Abstract
In the wild, male rhesus macaques disperse at sexual maturity. In captivity, however, males cannot disperse from their natal groups. Thus, the presence of natal males in captive rhesus social groups is unnatural and has the potential to negatively influence group dynamics and stability. A primary difference between natal males and non-natal (immigrant) males is that natal males have the opportunity to form long-term alliances with their maternal kin as well as nonkin. We investigated the factors associated with natal males’ kin alliances and the impact of these alliances on measures of natal male behavior, group dynamics, and group stability. We found that natal males more frequently formed alliances with maternal kin when they were from high-ranking matrilines, had more siblings, and were younger. More frequent kin alliances was associated with more frequent use of intense aggression, higher individual rank, and higher degree of integration within the male displacement network. Thus, it seems that natal males use their alliances to be more active and influential in the social group, which may affect group stability. It appears that juvenile natal males from high-ranking matrilines, in particular, have the largest impact on group stability. Younger natal males from high-ranking matrilines formed alliances with kin more frequently and used intense aggression more frequently than older or lower-ranking males. Furthermore, groups with a higher proportion of juvenile males from high-ranking matrilines also had higher rates of wounding. We suggest that the presence of natal males in rhesus groups may act in opposition to group stability.
Keywords: social network, coalition, primates, group stability, aggression
Introduction
In the wild, male rhesus macaques disperse at sexual maturity. In captivity, however, males cannot disperse from their natal groups, and males may have the opportunity to form long-term alliances with their maternal kin as well as nonkin. Thus, the presence of many/mostly natal males in a rhesus group is unnatural, and may potentially pose a problem for group stability, particularly in a captive setting. Our goal is two-fold: (1) identify factors associated with natal males’ alliances with maternal kin, and (2) determine whether there is there evidence that natal males’ alliances negatively impact group stability. We look for evidence of group instability in four areas: (1) natal males’ rank in the adult male hierarchy, since previous researchers report that some natal males use maternal kin coalitions to attain high rank prematurely, often above older and larger prime-age males [Chapais 1983; Koford 1963], which has the potential to upset the stability of the male hierarchy; (2) natal males’ degree of connectedness in the displacement network, because alliances with maternal kin may allow young natal males to be well integrated within the male hierarchy, and occupy an atypical social position; (3) natal males’ aggressive behavior, as more frequent intense aggression can lead to more frequent wounding, which is detrimental to the health and well-being of the animals and may be a symptom of instability [Flack et al. 2005a; Oates-O'Brien et al. 2010]; and (4) the group-level rate of wounding.
Male Dispersal vs. Natality
Female cercopithecine primates, such as baboons and macaques, typically remain in their natal groups, and thus have access to maternal kin allies. Females frequently form alliances with kin to establish/maintain their dominance rank and gain access to resources. Males, however, typically disperse from their natal groups at sexual maturity [Cheney and Seyfarth 1983; Drickamer and Vessey 1973; Packer 1979] and therefore do not have access to kin allies [but see Meikle and Vessey 1981]. In their new social group, male rank is more often determined by factors such as the male’s age, body size, competitive ability, and group tenure [Boelkins and Wilson 1972; Packer 1977; Sprague 1992] than by the formation of alliances as among females [Datta 1986]. When males do form coalitions, it is typically to help an unrelated male gain access to estrous females [Packer 1977] or to intervene on others’ conflicts [Boehm 1981; Flack et al. 2005a] and reinforce the existing hierarchy [Ehardt and Bernstein 1992].
Although male dispersal is the norm for cercopithecines, males in wild, free-ranging groups sometimes remain in their natal groups [Kaufman 1976; Koford 1963; Tilford 1982], but little is known about their behavior given the rarity of such cases. In captivity, the presence of natal males is even more common as dispersal may only be achieved if it is facilitated by management staff [Fairbanks et al. 2004], and in many cases, introduction of new males to an existing captive social group is problematic. Thus, males may frequently reside in their natal groups in captive settings, and face a different social situation than their wild counterparts typically do. However, precisely what factors govern natal male behavior under these unusual social circumstances is not well understood.
Natal Male Alliances with Kin
Female macaques defend both male and female immature offspring [Boelkins and Wilson 1972; Kawamura 1958; Sade 1967]; females continue to support daughters into adulthood, but continued support of sons may be precluded by their dispersal. We therefore predict that mothers continue to support their sons when those sons remain in their natal group. Most agonistic support may come from the mother, rather than other kin, and the mother’s presence may have a positive effect on the frequency of alliances between the natal male and his kin. Alternatively, the absence of a male’s mother may reduce his cooperative interactions with maternal kin since kin bias develops/persists via social transmission through the mother [Berman and Kapsalis 1999] such that sisters regard each other as kin by association with a common mother. Although the effect of being orphaned on dispersal behavior is variable [Drickamer and Vessey 1973; Koford 1963], being orphaned is more likely to have repercussions on kin alliances, even for older natal males as a result of the important role that alliances play in macaque societies. We predict that males whose mothers are absent have fewer kin alliances than those whose mothers are present.
On Cayo Santiago, natal male rhesus macaques from high-ranking matrilines have been reported to reach high ranks in the adult male hierarchy [Chapais 1983; Koford 1963] largely due to agonistic support from their high-ranking female kin. Males from low-ranking families typically do not achieve high ranks in their natal groups, as lower-ranking female kin are less able to provide meaningful support [but see Manson 1993]. We predict natal males from higher-ranking matrilines have more frequent alliances with kin during agonistic interactions.
Rhesus mothers preferentially support their youngest daughters in contests with older sisters, a pattern which results in the phenomenon of youngest ascendancy, in which a female’s youngest daughter outranks the older sisters [Missakian 1972; Sade 1972]. Although the reason for the emergence of this pattern is still debated [Hill 1999; Hill and Okayasu 1995], it is likely that (1) mothers preferentially support their youngest offspring, regardless of sex and (2) this preference may extend beyond agonistic interactions among siblings to agonistic interactions with other group members. We therefore predict that younger males have more frequent alliances with their maternal kin during agonistic interactions.
Among female macaques, alliances are important to rank acquisition and maintenance, particularly kin alliances [Datta 1986]. Variation in the number of available allies [Datta 1992], such as the size of one’s matriline or the number of close kin, may influence the frequency of alliances between natal males and their maternal kin. Indeed, natal males on Cayo Santiago dropped in rank following the loss of allies during the breeding season [Tilford 1982]. We predict that males from larger matrilines have more frequent kin alliances. However, males with more siblings may have more frequent kin alliances due to greater ally availability, or may have fewer kin alliances due to mother’s preoccupation with other offspring.
Natal Male Alliances and Rank
In wild, free-ranging groups of rhesus macaques, immigrant males attain an individual rank within the dominance hierarchy in one of two ways: (1) enter a new group at the bottom of the male hierarchy, and rise in rank when a higher-ranking male dies or leaves the group [Drickamer and Vessey 1973; Vessey and Meilke 1987] or (2) aggressively challenge the resident alpha male for his rank [Lindburg 1969; Neville 1968]. However, natal males appear to use a different strategy: forming alliances with their maternal kin to rise in rank more quickly and potentially attaining a rank above older, larger adult males [Chapais 1983; Kaufman 1976; Koford 1963]. We, therefore, predict that natal males with more frequent kin alliances attain higher ranks in the adult male hierarchy. Furthermore, males’ alliances with kin may be directly associated with their individual rank if kin alliances are more frequent in contests against other males. However, frequent kin alliances may indirectly influence group members’ perceptions of males’ competitive ability, regardless of the age/sex of the opponent.
Other factors are known to affect the rank of non-natal, immigrant males, and we predict these factors will affect natal males similarly. First, male rank is often correlated with age and body size, such that older, larger males are higher-ranking than younger, smaller males, although this pattern typically does not hold when only adult males are compared [Sprague 1998]. Although young natal males may use kin alliances to outrank older, larger males, and mothers may preferentially support young sons (see above), we predict that once these variables are taken into account, older males will be higher-ranking than younger males. Second, given that rank acquisition and maintenance among female macaques is dependent upon the number of available allies [Datta 1992], we predict that males from larger matrilines are higher-ranking.
Power Dynamics within the Male Displacement Network
Although most natal male rhesus macaques remain peripheral and appear to avoid interactions with adult males, those natal males that do attain a high individual rank become integrated into the social network of male dominance interactions, a social position that is apparently made possible by maternal kin alliances [Chapais 1983]. However, given the rarity of reports of natal males attaining ranks within the male hierarchy, we don’t know if the degree of integration is dependent upon the frequency of alliances with kin, nor what the effect of being high-ranking is on one’s degree of integration within the social network of dominance interactions. A male that is well-integrated in the network of dominance interactions has greater potential to be socially influential. We predict higher-ranking males are better integrated within the displacement network than lower-ranking males as a result of their ability to displace most other males. In addition, we predict that males with more alliances, both kin and nonkin, are better integrated than males with few alliances, and thus have greater potential for social influence other males in the dominance network. Bonacich power [Bonacich 1987] will be used to measure males’ degree of integration within social networks (see Methods).
Group Stability
The identification of factors influencing group stability is still in its infancy [Flack et al. 2005b]. In captivity, however, atypical social or demographic circumstances, such as the presence of natal males, have the potential to upset group stability. Group instability has been associated with increased rates of overall aggression, severe aggression, and wounding [Flack et al. 2005b; McCowan et al. 2008]. Natal males with access to kin allies may use their alliances to instigate aggression, thereby influencing group stability. We predict that natal males with more kin alliances start more fights and use severe aggression more frequently than males with few alliances. Furthermore, given our predictions that young males and males from high-ranking matrilines have more frequent kin alliances and that these alliances facilitate aggressive behavior, we further predict that groups with a greater proportion of males that are juveniles from high-ranking families also have higher rates of wounding as a result of their aggression.
Methods
Study Site and Groups
The study was conducted at the California National Primate Research Center (CNPRC) in Davis, CA from June 2008 through early December 2009. All research adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non Human Primates as well as all laws of the United States government. This research was approved by the University of California, Davis Institutional Animal Care and Use Committee, protocol #11843. The subjects of this study were 165 natal males (age range: 2.5 – 22 years old, mean = 6.1) from seven groups (Groups 1, 5, 8, 10, 14, 16 and 18) of rhesus macaques housed in 0.2 ha enclosures (Table 1). The majority of natal males were juveniles, subadults, and young adults.
Table 1.
Characteristics of study groups
| Group | Natal Males |
Unrelated Males |
Natal Juveniles (2.5 – 4.5 yrs) |
Natal Adults (6+ yrs) |
Group size mean (range) |
|---|---|---|---|---|---|
| 1 | 31 | 5 | 14 | 11 | 176.5 (167–182) |
| 5 | 25 | 1 | 16 | 7 | 137.1 (127–148) |
| 8 | 22 | 1 | 6 | 12 | 160.1 (147–169) |
| 10 | 11 | 0 | 4 | 4 | 164.4 (150–175) |
| 14 | 17 | 1 | 14 | 1 | 108.3 (105–110) |
| 16 | 29 | 1 | 24 | 2 | 150.3 (141–158) |
| 18 | 30 | 0 | 16 | 10 | 197.9 (170–210) |
All males were either born into the group, or were present at the date of group formation (most recent group formation: February 2004). Thus, no males were transferred into any group. Natal males were defined as those males with maternal kin present in the group. Unrelated males were those with no maternal kin present, either because males had no maternal kin when the group was formed or as a result of the death or permanent removal of maternal kin.
All enclosures were similar in having ten A-frame houses, multiple suspended barrels, swings and several perches. Groups were fed a standard monkey chow diet twice per day at approximately 0700 hours and again between 1430 and 1530 hours. Fresh fruit or vegetables were provided twice per week.
Rhesus macaques in this outdoor colony were managed with a minimal level of disturbance, and individuals of each group were free to interact with one another as they chose. Disturbances within the enclosure were typically limited to daily morning health checks, two round-ups per year to conduct health examinations on all animals and removal of injured or sick animals for medical treatment.
Sampling methods
Two observers (primary observers: BAB, MEJ) recorded aggressive interactions among juvenile, subadult, and adult males and females. Each group was observed from 0900h – 1200h and 1300h – 1600h, four days per week, on a 4-week rotating schedule. Groups 5, 8, and 14 were observed June 2008 – April 2009, and Groups 1, 10, and 18 were observed May – December 2009, which yielded 8–11 weeks of observation (192–264 hours) per group. Group 16 was observed only June – November 2008 due to a social overthrow that occurred December 3, 2008.
An event sampling design was used to collect data on agonistic interactions which were recorded as an ordered series of dyadic interactions. Both aggressive and submissive behaviors were categorized in increasing levels of severity. Aggression included threat, vocal threat or threat and follow, lunge or mild slap, chase < 3 meters, chase > 3 meters or grapple, bite < 5 seconds, chase and bite < 5 seconds, and bite > 5 seconds. Submission included silent bared teeth display (SBT), turn away, turn away with SBT, move out of arms’ reach, move out of arms’ reach with SBT, run away < 3 meters, run away < 3 meters with SBT, run away > 3 meters, run away > 3 meters with SBT, prolonged scream, crouch (animal stops resisting aggression and gives up, i.e. during mobbing events), and crouch with SBT. Intense aggressive interactions included bite < 5 seconds, chase and bite < 5 seconds, and bite > 5 seconds.
We collected a total of 76,168 aggressive dyadic interactions (from 18,920 fighting events) in the seven study groups, and 36,786 (48.3%) of these dyadic interactions involved males. Males could form an alliance during an agonistic interaction in one of two ways: (1) co-aggression, where a male and another individual simultaneously direct aggression at the same recipient and (2) third-party intervention, where another individual intervenes upon an on-going conflict (within 5 seconds of initiator’s aggression) to help a male or where a male intervenes upon an on-going conflict to help another monkey. These two types of alliances differ only in the timing of the ally’s assistance, and are otherwise identical in structure, especially since active recruitments were rarely recorded due to the difficulty in accurately identifying this subtle behavior. A total of 3453 alliances were recorded involving males. These data yielded a dataset of 1243 male × week counts of the following behavioral variables: alliances with kin, alliances with nonkin, fights started, and fights using intense aggression.
Matrilineal ranks and males’ individual ranks were determined from behavioral management staff records of weekly observations of displacements and aggressive interactions and were supplemented by observations of agonistic interactions from this study. Males in the top third of the dominance hierarchy were assigned a rank of high, those in the middle third a rank of middle, and those in the bottom third of the hierarchy were assigned a rank of low. Matrilineal ranks were assigned a number, where 1 = highest ranking matriline. All members of the same matriline generally held the same rank. However, in cases where some matriline members held a different rank from the rest of their family, the matrilineal rank assigned to the matriline was the rank held by the majority of the matriline members.
A male’s mother was recorded as present in the group if she resided within the group during the week of observation for which the behavioral counts were calculated. Temporary absence of a mother (e.g., hospital treatment for illness) was recorded as present because the mother was soon returned to the group. A male’s mother was recorded as absent from the group if she had been permanently removed from the group. Only two males’ mothers were permanently removed (due to old age) from a group during the study, and they were categorized as ‘mother absent’ one month after the mothers’ removal. Matriline size included all female and male matriline members older than 2.5 years, and total siblings included a male’s siblings older than 1 year that were present in the group.
Social Networks
Social networks were created for each group from the displacement interactions among males over an 8-month period of observation (groups 5, 8, 14: June 2008 – January 2009; groups 1, 10, 18:May – December 2009) using UCINET 6.247 [Borgatti et al. 2002]. The five months of displacement data from group 16 were not analyzed because the network was too sparse for comparison with the other groups. The displacement sociomatrix included all displacement (i.e., approach – move away) and status interactions (i.e., approach – silent-bared teeth display) among males of each group. We calculated the Boncich power [Bonacich 1987] score for each male in the network to assess his degree of integration in the network. Bonacich power is a network measure of the degree to which individuals are connected to other individuals with high connectedness. Note that a male may be highly connected by displacing many others or by being displaced by many others. Although Bonacich power is intended to be a measure of one’s power or influence over others, because it is an undirected measure, we use it here to assess how well integrated (or alternatively, how peripheral) a male is within the male social hierarchy. A well-integrated male has greater potential to exercise social influence over others in the network. Since Bonacich power values per node will vary depending upon the total nodes and edges in the network, we used normalized values of power in our analyses of all groups.
Statistical Analyses
We analysed the data using linear and generalized linear mixed-effects regression models [McCullagh and Nelder 1989]. Models were fit to the data for six dependent variables: (1) counts of males’ alliances with kin per week, (2) ordered categories of male individual rank (high, mid, low), (3) whether intervention alliances were with kin or nonkin, (4) normalized Bonacich power per male, (5) counts of fights started by each male per week, and (6) counts of fights using intense aggression by each male per week. We ran a series of models for each dependent variable using a stepwise procedure where a single predictor or interaction term was added to the model at each step. Akaike’s Information Criterion (AIC) scores were used to select the best fit model, i.e., the model with the lowest AIC score. Nested models having a difference in AIC less than or equal to two were considered equivalent, and AIC scores were corrected for small sample size (N/K<40) for the Bonacich models [Burnham and Anderson 2002]. A random effect for male was included in all models except those predicting male rank and Bonacich power. A random effect for group was attempted for all models, and explained a significant amount of variation in the Bonacich models only.
A generalized linear mixed-effects Poisson regression model was fit to the count of males’ kin alliances per week. Fixed effects included: a male’s total participation in agonistic interactions (total participation), male’s matriline rank, male’s individual rank, age, matriline size, total siblings, the presence or absence of a male’s mother, nonkin alliances per week, and interactions among these predictors. A generalized linear mixed-effects negative binomial regression model was fit to the counts of fights started per week and fights using intense aggression per week. Fixed-effects for these models included: a male’s total participation in agonistic interactions, male’s matriline rank, male’s individual rank, age, matriline size, kin alliances per week, nonkin alliances per week, season, and interactions among these predictors.
Male individual rank was determined from the sum of many agonistic interactions over the course of several months, and therefore a weekly count of male alliances and aggressive behavior was not appropriate for this analysis. A smaller dataset was created in which each male (N=165) appeared once, and alliances with kin and nonkin were summed for an equivalent period of observation time (June–November) for each male in all groups. A generalized linear regression model (Binomial family, ordinal logit link) was fit to the ordered categories of male rank. Fixed-effects included: male’s matriline rank, age, matriline size, presence or absence of a male’s mother, total kin alliances, and total nonkin alliances.
A logistic regression model was fit to the categorical variable of intervention alliance with kin or with nonkin. Fixed effects included age and sex of opponent, age and sex of intervener, natal male as the intervener, natal male as the ally, whether the original recipient was helped, and interactions among these predictors.
A linear mixed-effects model was fit to the normalized Bonacich power per male in his group’s displacement network. This dataset included males from all groups except group 16 (N = 116 males), and alliances with kin and nonkin were summed for an equivalent 8-month period per male. Fixed-effects included total kin alliances, total nonkin alliances, male age, matriline rank, male rank, and interactions among these predictors.
Finally, a linear regression model was fit to the wounding rate per group over the study period (N=7). Fixed effects included proportion of natal males in the group, proportion of males that are juveniles (2.5 – 4.5 years old), and proportion of males that are juveniles from high-ranking matrilines. Only one predictor was fit in each model due to small sample size. All analyses were performed using Stata (Stata 9; Stata Corporation, College Station, Texas) and the R statistical computing program [R Development Core Team 2008].
Results
Male’s Alliances with Maternal Kin
The best fit model explaining variation in natal males’ kin alliances included fixed-effects for male’s total participation in aggression, matriline rank, male age, mother’s presence/absence, total siblings, nonkin alliances, and the interaction terms mother’s presence × male age and mother’s presence × matriline rank (compared to the second best fit model, ΔAIC=5). Males had more alliances with maternal kin when: (1) they were from higher-ranking matrilines (β = −0.22, P <0.0001; Fig.1a), (2) they had more siblings present (β = 0.076, P = 0.02), and (3) they had more alliances with nonkin (β = 0.06, P <0.0001; Fig. 1b). Furthermore, there was a three-way interaction among matriline rank, mother’s presence, and male age such that younger males (<5 years old) had more kin alliances than older males, but only when their mothers were present in the group, and males were from higher-ranking matrilines (mother’s presence × age: β = −0.15, P = 0.0007; mother’s presence × matriline rank: β = 0.13, P = 0.008; Fig. 2). Finally, as expected, it was necessary to account for males’ total participation in agonistic events, since males who participated more frequently had more frequent alliances with kin (β = 0.018, P <0.0001).
Fig. 1.
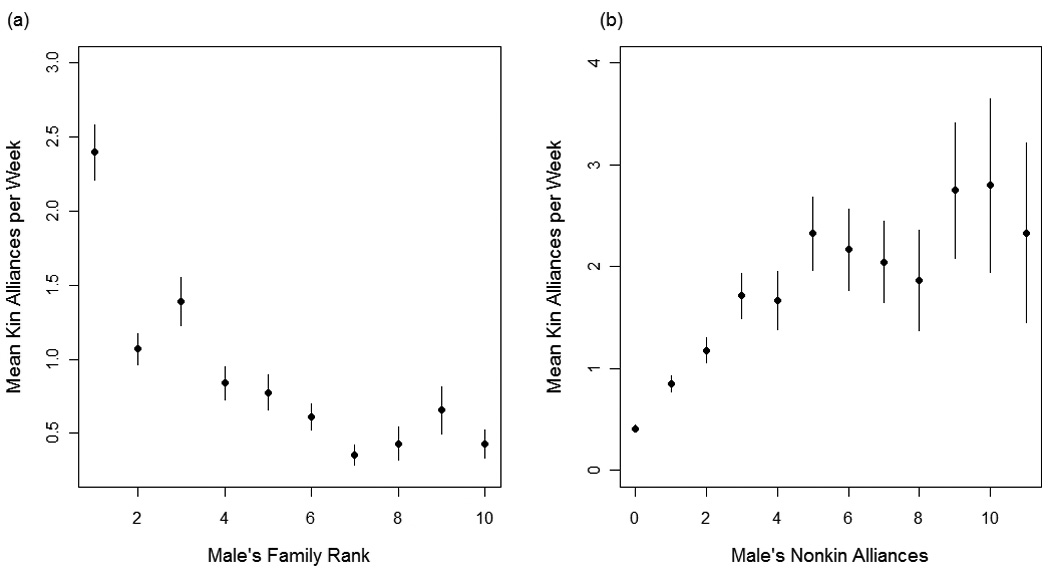
Mean alliances between natal males and their maternal kin plotted for (a) all males from matrilines of a given rank and (b) males having different frequencies of alliances with nonkin. The highest-ranking matriline is set to rank = 1. Vertical bars represent standard errors.
Fig. 2.
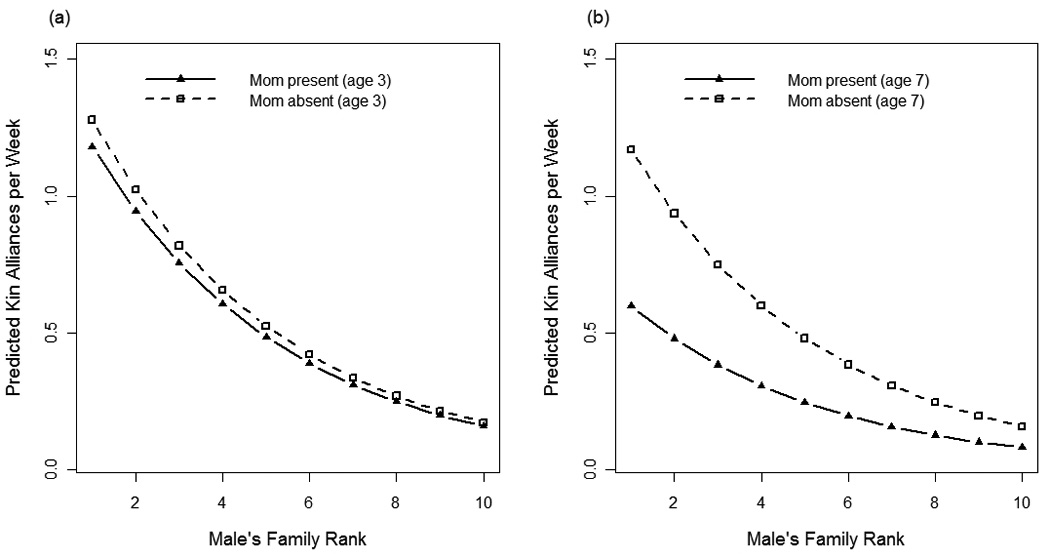
The predicted frequency of kin alliances per week, calculated from the best fit model, are plotted against natal males’ matriline ranks for males whose mothers were present in the group and males whose mothers were absent from the group. In plot (a), male age is held constant at 3 years old and in plot (b) 7 years old.
Male Individual Rank by Kin Alliances
The best fit model explaining variation in male rank included fixed-effects for matriline rank, matriline size, male age, total kin alliances, and the interaction terms matriline rank × male age and kin alliances × male age. However, there were two other models with ΔAIC ≤ 2, indicating that all three models are equally good at explaining the observed variation in male rank category. The second best fit model omitted the interaction term kin alliances × male age (ΔAIC=1.7) and the third best fit model included total siblings as a predictor, which did not have a significant effect on male rank (ΔAIC=1.9).
Males were more likely to have a high rank (high = 1, low = 3) when: (1) their matriline rank was high (β = 0.93, P <0.0001; Fig. 3a), (2) they had more kin alliances (β = −0.14, P = 0.001; Fig. 3b), and (3) when their matriline size was larger (β = 0.053, P = 0.07). Furthermore, an increase in male age reduced the effect of matriline rank on male individual rank (β = −0.09, P <0.0001; Fig. 4).
Fig. 3.
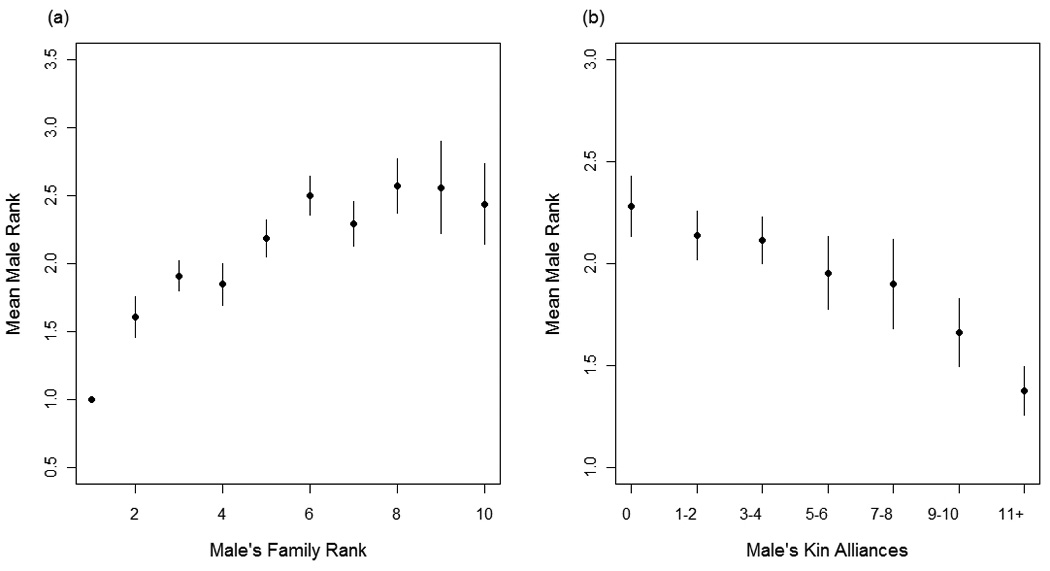
Mean male rank category, where high-rank = 1, mid-rank = 2 and low-rank =3, is plotted for: (a) all males from matrilines of a given rank and (b) males having different frequencies of kin alliances. The highest-ranking matriline is set to rank = 1. Vertical bars represent standard errors.
Fig. 4.
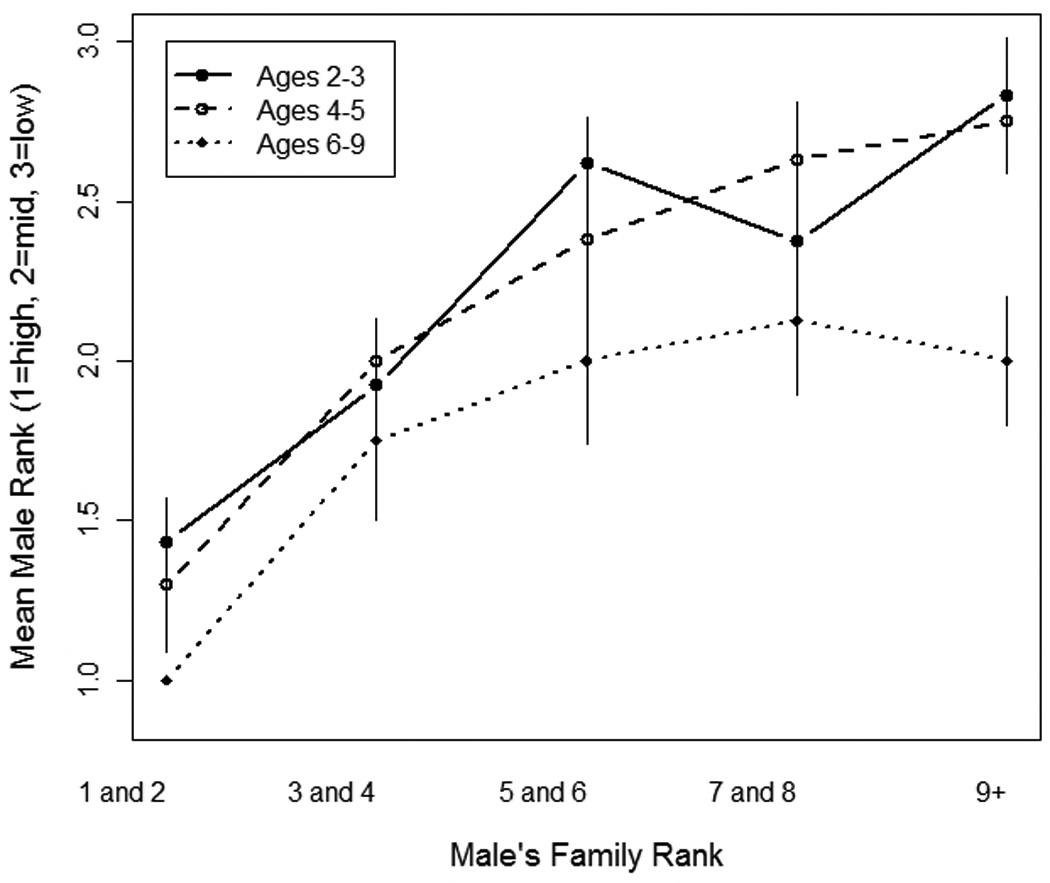
Mean male rank category, where high-rank = 1, mid-rank = 2 and low-rank =3, is plotted for all males from a given matriline rank for three age categories: males aged 2–3 years, 4–5 years, and 6–9 years.
Alliance with Kin or Nonkin by Age/Sex Class
Whether or not natal males’ intervention alliances were with kin or nonkin depended upon the age and sex classes of the participants and who was being helped. The best fit model included fixed effects for the natal male as the intervener, natal male as the ally, intervener age, opponent sex, help recipient of conflict (vs. join initiator), and the interaction terms help recipient × natal male intervener, help recipient × natal male ally, and intervener age × natal male intervener (compared to the second best fit model, ΔAIC=16.4). Thus, an intervention alliance was more likely to be between a natal male and his kin when: (1) the opponent was female (β = 0.27, P =0.003) and (2) the natal male was the recipient of intervention protection (help recipient × natal ally: β = 0.81, P =0.002). An intervention alliance was less likely to be between kin when: (1) the intervener was an older natal male (intervener age × natal intervener: β = 0.81, P =0.002) and (2) when the intervener was a natal male protecting the original recipient (help recipient × natal intervener: β = −0.83, P = 0.001).
Male Bonacich Power in Displacement Network by Kin Alliances
The best fit model explaining variation in male Bonacich power included fixed-effects for male rank, age, total kin alliances, total nonkin alliances, and the interaction terms kin alliances × age and nonkin alliances × male rank (compared to second best fit model, ΔAIC=2.7). Males were more connected within the displacement network when they were higher-ranking (high-rank vs. mid-rank: β = 3.9, P = 0.005; mid-rank vs. low-rank: β = 4.2, P = 0.003). Furthermore, having more nonkin alliances reduces the effect of high-rank on males’ connectedness in the network (nonkin × high-rank: β = −0.25, P = 0.007; nonkin × low-rank: β = 0.07, P = 0.7; Fig. 5b). Finally, the interaction kin alliances × male age indicates that older males having more kin alliances are also more connected within the displacement network (β = 0.06, P = 0.01; Fig. 5a). Male displacement networks for all groups are shown in Fig. 6.
Fig. 5.
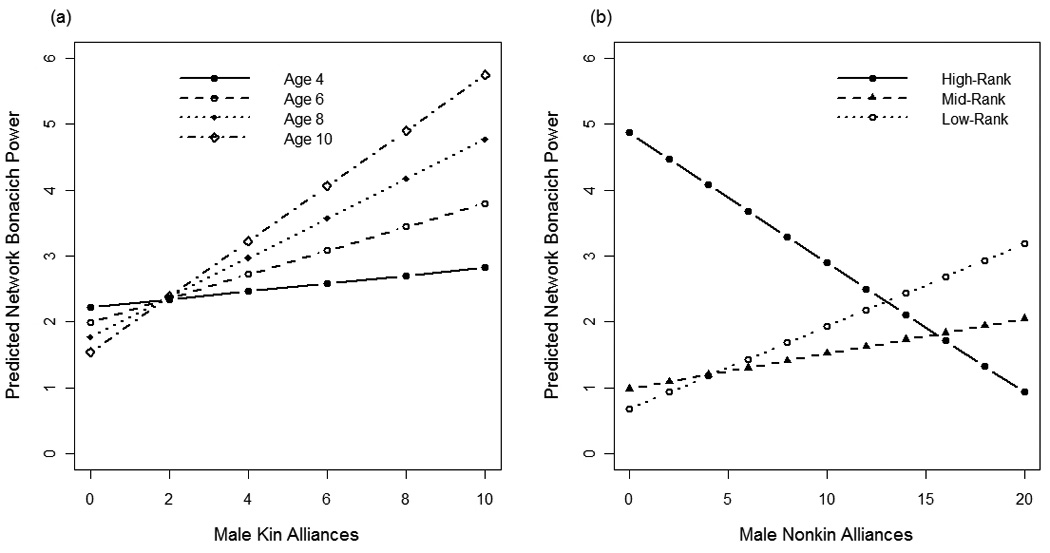
The predicted Bonacich power in the male displacement network, calculated from the best fit model, is plotted against (a) males’ kin alliances for four different age categories (males aged 4, 6, 8, or 10 years) and (b) males’ nonkin alliances for each male rank category.
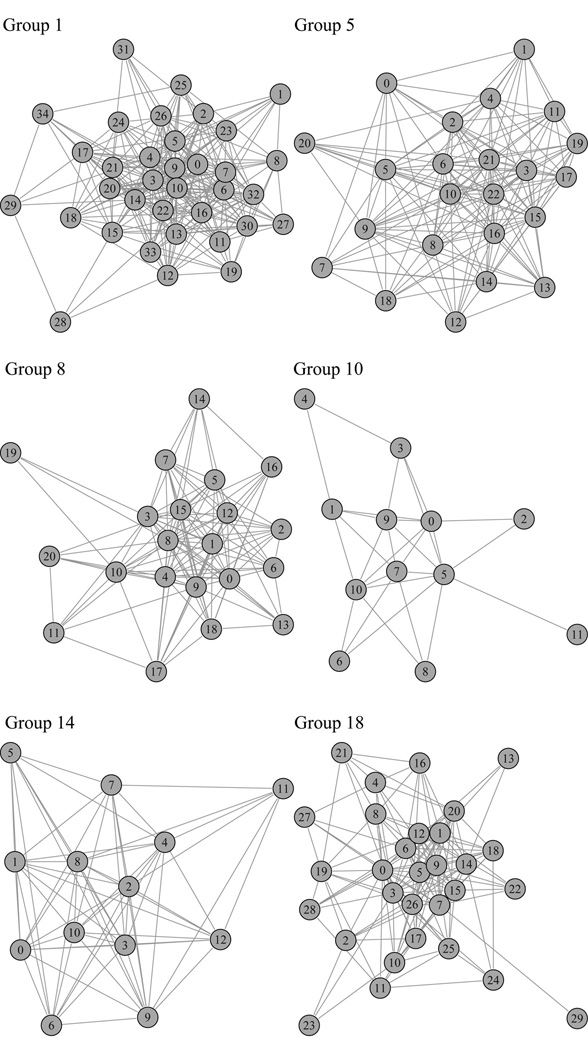
Fig. 6.
Male displacement network plots for six of the seven study groups. Group 16 was not included because only five months of data were collected on this group, compared to eight months of observation for the other six groups.
Starting Fights by Kin Alliances
The best fit model explaining variation in how frequently males start fights included fixed effects for kin alliances per week, nonkin alliances per week, male rank, season, male’s total participation in aggression, and the interaction terms kin alliances × nonkin alliances, season × nonkin alliances, and total participation × male rank (compared to second best fit model, ΔAIC=5.8). Males started fights more frequently when: (1) they were higher-ranking (high vs. low rank: β = 1.96, P < 0.0001, mid vs. low rank: β = 1.04, P < 0.0001), (2) they had more alliances with kin (β = 0.076, P < 0.0001; Fig. 7a), (3) they had more alliances with nonkin (β = 0.036, P = 0.001), (4) during the autumn breeding season (autumn vs. summer: β = 0.28, P < 0.001; autumn vs. winter: β = −0.1, P = 0.22; autumn vs. spring: β = 0.11, P = 0.18), and (5) they participated in fights more frequently (β = 0.05, P <0.001).
Fig. 7.
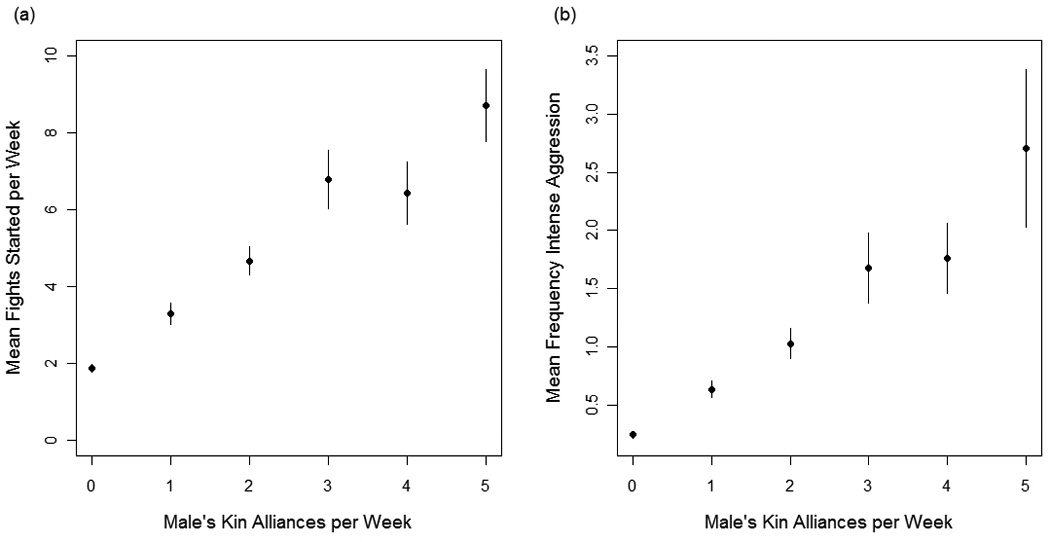
The effect of males’ kin alliances on (a) mean fights started per week and (b) mean frequency of intense aggression used per week. Vertical bars represent standard errors.
The three interaction terms qualify these main effects. The interaction between kin alliances and nonkin alliances indicates that having more kin alliances reduces the effect size of nonkin alliances (β = −0.015, P <0.0001). More nonkin alliances during spring and summer increased the number of fights started by a male relative to the autumn breeding season (spring × nonkin alliances: β = 0.04, P = 0.008; summer × nonkin alliances: β = 0.03, P = 0.06). Finally, being higher-ranking reduced the effect of total participation in agonistic interactions on the frequency of starting fights (total participation × high-rank: β = −0.03, P < 0.0001; total participation × mid-rank: β = −0.03, P <0.0001).
Intense Aggression by Kin Alliances
The best fit model explaining variation in males’ use of intense aggression included fixed-effects for male rank, male age, alliances with kin per week, alliances with nonkin per week, total participation in agonistic interactions, and the interaction terms male rank × age and male rank × nonkin alliances (compared to second best fit model, ΔAIC=9.1). Males used intense aggression (biting) more frequently when: (1) they were younger (β = −0.97, P = 0.001), (2) they had more kin alliances (β = 0.17, P < 0.0001; Fig. 7b), (3) they had more nonkin alliances (β = 0.44, P < 0.0001), and (4) they participated in fights more frequently (β = 0.01, P < 0.001). Use of intense aggression was further influenced by three interaction terms. Having more kin alliances reduces the effect size of nonkin alliances (β = −0.02, P = 0.001; Fig. 8a). Being higher-ranking amplifies the effect of age (high-rank × age: β = 0.92, P = 0.002; mid-rank × age β = 0.76, P = 0.01; Fig. 8b) such that young, higher-ranking males use intense aggression more frequently than young, lower-ranking males. Finally, being higher-ranking reduces the effect of nonkin alliances (high-rank × nonkin alliances: β = −0.34, P = 0.005; mid-rank × nonkin alliances: β = −0.24, P = 0.05).
Fig. 8.
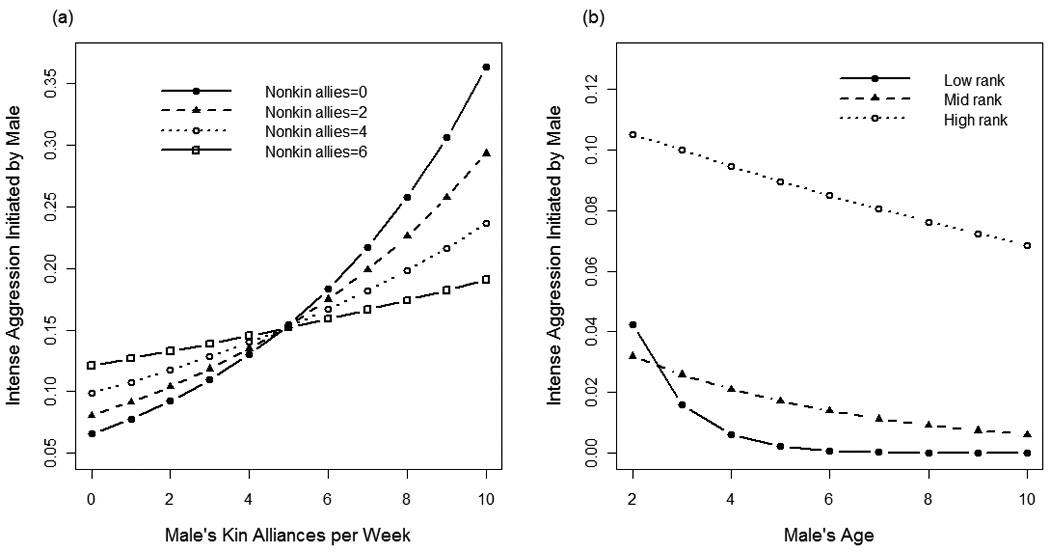
The predicted frequency of intense aggression initiated by males per week, calculated from the best fit model, is plotted against (a) males’ frequency of kin alliances for four categories of nonkin alliances and (b) males’ age for each male rank category.
Group-level Rate of Wounding by Natal Male Presence
We fit linear regression models to the rates of wounding per group (N=7 groups) using a single predictor. Groups with higher proportions of males that were juveniles from high-ranking matrilines had higher rates of wounding (β = 0.02, P=0.002). However, the group proportion of natal males and the proportion of males that were juveniles were not associated with wounding.
Discussion
Our results suggest that several demographic factors influence the frequency of alliances between natal males and their maternal kin. These results support the relationship between matriline rank and frequency of kin alliances reported for natal males on Cayo Santiago [Chapais 1983; Koford 1963], and further demonstrate that males from lower-ranking matrilines also form alliances with kin, but less frequently than those from high-ranking matrilines. Younger males generally formed alliances more frequently with kin than older males, which suggests that mothers and other maternal kin preferentially support younger sons. Unexpectedly, males whose mothers were absent from the group formed more kin alliances than males whose mothers were present, particularly among older males. This suggests that a male’s mother, when present, may be his primary kin ally, but when his mother is absent, the male may actively establish alliances with other kin. Finally, the positive relationship between natal males’ nonkin and kin alliances suggests that having one type of alliance may facilitate the establishment of other alliances.
Natal Male Alliances and Rank
Natal males in our study groups had higher individual ranks when their matriline rank was higher, matriline size was larger, and they had more kin alliances which supports previous findings that matriline rank is positively associated with natal male individual rank [Chapais 1983; Koford 1963; but see Manson 1993]. However, since the frequency of kin alliances is associated with natal male rank when matriline rank is held constant, it may not be sufficient to be from a high-ranking family. Frequent help from maternal kin may also be necessary for natal males to attain high rank.
An intervention alliance was more likely to be with kin than nonkin when the natal male’s opponent was female and when the intervener was protecting the male from an aggressor. These results suggest that, while males may not directly use their kin alliances to gain rank, kin are more significant allies to males than vice versa. The positive relationship between male rank and kin alliances may reflect and indirect influence of alliances on others’ perceptions of male competitive ability.
Natal and immigrant males appear to differ in their rank acquisition strategies, which may create conflict and instability within the male hierarchy. Immigrant males enter a new group and either gradually rising in rank as higher-ranking males leave [Drickamer and Vessey 1973; Missakian 1972] or challenge the resident alpha male for his position [Lindburg 1969; Neville 1968]. If natal male rank is more dependent upon alliances than age, competitive ability, or group tenure, then natal male ranks are more susceptible to changes in alliances than are non-natal males. Natal males on Cayo Santiago dropped in rank when their alliances changed, while non-natal males did not experience a similar decrease in rank [Tilford 1982]. In addition, animals in captive groups are temporarily removed for hospitalization when injuries occur. A temporary absence from the group may change the alliance structure and result in a decrease in rank upon re-entry [Manson 1993]. Alternatively, natal male ranks may be more stable if their alliances persist for long periods of time with respect to the age and competitive abilities of non-natal males. Regardless, the presence of multiple methods of rank acquisition may create instability in the male hierarchy.
Power Dynamics in the Male Displacement Network
In our study groups, the most highly integrated males in the displacement networks were the alpha males, which is unsurprising given that alpha males are capable of displacing all other males in the network and may actively reinforce their rank by doing so. However, some natal males were also well-integrated in the network, a reflection of their active participation in dominance interactions, which may be atypical as natal males in wild, free-ranging groups become increasingly peripheral prior to dispersal and may even avoid interactions with adult males [Koford 1963; Pusey and Packer 1987; Tilford 1982]. It appears that alliances with kin and nonkin facilitate a natal male’s active participation in dominance interactions, which may be an alternate strategy for achieving social influence within the group. As with male rank acquisition, the presence of multiple strategies for achieving high-degree of integration and social influence may conflict with one another and negatively impact social dynamics and stability.
Natal Males, Aggressive Behavior, and Stability
Natal males with more frequent kin alliances started more fights and used intense aggression more frequently. Higher-ranking younger males especially used more intense aggression. As kin alliances were more frequent among males from higher-ranking families, young natal males from high-ranking matrilines may have a negative impact on group dynamics and stability as a result of their kin alliances. Indeed, those study groups with a higher proportion of juvenile natal males from high-ranking matrilines had higher rates of wounding.
Although rhesus macaques are characterized by frequent severe aggression [Thierry 2004], rates of aggression are higher in captive than in wild, free-ranging groups, and increased rates of severe aggression are associated with more wounding, instability, and higher likelihood of social overthrow [Flack et al. 2005b; McCowan et al. 2008; Oates-O'Brien et al. 2010]. Furthermore, rates of contact aggression, wounding, and group stability among the rhesus groups at the CNPRC are influenced by the presence of unrelated alpha and beta males [McCowan et al. 2008]. Groups having unrelated alpha and beta males (unrelated to any of the group’s matrilines) had greater cohesion in the group displacement network, higher social power, less contact aggression and wounding, and lower likelihood of cage war. Thus, it seems that unrelated adult males promote group stability by reducing rates of severe aggression and promoting group cohesion, whereas juvenile natal males use severe aggression frequently, and are associated with higher rates of wounding. Given these results, we suggest that young natal males, particularly those from high-ranking families, act in opposition to group stability.
Young natal males from high-ranking families may oppose group stability by upsetting the power dynamics of the group. In groups with high variance in social power, the highest ranking males have the highest social power and are able to successfully police others’ conflicts [Flack et al. 2005a; Flack et al. 2005b]. In addition to their frequent aggression, young natal males from high-ranking matrilines have frequent kin alliances, which they may use to prematurely attain high rank and a high degree of social influence in the male network, but they may lack the competitive ability and social power (group consensus of competitive ability) to effectively police others’ conflicts, leading to group instability.
Acknowledgments
This project was supported by NIH grant #PR51 RR000169 and R24 RR024396, and conducted under IACUC protocol #11843. We thank John Capitanio, Jessica Flack, David Krakauer, Shannon Seil, and Allison Heagerty for many helpful conversations throughout this study.
References
- Berman C, Kapsalis E. Development of kin bias among rhesus monkeys: maternal transmission or individual learning? Animal Behaviour. 1999;58:883–894. doi: 10.1006/anbe.1999.1221. [DOI] [PubMed] [Google Scholar]
- Boehm C. Parasitic selection and group selection: a study of conflict interference in rhesus and Japanese macaque monkeys. In: Chiarelli AB, Corruccini RS, editors. Primate Behavior and Sociobiology. New York: Springer Verlag; 1981. pp. 161–182. [Google Scholar]
- Boelkins RC, Wilson AP. Intergroup social dynamics of the Cayo Santiago rhesus. Primates. 1972;13:125–140. [Google Scholar]
- Bonacich P. Power and centrality: a family of measures. American Journal Of Sociology. 1987;92:1170–1182. [Google Scholar]
- Borgatti SP, Everett MG, Freeman LC. Ucinet for Windows: Software for Social Network Analysis. Harvard, MA: Analytic Technologies; 2002. [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer-Verlag; 2002. [Google Scholar]
- Chapais B. Matriline membership and male rhesus reaching high ranks in the natal troops. In: Hinde RA, editor. Primate Social Relationships: An Integrated Approach. Sunderland, MA: Blackwell Scientific Publications; 1983. pp. 171–175. [Google Scholar]
- Cheney DL, Seyfarth RM. Nonrandom dispersal in free-ranging vervet monkeys: social and genetic consequences. The American Naturalist. 1983;122:392–412. [Google Scholar]
- Datta SB. The role of alliances in the acquisition of rank. In: Else JG, Lee PC, editors. Primate Ontogeny, Cognition, and Social Behaviour. New York: Cambridge University Prress; 1986. pp. 219–225. [Google Scholar]
- Datta SB. Effects of availability of allies on female dominance structure. In: De Waal FBM, Harcourt AH, editors. Coalitions and Alliances in Humans and Other Animals. Oxford: Oxford University Press; 1992. pp. 61–82. [Google Scholar]
- Drickamer LC, Vessey SH. Group changing in free-ranging male rhesus monkeys. Primates. 1973;14:359–368. [Google Scholar]
- Ehardt CL, Bernstein IS. Conflict intervention behavior by adult male macaques: structural and functional aspects. In: Harcourt AH, De Waal FBM, editors. Coalitions and Alliances in Humans and other Animals. Oxford: Oxford University Press; 1992. pp. 83–111. [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung Y-Y, Mann JJ. Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. American Journal of Primatology. 2004;64:1–17. doi: 10.1002/ajp.20057. [DOI] [PubMed] [Google Scholar]
- Flack JC, de Waal FBM, Krakauer DC. Social structure, robustness, and policing cost in a cognitively sophisticated species. The American Naturalist. 2005a;165:E000. doi: 10.1086/429277. [DOI] [PubMed] [Google Scholar]
- Flack JC, Krakauer DC, de Waal FBM. Robustness mechanisms in primate societies: a perturbation study. Proceedings of the Royal Society Biological Sciences Series B. 2005b;272:1091–1099. doi: 10.1098/rspb.2004.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. Effects of provisioning on the social behavior of Japanese and rhesus macaques: Implications for socioecology. Primates in Medicine. 1999;40:187–198. doi: 10.1007/BF02557710. [DOI] [PubMed] [Google Scholar]
- Hill D, Okayasu N. Absence of youngest ascendancy in the dominance relations of sisters in wild Japanese macaques (Macaca fuscata yakui) Behaviour. 1995;132:367–379. [Google Scholar]
- Kaufman JH. Social relations of adult males in a free-ranging band of rhesus monkeys. In: Altmann SA, editor. Social Communication among Primates. University of Chicago Press; 1976. pp. 73–98. [Google Scholar]
- Kawamura S. Matriarchal social ranks in the Minoo-B troop: a study of the rank system of Japanese monkeys. Primates. 1958;1:149–156. [Google Scholar]
- Koford CB. Rank of mothers and sons in bands of rhesus monkeys. Science. 1963;141(3578):356–357. doi: 10.1126/science.141.3578.356. [DOI] [PubMed] [Google Scholar]
- Lindburg DG. Rhesus monkeys: mating season mobility of adult males. Science. 1969;166:1176–1178. doi: 10.1126/science.166.3909.1176. [DOI] [PubMed] [Google Scholar]
- Manson JH. Sons of low-ranking female rhesus macaques can attain high dominance ranks in their natal groups. Primates. 1993;34:285–288. [Google Scholar]
- McCowan B, Anderson K, Heagarty A, Cameron A. Utility of social network analysis for primate behavioral management and well-being. Applied Animal Behaviour Science. 2008;109:396–405. [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman and Hall; 1989. [Google Scholar]
- Meikle DB, Vessey SH. Nepotism among rhesus monkey brothers. Nature. 1981;294:160–161. doi: 10.1038/294160a0. [DOI] [PubMed] [Google Scholar]
- Missakian EA. Genealogical and cross-genealogical dominance relations in a group of free-ranging rhesus monkeys (Macaca mulatta) on Cayo Santiago. Primates. 1972;13:169–180. [Google Scholar]
- Neville MK. Male leadership change in a free-ranging troop of Indian rhesus monkeys (Macaca mulatta) Primates. 1968;9:13–27. [Google Scholar]
- Oates-O'Brien RS, Farver TB, Anderson-Vicino KC, McCowan B, Lerche NW. Predictors of matrilineal overthrows in large captive breeding groups of rhesus macaques (Macaca mulatta) Journal of the American Association for Laboratory Animal Science. 2010;49:196–201. [PMC free article] [PubMed] [Google Scholar]
- Packer C. Reciprocal altruism in Papio anubis. Nature. 1977;265:441–443. [Google Scholar]
- Packer C. Inter-troop transfer and inbreeding avoidance in Papio anubis. Animal Behaviour. 1979;27:1–36. doi: 10.1016/0003-3472(79)90127-1. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Packer C. Dispersal and philopatry. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 250–266. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- Sade DS. Determinants of dominance in a group of free-ranging rhesus monkeys. In: Altmann SA, editor. Social Communication Among Primates. Chicago: University of Chicago Press; 1967. [Google Scholar]
- Sade DS. A longitudinal study of social behavior of rhesus monkeys. In: Tuttle R, editor. The Functional and Evolutionary Biology of Primates. New York: Aldine Atherton; 1972. pp. 378–398. [Google Scholar]
- Sprague DS. Life history and male intertroop mobility among Japanese macaques (Macaca fuscata) International Journal of Primatology. 1992;13:437–454. [Google Scholar]
- Sprague DS. Age, dominance rank, natal status and tenure among male macaques. American Journal Of Physical Anthropology. 1998;105:511–521. doi: 10.1002/(SICI)1096-8644(199804)105:4<511::AID-AJPA8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Thierry B. Social epigenesis. In: Thierry B, Singh M, Kaumanns W, editors. Macaque Societies. Cambridge: Cambridge University Press; 2004. pp. 267–290. [Google Scholar]
- Tilford BL. Seasonal rank changes for adolescent and subadult natal males in a free-ranging group of rhesus monkeys. International Journal of Primatology. 1982;3:483–490. [Google Scholar]
- Vessey SH, Meilke DB. Factors affecting social behavior and reproductive success of male rhesus monkeys. International Journal of Primatology. 1987;8:281–292. [Google Scholar]