suggest that levels of FGF2 within the hippocampus signal overall allostatic load (11), or a sum of an individual’s net vulnerability and resilience to morbidity for anxiety or mood disorders (Figure 1). This is consistent with FGF2 being increased by neuroprotective events, acute stress, escapable shock and ADT treatment, suggesting that levels of this factor are regulated in postnatal life to maintain homeostasis through mechanisms that are still unknown. Conversely, chronic stress, chronic corticosteroid administration and inescapable shock all decrease FGF2 in the hippocampus and are associated with increased vulnerability and decreased resilience to anxiety and mood disorders. Of course, allostatic load is not only modulated by postnatal environmental events but also incorporates genetic vulnerability, prenatal events and epigenetic modifications, among others, that can all play contributory roles in disease morbidity. Interestingly, there is some evidence that FGF2 may also be related to these predisposing factors, as baseline levels of FGF2 have also been shown to differ between strains of rats that show differential anxiety levels, such that highly anxious animals (presumably as a result of strain differences in genetic and developmental factors) show lower levels of FGF2.
Figure 1.
Hippocampal FGF2 levels are correlated with events that mediate allostatic load for anxiety and mood disorder morbidity. Genetic predisposition, prenatal and postnatal environmental events all contribute to any given individual’s allostatic load, and, in addition, they may also modulate hippocampal FGF2 levels. FGF2 modulates allostatic load through unknown factors, which might include hippocampal neurogenesis, gliogenesis and synaptogenesis, all of which have been implicated in the regulation of learning and memory, and perhaps in certain aspects of ADT.
What could be linking these different roles of FGF2 in pre- and postnatal development? Biologically, FGF2 promotes the self-renewal of neural stem cells increasing the size of neural stem cell pools (12) and thus promotes neurogenesis and gliogenesis, both during embryogenesis and in adulthood (2, 6, 9, 12, 13). Perhaps it is not by chance that the hippocampus, in which FGF2 has been implicated in the regulation of anxiety, is also the region that plays a fundamental role in learning and memory through adult neurogenesis. Hippocampal neurogenesis has been also implicated in the pathophysiology of mood disorders. For example, ablation of hippocampal neurogenesis using focal irradiation blocks the effects of ADT treatment on anxious and depressive-like behavior in mice, an effect that appears to be inextricably linked to changes in learning and memory (14).
The specific biological mechanisms through which FGF2 manifests its effects on mood and anxiety are still to be explored. Besides the role of FGF2 in neural stem cell self-renewal and increased new cell survival, this factor may potentially have other related or independent effects, any of which could presumably decrease vulnerability and increase resilience to anxiety/mood disorders. For example, FGF ligands and receptors promote synaptogenesis in the early postnatal period (15), and presumably some FGFs can continue to play a role in synaptic plasticity in adulthood. FGF2 has been shown to be instrumental in facilitating long-term learning and memory and potentiation of synapses, and in a recent paper, a role for FGF2 in mediating NMDAr dependant and independent extinction of conditioned fear has been proposed (16). Conditioned fear is at the core of many dysfunctional cognitive components involved in the development of mood and anxiety disorders. Extinction of conditioned fears (or at the very least, a decrease in salience) must occur during recovery. Therefore, it remains possible that the observed correlation of hippocampal FGF2 levels with behavioral tests of anxiety do not underlie the direct expression of anxiety behavior per se. Rather, FGF2 may also regulate the flexibility of learning and memory in the hippocampus, and facilitate recovery through extinction of cognitive changes associated with psychopathology and ultimately the learning of new associations. Despite the potential implications of the current study from Eren-Kocak et al., it is important to note that this study only examined the effects of blocking FGF2 on the elevated plus maze. Therefore, future studies will need to verify whether the behavioral effects of blocking FGF2 currently observed will generalize to other behavioral tests, such as novelty-suppressed feeding, open field, and light-dark box. Moreover, despite the indisputable wealth of knowledge that is obtained from animal models of mental illness, these models also have considerable limitations, particularly when assessing cognitive and emotional processes indirectly from behavioral performance. To date, all the work that has been done in humans has been largely correlative, and new pharmacological tools need to be established to test an etiological role for FGF2 in mood and anxiety disorders.
In sum, the Akil group has demonstrated some first experimental evidence that FGF2 is causally implicated in the process of anxiety development, and theirs and previous data would suggest that FGF2 levels within the hippocampus are negatively correlated with expression of anxiety behavior; however, the mechanisms by which FGF2 exerts its effects on anxiety behavior remain to be determined. Given the substantial role for FGF2 in modulating stem cells self-renewal and neurogenesis within the hippocampus and the integral role for changes in hippocampal neurogenesis in regulating learning and memory processes, it is likely that FGF2 may act on anxiety behavior through stimulating or decreasing hippocampal neurogenesis. However, the study of FGFs is an ever-evolving field; future studies ablating FGF2 gene expression at different stages of development or adulthood will answer these questions.
Footnotes
Financial Disclosure
Drs. Salmaso and Vaccarino report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000;20:5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens HE, Smith KM, Rash BG, Vaccarino FM. Neural stem cell regulation, fibroblast growth factors, and the developmental origins of neuropsychiatric disorders. Front Neurosci. 2010:4. doi: 10.3389/fnins.2010.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Turner CA, Akil H, Watson SJ, Evans SJ. The fibroblast growth factor system and mood disorders. Biol Psychiatry. 2006;59:1128–1135. doi: 10.1016/j.biopsych.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Molteni R, Fumagalli F, Magnaghi V, Roceri M, Gennarelli M, Racagni G, et al. Modulation of fibroblast growth factor-2 by stress and corticosteroids: from developmental events to adult brain plasticity. Brain Res Brain Res Rev. 2001;37:249–258. doi: 10.1016/s0165-0173(01)00128-x. [DOI] [PubMed] [Google Scholar]
- 7.Ganat Y, Soni S, Chacon M, Schwartz ML, Vaccarino FM. Chronic hypoxia up-regulates fibroblast growth factor ligands in the perinatal brain and induces fibroblast growth factor-responsive radial glial cells in the sub-ependymal zone. Neuroscience. 2002;112:977–991. doi: 10.1016/s0306-4522(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 8.Fagel DM, Ganat Y, Cheng E, Silbereis J, Ohkubo Y, Ment LR, et al. Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia. J Neurosci. 2009;29:1202–1211. doi: 10.1523/JNEUROSCI.4516-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eren-Kocak E, Turner CA, Watson SJ, Akil H. shRNA Silencing of Endogenous FGF2 in Rat Hippocampus Increases Anxiety Behavior. Biological Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 12.Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26:181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]
- 13.Bland ST, Schmid MJ, Greenwood BN, Watkins LR, Maier SF. Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport. 2006;17:593–597. doi: 10.1097/00001756-200604240-00008. [DOI] [PubMed] [Google Scholar]
- 14.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 15.Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Graham BM, Richardson R. Fibroblast growth factor-2 alters the nature of extinction. Learn Mem. 2011;18:80–84. doi: 10.1101/lm.2006511. [DOI] [PubMed] [Google Scholar]