Abstract
Little is known about the association between prenatal cocaine exposure and obesity. We tested whether prenatal cocaine exposure increases the likelihood of obesity in 561 9-year-old term children from the Maternal Lifestyle Study (MLS). Overall, 21.6% of children met criterion for obesity (body mass index [BMI] ≥ 95th percentile, age and sex-specific). While there was no overall cocaine effect on obesity, multivariate logistic analysis revealed that children exposed to cocaine but not alcohol were 4 times more likely to be obese (OR 4.11, CI 2.04–9.76) than children not exposed to either drug. No increase in obesity prevalence was found in children exposed to alcohol but not cocaine (OR 1.08, CI .59–1.93) or both (OR 1.21, CI 0.66–2.22). Alcohol exposure may attenuate the effect of cocaine exposure on obesity. Increased obesity associated with cocaine but not alcohol exposure was first observed at 7 years. BMI was also elevated from 3 to 9 years in children exposed to cocaine but not alcohol, due to increasing weight but normal height. Prenatal exposure to cocaine may alter the neuroendocrine system and metabolic processes resulting in increased weight gain and childhood obesity.
Keywords: Prenatal cocaine exposure, prenatal alcohol exposure, childhood obesity, growth, fetal origins
1. Introduction
Studies of perinatal growth in children with prenatal cocaine exposure have generally reported deficits in birth weight, length and head circumference [6,33,42] with increased prevalence of small for gestational age [33,47] and rapid early weight gain [23,33]. Reports of exposure effects on long term growth up to 10 years of age are inconsistent including no effects [33,37], increased weight [23] and deficits in height but not weight gain [13,35]. The 1 study that tested and found no effects of cocaine exposure on obesity (≥ 95th percentile, age and sex-specific) did not have enough power to adjust for confounding factors including other drugs of abuse likely to co-occur [33]. Of the other drugs of abuse commonly used with cocaine, increased obesity in children has only been reported with prenatal nicotine exposure [14,32,43]. In other studies, the association of prenatal nicotine exposure and standard growth parameters vary from increased body mass index (BMI) [20,28] to no differences in weight or height [33,42]. Prenatal alcohol exposure, on the other hand, has been is generally associated with long-term growth deficits [20,33,37]. No long term growth deficits or increases have been associated with prenatal marijuana exposure [17,42]. In our large sample, we found evidence that cocaine exposure was associated with increased BMI and hypertension in 9-year-old term children[46] with adjustment for confounding factors. The prevalence of obesity per se is not apparent by evaluation of BMI. Thus the current study examines prenatal cocaine exposure and obesity directly in the context of other prenatal exposures, early growth and behavioral factors, some of which are also associated with prenatal cocaine exposure.
Obesity in children is a serious problem related to increased metabolic risk factors in childhood [12,34] as well as increased likelihood of adult obesity, which is a key risk factor for type 2 diabetes and cardiovascular disease, the leading causes of death, disease and disability in the US [39,41]. The increased prevalence of childhood obesity from 1980 to 2004 is well-documented [38]. Recent analyses show no further overall increase in obesity from 2003–2006. Relevant to this study, Black girls were more likely to be obese than White girls, but no differences were found between Black and White boys [40]. Also alarming are indicators that most children of all races identified as obese by the ≥ 95th percentile would also meet the higher cutoff of ≥ 97th percentile, suggesting that the heaviest children are getting heavier [40]. Poverty which affects many minority children as well as children in the current study has been associated with conditions that contribute to obesity in children as young as 6 years such as high caloric diet, inadequate exercise and sedentary behavior (excessive television watching) [54–56].
Early identification of children at risk for obesity is important to the development of effective interventions with impact on current and long term public health. BMI and obesity in middle childhood has been related to higher birth weight [22,48] and rapid early weight gain during the first year [4,18,50], but not intrauterine growth retardation or small for gestational age (SGA). Studies of children ages 6 and 9 years reported that children with SGA continue to be smaller than children with normal weight [10,47]. On the other hand, SGA has been associated with increased prevalence of hypertension at 6 years [47] as well as obesity and metabolic syndrome in adults [48]. These findings have been explained as a consequence of fetal programming that alters metabolic pathways [48]. Although original studies on fetal programming focused on low birth weight as the precipitating condition, it is generally accepted that low birth weight per se is not at the heart of these disorders, but a proxy for other factors that influence intrauterine growth [30,57], one of which could be prenatal cocaine exposure [30,57].
The mechanisms of action of cocaine have been well described in terms of neurochemical and vasoconstrictive effects. However, there may be a “third pathophysiology” in which cocaine acts as an intrauterine stressor that alters fetal programming [30]. Stress hormones such as catecholamines and glucocorticoids, which are elevated in the fetus exposed to cocaine, can alter regulation of the neuroendocrine environment by acting on the hypothalamic-pituitary-adrenal axis which results in an altered set point for physiologic and metabolic outcomes including obesity [7]. As a stressor that alters the neuroendocrine environment, prenatal cocaine exposure could reprogram metabolic pathways of the fetus increasing the likelihood of rapid early weight gain and childhood obesity.
The goal of this study was to test the hypothesis that prenatal cocaine exposure is associated with increased prevalence of obesity at 9 years of age.
2. Methods
2.1. Design
MLS subjects were recruited postpartum at 4 participating hospital sites from 1993 to 1995. The study was approved by the institutional review board at each site. A Certificate of Confidentiality issued by the National Institute on Drug Abuse assured confidentiality regarding subjects’ drug use and the mother provided informed consent. The study was conducted in 2 phases: an acute phase [8,9], which extended through hospital discharge, and the longitudinal follow-up of a subset of subjects, which began at the 1-month visit (age corrected for prematurity) [31]. Here we report obesity at 9 years from the longitudinal phase.
The study definition of exposure was maternal report of any cocaine or opiate use during pregnancy during a postpartum interview at recruitment and/or gas chromatography-mass spectrometry (GC/MS) confirmation of presumptive positive screens for cocaine or opiate metabolites [15,29]. Opiates were included because hospital reports indicated that many cocaine users were also using opiates, however only 10% of cocaine users in MLS also used opiates. “Unexposed” was defined as denial of prenatal cocaine and opiate use and a negative enzyme-multiplied immunoassay technique (EMIT) screen for both drugs. Complete details of eligibility are reported elsewhere [8,9]. A brief summary follows. Maternal exclusion criteria included: Age <18 years; identified psychosis or history of institutionalization for retardation or emotional problems; language barriers that prevented her from giving informed consent or understanding the study; plans to move out of the catchment area. Infant exclusion criteria included: Outborn birth; multiple gestation; birth weight <501 grams; gestational age >42 weeks; unlikely to survive, chromosomal abnormality or TORCH infection.
2.2. Subjects
There were 1,388 mother/infant dyads (658 in the exposed group and 730 in the unexposed or comparison group) enrolled in the longitudinal study at the 1 month visit. The exposed and unexposed groups were group matched within site on race/ethnicity, child’s sex, and gestational age categories (<32 weeks, 33–36 weeks, and >36 weeks) [31]. Prenatal use of alcohol, marijuana, and nicotine determined by maternal report during the hospital interview was included as background drugs in both the exposed and unexposed groups.
MLS subjects were included in this study if the subject was term, ≥ 37 weeks gestational age at birth (n=811 of 1388 initially enrolled), and had weight and height measures at the 9-year visit (n=561). As the focus of this study was childhood obesity, we selected children at 9 years of age to capture obesity status prior to increases in obesity associated with advanced maturation [21]. At 9 years, Tanner Stage for boys was less than stage 3 in 99.7% and 100% for pubic hair and genital growth, respectively, and for girls was 92.4% and 95.6% for pubic hair and breast development, respectively. Preterm infants were excluded because their early growth trajectories differ from term infants and may be affected by medical status, treatment, and type of feeding. The final sample had 238 children exposed to cocaine and 323 children not exposed to cocaine during pregnancy.
2.3. Procedures
2.3.1. Subject characteristics
Maternal and child characteristics were collected postpartum at recruitment by maternal interview (race and ethnicity, maternal age, marital status, any prenatal use of cocaine, opiates, alcohol, tobacco, and marijuana) and by medical chart review (Medicaid recipient, birth weight, length, and head circumference, gestational age, infant’s sex). At the 1-month visit, mothers reported quantity and frequency information about use of legal and illegal drugs during pregnancy. Indicators of heavy use were derived based on previous studies [25,31]: Heavy cocaine use was defined as ≥ 3 days per week in the first trimester. Calculated across pregnancy, heavy alcohol use was ≥ 0.5 oz. of absolute alcohol per day; heavy tobacco use was ≥ 10 cigarettes per day; and heavy marijuana use was ≥ 0.5 joints per day. Some use was any use not meeting the criterion for heavy use. No use was denial of use during pregnancy. The infrequent use of opiates during pregnancy did not permit calculation of heavy use.
2.3.2. Environment
At the 1-month visit and every annual visit, the relationship of the caretaker to the child was recorded (e.g., biological mother or other related or unrelated adult), which provides information on the number of changes in caretakers, which is an indicator of disrupted and unstable family and neighborhood environments. The caretaker also reported educational level and occupation for the primary contributors to the household from which was calculated the 5-group Hollingshead Index of Social Position or socioeconomic status (SES) [11,27]. We dichotomized the SES groups into low (group 5) versus middle to high (groups 1 to 4). At each annual visit, caretakers reported any physical and sexual abuse to themselves during the previous year. Domestic violence was scored as 1 for any report of abuse occurring across the childhood years.
2.3.3. Early weight gain
SGA was defined as <10th percentile of birth weight for gestational age and sex [5]. Early weight gain was calculated as weight (grams) increase per month from birth to 1 year. For 91 cases with missing data, weights at adjacent visits, 8 months and 2 years, were used to estimate the 1-year weight. The mean for the actual weight gain per month from birth to 1 year for non-missing cases is 544.84 (SD 94.07) versus 544.14 (SD 91.56) for the estimated value. For analysis we grouped the data into 100 g units for ease of interpretation.
Prepregnancy BMI of the biological mother was based on maternal self report of height and prepregnancy weight during the postpartum interview at recruitment then calculated according to the Centers for Disease Control and Prevention (CDC) [1]. Maternal prepregnancy BMI is a consistent predictor of birth weight and obesity in children [48]. Its inclusion in the analysis accounts for familial influence (genetic and lifestyle effects) on obesity.
2.3.4. Energy imbalance
There have been many studies examining the logical link between obesity and sedentary behavior and/overeating, although evidence is inconsistent across samples and methodologies [36]. In our study, we evaluated excessive TV watching, a measure of sedentary behavior, and inadequate exercise. Exercise was measured by caretaker report of the child’s number of hours of vigorous physical activity per day at age 7 years and days per week with more than 0.5 hour of regular exercise (e.g., sports, jog, swim) at ages 8 and 9 years. Inadequate exercise was defined as less than ½ hour per day at 7, 8 or 9 years. TV watching was measured by caretaker report of the amount of time per day the child watches television at ages 7 to 9 years with excessive TV watching defined as greater than 4 hours per day at 7, 8, or 9 years. Both these criteria are less stringent than national recommendations as too few children met cutoffs. The American Association of Pediatrics recommends < 2 hours daily of screen time [2] and the Office of Disease Prevention and Health Promotion recommends 60 minutes daily of moderate to vigorous activity [3].
Caloric intake was calculated by the NutritionistPro software based on a 3-day diary of all foods ingested. The diary was completed prior to the 9-year clinic visit by the child with assistance of the parent. At the visit, nurses trained on the procedure reviewed the diary with parent and child to clarify portion size (using props) and ingredients of reported food [16] and entered the information into NutritionistPro. If the diary was not completed prior to the clinic visit, a blank diary was sent home with the family and the nurse conducted a home visit to obtain current information. In this study, we included the average calories per day as our measure of caloric intake.
2.3.5 Obesity and growth
In MLS height and weight were obtained during a physical exam by medical staff at 4 and 8 months then at each annual visit from 1 to 9 years. For analysis of obesity in children ages 3 to 9 years, BMI was calculated [weight (kg)/height (m)2] and the BMI percentiles by age and sex were derived from the CDC based on the 2000 growth charts [26]. Obesity was defined as BMI ≥ 95th percentile [38]. A higher criterion for obesity, BMI ≥ 97th percentile, was also calculated to further describe the sample. In children from birth to 2 years, weight-for-length percentiles by age and sex were derived from the CDC. Obesity was defined as weight-for-length ≥ 95th percentile. Growth analyses used z-scores by age and sex for weight; stature (3–9 years) or length (0 to 2 years); and BMI (3–9 years) or weight-for-length (birth to 2 years), which were also derived from the CDC.
2.4. Statistical Methods
Unadjusted bivariate analysis was used to test for selective attrition by comparing children who were included in the study versus those not included and to examine the association of cocaine exposure with subject characteristics and behavioral risk factors. Chi square was used for dichotomized variables and ANOVA was used for continuous variables. Logistic regression (SAS 9.1.3) was used to determine covariates and for the final model of obesity at age 9 years.
General linear mixed models (GLMM), specifically PROC GLIMMIX from SAS version 9.1.3, was used for longitudinal analyses of the prevalence of obesity as well as z-scores for related growth parameters (BMI, weight, and height) at 12 age points from birth to 9 years. GLMM are useful for modeling correlated or repeated measures data where the underlying distribution is not necessarily normal. GLMM can accommodate both continuous and categorical outcome variables. All available observations for the 561 children are included in GLMM. Missing observations are not imputed nor are cases dropped due to missing observations. The number of subjects contributing growth data at each age point are: Birth n=538; 4 months n=464; 8 months n=454; 1 year n=437; 2 years n=451; 3 years n=447; 4 years n=443; 5 years n=439; 6 years n=468; 7 years n=481; 8 years n=520; 9 years n=561. Specific a priori hypotheses were tested within the mixed model framework using estimable linear combinations of the exposure effects from the primary analysis of obesity at 9 years. Follow-up analyses targeting specific ages or intervals use all observations available at that age point or interval. All GLMM models were adjusted for the prenatal and infancy covariates used in the final model of obesity at 9 years.
2.4.1 Determination of covariates and other predictors
Covariates were selected in a 2-step process. The initial selection of covariates included all growth and behavioral risk factors in Table 3 and site as well as variables in Table 2 that had been previously associated with increased obesity including prenatal nicotine exposure, birth weight, female sex, and SES or the potential for decreased obesity due to persistent growth deficits such as prenatal alcohol exposure. SES at 1 month and 9 years were highly correlated (r = 0.655). We selected the 9-year measure to represent the SES of the family during childhood. We examined the other subject characteristics in Table 2 as possible confounding factors between cocaine exposure and obesity if significantly associated with both cocaine exposure and obesity but not highly correlated with a variable already selected above the cutoff, r > 0.60. The 2 possible confounded factors that were also highly correlated with another factor hence not included were birth length, r = 0.75 with birth weight; maternal education, r=0.61 with SES.
Table 3.
Growth and behavioral risk factors by cocaine exposure status and obesity (> 95th percentile for age and sex) at age 9 years.
| n(%) or Mean (SD) | Exposed (n = 238) | Not Exposed (n = 323) | Obese (n=121) | Not Obese (n=440) |
|---|---|---|---|---|
| Early weight gain (g/mo.) | 554.82 (89.4)* | 536.26 (92.3) | 574 (89.6)*** | 535 (90.3) |
| SGA | 85 (36%)*** | 64 (20%) | 16 (13%)** | 133 (30%) |
| Inadequate exercise | 135 (57%) | 173 (54%) | 79 (65%)** | 229 (52%) |
| Excessive TV watching | 149 (64%) | 197 (63%) | 76 (63%) | 270 (63%) |
| Caloric intake (avg. per day) | 2236 (577) | 2240 (656) | 2367 (687)* | 2202 (600) |
| Maternal prepregnancy BMI | 23.49 (4.89)*** | 26.56 (6.96) | 27.68 (7.28)*** | 24.62 (5.91) |
p<.001,
p<.01,
p<.05
Table 2.
Sample characteristics by cocaine exposure status and obesity.
| n(%) or Mean (SD) | Exposed (n = 238) | Not Exposed (n = 323) | Obese (n=121) | Not Obese (n=440) |
|---|---|---|---|---|
| CHARACTERISTICS OF THE BIOLOGICAL MOTHER | ||||
| Race | ||||
| Black | 195 (82%) | 247 (76%) | 92 (76%) | 350 (80%) |
| White | 24 (10%) | 47 (15%) | 19 (16%) | 52 (12%) |
| Hispanic/Other | 19 (8%) | 29 (9%) | 10 (8%) | 38 (9%) |
| Maternal age, yr | 30.49 (5.13)*** | 26.78 (5.91) | 28.4 (5.51) | 28.3 (5.98) |
| Single | 215 (91%)*** | 246 (76%) | 99 (82%) | 362 (82%) |
| < High school | 110 (46%)** | 108 (34%) | 33 (27%)** | 185 (42%) |
| Medicaid recipient | 209 (88%)** | 253 (78%) | 102 (84%) | 360 (82%) |
| Prenatal Cocaine Use | - | - | 55 (45%) | 183 (42%) |
| Heavy (≥3 days/week) | - | - | 14 (12%) | 46 (11%) |
| Prenatal opiate use | 23 (10%) | 21 (7%) | 16 (13%)* | 28 (6%) |
| Prenatal alcohol use | 184 (77%)*** | 175 (54%) | 70 (58%) | 289 (66%) |
| Heavy (≥0.5 oz abs. alc./day) | 60 (29%)*** | 15 (5%) | 18 (16%) | 57 (14%) |
| Prenatal tobacco use | 200 (84%)*** | 96 (30%) | 67 (55%) | 229 (52%) |
| Heavy (≥10 cigarettes/day) | 87 (43%)*** | 39 (12%) | 31 (27%) | 95 (23%) |
| Prenatal marijuana use | 93 (39%)*** | 31 (10%) | 22 (18%) | 102 (23%) |
| Heavy (≥0.5 joints/day) | 14 (7%)*** | 6 (2%) | 1 (1%) | 19 (5%) |
| CHILD CHARACTERISTICS | ||||
| Mean gestational age | 38.86 (1.34) | 39.02 (1.33) | 39.0 (1.33) | 38.9 (1.33) |
| Birth weight (g) | 2954 (500)*** | 3228 (525) | 3265 (537)** | 3070 (523) |
| Birth length (cm) | 48.63 (2.81)*** | 50.00 (2.66) | 49.97 (2.59)* | 49.27 (2.84) |
| Birth head circum. (cm) | 33.26 (1.62)*** | 34.04 (1.43) | 33.91 (1.58) | 33.66 (1.55) |
| Female sex | 109 (46%) | 144 (45%) | 59 (49%) | 194 (44%) |
| Obesity at 9 years | 55 (23%) | 66 (20%) | - | - |
| BMI ≥ 97th percentile | 48 (20%) | 52 (16%) | 100 (83%) | - |
| POSTNATAL ENVIRONMENTAL CHARACTERISTICS | ||||
| >2 Caretaker changes | 87 (37%)*** | 26 (8%) | 28 (24%) | 85 (19%) |
| Low SES at 1 month | 62 (28%) | 67 (21%) | 17 (15%)* | 112 (26%) |
| Low SES at 9 years | 55 (23%)** | 41 (13%) | 16 (13%) | 80 (18%) |
| Domestic violence | 68 (29%)* | 64(20%) | 30 (25%) | 102 (23%) |
p<.001,
p<.01,
p<.05
In the second step, backward logistic regression was used to test the contribution of the previously selected covariates as well as potential interactions between cocaine exposure and the covariates. Variables unrelated to obesity at the p value > 0.10 were systematically removed (beginning with the largest p value) and the analysis repeated with the variable excluded. If results of the other variables and overall fit of the model remained largely unchanged, the variable was excluded from further testing. If the interaction was significant, the main effect of the covariate was maintained regardless of significant value. Variables excluded by this process were birth weight, excessive TV watching, prenatal exposure to tobacco, SES and site. The second step was repeated substituting heavy use (heavy, some, no use) for any use (Y/N) of cocaine, alcohol, and tobacco during pregnancy.
In longitudinal analyses, covariates were selected from the final logistic model but were limited to prenatal or infancy measures that that could be related to all age points including SGA, early weight gain, maternal prepregnancy BMI, and gender.
2.4.2 Imputation of missing values
There were 11% missing values for caloric intake. Rather than eliminating these subjects, we applied multiple imputations [44,45,52] using SAS PROC MI and MIANALYZE. The results were very similar to analysis without this measure included. Further, the results in the logistic model for caloric intake were the same with and without imputation. Thus we present the final model from the imputed dataset to retain the largest sample size.
3. Results
3.1. Selective attrition
Comparison of the characteristics of the 561 subjects in this study to the 250 subjects excluded due to missing follow-up at 9 years (Table 1) showed that included subjects were more likely to be Black (P<0.001), single (P<0.01), low SES (P<0.01), and users of alcohol during pregnancy (P<0.05). No differences in prenatal use including heavy use of cocaine, opiates, tobacco or marijuana or indicators of poverty were observed (P>0.05), suggesting that higher risk families remained in the study including cocaine-exposed children. Further, there was no selective attrition due to infant characteristics (P>0.05) (Table 1). We also examined selective attrition by cocaine exposure group. In both the exposed and not exposed groups, included subjects were more likely to be Black (P<0.001, P=0.026, respectively) and single (P=0.017, P=0.045, respectively). There were no selective attrition in either group due to low SES (P=0.109, P=0.113, respectively) or to prenatal alcohol use (P=0.069, P=0.237, respectively). However, in the exposed group, more heavy alcohol users were included in the study (29% versus 17%, P=0.039).
Table 1.
Comparison of dyads included and not included in the study.
| n(%) or Mean (SD) | Included N=561 | Not Included n=250 |
|---|---|---|
| CHARACTERISTICS OF THE BIOLOGICAL MOTHER | ||
| Race | ||
| Black | 442 (79%)*** | 160 (64%) |
| White | 71 (13%) | 65 (26%) |
| Hispanic/Other | 48 (8%) | 25 (10%) |
| Maternal age, yr | 28.35 (5.88) | 27.63 (5.75) |
| Single | 461 (82%)** | 183 (73%) |
| < High school | 218 (39%) | 92 (37%) |
| Low SES at 1 month | 129 (24%)* | 40 (17%) |
| Medicaid recipient | 462 (82%) | 198 (79%) |
| Prenatal cocaine use | 238 (42%) | 103 (41%) |
| Heavy cocaine use (≥ 3 days/wk) | 60 (11%) | 32 (14%) |
| Prenatal opiate use | 44 (8%) | 28 (11%) |
| Prenatal alcohol use | 359 (64%)* | 141 (56%) |
| Heavy (≥0.5 oz absolute alcohol/day) | 75 (14%) | 21 (9%) |
| Prenatal tobacco use | 296 (53%) | 138 (55%) |
| Heavy (≥10 cigarettes/day) | 126 (24%) | 57 (25%) |
| Prenatal marijuana use | 124 (22%) | 60 (24%) |
| Heavy (≥0.3 joints/day) | 20 (4%) | 11 (5%) |
| CHILD CHARACTERISTICS | ||
| Mean gestational age | 38.95 (1.34) | 38.98 (1.42) |
| Small for gestational age (SGA) | 149 (27%) | 59 (24%) |
| Birth weight (g) | 3112 (531) | 3125 (494) |
| Birth length (cm) | 49.42 (2.80) | 49.40 (2.74) |
| Birth head circumference (cm) | 33.71 (1.56) | 33.83 (1.53) |
| Female sex | 253 (45%) | 109 (44%) |
p<.001,
p<.01,
p<.05
3.2. Univariate analysis by prenatal cocaine exposure and obesity
Table 2 shows separate univariate analyses of sample characteristics by cocaine exposure and obesity at 9 years. Overall, the sample was predominantly single parent, Medicaid recipients and minority (79% Black, 8% Hispanic versus 13% White). The 4 subjects who reported mixed ancestry were too few to be tested as a separate group. They were included with Hispanics as this group is also diverse in race as well as country of origin. There were no differences in race by cocaine exposure or obesity.
Biological mothers in the cocaine exposure group were older (P<0.001), more likely to be single (P<0.001), without a high school diploma or GED (P<0.01), Medicaid recipients (P<0.01) and more likely to use alcohol, tobacco and marijuana including heavy use of these drugs during pregnancy (all P’s <0.001). Infants with prenatal cocaine exposure were lighter, shorter, and had a smaller head circumference at birth (all P’s<0.001). By 9 years, 37% of the children with cocaine exposure had experienced 2 or more changes in caretaker with cocaine-exposed children experiencing multiple placements more than 4 times as often as children who were not exposed (P<0.001). Cocaine-exposed children were more likely to live in low SES households (P<0.01) than children who were not exposed. More domestic violence was reported by caretakers in the exposed group than in the comparison group (P<0.05). There were no differences between exposed and not exposed groups in race, gestational age and sex (all Ps>0.05), which were the MLS matching criteria at enrollment. Overall, 21.6% (n=121) of the children at age 9 years met criterion for obesity and the percentage of children with obesity did not differ by cocaine exposure group (P>0.05). Further, 83% (n=100) of obese children had a BMI ≥ 97th percentile with no significant difference by cocaine exposure group (P>.05). Mothers of children in the obese group were more likely to have at least a high school education (P<0.01) and more likely to use opiates during pregnancy (P<0.05). Further, children with obesity are less likely to be low SES during early infancy (P<0.05). Obese children were heavier (P<0.01) and longer (P<0.05) at birth than non obese children.
Table 3 shows univariate analysis of growth and behavioral risk factors for obesity by cocaine exposure and obesity. Most of these factors have previously been reported including early weight gain [49] and energy imbalance [36]. The selected risk factors provide a context in which to determine whether prenatal cocaine exposure contributes new information about the development of obesity and recognizes that multiple risk factors are involved. Cocaine exposure was associated with rapid early weight gain (P<0.05), increased likelihood of SGA (P<0.001) but lower maternal prepregnancy BMI (P<0.001) compared to not exposed children. There was no association between cocaine exposure and inadequate exercise, excessive TV watching, and caloric intake (all Ps>.05). Obesity was associated with faster early weight gain (P<0.001), inadequate exercise (P<0.01), higher caloric intake (P<0.05), higher maternal prepregnancy BMI (P<0.001), but less SGA (P<0.01) compared to non obese children. Excessive TV watching was not associated with obesity (P >0.05).
3.3. Cocaine and obesity
While univariate analysis did not show significant differences in obesity by cocaine exposure (Table 2), multiple logistical analysis revealed an interaction between cocaine and alcohol exposures (P=0.017). The logistic regression was repeated replacing the interaction with contrast tests between each combination of cocaine and alcohol exposure versus the group with no exposure to either drug (Table 4). Children exposed to cocaine but not alcohol (n=54) were 4 times more likely to be obese, but children exposed to cocaine and alcohol (n=184) or exposed to alcohol but not cocaine (n=175) did not show increased obesity rates over the group not exposed to cocaine or alcohol (n=148). There were also 2 effects of early growth. Obesity was more likely with each 100 g increase in early weight gain but was two-thirds less likely if SGA at birth. There were 2 effects related to energy imbalance. Obesity was twice as likely if the child did not exercise regularly and the odds of obesity increased slightly with each additional 100 calories per day. Further, obesity was 1.7 times more likely if the child was female versus male. Obesity was also more likely with each increase in maternal BMI before pregnancy.
Table 4.
Odds ratios for obesity at 9 years
| Risk Factor | Odds Ratio (95th Confidence Interval) |
|---|---|
| Exposed to cocaine, not alcohol | 4.11 (1.90–8.89) |
| Exposed to alcohol, not cocaine | 1.08 (0.59–1.93) |
| Exposed to cocaine & alcohol | 1.21 (0.66–2.22) |
| Early weight gain, per 100 g/mo. | 1.75 (1.35–2.27) |
| SGA | 0.35 (0.19–0.64) |
| Inadequate exercise | 1.98 (1.30–3.31) |
| Caloric intake, per 100 calorie unit | 1.10 (1.02–1.19) |
| Female sex | 1.78 (1.12–2.85) |
| Prepregnancy maternal BMI per unit | 1.08 (1.05–1.12) |
3.4. SGA, early weight gain and cocaine exposure
The inverse relationship between SGA and obesity could be an artifact of the inclusion of early weight gain in the model. To test a potential ‘reversal paradox’ [53], we reanalyzed the final model without early weight gain. SGA remained a significant inverse predictor of obesity (P<0.001). The positive association of prenatal cocaine exposure with SGA and early weight gain and their opposite effects on obesity suggests the possibility of an interaction among these 3 factors. We added 2-way interactions including cocaine exposure by SGA, cocaine exposure by early weight gain, early weight gain by SGA and a 3-way interaction and found no statistically significant interaction effects. Thus, there were no significant interactions observed that would identify specific subgroups of cocaine-exposed children who were more vulnerable to obesity than others based on fetal and early growth.
3.5. Heavy cocaine exposure
To determine if heavy prenatal exposure to cocaine was associated with obesity, the original logistic regression model was reanalyzed substituting measures of heavy cocaine exposure for any exposure and heavy use of alcohol and tobacco for any exposure to these drugs. There was no significant effect of heavy cocaine exposure on obesity (P =0.082). Compared to no exposure, the parameter estimates for some and heavy exposure were similar (0.665 and 0.659, respectively). There was no significant effect for heavy alcohol use (P=0.739). Due to small cell sizes, the interaction of heavy use of cocaine and alcohol could not be tested.
3.6. Longitudinal analysis of obesity and growth
To examine the patterns of growth underlying obesity, we analyzed the prevalence of obesity and z-scores for BMI, weight, and height at 12 age points from birth to 9 years, adjusted for covariates from the prenatal period or infancy from the analysis of obesity at 9 years (section 3.3). All available growth data for the 561 participants are included in longitudinal analyses of the 4 cocaine-by-alcohol exposure groups described in section 3.3. (exposed to cocaine not alcohol; exposed to alcohol not cocaine; exposed to cocaine & alcohol; not exposed to cocaine or alcohol). There were no group differences in the mean number of visits from birth to 9 year (range from 9.82 visits in the group exposed to cocaine and alcohol to 10.44 visits in the group not exposed to either drug, P=0.328). Overall 75% cases had ≥ 10 of 12 visits.
GLMM applied to analysis of growth parameters included 3 a priori group contrasts based on the findings of obesity at 9 years, testing differences between (1) the group exposed to cocaine not alcohol versus the group not exposed to either drug; (2) the group exposed to alcohol not cocaine versus the group not exposed to either drug; (3) the group exposed to cocaine and alcohol versus the group exposed to both alcohol and cocaine. Further, we included a fourth contrast test comparing the group exposed to cocaine not alcohol versus the 3 other groups combined. Specific age points or time intervals were tested for each growth parameter.
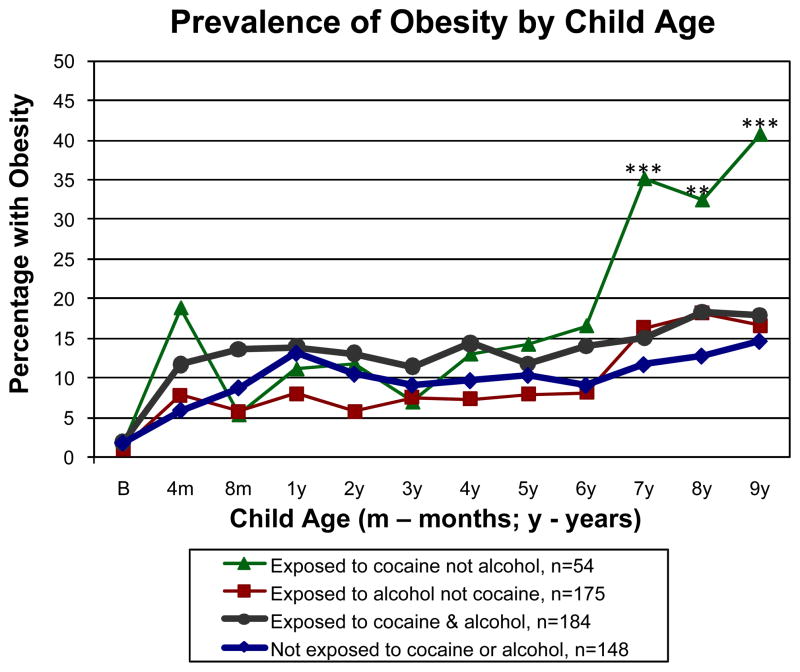
Fig. 1 shows the prevalence of obesity by child age in the 4 cocaine-by-alcohol exposure groups. To determine the age at which cocaine exposure is first associated with obesity, we analyzed the prevalence of obesity from birth to 9 years by the 4 groups, adjusted for SGA, early weight gain, maternal prepregnancy BMI, and gender. Overall (n=561), there was a significant effect for age (P<0.001), but not for group (P=0.070) or group contrasts (P values range from 0.130 to 0.473). From birth to 6 years (range of n=437 to 538), the 4 groups did not differ in the prevalence of obesity (P values range from 0.736 at birth to 0.152 at 6 years). However by 7 years (n=481), children who were exposed to cocaine but not alcohol were more likely to be obese than those not exposed to either drug (P<0.001) as well as those in the other 3 groups combined (P<0.001), continuing the pattern at 8 years (n=520, P=0.006, P=0.012), respectively), and 9 years (n=561, P<0.001 in both cases). No other group contrasts were significant from 7 to 9 years (P values range from 0.198 to 0.810).
Fig. 1.
Longitudinal analysis of the prevalence of obesity (BMI ≥95th percentile) from birth to 9 years by 4 cocaine-by-alcohol exposure groups, adjusted for SGA, early weight gain from birth to 1 year, sex, and maternal prepregnancy BMI. ***P<0.001, **P<0.01 to P<0.05, respectively, for significant differences between the group exposed to cocaine but not alcohol versus the group not exposed to cocaine or alcohol and the other 3 groups combined.
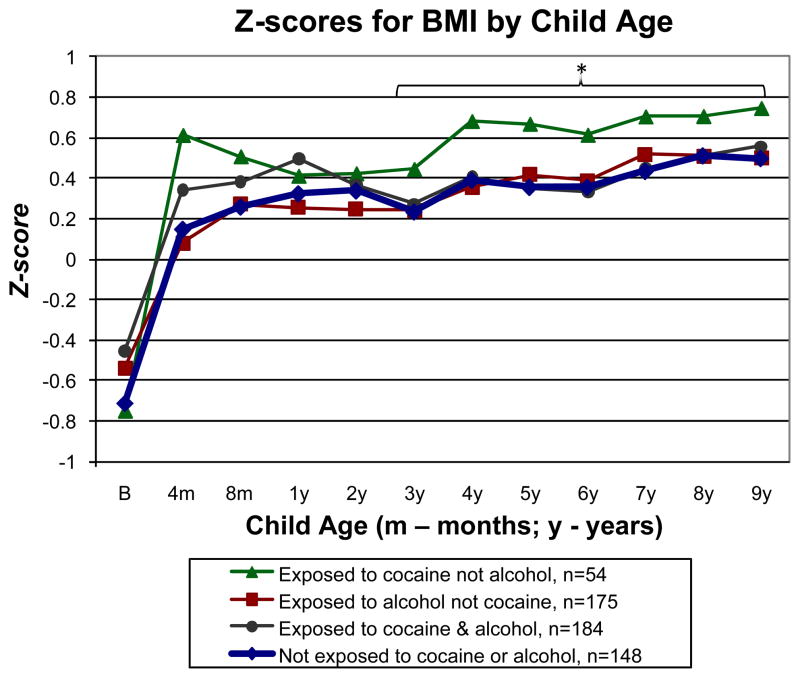
Z-scores by age and sex for BMI from birth to 9 years are shown in Fig. 2 for the 4 cocaine-by-alcohol exposure groups. Overall, there was a significant effect for age (P<0.001) but not for group (P=0.197). The group exposed to cocaine but not alcohol had higher BMI than the group not exposed to cocaine or alcohol (P=0.047). No other group contrasts were significant (P values from 0.390 to .910). Due to the bimodal curve shown in Fig. 2, we conducted follow-up analyses of group differences from ages 4 to 8 months then from 3 to 9 years. There were no significant group contrasts (P values from 0.102 to 0.418) in the 4- to 8-month interval (n=545). Analysis of the 3- to- 9-year interval (n=561) showed higher BMI in the group exposed to cocaine but not alcohol versus the group not exposed to either drug (P=0.042) and the other 3 groups combined (P=0.044) with no other significant group contrasts (P values from 0.431 to 0.609).
Fig. 2.
Longitudinal analysis of z-scores by age and sex for BMI from birth to 9 years by the 4 cocaine-by-alcohol exposure groups, adjusted for SGA, early weight gain from birth to 1 year, sex, and maternal prepregnancy BMI. *P<0.05 for significant differences between the group exposed to cocaine but not alcohol versus the group not exposed to cocaine or alcohol or the other 3 groups combined. Percentiles for age and sex from the CDC growth charts are included on the right Y axis for reference.
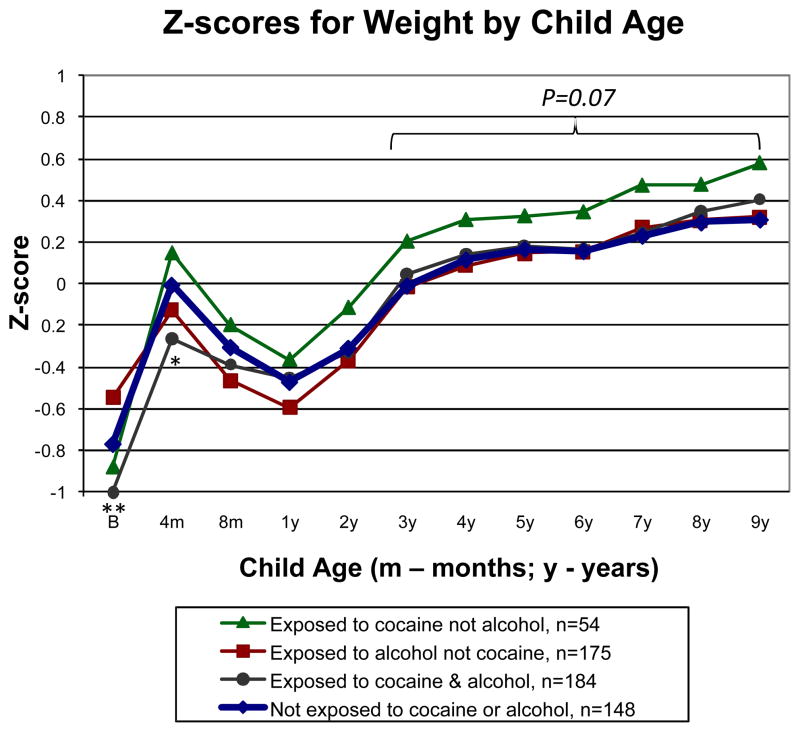
Z-scores by age and sex for weight from birth to 9 years are shown in Fig. 3 for the 4 groups. Overall, there was a significant effect for age (P<0.001), but not for group (P=0.265) or group contrasts (P values from 0.056 to 0.717). Based on the observed deviation between the groups by age 3 similar to BMI, we conducted follow-up analysis of the 3- to- 9-year interval (n=561). There was a trend toward increased weight in the group exposed to cocaine but not alcohol versus the group not exposed to cocaine or alcohol (P=0.065) and the other 3 groups combined (P=0.064). Significance levels for other group contrasts ranged from P=0.488 to P=0.899. Noting divergence in z-scores for weight at birth by group, we tested group differences by age beginning at birth. At birth (n=538), the group with cocaine and alcohol exposure had lower weight than the group with no exposure to either drug (P=0.005). At 4 months (n=464), the group with cocaine and alcohol exposure remained lower weight compared to the group not exposed to either drug (P=0.019). No group contrasts were significant beginning at 8 months (P values from 0.123 to 0.983), with the exception of 7 years (n=481), when the group exposed to cocaine but not alcohol was heavier than the other 3 groups combined (P=0.037).
Fig. 3.
Longitudinal analysis of z-scores by age and sex for weight from birth to 9 years by the 4 cocaine-by-alcohol exposure groups, adjusted for SGA, early weight gain from birth to 1 year, sex, and maternal prepregnancy BMI. **P<0.01, *P<0.05 between the group exposed to cocaine and alcohol versus the group not exposed to cocaine or alcohol. In the interval from 3 to 9 years, there was a trend for increased weight in the group exposed to cocaine but not alcohol versus the group not exposed to cocaine or alcohol and the other 3 groups. Percentiles for age and sex from the CDC growth charts are included on the right Y axis for reference.
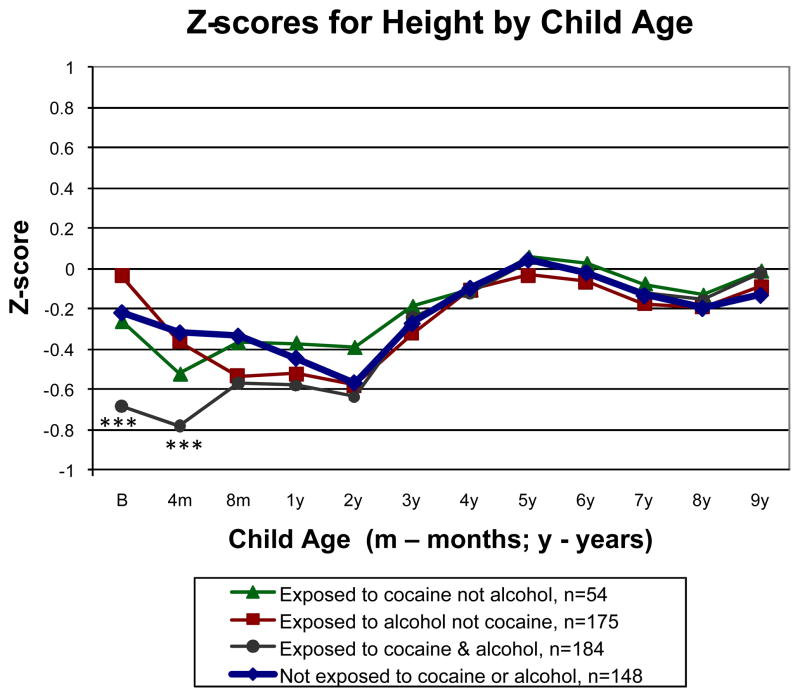
Z-scores by age and sex for height from birth to 9 years are shown in Fig. 4 for the 4 groups. Overall, there was a significant effect for age (P<0.001) but not for group (P=0.499) or group contrasts (P values from 0.202 to 0.802). Similar to weight, we tested group differences by age beginning at birth. At birth (n=538), the group exposed to cocaine and alcohol was shorter than the group not exposed to either drug (P<0.001). At 4 months (n=464), the group exposed to cocaine and alcohol remained shorter than the group not exposed to either drug (P<0.001). No group contrasts were significant beginning at 8 months through 9 years of age (P values from 0.059 to 0.973).
Fig. 4.
Longitudinal analysis of z-scores by age and sex for height from birth to 9 years by the 4 cocaine-by-alcohol exposure groups, adjusted for SGA, early weight gain from birth to 1 year, sex, and maternal prepregnancy BMI. ***P<0.001 between the group exposed to cocaine and alcohol versus the group not exposed to cocaine or alcohol. Percentiles for age and sex from the CDC growth charts are included on the right Y axis for reference.
A key predictor of obesity at 9 years is rapid weight gain from birth to 1 year. We examined whether early weight gain continues to make a contribution to obesity from 2 to 9 years of age. Overall, there was a significant effect for early weight gain on the prevalence of obesity (P<0.001). Further, there was a significant interaction of age and weight gain on obesity from 2 to 3 years (P=0.003), but not beyond (P=0.747). That is, the association between weight gain and the prevalence of obesity increased across the second and third year that then remained stable from 4 to 9 years, neither increasing nor decreasing.
The longitudinal analyses were restricted to the 561 children who had obesity data at 9 years. Given the total full-term sample of 811 at birth, 250 additional children contributed growth data to 1 or more of previous visits (ranging from 240 at birth to 60 at the 8-year visit). To examine the impact of these cases, we calculated the difference in each growth parameter between the total full-term sample less the study sample at each visit age from birth to 8 years. The average difference across age was −0.55% (range −1.54% to 0.26%) for obesity; −0.016 standard deviation unit (SD) (range −0.045 to 0.022) for BMI z-scores; −0.19 SD (range −0.046 to 0.018) for weight z-scores; and −0.009 SD (range −0.038 to 0.020) for height z-scores. The age with the largest difference occurred at 1 year for obesity and BMI, 3 years for height and 4 years for weight. Overall, there were small differences between the full sample available at each age and the 561 in the study sample.
The longitudinal analyses were also restricted to the covariates from the final logistic model of obesity at age 9 years further limited to measures from the prenatal or infancy period that could apply to all age points. Prenatal exposures to tobacco and marijuana and SES had been excluded from analysis of obesity at 9 years, but could contribute to the longitudinal models of obesity as well as BMI, weight and height. Thus, we reanalyzed the longitudinal models with these drugs and SES (measured at the 1-month visit) included. For tobacco and marijuana exposure, we tested the main effect, the 2-way interaction with age, and 2 3-way interactions with cocaine exposure and age and alcohol exposure and age for each drug. The only finding was a significant tobacco-by-age interaction on height (P=0.008). Tobacco-exposed children were shorter than those not exposed to tobacco at 4 months (n=464, mean z-score −0.781, SE 0.10 versus mean z-score −0.51, SE 0.11, respectively, P=0.019) and 8 months (n=454, mean z-score −0.670, SE 0.10 versus mean z-score −0.405, SE 0.11, respectively, P=0.023). There were no significant effects of tobacco exposure at birth (P=0.141) or from ages 1 to 9 years (P values from 0.141 to 0.987). No other significant main or interaction effects were found for either drug on growth measures (all P values > 0.05). Further, there were no significant 3-way interactions of cocaine, alcohol and tobacco or cocaine, alcohol and marijuana on any growth measure (all P values > 0.05). SES was also not significant in any growth model (P values > 0.05). In addition, we reanalyzed the longitudinal models including opiate exposure. There were no significant main or interaction effects with age for opiate exposure on any growth measure (all P values > 0.05).
4. Discussion
This is the first study to show a unique effect of prenatal cocaine exposure on obesity adjusted for alcohol exposure and other factors. The association of cocaine exposure and obesity was strongest in a subgroup of exposed children. Cocaine-exposed children were 4 times as likely to become obese at 9 years of age if they were not exposed to alcohol as well. Prenatal exposure to cocaine and alcohol did not increase the prevalence of obesity compared to exposure to neither drug or to alcohol alone. Others have found persistent growth decrements associated with alcohol exposure [20,33,37] when cocaine and other drugs of abuse are adjusted. But this is the first report of a subgroup of children who were exposed to both cocaine and alcohol with findings that alcohol exposure may attenuate the effect of cocaine exposure on obesity and growth. This also suggests that failure to show a positive association between cocaine exposure and obesity may be in part be due to the practice of using both drugs together during pregnancy and to samples smaller than the MLS that may not permit analysis of interactions.
Longitudinal analysis revealed increased prevalence of obesity in the group with prenatal exposure to cocaine but not alcohol beginning at 7 years then continuing through 8 and 9 years, relative to the 3 other cocaine-by-alcohol exposure groups. Also found was increased BMI from 3 to 9 years in children with cocaine but not alcohol exposure versus the other 3 groups with the mean BMI at approximately the 77th percentile (z-score 0.78) by 9 years. The 3 other groups showed similar mean BMI levels around the 69th percentile (z-score 0.50) at 9 years. The mean weight increased from 1 to 9 years in all 4 groups. In the group exposed to cocaine but not alcohol, the mean weight at 9 years was at the 73rd percentile (z-score 0.60) compared to the 63rd to 66th percentiles (z-scores 0.30 to 0.40) in the other 3 groups. Unlike BMI, the increased weight associated with cocaine but not alcohol exposure was not statistically significant from 3 to 9 years except for 7 years. Height, on the other hand, was nearly identical for all 4 groups from 1 to 9 years, reaching the 50% percentile (z-score 0) at 5 years then staying between the 42nd and 50th percentiles (z-scores −0.20 to 0) until 9 years. A decrease in stature gain associated with cocaine exposure had been reported [13,35], but similar to other studies [33,42] we did not find this effect. Taken together, the increase in obesity associated with exposure to cocaine but no alcohol appears to be related to increasing weight combined with small reductions in height percentiles albeit all close to average in all 4 groups.
Childhood obesity has previously been linked to higher birth weight, rapid early weight gain during early infancy [18] independent of birth weight [50], but not to SGA [10]. In our study, early weight gain over the first year predicted obesity at 9 years, with 39 g/month higher weight gain in obese than non-obese children. Further, longitudinal analyses showed a continued association between early weight gain and obesity from 2 to 9. On the other hand, SGA children were less likely to be obese than children who were appropriate for gestational age, supporting the previously reported negative association with obesity in mostly term children [19]. The possibility that the relationship between SGA and obesity is reversed due to its association with early weight gain was not supported and persisted with early weight gain excluded from the analysis. Our findings do not link SGA and early weight gain to each other in the development of obesity at 9 years. The positive relationship between SGA and obesity predicted by the fetal origins hypothesis may emerge in a longer follow-up than 9 years as found in older adolescents and adults [48].
Although both early weight gain and SGA are associated with cocaine exposure, each makes a unique contribution to the prevalence of obesity independent of cocaine exposure. This pattern of effects is not consistent with catch-up growth due to in utero growth restriction from cocaine exposure, but could be explained by the perspective of cocaine as an intrauterine stressor that disrupts the neuroendocrine environment, which impacts pre- and postnatal metabolic processes resulting in obesity [7].
Early weight gain in infants with SGA may reflect catch-up growth, that is, rapid weight gain following growth restriction in utero, resulting in postnatal ‘catching up’ to a normative, comparison sample or appropriate-for-age percentiles. This pattern has been reported in infants exposed to cocaine and tobacco but not with alcohol exposures [33,37]. The reported ages when cocaine-exposed infants catch-up in weight range from 6 months to 2 years and in height from 1 to 7 years [13,33,35]. Due in part to adjustment for SGA and early growth, our longitudinal analyses did not show the expected decrement in growth associated with cocaine exposure when alcohol exposure was not involved. However, the group exposed to both cocaine and alcohol had lower weight and height at birth and 4 months compared to the group not exposed to either drug, catching-up to the other groups by 8 months on both growth measures, which is within or close to the range reported for children with cocaine exposure. Most cocaine-exposed children are also exposed to alcohol. This is the first study to parse out the effects of cocaine and alcohol exposure. Our findings show early growth deficits with exposure to both drugs, but not with exposure to either cocaine or alcohol alone. On the other hand, all cocaine-by-alcohol groups show a pattern of increased weight by 4 months (range z-scores −0.28 to 0.20) while height remained less than the 50th percentile (z-score 0) until 5 years, continuing between the 42nd and 50th percentile (z-scores −0.20 to 0) until 9 years.
Nicotine has been linked with increased risk of obesity. Our null findings of prenatal tobacco exposure and obesity at 9 years were inconsistent with several other reports [14,32,43]. To examine whether prenatal exposure to tobacco could impact earlier ages, we reanalyzed the longitudinal models including tobacco exposure as well as the interaction of tobacco and cocaine and alcohol. There was no association of tobacco exposure and increased obesity, BMI or weight from birth to 9 years. The only effects were decreased height at 4 and 8 months only. In other reports of increased obesity cited above, the studies did not include cocaine as well as nicotine and the participants were not as disadvantaged as MLS. This suggests that the impact of prenatal tobacco exposure on obesity may be less salient in high risk samples with illicit drug use and greater poverty as found by Lumeng and colleagues [33]. Similarly, we analyzed for prenatal marijuana effects, but found no significant association with the 4 growth measures at any age.
Others have reported decreased BMI related to prenatal alcohol exposure [20,33]. However, to our knowledge no one has examined the interaction of prenatal exposure to alcohol and cocaine. The rate of obesity when exposed to both cocaine and alcohol was similar to exposure to alcohol alone, suggesting that prenatal alcohol exposure may act to constrain weight gain in children despite other drug exposures. It is also possible that the neuroendocrine pathways that drive cocaine addiction also drive obesity. Eating is a behavior, which is primarily regulated by neuroendocrine pathways. We can speculate that families who are genetically predisposed to pure cocaine addiction (i.e. not cocaine and alcohol addiction) may also be predisposed to “comfort food consumption” for the same underlying neurobiological reasons. The observation that the group with cocaine but no alcohol exposure does not diverge until about age 7 years (and not earlier) may reflect greater autonomy in eating behavior that could be driven by affect due to underlying neurobiology.
In our study, inadequate physical activity and high caloric intake, but not excessive TV watching, were significant predictors of obesity. In children who were obese, 65% did not meet the minimum criterion of more than 30 minutes of regular exercise compared to 52% in those who were not obese. Even though the criterion for inadequate exercise in this study was less stringent than the national recommendations of 60 minutes per day [51], the majority of children met the study criterion. Further, children who were obese consumed 166 calories more per day on average than those who were not obese, which is a modest but significant effect size. Surprisingly, excessive TV watching was not related to obesity in this study with 63% of children in both the obese and non-obese groups exceeding the criterion of 4 hours a day. A better discriminator of sedentary behavior was inadequate exercise.
This study has several limitations. The MLS was not designed to study obesity. Although the MLS has an extensive protocol with health-related concerns and growth examined on annual visits, the selection of predictor variables for this study was dependent on the existing dataset. Our measures of exercise and TV watching were straightforward questions that required the parent to recall and summarize across various contexts for amount and frequency of exercise and TV watching. We also did not have multiple respondents available to increase validity of these reports. Food diaries are difficult for both children and parents. We attempted to standardize the interview and data collection process as much as possible. Further, MLS recruited postpartum in order to enroll a community rather than a clinical sample of cocaine using mothers. It could be argued that self-report during pregnancy is more reliable than postnatal recall. However retrospective report appears to be as valid as prenatal report of cocaine use during pregnancy in terms of predicting outcomes such as birth size [24]. As a subset of the MLS, the current study may not be representative of the original MLS term cohort. However, we found few differences in demographic and prenatal drug use variables and none in newborn growth measures between subjects included in this study and those excluded due to missing the 9-year visit. Further, there was minimal impact on growth measures from birth to 8 years when data from subjects who missed the 9-year visit were included at the previous ages. Our focus on obesity adjusting for other covariates including SGA and early weight gain may have contributed to the failure to observe prenatal insult linked to catch-up growth in cocaine-exposed infants as reported by others. Despite the large sample size of MLS, we were unable to test the interaction of heavy use of cocaine and alcohol due to small cell sizes. Dose-response analyses could have been more informative to clinical practice as well as public policy than any use of these drugs. Further, classification in the alcohol use category had a large range from 8% reporting 1 drink across pregnancy to 15% reporting 1 drink per day across pregnancy.
Finally, we have not measured genetic influences in MLS and epigenetic mechanisms could also explain associations between prenatal cocaine exposure and obesity. Our findings are consistent with a fetal origins model of cocaine as a prenatal stressor affecting placental gene expression, altering metabolic pathways, resulting in rapid postnatal early weight gain and childhood obesity [30]. Others have reported epigenetic mechanisms (DNA methylation), showing that cocaine reduces placental norepinephrine transporter gene expression leading to increased circulating catecholamines and downregulation of a steroid metabolic enzyme [11β-HSD-2] that protects the fetus from exposure to high levels of maternal cortisol [30]. Such exposure could affect the fetal neuroendocrine system and alter metabolic pathways leading to the development of later childhood obesity.
In summary, we have known for a long time that prenatal factors contribute to the origins of childhood obesity. We present the first evidence that prenatal cocaine exposure is one of those factors. But the findings must be understood in the context of alcohol exposure. Most cocaine users are polydrug users of alcohol, tobacco and marijuana as well. Although all these drugs have been associated with deficits in birth weight, height and head circumference, infants exposed to cocaine, tobacco or marijuana will catch-up to non-exposed peers, but alcohol-exposed infants do not catch-up. We find that cocaine exposure quadruples the prevalence of obesity but only for the subset of children who are not also alcohol-exposed. While providing a new direction for research on prenatal cocaine exposure, the challenge is to elucidate the mechanisms and pathways that lead from prenatal cocaine exposure to obesity in the context of alcohol exposure. One direction is to “drill down” and investigate neuroendocrine, metabolic and epigenetic factors, which may have prenatal origins and increased risk for obesity.
Acknowledgments
This study is part of the MLS which was conducted with support from the National Institute on Drug Abuse (NIDA) through cooperative agreements with the National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). Participating institutions, grant awards, investigators and key research personnel include: Warren Alpert Medical School of Brown University, U10-DA-024119, U10-HD-27904, N01-2-3159(Barry M. Lester, PhD., Cynthia Miller-Loncar PhD, Linda LaGasse, PhD., and Jean Twomey, PhD); Wayne State University, U10-DA-024117, U10-HD-21385 (Seetha Shankaran, MD, Eunice Woldt, MSN, and Jay Ann Nelson, BSN);University of Tennessee, U10-DA-024118, U10-HD-21415 (Henrietta S. Bada, MD, Toni Whitaker, MD, Charlotte Bursi, MSSW, Leann Pollard BA and Jonathan Rowland, BS; University of Miami, U10-DA-024118, U10-HD-21397 (Charles R. Bauer, MD, Ann L. Graziotti ARNP and Susan Gautier, MS); RTI International, U01-HD-36790 (W. Kenneth Poole, PhD, Abhik Das, PhD, Jane Hammond, PhD, Debra Fleischmann BS); National Institute on Drug Abuse (Nicolette Borek PhD and Vincent L. Smeriglio PhD); National Institute of Child Health and Human Development (Rosemary D. Higgins MD). The MLS is a funded as a cooperative agreement and as such the funding sponsors (NIDA, NICHD, NIMH) have input into the design and conduct of the study.
Abbreviation
- DNA
Deoxyribonucleic acid
- BMI
body mass index
- SGA
Small for gestational age
- 11β-HSD-2
11 beta hydroxysteroid dehydrogenase-type 2
Footnotes
Conflict of interest statement
The authors declare there are no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Healthy Weight-It’s not a diet, it’s a lifestyle. Centers for Disease Control and Prevention(CDC); 2008. [Accessed Dec 18, 2008.]. About BMI for Adults. http://www.cdc.gov/nccdphp/dnpa/healthyweight/assessing/bmi/adult_BMI/about_adult_BMI.htm. Updated Jun 20 2008. [Google Scholar]
- 2.American Academy of Pediatrics. Children, adolescents, and television. Pediatrics. 2001;107:423–426. doi: 10.1542/peds.107.2.423. [DOI] [PubMed] [Google Scholar]
- 3.Physical Activities Guidelines for Americans At-A-Glance: A Fact Sheet for Professionals. Office of Disease Prevention & Health Promotion, U.S. Department of Health and Human Services; 2008. [Accessed Jun 02 2010.]. http://www.ealth.gov/paguidelines/factsheetprof.aspx. Updated Aug 21, 2009. [Google Scholar]
- 4.Agras WS, Mascola AJ. Risk factors for childhood overweight. Curr Opin Pediatr. 2005;17:648–652. doi: 10.1097/01.mop.0000172818.87261.ab. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 6.Bada HS, Das A, Bauer CR, Shankaran S, Lester B, Wright LL, Verter J, Smeriglio VL, Finnegan LP, Maza PL. Gestational cocaine exposure and intrauterine growth: Maternal Lifestyle Study. Obstet Gynecol. 2002;100:916–924. doi: 10.1016/s0029-7844(02)02199-3. [DOI] [PubMed] [Google Scholar]
- 7.Barat P, Gayard-Cros M, Andrew R, Corcuff JB, Jouret B, Barthe N, Perez P, Germain C, Tauber M, Walker BR, et al. Truncal distribution of fat mass, metabolic profile and hypothalamic-pituitary adrenal axis activity in prepubertal obese children. J Pediatr. 2007;150:535–539. 539, e531. doi: 10.1016/j.jpeds.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Bauer CR, Langer JC, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, Smeriglio VL, Finnegan LP, Maza PL, et al. Acute neonatal effects of cocaine exposure during pregnancy. Arch Pediatr Adolesc Med. 2005;159:824–834. doi: 10.1001/archpedi.159.9.824. [DOI] [PubMed] [Google Scholar]
- 9.Bauer CR, Shankaran S, Bada HS, Lester B, Wright LL, Krause-Steinrauf H, Smeriglio VL, Finnegan LP, Maza PL, Verter J. The Maternal Lifestyle Study: drug exposure during pregnancy and short-term maternal outcomes. Am J Obstet Gynecol. 2002;186:487–495. doi: 10.1067/mob.2002.121073. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty S, Joseph DV, Bankart MJ, Petersen SA, Wailoo MP. Fetal growth restriction: relation to growth and obesity at the age of 9 years. Arch Dis Child Fetal Neonatal Ed. 2007;92:F479–483. doi: 10.1136/adc.2006.109728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment. 2002;9:145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- 12.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr. 2008;152:165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Covington CY, Nordstrom-Klee B, Ager J, Sokol R, Delaney-Black V. Birth to age 7 growth of children prenatally exposed to drugs: A prospective cohort study. Neurotoxicol Teratol. 2002;24:489–496. doi: 10.1016/s0892-0362(02)00233-7. [DOI] [PubMed] [Google Scholar]
- 14.Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes. 2006;30:610–617. doi: 10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- 15.ElSohly MA, Stanford DF, Murphy TP, Lester BM, Wright LL, Smeriglio VL, Verter J, Bauer CR, Shankaran S, Bada HS, et al. Immunoassay and GC-MS procedures for the analysis of drugs of abuse in meconium. J Anal Toxicol. 1999;23:436–445. doi: 10.1093/jat/23.6.436. [DOI] [PubMed] [Google Scholar]
- 16.Falkner B, Sherif K, Michel S, Kushner H. Dietary nutrients and blood pressure in urban minority adolescents at risk for hypertension. Arch Pediatr Adolesc Med. 2000;154:918–922. doi: 10.1001/archpedi.154.9.918. [DOI] [PubMed] [Google Scholar]
- 17.Fried PA, James DS, Watkinson B. Growth and pubertal milestones during adolescence in offspring prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2001;23:431–436. doi: 10.1016/s0892-0362(01)00161-1. [DOI] [PubMed] [Google Scholar]
- 18.Gardner DSL, Hosking J, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ. Contribution of early weight gain to childhood overweight and metabolic health: A longitudinal study (Early Bird 36) Pediatrics. 2009;123:e67–73. doi: 10.1542/peds.2008-1292. [DOI] [PubMed] [Google Scholar]
- 19.Hediger ML, Overpeck MD, McGlynn A, Kuczmarski RJ, Maurer KR, Davis WW. Growth and fatness at three to six years of age of children born small- or large-for-gestational age. Pediatrics. 1999;104:e33. doi: 10.1542/peds.104.3.e33. [DOI] [PubMed] [Google Scholar]
- 20.Hill SY, Shen S, Locke Wellman J, Rickin E, Lowers L. Offspring from families at high risk for alcohol dependence: increased body mass index in association with prenatal exposure to cigarettes but not alcohol. Psychiatry Res. 2005;135:203–216. doi: 10.1016/j.psychres.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Himes JH, Obarzanek E, Baranowski T, Wilson DM, Rochon J, McClanahan BS. Early sexual maturation, body composition, and obesity in African-American girls. Obes Res. 2004;12(Suppl):64S–72S. doi: 10.1038/oby.2004.270. [DOI] [PubMed] [Google Scholar]
- 22.Hui LL, Schooling CM, Leung SS, Mak KH, Ho LM, Lam TH, Leung GM. Birth weight, infant growth, and childhood body mass index: Hong Kong’s children of 1997 birth cohort. Arch Pediatr Adolesc Med. 2008;162:212–218. doi: 10.1001/archpediatrics.2007.62. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson JL, Jacobson SW, Sokol RJ. Effects of prenatal exposure to alcohol, smoking, and illicit drugs on postpartum somatic growth. Alcohol Clin Exp Res. 1994;18:317–323. doi: 10.1111/j.1530-0277.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM. New evidence for neurobehavioral effects of in utero cocaine exposure. J Pediatr. 1996;129:581–590. doi: 10.1016/s0022-3476(96)70124-5. [DOI] [PubMed] [Google Scholar]
- 26.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States, Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 27.LaGasse LL, Seifer R, Wright LL, Lester BM, Tronick EZ, Bauer CR, Shankaran S, Bada HS, Smeriglio V. The Maternal Lifestyle Study (MLS): The caretaking environment of infants exposed to cocaine/opiates. Pediatr Res. 1999;45(No 4 part 2):247A. [Google Scholar]
- 28.Leary SD, Smith GD, Rogers IS, Reilly JJ, Wells JC, Ness AR. Smoking during pregnancy and offspring fat and lean mass in childhood. Obesity (Silver Spring) 2006;14:2284–2293. doi: 10.1038/oby.2006.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lester BM, ElSohly M, Wright LL, Smeriglio VL, Verter J, Bauer CR, Shankaran S, Bada HS, Walls HH, Huestis MA, et al. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 30.Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Dev Neurosci. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- 31.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J, et al. The Maternal Lifestyle Study: Effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110:1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 32.Li C, Goran MI, Kaur H, Nollen N, Ahluwalia JS. Developmental trajectories of overweight during childhood: role of early life factors. Obesity (Silver Spring) 2007;15:760–771. doi: 10.1038/oby.2007.585. [DOI] [PubMed] [Google Scholar]
- 33.Lumeng JC, Cabral HJ, Gannon K, Heeren T, Frank DA. Pre-natal exposures to cocaine and alcohol and physical growth patterns to age 8 years. Neurotoxicol Teratol. 2007;29:446–457. doi: 10.1016/j.ntt.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messiah SE, Arheart KL, Luke B, Lipshultz SE, Miller TL. Relationship between body mass index and metabolic syndrome risk factors among US 8- to 14-year-olds, 1999 to 2002. J Pediatr. 2008;153:215–221. doi: 10.1016/j.jpeds.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Minnes S, Robin NH, Alt AA, Kirchner HL, Satayathum S, Salbert BA, Ellison L, Singer LT. Dysmorphic and anthropometric outcomes in 6-year-old prenatally cocaine-exposed children. Neurotoxicol Teratol. 2006;28:28–38. doi: 10.1016/j.ntt.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newby PK. Are dietary intakes and eating behaviors related to childhood obesity? A comprehensive review of the evidence. J Law Med Ethics. 2007;35:35–60. doi: 10.1111/j.1748-720X.2007.00112.x. [DOI] [PubMed] [Google Scholar]
- 37.Nordstrom-Klee B, Delaney-Black V, Covington C, Ager J, Sokol R. Growth from birth onwards of children prenatally exposed to drugs: A literature review. Neurotoxicol Teratol. 2002;24:481–488. doi: 10.1016/s0892-0362(02)00232-5. [DOI] [PubMed] [Google Scholar]
- 38.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 39.Ogden CL, Carroll MD, Flegal KM. Epidemiologic trends in overweight and obesity. Endocrinol Metab Clin North Am. 2003;32:741–760. doi: 10.1016/s0889-8529(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 40.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 41.Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, Kelnar CJ. Health consequences of obesity. Arch Dis Child. 2003;88:748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson GA, Goldschmidt L, Larkby C. Effects of Prenatal Cocaine Exposure on Growth: A Longitudinal Analysis. Pediatrics. 2007;120:e1017–1027. doi: 10.1542/peds.2006-3482. [DOI] [PubMed] [Google Scholar]
- 43.Salsberry PJ, Reagan PB. Dynamics of early childhood overweight. Pediatrics. 2005;116:1329–1338. doi: 10.1542/peds.2004-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychol Meth. 2002;7:147–177. [PubMed] [Google Scholar]
- 45.Schiattino I, Villegas R, Cruzat A, Cuenca J, Salazar L, Aravena O, Pesce B, Catalan D, Llanos C, Cuchacovich M, et al. Multiple imputation procedures allow the rescue of missing data: an application to determine serum tumor necrosis factor (TNF) concentration values during the treatment of rheumatoid arthritis patients with anti-TNF therapy. Biol Res. 2005;38:7–12. doi: 10.4067/s0716-97602005000100002. [DOI] [PubMed] [Google Scholar]
- 46.Shankaran S, Bann C, Bauer CR, Lester B, Bada H, Das A, Higgins R, Poole K, LaGasse L, Hammond J, et al. Prenatal cocaine exposure and body mass index and blood pressure at 9 years of age. J Hypertens. in press. [PMC free article] [PubMed] [Google Scholar]
- 47.Shankaran S, Das A, Bauer CR, Bada H, Lester B, Wright L, Higgins R, Poole K. Fetal origin of childhood disease: intrauterine growth restriction in term infants and risk for hypertension at 6 years of age. Arch Pediatr Adolesc Med. 2006;160:977–981. doi: 10.1001/archpedi.160.9.977. [DOI] [PubMed] [Google Scholar]
- 48.Simmons R. Perinatal programming of obesity. Semin Perinatol. 2008;32:371–374. doi: 10.1053/j.semperi.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh G, Kogan M, van Dyck P. A Multilevel Analysis of State and Regional Disparities in Childhood and Adolescent Obesity in the United States. J Community Health. 2008;33:90–102. doi: 10.1007/s10900-007-9071-7. [DOI] [PubMed] [Google Scholar]
- 50.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194–199. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 51.Strong WB, Malina RM, Blimkie CJ, Daniels SR, Dishman RK, Gutin B, Hergenroeder AC, Must A, Nixon PA, Pivarnik JM, et al. Evidence based physical activity for school-age youth. J Pediatr. 2005;146:732–737. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 52.Tang L, Song J, Belin TR, Unutzer J. A comparison of imputation methods in a longitudinal randomized clinical trial. Stat Med. 2005;24:2111–2128. doi: 10.1002/sim.2099. [DOI] [PubMed] [Google Scholar]
- 53.Tu YK, West R, Ellison GT, Gilthorpe MS. Why evidence for the fetal origins of adult disease might be a statistical artifact: the “reversal paradox” for the relation between birth weight and blood pressure in later life. Am J Epidemiol. 2005;161:27–32. doi: 10.1093/aje/kwi002. [DOI] [PubMed] [Google Scholar]
- 54.Vandewater EA, Shim MS, Caplovitz AG. Linking obesity and activity level with children’s television and video game use. J Adolesc. 2004;27:71–85. doi: 10.1016/j.adolescence.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Zhang Q. Are American children and adolescents of low socioeconomic status at increased risk of obesity? Changes in the association between overweight and family income between 1971 and 2002. Am J Clin Nutr. 2006;84:707–716. doi: 10.1093/ajcn/84.4.707. [DOI] [PubMed] [Google Scholar]
- 57.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]