Abstract
Background
CHRNA5-CHRNA3-CHRNB4 and TTC12-ANKK1-DRD2 gene-clusters influence smoking behavior. Our aim was to test developmental changes in their effects as well as the interplays between them and with non-genetic factors.
Methods
Participants included 4,762 subjects from a general population based prospective Northern Finland 1966 Birth Cohort (NFBC 1966). Smoking behavior was collected at age 14 and 31 years(y). Information on maternal smoking, socio-economic status, and novelty seeking were also collected. Structural equation modeling was used to construct an integrative etiological model including genetic and non-genetic factors.
Results
Several SNPs in both gene-clusters were significantly associated with smoking. The most significant were in CHRNA3 (rs1051730, P=1.1×10−5) and in TTC12 (rs10502172, P=9.1×10−6). CHRNA3-rs1051730[A] was more common among heavy/regular smokers than non-smokers with similar effect-sizes at age 14y [OR(95%CI):1.27(1.06–1.52)] and 31y [1.28(1.13–1.44)]. TTC12-rs10502172[G] was more common among smokers than non-smokers with stronger association at 14y [1.33(1.11–1.60)] than 31y [1.14(1.02–1.28)]. In adolescence, carriers of three-four risk alleles at either CHRNA3-rs1051730 or TTC12-rs10502172 had almost 3-fold odds of smoking regularly than subjects with no risk alleles. TTC12-rs10502172 effect on smoking in adulthood was mediated by its effect on smoking in adolescence and via novelty seeking. Effect of CHRNA3-rs1051730 on smoking in adulthood was direct.
Conclusions
TTC12-ANKK1-DRD2s seemed to influence smoking behavior mainly in adolescence and its effect is partially mediated by personality characteristics promoting drug-seeking behavior. In contrast, CHRNA5-CHRNA3-CHRNB4 is involved in the transition towards heavy smoking in mid-adulthood and in smoking persistence. Factors related to familial and social disadvantages were strong independent predictors of smoking.
Keywords: TTC12, ANKK1, DRD2, CHRNA5, CHRNA3, CHRNB4, nicotine, gene, adolescence, smoking
INTRODUCTION
Tobacco smoking is the largest avoidable cause of morbidity and mortality in developed countries. Genetic factors influence risk of initiating and persisting in smoking(1, 2) as well as the susceptibility to develop tobacco-related diseases(3–5).
Although a large number of genetic loci potentially moderating smoking have been tested, only a few have been convincingly replicated. So far, the strongest evidence of association is for CHRNA5-CHRNA3-CHRNB4 gene-cluster on chromosome 15q25(3, 6–10). Another chromosome region that has been linked with nicotine use is the TTC12-ANKK1-DRD2 gene-cluster on chromosome 11q23(11–13), although evidence here is less consistent.
The CHRNA5-CHRNA3-CHRNB4 gene-cluster encodes for the nicotinic acetylcholine receptor subunits alpha five, three, and beta four. In this region, single nucleotide polymorphisms (SNPs) repeatedly associated with smoking include a non-synonymous (aspartic acid [D] to asparagine [N]; rs16969968) SNP at codon 398 of CHRNA5 and a synonymous SNP (rs1051730) in CHRNA3. The asparagine allele at CHRNA5-rs16969968 has been associated with nicotine dependence/heavy smoking(7, 8), pleasurable response to smoking(9), and decreased response to nicotine agonists in vitro(14). The CHRNA3-rs1051730[A] has been associated with nicotine dependence(3, 15), smoking quantity(3), and increased susceptibility to develop lung cancer and vascular disease among smokers(3–5). According to a recent meta-analysis, each copy of the CHRNA3-rs1051730[A] allele accounts only for ~0.5% of the variance in number of cigarettes smoked/day. Nevertheless the overall P value for this large meta-analysis including >74,000 participants was ~10−73, making this finding one of the most consistent across the entire panorama of genetic-association studies on human behavior(16). Of note, CHRNA3-rs1051730[A] is more common in heavy-smokers (≥10cigarette/day) than non-smokers. In contrast, frequency of this allele appears to be reduced, rather than increased, among light-smokers than non-smokers (3, 17).
The dopamine receptor 2 (DRD2), the ankyrin repeat and kinase domain containing 1 (ANKK1), and tetratricopeptide repeat domain 12 (TTC12) gene-cluster on chromosome 11q23 has been linked to nicotine(13, 18–20), alcohol(21), and opiate(22) dependencies indicating that genetic variation within this region might contribute to the shared liability between different types of addictions. DRD2 has a central role in modulating the dopamine reward system that mediates the reinforcing effect of all known addictive substances(23). In addition, polymorphisms within this region might influence personality characteristics such as novelty seeking(24) that are associated with increased vulnerability to different types of addictions(25). For DRD2, the majority of studies have focused on a polymorphism known as Taq1A (rs1800497), which has been eventually shown to map in the neighbouring ANKK1. Studies testing association between Taq1A and smoking have reported positive(26, 27) as well as negative results(28, 29).
Up to date, most of the genetic studies on smoking have focused on adulthood and little is known about genetic factors influencing smoking in adolescence, which is a critical period for smoking initiation(30, 31). Aproximately 80–90% of adult-smokers initiate use before age 18 years(31) and ~40% before age 14(32). Early initiation (before age 16 years) of tobacco use is associated with increased risk of developing nicotine(33, 34) and other drug dependencies(35–37). Genetic influences moderating smoking behaviour are likely to vary both quantitatively and qualitatively during the lifespan(38) and most of the studies performed so far have not tested for developmental changes in gene effects. Finally, although several whole genome association studies on smoking have been performed on very large study samples, these studies have not taken into account gene-gene and gene-environment interplays. In the present study, we tested the independent and combined effects of common polymorphisms within CHRNA5-CHRNA3-CHRNB4 and TTC12-ANKK1-DRD2 gene-clusters on smoking behaviour during adolescence and mid-adulthood in a prospective general population based cohort including 4,762 Finns (Northern Finland 1966 Birth Cohort, NFBC 1966). We tested whether the genetic effects of these two loci on smoking changed moving from adolescence to mid-adulthood. Finally, we tested the joint effects of the most significantly associated loci within these two chromosome regions, namely CHRNA3-rs1051730 and TTC12-rs10502172, and other factors that have been previously associated with smoking in the NFBC 1966, such as maternal smoking(39), novelty seeking(40), and socio-economic status(41–43), and we attempted to construct an integrative, albeit incomplete, etiological model testing specific life-course pathways leading to smoking behavior.
METHODS
Participants
We studied a subset of 4,762 individuals with phenotypic/genotypic data available from the general population-based Northern Finland 1966 Birth Cohort (NFBC 1966) (for more details see(44) and Supplement 1). Briefly, 12,068 pregnant women with expected dates of delivery in 1966 were recruited from the provinces of Oulu and Lapland. Their 12,058 live-born children, 96.3% of all eligible births, were prospectively followed until the age of 31 years. Phenotypic data were collected mainly during pregnancy/birth in 1965–67, at follow-up in 1980 (age 14), and in 1997–8 (age 31).
Smoking-related variables
At age 14, participants reported via a postal questionnaire if they smoked and how much using eight options: ‘Never’; ‘I have tried once’; ‘I have tried twice or more’; ‘I smoke occasionally’; ‘I smoke about twice a week’; ‘I smoke between 1–5 cigarettes a day’; ‘I smoke between 6–10 cigarettes a day’; ‘I smoke more than 10 cigarettes a day’. As before(41), adolescents who never smoked or had smoked once/twice in their lives were classified as non-smokers; subjects reporting they had been smoking occasionally or about twice per week as occasional-smokers; and the rest as regular-smokers.
At age 31 participants reported via postal questionnaire if they had ever smoked, and the number of cigarettes/day they were currently smoking or used to smoke if they had quitted during the past year. Participants were classified as: non-smokers; light-smokers (1–10 cigarettes/day); and heavy-smokers (>10 cigarettes/day).
Although smoking at 14 and 31 years were collected using different questions, there was a stepwise association between the degrees of smoking severity at the two time points. Occasional-smokers at age 14, as compared to non-smokers, were almost four-times more likely to become heavy-smokers at 31 years [OR (95%CI): 4.01 (3.17–5.08), Wald-Chi-square=78.2, df=1, p<0.0001]. Regular-smokers at age 14, as compared to non-smokers, were almost ten-times more likely to become heavy-smokers at 31 years [OR (95%CI): 9.86 (7.12–13.66), Wald-Chi-square=189.3, df=1, p<0.0001]
Participants were classified based on change/persistence of their smoking habits from 14 to 31 years as(41):
Persistent-smokers (regular-smokers at age 14; heavy-smokers at age 31);
Late onset-smokers (non-smokers at 14; heavy-smokers at age 31);
Ex-smokers (regular-smokers at age 14; non-smokers at age 31);
Non-smokers/infrequent-smokers (non-smokers or occasional/light smokers at both age 14 and 31).
Participants’ mothers’ smoking habits were assessed during pregnancy(39). Mothers were classified based on the number of cigarettes they were smoking during the 2nd month of pregnancy as: non-smokers; light-smokers (1–10 cigarettes/day); and heavy-smokers (>10 cigarettes/day). Mothers were also asked if and how much they were smoking during the year preceding pregnancy (Supplement 1).
Novelty seeking
Novelty seeking was assessed at age 31 using the Temperament and Character Inventory questionnaire(45).
Socio-demographic factors
Family socio-economic status, based on father’s occupation, was collected during pregnancy and at age 14 years and was classified as: professionals; skilled-workers; unskilled-workers; and farmers(46).
Cohort members’ socio-economic status based on occupation was collected at 31 years and classified as: professionals; skilled-workers; unskilled-workers; farmers; and others (students, pensioners, unemployed, and unknown).
Mothers’ marital status during pregnancy was dichotomised into married versus unmarried (singles, divorced or widows).
Genotyping
Genome-wide genotyping was performed at the Broad Institute Biological Sample Repository in ~5,000 participants with DNA available using the Illumina Infinium 370cnvDuo array(47). 4,762 subjects passed quality control. All the SNPs mapping in the two candidate chromosome regions and passing quality control(33) were used for the current analyses.
Distributions of the different variables in the study sample and in participants who were excluded because DNA was unavailable are shown in Table S1 (see Supplement 1). The proportions of smoking and unmarried mothers and of unskilled-worker families at birth/14 years were greater in non-participants. Females were more eager to participate than males. No differences were found for smoking at 14/31 years, novelty seeking, and socio-economic status at 31 years (Table S1 in Supplement 1).
Statistical analyses
Primary association analyses were performed between all SNPs in the two chromosome regions and smoking behavior using logistic regression run in PLINK v1.06. Smoking at age 14/31 were dichotomized to increase statistical power and entered as dependent variables. Previous studies(3, 7, 17) have consistently shown an association between genetic variation within 15q25 and heavy-smoking/dependence, but not with light-smoking(3, 17, 48). Therefore, for CHRNA5-CHRNA3-CHRNB4, smoking behavior was dichotomized as: 1) Smoking at 14: regular-smokers Vs. occasional + non-smokers 2) Smoking at 31: heavy-smokers Vs. light-smokers + non-smokers (i.e. in both time points we compared heavier smokers with the rest of the sample). In contrast, for the TTC12-ANKK1-DRD2 gene-cluster, smoking behavior was dichotomized as smokers Vs. non-smokers, because we hypothesized that genetic variation at this site might be relevant for smoking use regardless of smoking severity.
The most statistically significant SNPs, namely CHRNA3-rs1051730 and TTC12-rs10502172, were selected for further analyses. Main effects of CHRNA3-rs1051730 and TTC12-rs10502172 on smoking behaviours were tested using multinomial-logistic regression run in SAS v9.2. Smoking at 14 and 31 years were coded using three categories (age 14: regular/occasional/non-smokers; age 31: heavy/light/non-smokers) with non-smokers being the reference category.
The combined effect of TTC12-rs10502172 and CHRNA3-rs1051730 on smoking was tested under a multiplicative as well as an additive model by using regression analyses. For the additive model, the cumulative number of risk alleles at either TTC12-rs10502172 or CHRNA3-rs1051730 was used as a predictor.
Linear regression was used to test the two SNPs’ effects on novelty seeking.
In all the above-described analyses covariates entered in the regression models included: sex, maternal smoking, socio-economic status at 14/31 years and principal components computed from the genome-wide data to control for population stratification(47, 49) (Supplement 1).
Haplotype-structures of the two regions were computed with Haploviewv3(50) and haplotype-association analyses were performed using χ2-test in PLINK (Supplement 1).
At age 31 years, the inclusion among non-smokers of participants who were not current-smokers but used to smoke in the past might have potentially confounded our results. Analyses performed excluding these participants left results substantially unchanged (Tables S7-S9 in Supplement 1).
Structural equation modeling (SEM) was used to construct an integrative model including both genetic and non-genetic risk factors. Probit regression was fitted using the robust weighted least squares estimation. To increase statistical power, all smoking-related variables were dichotomized. To keep for multiple testing at a minimum, only variables with significant effects on smoking at either age 14 or 31 were entered into the model. Maternal smoking and smoking at 14 years were coded as smokers Vs. non-smokers (due to the relative low number of heavy/regular-smokers in these two groups); smoking at 31 years was coded as heavy-smokers vs. light+non-smokers (to allow the detection of CHRNA3-rs1051730 effect on smoking). Effect sizes of the predictors on outcome variables are expressed as un-standardized and standardized beta estimates. The beta coefficients are interpreted in z-score metrics since the probit analysis is based on the cumulative normal probability distribution. The standardized beta coefficients are used to compare the relative importance of the different predictors as they describe the change in standard deviation (SD) units of the outcome variable per each SD change in a continuous predictor and per change from 0 to 1 for a binary predictor. For binary outcomes, a latent underlying continuous variable is assumed. Total effects of predictors on outcomes were computed by adding indirect and direct effects together. The overall model fit was evaluated in terms of the comparative fit index(CFI) and root mean square error of approximation(RMSEA). A CFI>0.95(51) and RMSEA<0.05(52) were used as indicative of good fit. SEM was performed using Mplus v.3.12(53)
RESULTS
Several SNPs in both chromosome regions were significantly associated with smoking (Figure-1, Tables S2 and S3 in Supplement 1).
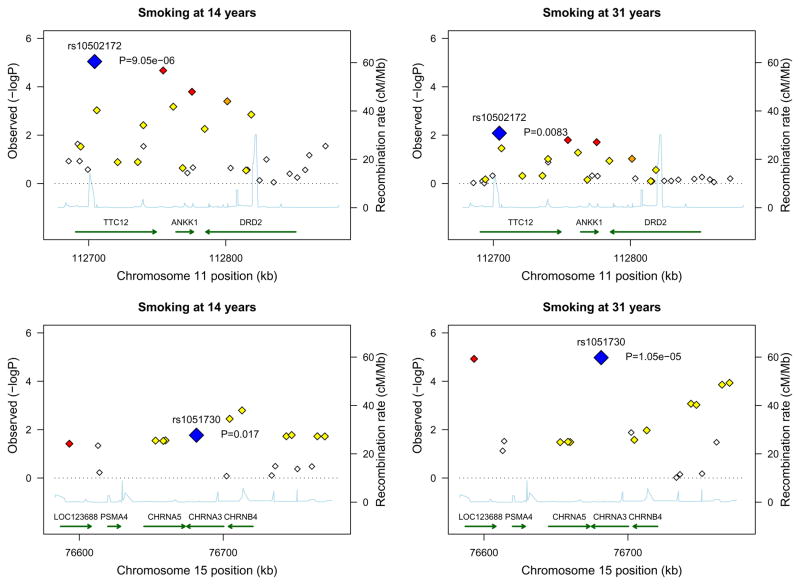
Figure 1.
Association between smoking behavior during adolescence and adulthood and genetic variation in the two candidate chromosome regions. A and B: the 11q23 region; C and D: the 15q25 region. Blue diamond indicates the most significantly associated SNP. For other SNPs, diamonds are colored in a white-to-red scale corresponding to r2 values from 0 to 1 with either rs1051730 or rs10502172. SNP position refers to NCBI build 35. Estimated recombination rates are from Hapmap and gene annotations from UCSC genome browser using build 35 coordinates.
For CHRNA5-CHRNA3-CHRNB4, the most significant SNP was rs1051730 (P=1.1×10−5) mapping in CHRNA3 (Figure-1, Table S2 in Supplement 1). Carriers of rs1051730[A] were at increased risk of being heavy/regular smokers than non-carriers with similar effect-size at age 14 [OR(95%CI):1.27(1.06–1.52)] and 31 years [OR(95%CI):1.28(1.13–1.44)]. As expected(3, 48), carriers of rs1051730[A] were not at increased risk of being light/occasional smokers compared with non-carriers (Table 1)(3).
Table 1.
Association between TTC12-rs10502172, CHRNA3-rs1051730, maternal smoking, socio-economic status, and gender with smoking behavior at 14 and 31 years. Analyses are computed by using multinomial logistic regression. Non-smokers are the reference category both at age 14 and 31 years. Effect sizes for each variable are adjusted for the effect of all other variables listed in the table and for principal components (PCs) to correct for population stratification. Genetic effect is estimated assuming an additive model of number of risk alleles for each locus.
| Smoking at 14 yrs |
Smoking at 31 yrs |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Occasional-smokers (N=491) vs. non-smoker (N= 3,787) |
Regular-smokers (N= 279) vs. non-smokers (N= 3,787) |
Light-smokers (N=947) vs. non-smokers (N=2,589) |
Heavy-smokers (N=932) vs. non-smokers (N=2,589) |
|||||||||
| OR | (95% CI) | P Value | OR | (95% CI) | P Value | OR | (95% CI) | P Value | OR | (95% CI) | P Value | |
| TTC12-rs10502172[G] | 1.29 | (1.12–1.48) | <0.001 | 1.33 | (1.11–1.60) | 0.002 | 1.13 | (1.01–1.26) | 0.03 | 1.14 | (1.02–1.28) | 0.02 |
| CHRNA3-rs1051730[A] | 1.06 | (0.91–1.22) | 0.45 | 1.27 | (1.06–1.52) | 0.01 | 0.95 | (0.84–1.06) | 0.35 | 1.28 | (1.13–1.44) | <0.001 |
| Gender | ||||||||||||
| Female (N=2,476) | 1 | 1 | 1 | 1 | ||||||||
| Male (N=2,286) | 1.01 | (0.84–1.23) | 0.89 | 0.82 | (0.64–1.06) | 0.12 | 1.01 | (0.86–1.19) | 0.89 | 3.16 | (2.65–3.77) | <0.001 |
| Maternal smoking | ||||||||||||
| Non-smoker (N=4,000) | 1 | 1 | 1 | 1 | ||||||||
| Light-smokers (N=554) | 1.25 | (0.94–1.66) | 0.12 | 2.04 | (1.49–2.79) | <0.001 | 1.26 | (1.00–1.60) | 0.05 | 1.74 | (1.38–2.19) | <0.001 |
| Heavy-snokers (N=96) | 0.92 | (0.44–1.94) | 0.83 | 1.84 | (0.90–3.76) | 0.10 | 0.96 | (0.55–1.69) | 0.89 | 1.54 | (0.91–2.59) | 0.11 |
| Socio-economic statusa | ||||||||||||
| Professionals (N=1,341 at 14 yrs; N=1,098 at 31 yrs) | 1 | 1 | 1 | 1 | ||||||||
| Skilled-workers (N=1,573 at 14 yrs; N=1,481 at 31 yrs) | 1.17 | (0.92–1.49) | 0.20 | 1.57 | (1.12–2.19) | 0.01 | 1.56 | (1.26–1.94) | <0.001 | 1.59 | (1.24–2.04) | <0.001 |
| Unskilled-workers (N=987 at 14 yrs; N=1,215 at 31 yrs) | 1.13 | (0.86–1.49) | 0.38 | 2.31 | (1.63–3.27) | <0.001 | 1.89 | (1.51–2.38) | <0.001 | 3.04 | (2.41–3.82) | <0.001 |
| Farmers (N=674 at 14 yrs; N=164 at 31 yrs) | 0.89 | (0.64–1.22) | 0.46 | 0.88 | (0.54–1.44) | 0.62 | 0.86 | (0.53–1.40) | 0.55 | 0.75 | (0.44–1.29) | 0.29 |
| Others (N=747) | NA | NA | 1.68 | (1.30–2.17) | <0.001 | 2.31 | (1.76–3.02) | <0.001 | ||||
Abbreviations: OR, odds ratio; CI, confidence interval; NA: not applicable
Family socio-economic status measured at 14 was used to predict smoking at 14 years and socio-economic status measured at 31 was used to predict smoking at 31.
For TTC12-ANKK1-DRD2, the most significantly associated SNP was rs10502172 (P=9.1×10−6) mapping in TTC12. Also ANKK1-rs2734849 and DRD2-rs1076563 were significantly associated (Table S3 in Supplement 1). In adolescence, TTC12-rs10502172[G] conferred increased risk of both smoking regularly [OR(95%CI):1.33(1.11–1.60)] and occasionally [OR(95%CI):1.29(1.12–1.48)]. TTC12-rs10502172[G] effect was weaker at age 31 [ORs~1.13–1.14] than 14, but still significant (Table 1).
Patterns of association observed at age 14 were similar to patterns observed at 31 and very different for the two loci. For CHRNA3-rs1051730[A], at both time points, association was driven by the category including the heaviest smokers. In contrast, TTC12-rs10502172[G] is equally overrepresented in low- and high-quantity smokers than non-smokers at both time points (see Table 1).
Of note, the most significant associations survived Bonferroni correction (Bonferroni-corrected level of significance for 49 SNPs is 0.001).
Haplotype analysis
For chromosome 15q25 we identified two haplotype-blocks (Figure S2 in Supplement 1). Significant differences in haplotype frequencies between heavy-smokers and light/non-smokers were found in both blocks mainly at age 31 (Table 2).
Table 2.
Frequencies of Haplotypes Within 15q25. High-quantity smokers are compared to Low-quantity/non-smokers in Adolescence and Mid-adulthood (χ2-test).*
| Smoking at 14 years | Smoking at 31 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Haplotype | df | Hap frequency Regular-smokers (N=279) | Hap frequency Occasional+non-smokers (N=4278) | χ2 | p | Hap frequency Heavy-smokers (N=932) | Hap frequency Light+non-smokers (N=3,536) | χ2 | p | |
| Block 1a | Global test | 8 | NA | NA | 14.44 | 0.07 | NA | NA | 21.39 | 0.006 |
| GAAAGAAGGA | 1 | 0.28 | 0.26 | 1.44 | 0.23 | 0.29 | 0.25 | 11.73 | 0.0006 | |
| AAAGAGGGGA | 1 | 0.05 | 0.03 | 3.24 | 0.07 | 0.04 | 0.04 | 0.90 | 0.34 | |
| AAAGAGGGAG | 1 | 0.25 | 0.31 | 8.18 | 0.004 | 0.29 | 0.31 | 3.49 | 0.06 | |
| AAGAGAGAGA | 1 | 0.25 | 0.26 | 0.28 | 0.59 | 0.24 | 0.27 | 5.28 | 0.02 | |
| AAAAGAGAGA | 1 | 0.02 | 0.02 | 1.16 | 0.28 | 0.01 | 0.02 | 0.88 | 0.35 | |
| AAAGAGGGGG | 1 | 0.01 | 0.01 | 0.00 | 0.96 | 0.01 | 0.01 | 1.96 | 0.16 | |
| GGAAGAAGGA | 1 | 0.08 | 0.06 | 2.95 | 0.08 | 0.07 | 0.06 | 1.80 | 0.18 | |
| AAAGAGGGAA | 1 | 0.03 | 0.03 | 0.31 | 0.58 | 0.03 | 0.03 | 0.00 | 0.97 | |
| AAGAGAGGGA | 1 | 0.02 | 0.02 | 0.16 | 0.69 | 0.02 | 0.02 | 1.03 | 0.31 | |
| Block 2b | Global test | 6 | NA | NA | 12.20 | 0.06 | NA | NA | 23.85 | 0.0006 |
| GAGCGGAA | 1 | 0.38 | 0.33 | 4.55 | 0.03 | 0.37 | 0.33 | 10.90 | 0.001 | |
| GAAAGAGG | 1 | 0.26 | 0.28 | 0.98 | 0.32 | 0.25 | 0.28 | 4.56 | 0.03 | |
| GGAAAGGG | 1 | 0.12 | 0.14 | 1.79 | 0.18 | 0.14 | 0.15 | 0.10 | 0.76 | |
| AAAAAGGG | 1 | 0.16 | 0.14 | 1.64 | 0.20 | 0.15 | 0.14 | 1.62 | 0.20 | |
| GAAAAGGG | 1 | 0.01 | 0.14 | 0.95 | 0.03 | 0.01 | 0.03 | 9.27 | 0.002 | |
| GAGCGGGG | 1 | 0.02 | 0.02 | 0.00 | 0.97 | 0.01 | 0.02 | 0.55 | 0.16 | |
| AAAAGGGG | 1 | 0.06 | 0.07 | 0.73 | 0.39 | 0.07 | 0.07 | 2.39 | 0.12 | |
SNPs in Block 1: rs8034191, rs3885951, rs2036534, rs6495306, rs680244, rs621849, rs1051730, rs6495309, rs1948, rs950776
SNPs in Block 2: rs12594247, rs12900519, rs1996371, rs6495314, rs8032156, rs8038920, rs4887077, rs11638372
P<0.05 are in bold
For chromosomes 11q23 we identified three haplotype-blocks (Figure S3 in Supplement 1). Significant differences in haplotype frequencies between smokers and non-smokers were found only in Block-2 (Table 3). Haplotype AAGGGAAAAGGGACGGGA appeared to be protective with stronger effect at age 14 than 31. In the same block, haplotype GGGGAGGGAGAGGAGGGG was associated with increased risk of smoking at age 14 but not 31.
Table 3.
Frequencies of Haplotypes Within 11q23. Smokers are compared to non-smokers in adolescence and mid-adulthood (χ2-test) (see text for more details)*
| Haplotype | Smoking at 14 years | Smoking at 31 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | Hap frequencies Occasional+regular-smokers (N=770) | Hap frequencies Non-smokers (N=3,787) | χ2 | p | Hap frequencies Light+heavy-smokers (N=1,879) | Hap frequencies Non-smokers (N=2,589) | χ2 | p | ||
| Block 1a | Global test | 4 | NA | NA | 9.16 | 0.06 | NA | NA | 2.21 | 0.68 |
| AAACC | 1 | 0.17 | 0.19 | 5.32 | 0.02 | 0.19 | 0.19 | 0.02 | 0.88 | |
| AAAAC | 1 | 0.25 | 0.24 | 0.54 | 0.46 | 0.24 | 0.24 | 0.14 | 0.71 | |
| GGGAA | 1 | 0.32 | 0.29 | 5.09 | 0.02 | 0.30 | 0.30 | 0.14 | 0.71 | |
| GAGAA | 1 | 0.19 | 0.20 | 0.32 | 0.57 | 0.20 | 0.20 | 0.11 | 0.74 | |
| AAAAA | 1 | 0.08 | 0.08 | 0.64 | 0.42 | 0.08 | 0.09 | 2.08 | 0.15 | |
| Block 2b | Global test | 9 | NA | NA | 21.96 | 0.009 | NA | NA | 10.47 | 0.31 |
| GGGGAAGGAAAAAAAGGA | 1 | 0.16 | 0.14 | 2.74 | 0.09 | 0.14 | 0.14 | 0.78 | 0.38 | |
| GAAAGAGACGAGGAGAAG | 1 | 0.21 | 0.19 | 2.63 | 0.10 | 0.20 | 0.20 | 0.33 | 0.57 | |
| AAGGGAAAAGGGACGGGA | 1 | 0.42 | 0.48 | 17.78 | 2.8×10-5 | 0.46 | 0.48 | 4.81 | 0.03 | |
| GAGAGAGACGAGGAGAAG | 1 | 0.01 | 0.01 | 0.00 | 0.96 | 0.01 | 0.01 | 0.49 | 0.49 | |
| GGGGAGGGAGAGGAGGGG | 1 | 0.11 | 0.09 | 6.22 | 0.01 | 0.10 | 0.09 | 3.59 | 0.06 | |
| GAGAAAGACGAGGAGAGG | 1 | 0.02 | 0.02 | 0.02 | 0.90 | 0.02 | 0.02 | 0.02 | 0.88 | |
| GGGGAGGGAAAAAAAGGA | 1 | 0.01 | 0.01 | 0.25 | 0.62 | 0.01 | 0.02 | 0.45 | 0.50 | |
| GAAAGAGACGAAAAAGGA | 1 | 0.02 | 0.01 | 0.20 | 0.65 | 0.01 | 0.01 | 0.02 | 0.89 | |
| AAGGGAAAAGGGACGGGG | 1 | 0.02 | 0.01 | 1.97 | 0.16 | 0.01 | 0.01 | 0.14 | 0.29 | |
| GGGGAGGGAGAGGCGGGA | 1 | 0.04 | 0.04 | 0.01 | 0.93 | 0.04 | 0.03 | 1.71 | 0.19 | |
| Block 3c | Global test | 7 | NA | NA | 7.11 | 0.42 | NA | NA | 8.47 | 0.29 |
| AACGAAAA | 1 | 0.17 | 0.16 | 0.84 | 0.36 | 0.16 | 0.16 | 0.07 | 0.80 | |
| AAAAACAG | 1 | 0.44 | 0.45 | 0.52 | 0.47 | 0.44 | 0.45 | 1.03 | 0.31 | |
| GACAACGG | 1 | 0.13 | 0.15 | 3.41 | 0.06 | 0.15 | 0.14 | 0.78 | 0.38 | |
| AACAACAG | 1 | 0.02 | 0.02 | 0.04 | 0.84 | 0.01 | 0.02 | 2.98 | 0.08 | |
| GACAACGA | 1 | 0.03 | 0.03 | 0.04 | 0.85 | 0.02 | 0.03 | 2.65 | 0.10 | |
| AACAACAA | 1 | 0.08 | 0.07 | 0.97 | 0.32 | 0.08 | 0.07 | 0.78 | 0.38 | |
| AACGGAAA | 1 | 0.08 | 0.08 | 0.27 | 0.61 | 0.08 | 0.08 | 0.62 | 0.43 | |
| GGAAACAA | 1 | 0.06 | 0.05 | 2.09 | 0.15 | 0.06 | 0.05 | 0.40 | 0.53 | |
SNPs in Block 1: rs4517559, rs2236709, rs7927508, rs2156486, rs723077
SNPs in Block2: rs10502172, rs2303380, rs4987094, rs2276070, rs719802, rs719804, rs754672, rs877138, rs4590907, rs7118900, rs2734849, rs1800497, rs2242592, rs1076563, rs2471857, rs4620755, rs7125415, rs4648318
SNPs in Block 3: rs4274224,rs4581480, rs7131056,rs4938019, rs12364283, rs10891556, rs6589377, rs10736466
p<0.05 are in bold
None of the haplotypes tested displayed a stronger association signal as compared to rs1051730[A] and rs10502172[G] alone. Therefore, for all the following analyses genetic effects were modeled as rs1051730[A] and rs10502172[G].
Combined effect of TTC12-rs10502172 and CHRNA3-rs1051730
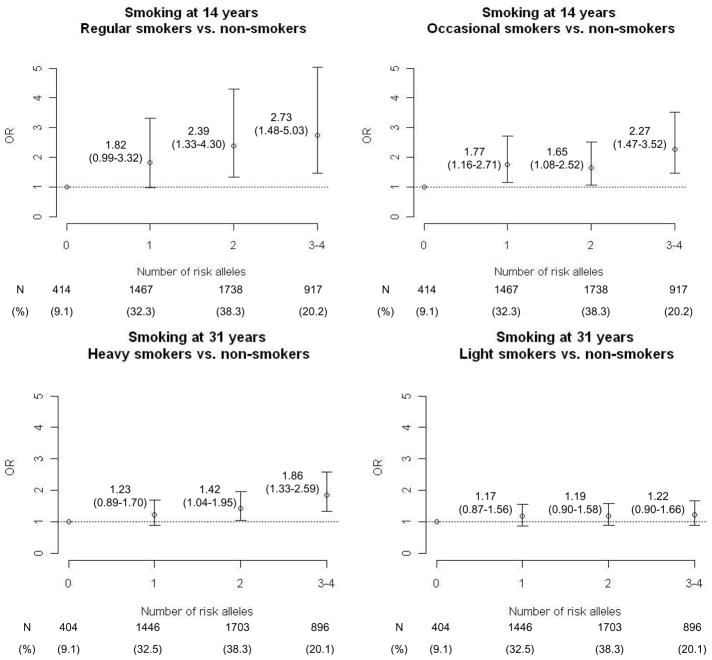
Applying a multiplicative model, we found no significant interaction between TTC12-rs10502172 and CHRNA3-rs1051730 (Age 14: Wald-Chi-square=1.26, df=2, P=0.53; Age 31: Wald-Chi-square=0.81, df=2, P=0.67). In adolescence, the combined effect of these two loci seemed additive (Figure 2). Carriers of three/four risk alleles (20% of the population) had almost 3-fold increased odds of smoking regularly and almost 2.5-fold increased odds of being occasional-smokers as compared to subjects with no risk alleles (9% of the population). Carriers of 1–2 risk alleles had an intermediate risk pattern.
Figure 2.
Combined effect of CHRNA3-rs1051730 and TTC12-rs10502172 on smoking behavior in adolescence and mid-adulthood. Odds ratios (OR) and 95% confidence intervals (CI) are computed using multinomial logistic regression and adjusted for principal components, gender, maternal smoking during pregnancy, and socio-economic status.
Impact of TTC12-rs10502172 and CHRNA3-rs1051730 on change/persistence of smoking habits
Carriers of TTC12-rs10502172[G] had 1.64 increased odds (95%CI: 1.10–2.45) of being ex-smokers than non-carriers. In contrast, carriers of CHRNA3-rs1051730[A] were more likely to be persistent-smokers [OR(95%CI):1.58(1.23–2.04)] and late onset-smokers [OR(95%CI):1.16(1.01–1.34)] than non-carriers (Table 4).
Table 4.
Association between smoking habits at age 14 and 31 years and genotypes at TTC12-rs10502172 and CHRNA3-rs1051730[A]. Anaysis are computed with Multinomial logistic regression, ‘Non-smokers/Infrequent smokers is the reference categories, allelic effect a each locus is modeled as additive (df=1). P<0.05 are in bold. Participants with missing data at either age 31 yrs or 14 yrs have been excluded from this analysis. The analyses were adjusted for maternal smoking at the 2nd month of pregnancy, gender, socio-economic status at 31 years and the principal components that associated with the outcome.
| Persistent-Smokers (N=137) | Late onset-smokers (N=577) | Ex-smokers (N=55) | Non-smoker/Infrequent-smokers (N=3512) | |
|---|---|---|---|---|
| TTC12-rs10502172[G] | ||||
| 0R (95%CI) | 1.24 (0.95–1.60) | 1.05 (0.92–1.21) | 1.64 (1.10–2.45) | 1 |
| χ2 | 2.53 | 0.59 | 5.84 | NA |
| p | 0.11 | 0.44 | 0.02 | NA |
| CHRNA3-rs1051730[A] | ||||
| 0R (95%CI) | 1.58 (1.23 – 2.04) | 1.16 (1.01–1.34) | 1.06 (0.71–1.58) | 1 |
| χ2 | 12.37 | 4.56 | 0.08 | NA |
| p | 0.0004 | 0.03 | 0.78 | NA |
Impact of maternal smoking
Offspring of mothers who were smoking 1–10 cigarettes/day in pregnancy were at increased risk of being regular smokers at age 14 [OR(95%CI):2.04(1.49–2.79)] and heavy-smoker at age 31 [OR(95%CI):1.74(1.38–2.19)](Table 1). The same trend was observed for offspring of mothers smoking ≥10 cigarettes [OR(95%CI):1.84(0.90–3.76) for age 14 and 1.54(0.91–2.59) for age 31], although in this case differences were non-significant, probably because of the low number of heavily smoking mothers (N=96). The effect of maternal smoking during pregnancy became non-significant after adjusting for maternal smoking before pregnancy (Age 14:P=0.50; Age 31:P= 0.59).
Offspring of mothers smoking heavily during pregnancy more likely carried CHRNA3-rs1051730[A] than offspring of non-smoking mothers [OR(95%CI):1.39(1.04–1.87), P=0.03]. No differences in frequency of CHRNA3-rs1051730[A] were found when comparing offspring of mothers smoking 1–10 cigarettes/day with offspring of non-smoking mothers. No significant correlation was found between maternal smoking and genotype at TTC12-rs10502172[G] (see Supplement 1).
No significant multiplicative interactive effects were found between maternal smoking and genotype at TTC12-rs10502172/CHRNA3-rs1051730[A] except for a significant interaction between maternal smoking and CHRNA3-rs1051730[A] on occasional-smoking at 14 (see Supplement 1).
Novelty seeking
Novelty seeking was positively correlated with smoking behavior both at age 14 and 31 as previously reported(40) (Age 14: Spearman correlation coefficient=0.11, P<0.001; Age 31 years: Spearman correlation coefficient =0.16, P<0.001).
TTC12-rs10502172[G] was associated with increased novelty seeking (beta=0.27, SE=0.13, P=0.04). No association was found for CHRNA3-rs1051730[A] (beta=0.005, SE=0.14, P=0.97).
Structural Equation modeling
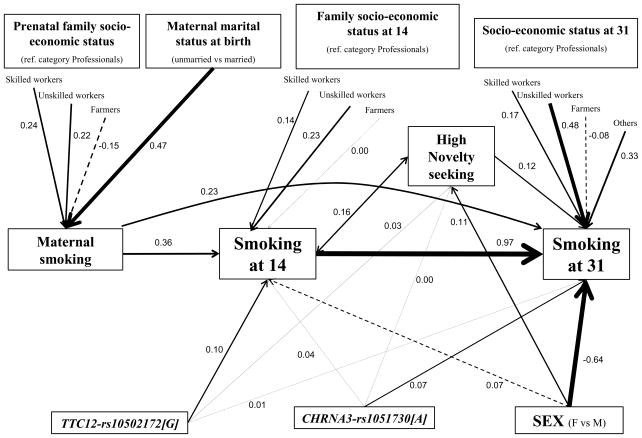
The structural equation model including the different genetic and non-genetic predictors of smoking behavior is shown in Figure 3 (see also Table 5, and Table S8 in Supplement 1). The model fitted the data quite well (RMSEA=0.022, CFI=0.85).
Figure 3.
Integrative etiological model for smoking behavior from adolescence to mid-adulthood. Path coefficients are standardized probit regression estimates computed via structural equation modeling, and thicknesses of path lines are proportional to the estimates. Non-significant (p>0.05) paths are shown with dashed lines. Socio-economic status at 31: Others: students, pensioners, long-term unemployed, not defined. Maternal smoking: smokers are compared to non-smokers; smoking at 14 years: smokers are compared to non-smokers; smoking at 31 years: heavy-smokers are compared to light/non-smokers.
Table 5.
Total, Direct, and Indirect Effects of TTC12-rs10502172 and CHRNA3-rs1051730 on Smoking at 14 and 31 Years (see also Table S10 in Supplement 1)
| β (SE)a | Standardized βa | P Value | ||
|---|---|---|---|---|
| TTC12-rs10502172[G]to smoking at 14 | ||||
| Total | 0.152 | (0.034) | 0.104 | <0.001 |
| CHRNA3-rs1051730[A]to smoking at 14 | ||||
| Total | 0.063 | (0.034) | 0.041 | 0.07 |
| TTC12-rs10502172[G]C to smoking at 31 | ||||
| Total | 0.091 | (0.037) | 0.052 | 0.01 |
| Total indirect | 0.073 | (0.017) | 0.042 | <0.001 |
| 1) TTC12→NS→Smoking31 | 0.007 | (0.004) | 0.004 | 0.06 |
| 2) TTC12→Smoking14→Smoking31 | 0.066 | (0.016) | 0.038 | <0.001 |
| Direct | 0.018 | (0.038) | 0.010 | 0.63 |
| CHRNA3-rs1051730[A] to smoking at 31 | ||||
| Total | 0.158 | (0.038) | 0.086 | <0.001 |
| Total indirect | 0.026 | (0.016) | 0.014 | 0.10 |
| 1) CHRNA3→NS→Smoking31 | −0.001 | (0.003) | 0.000 | 0.78 |
| 2) CHRNA3→Smoking14→Smoking31 | 0.027 | (0.015) | 0.015 | 0.07 |
| Direct | 0.131 | (0.037) | 0.071 | <0.001 |
Abbreviations: NS, Novelty Seeking.
The Estimates are probit regression estimates from the SEM model fitted to the data.
The effect of TTC12-rs10502172[G] on smoking at age 31 was mainly indirect and mediated via its effect on smoking at 14 and novelty seeking. In contrast, the effect of CHRNA3-rs1051730[A] on smoking in adulthood was mainly direct and not mediated by smoking in adolescence or novelty seeking. Low socio-economic status and unmarried status during pregnancy were associated with maternal smoking, which in turn was positively associated with smoking behaviors in the offspring both at age 14 and 31. Low socio-economic status was independently associated with increased smoking behavior in the offspring both in adolescence and adulthood. High novelty seeking was associated with increased smoking behavior at both 14 and 31 years. Men as compared to women were at increased risk of smoking at 31 but not at 14 years.
DISCUSSION
Our data provide further support for the association between smoking and common genetic variation within CHRNA5-CHRNA3-CHRNB4 and TTC12-ANKK1-DRD2 gene-clusters and indicate that these two loci are likely to be involved in different pathways leading to smoking behavior from adolescence to mid-adulthood.
As previously found(3, 4, 15, 16, 54), for CHRNA5-CHRNA3-CHRNB4, the most significantly associated allele was rs1051730[A]. Each copy of rs1051730[A] was associated with a 1.27 increased odds of being a heavy/regular-smoker with a similar effect size in adolescence and mid-adulthood. In line with previous studies(3), frequency of rs1051730[A] was not increased in low-quantity smokers compared to non-smokers(3). This pattern of association suggests that rs1051730 is unlikely to influence smoking initiation, but is more likely to influence smoking severity among subjects who have been already exposed to smoking(3, 17). Indeed, if rs1051730 was associated with increased risk of smoking initiation we would have expected an excess of the A allele also among low quantity-smokers. In addition, most of the CHRNA3-rs1051730[A] effect on smoking behavior in adulthood appears to be direct and not mediated by smoking in adolescence, which is the period of life when smoking initiation occurs in 80–90% of the cases(30, 55). We have also shown that rs1051730[A] is associated with increased risk of smoking persistence. CHRNA3-rs1051730 is located in a region of high linkage disequilibrium. In this regard, genotype at rs1051730 is highly correlated (r2=0.9 in Caucasians) with genotype at a non-synonymous (aspartic acid [D] to asparagine [N]; rs16969968) SNP mapping in CHRNA5. The asparagine allele at rs16969968 has been shown to moderate nicotine response both in vitro(14) and in vivo(9). Of note, the asparagine allele is on the same haplotype-background of 1051730[A] and might therefore be the ‘risk’ variant in this region.
Positive associations between variation in TTC12-ANKK1-DRD2 and smoking have been already reported(8, 11, 12, 19, 20). In our study, the most significantly associated allele was rs10502172[G] mapping in TTC12. Additional alleles that were significantly overrepresented in smokers than non-smokers, were ANKK1-rs2734849[A] and TTC12-rs2303380[G]. The same alleles at these two loci were associated with nicotine dependence in two previous studies(11, 12). Haplotype-analyses were conducted to help narrow the location of a susceptibility locus, although the high linkage disequilibrium across the entire region limits the possibility to exactly localize the association signal. A protective haplotype, namely AAGGGAAAAGGGACGGGA, spanning block-two, was significantly associated with smoking. Interestingly, haplotype GGGGAGGGAGAGGCGGGA, which shares the same allelic configuration as AAGGGAAAAGGGACGGGA at the last five markers, was found to have the same frequency in smokers and non-smokers. This result indicates that the potential ‘causal locus’ might map within the first part of block-two that covers the 3′ region of TTC12, ANKK1, and the 5′ region of DRD2. A possible functional locus here is rs2734849, a missense variation leading to a Histidine to Arginine substitution at codon 490 of the ANKK1 gene that appears to be involved in regulation of DRD2 expression(11).
The impact of the TTC12-ANKK1-DRD2 on smoking behavior was stronger in adolescence (OR for TTC12-rs10502172[G] ~1.30) than mid-adulthood (OR~1.15). This result suggests that TTC12-ANKK1-DRD2 impact on smoking behavior tends to decrease over time. Further supporting this hypothesis, TTC12-rs10502172[G] effect in adulthood was entirely mediated by its effect on smoking in adolescence and by high novelty seeking, a temperamental trait that is characterized by increased tendency to experiment psychotropic substances and that tends to decline with age(25, 56). Indeed, at age 31, TTC12-rs10502172[G] was found in excess among ex-smokers, indicating that not all individuals carrying this risk variant and initiating smoking in adolescence continue smoking in adulthood and that other genetic and/or environmental factors are likely to influence smoking persistence.
In contrast with our finding, recent genome wide association studies on smoking have found no evidence for association within TTC12-ANKK1-DRD2(3, 6, 48). A plausible explanation for this discrepancy is the difference in age in the conducted studies as compared to our study. In Thorgeirsson et al(3) the mean ages of samples included were older than 50 years. Similarly, in Caporaso et al(48) more than 80% of participants were older than 60 years. According to our results, TTC12-ANKK1-DRD2 effect on smoking decreases from age 14 to age 31 years and might become undetectable at later ages. This result also underlies that more studies conducted in young cohorts are needed. Genes influencing smoking behavior in adolescence are likely to partially differ from genes influencing smoking in adulthood and old age(38) and whole genome association studies in adolescents might therefore enable the discovery of novel risk variants for smoking.
As previously found, maternal smoking during pregnancy was a strong risk factor for smoking behavior in the offspring and it was linked with multiple indices of social disadvantage(39). The effect of maternal smoking in pregnancy on offspring’s smoking habits is likely to reflect a mixture of genetic and environmental contributors to liability. A genetic mechanism might be due to the fact that mothers who smoke during pregnancy might be more likely to carry and transmit risk alleles to their offspring. Although we could not directly test this hypothesis because DNA from mothers was unavailable, rs1051730[A] has been previously associated with inability to quit smoking in pregnancy(57). In our study, offspring of mothers smoking heavily during pregnancy were more likely to carry CHRNA3-rs1051730[A] as compared to the offspring of non-smoking mothers. This association might reflect an excess of A alleles in mothers smoking during pregnancy. Besides a gene-mediated effect, maternal smoking during pregnancy might increase the risk of smoking in the offspring via intrauterine exposure to nicotine(58). In our data, the effect of maternal smoking in pregnancy became non-significant after adjusting for maternal smoking before pregnancy. This indicates that the observed association between maternal and offspring smoking behaviors is not likely to reflect a prenatal exposure, but more likely a postnatal mechanism [i.e. mothers smoking during pregnancy are probably more likely to smoke also after delivery exposing their offspring to a tobacco enriched environment(58)].
In our study, men were three-times more likely to smoke heavily than women at age 31. In contrast, sex differences were not found in adolescence. This is in line with findings showing overlapping smoking rates in boys and girls younger than 16 years(59) and increased rates among boys than girls aged >16 years(59) and among adult men than women(60). A plausible explanation is that sex differences in smoking habits might become evident only after puberty because they are mediated by differences in sex-hormone levels(61, 62).
Results from this study should be interpreted in the context of some limitations: smoking behavior was collected only at two time points; since our sample is a community-ascertained sample we did not include many heavy-smokers; we are missing some psychosocial risk factors for smoking behavior (e.g. parental attitudes, peer influences); our sample includes only Finns limiting the generalizability of our findings to non-Finnish populations. Specific strengths of this study include: longitudinal design with all the phenotypic characteristics collected prospectively limiting recall bias; our study sample is general population-based increasing the ecological validity of our findings.
In conclusion, our study further supports the role of genetic variation within CHRNA5-CHRNA3-CHRNB4 and TTC12-ANKK1-DRD2 gene-clusters on smoking and suggests that they are involved in different stages of the multi-step process leading to nicotine addiction. Variation at TTC12-ANKK1-DRD2 seems to influence risk of smoking in adulthood via its effect on smoking in adolescence and via increased novelty seeking. In contrast, genetic variation at CHRNA5-CHRNA3-CHRNB4 seems mainly involved in the transition towards heavy use in mid-adulthood and in smoking persistence. Further studies are needed to identify new genetic loci moderating smoking behavior in adolescence and early adulthood.
Supplementary Material
Acknowledgments
We thank Professor (Emerita) Paula Rantakallio who initiated the NFBC studies. Recently we lost our distinguished colleague and co-author Professor Leena Peltonen. We acknowledge her major contributions in human genetics. This work was supported by the EC-FP6 Integrated Project IMAGEN (LSHM-CT-2007-037286) (GS), the EC-FP7 Project ADAMS (242257) (GS), the MRC-Addiction Research Cluster “Genomic Biomarkers” (GS, PE, MRJ), the NIHR Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, Kings College London’ (GS, FD) and the NIHR ACL at St George’s University, London (FD). Financial support for NFBC was received from the Academy of Finland (project grants 104781, 120315, 1110143), University Hospital Oulu, Biocenter, University of Oulu, Finland, and NIH/NHLBI grant 5R01HL087679-02 through the STAMPEED program and NIH/NIMH grant 1RL1MH083268-01. The current study is a component project of EU funded ENGAGE programme (HEALTH-F4-2007-201413). The analyses were also supported by the Medical Research Council (studentship grant G0500539), UK, Sigrid Juselius Foundation, and NARSAD. The DNA extractions, sample quality controls, biobank up-keeping and aliquotting were performed in the national Public Health Institute, Biomedicum Helsinki, Finland and supported financially by the Academy of Finland and Biocentrum Helsinki.
Footnotes
Location of work and address for reprints: School of Public Health, Department of Epidemiology and Biostatistics, Imperial College London, London W2 1PG United Kingdom And Institute of Psychiatry, Kings College, De Crespigny Park, London, United Kingdom, SE5 8AF
Parts of the article were presented as a poster at the XVII World Congress of Psychiatric Genetics, San Diego, California, November 4–8, 2009, and the poster presentation was awarded with Early career investigator award within the category “alternative phenotypes, gene-gene, and gene-environment interaction”.
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest. The funding sources played no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 2.Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–1428. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 6.Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103:1544–1552. doi: 10.1111/j.1360-0443.2008.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, et al. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang W, Payne TJ, Ma JZ, Beuten J, Dupont RT, Inohara N, et al. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology. 2009;34:319–330. doi: 10.1038/npp.2008.37. [DOI] [PubMed] [Google Scholar]
- 12.Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang BZ, et al. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet. 2006;15:3498–3507. doi: 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- 13.Morley KI, Medland SE, Ferreira MA, Lynskey MT, Montgomery GW, Heath AC, et al. A possible smoking susceptibility locus on chromosome 11p12: evidence from sex-limitation linkage analyses in a sample of Australian twin families. Behav Genet. 2006;36:87–99. doi: 10.1007/s10519-005-9004-0. [DOI] [PubMed] [Google Scholar]
- 14.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, et al. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009 doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, et al. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelernter J, Panhuysen C, Weiss R, Brady K, Poling J, Krauthammer M, et al. Genomewide linkage scan for nicotine dependence: identification of a chromosome 5 risk locus. Biol Psychiatry. 2007;61:119–126. doi: 10.1016/j.biopsych.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Laucht M, Becker K, Frank J, Schmidt MH, Esser G, Treutlein J, et al. Genetic variation in dopamine pathways differentially associated with smoking progression in adolescence. J Am Acad Child Adolesc Psychiatry. 2008;47:673–681. doi: 10.1097/CHI.0b013e31816bff77. [DOI] [PubMed] [Google Scholar]
- 20.Bergen AW, Conti DV, Van Den Berg D, Lee W, Liu J, Li D, et al. Dopamine genes and nicotine dependence in treatment-seeking and community smokers. Neuropsychopharmacology. 2009;34:2252–2264. doi: 10.1038/npp.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble EP. Polymorphisms of the D2 dopamine receptor gene and alcoholism and other substance use disorders. Alcohol Alcohol Suppl. 1994;2:35–43. [PubMed] [Google Scholar]
- 22.Xu K, Lichtermann D, Lipsky RH, Franke P, Liu X, Hu Y, et al. Association of specific haplotypes of D2 dopamine receptor gene with vulnerability to heroin dependence in 2 distinct populations. Arch Gen Psychiatry. 2004;61:597–606. doi: 10.1001/archpsyc.61.6.597. [DOI] [PubMed] [Google Scholar]
- 23.Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- 24.Nyman ES, Loukola A, Varilo T, Ekelund J, Veijola J, Joukamaa M, et al. Impact of the dopamine receptor gene family on temperament traits in a population-based birth cohort. Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30908. [DOI] [PubMed] [Google Scholar]
- 25.Ducci F, Enoch MA, Funt S, Virkkunen M, Albaugh B, Goldman D. Increased anxiety and other similarities in temperament of alcoholics with and without antisocial personality disorder across three diverse populations. Alcohol. 2007;41:3–12. doi: 10.1016/j.alcohol.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D. The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics. 1996;6:73–79. doi: 10.1097/00008571-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Stress-induced cigarette craving: effects of the DRD2 TaqI RFLP and SLC6A3 VNTR polymorphisms. Pharmacogenomics J. 2004;4:102–109. doi: 10.1038/sj.tpj.6500227. [DOI] [PubMed] [Google Scholar]
- 28.Bierut LJ, Rice JP, Edenberg HJ, Goate A, Foroud T, Cloninger CR, et al. Family-based study of the association of the dopamine D2 receptor gene (DRD2) with habitual smoking. Am J Med Genet. 2000;90:299–302. doi: 10.1002/(sici)1096-8628(20000214)90:4<299::aid-ajmg7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 29.Johnstone EC, Yudkin P, Griffiths SE, Fuller A, Murphy M, Walton R. The dopamine D2 receptor C32806T polymorphism (DRD2 Taq1A RFLP) exhibits no association with smoking behaviour in a healthy UK population. Addict Biol. 2004;9:221–226. doi: 10.1080/13556210412331292226. [DOI] [PubMed] [Google Scholar]
- 30.Youth tobacco surveillance--United States, 1998–1999. MMWR CDC Surveill Summ. 2000;49:1–94. [PubMed] [Google Scholar]
- 31.Marshall L, Schooley M, Ryan H, Cox P, Easton A, Healton C, et al. Youth tobacco surveillance--United States, 2001–2002. MMWR Surveill Summ. 2006;55:1–56. [PubMed] [Google Scholar]
- 32.Wittchen HU, Behrendt S, Hofler M, Perkonigg A, Lieb R, Buhringer G, et al. What are the high risk periods for incident substance use and transitions to abuse and dependence? Implications for early intervention and prevention. Int J Methods Psychiatr Res. 2008;17(Suppl 1):S16–29. doi: 10.1002/mpr.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van De Ven MO, Greenwood PA, Engels RC, Olsson CA, Patton GC. Patterns of adolescent smoking and later nicotine dependence in young adults: a 10-year prospective study. Public Health. 124:65–70. doi: 10.1016/j.puhe.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Breslau N, Fenn N, Peterson EL. Early smoking initiation and nicotine dependence in a cohort of young adults. Drug Alcohol Depend. 1993;33:129–137. doi: 10.1016/0376-8716(93)90054-t. [DOI] [PubMed] [Google Scholar]
- 35.Grant BF. Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:59–73. doi: 10.1016/s0899-3289(99)80141-2. [DOI] [PubMed] [Google Scholar]
- 36.Riala K, Hakko H, Isohanni M, Jarvelin MR, Rasanen P. Teenage smoking and substance use as predictors of severe alcohol problems in late adolescence and in young adulthood. J Adolesc Health. 2004;35:245–254. doi: 10.1016/j.jadohealth.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Isohanni M, Oja H, Moilanen I, Rantakallio P, Koiranen M. The relation between teenage smoking and drinking, with special reference to non-standard family background. Scand J Soc Med. 1993;21:24–30. doi: 10.1177/140349489302100105. [DOI] [PubMed] [Google Scholar]
- 38.Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isohanni M, Oja H, Moilanen I, Koiranen M, Rantakallio P. Smoking or quitting during pregnancy: associations with background and future social factors. Scand J Soc Med. 1995;23:32–38. doi: 10.1177/140349489502300107. [DOI] [PubMed] [Google Scholar]
- 40.Sovio U, King V, Miettunen J, Ek E, Laitinen J, Joukamaa M, et al. Cloninger’s Temperament dimensions, socio-economic and lifestyle factors and metabolic syndrome markers at age 31 years in the Northern Finland Birth Cohort 1966. J Health Psychol. 2007;12:371–382. doi: 10.1177/1359105307074301. [DOI] [PubMed] [Google Scholar]
- 41.Isohanni I, Jarvelin MR, Rantakallio P, Jokelainen J, Jones PB, Nieminen P, et al. Juvenile and early adulthood smoking and adult educational achievements--a 31-year follow-up of the Northern Finland 1966 Birth Cohort. Scand J Public Health. 2001;29:87–95. doi: 10.1177/14034948010290020501. [DOI] [PubMed] [Google Scholar]
- 42.Isohanni M, Moilanen I, Rantakallio P. Determinants of teenage smoking, with special reference to non-standard family background. Br J Addict. 1991;86:391–398. doi: 10.1111/j.1360-0443.1991.tb03416.x. [DOI] [PubMed] [Google Scholar]
- 43.Rantakallio P. Family background to and personal characteristics underlying teenage smoking. Background to teenage smoking. Scand J Soc Med. 1983;11:17–22. doi: 10.1177/140349488301100104. [DOI] [PubMed] [Google Scholar]
- 44.Rantakallio P. The longitudinal study of the northern Finland birth cohort of 1966. Paediatr Perinat Epidemiol. 1988;2:59–88. doi: 10.1111/j.1365-3016.1988.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 45.Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- 46.Makikyro T, Isohanni M, Moring J, Oja H, Hakko H, Jones P, et al. Is a child’s risk of early onset schizophrenia increased in the highest social class? Schizophr Res. 1997;23:245–252. doi: 10.1016/s0920-9964(96)00119-3. [DOI] [PubMed] [Google Scholar]
- 47.Sabatti C, Service SK, Hartikainen AL, Pouta A, Ripatti S, Brodsky J, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet. 2009;41:35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, et al. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One. 2009;4:e4653. doi: 10.1371/journal.pone.0004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ducci F, Roy A, Shen PH, Yuan Q, Yuan NP, Hodgkinson CA, et al. Association of substance use disorders with childhood trauma but not African genetic heritage in an African American cohort. Am J Psychiatry. 2009;166:1031–1040. doi: 10.1176/appi.ajp.2009.08071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 51.Hu LTBP. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modelling: A Multidisciplinary Journal. 1999;6:1. [Google Scholar]
- 52.Browne MWCR. Alternative ways of assessing model fit. In: Bollen KALJ, editor. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 445–455. [Google Scholar]
- 53.Muthén LMaB. Mplus: statistical analysis with latent variables. Los Angeles, CA: Muthén and Muthén; 1998. [Google Scholar]
- 54.Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 42:436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Youth tobacco surveillance--United States, 2000. MMWR CDC Surveill Summ. 2001;50:1–84. [PubMed] [Google Scholar]
- 56.Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 57.Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am J Psychiatry. 2003;160:1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- 59.Nelson DE, Mowery P, Asman K, Pederson LL, O’Malley PM, Malarcher A, et al. Long-term trends in adolescent and young adult smoking in the United States: metapatterns and implications. Am J Public Health. 2008;98:905–915. doi: 10.2105/AJPH.2007.115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tilloy E, Cottel D, Ruidavets JB, Arveiler D, Ducimetiere P, Bongard V, et al. Characteristics of current smokers, former smokers, and secondhand exposure and evolution between 1985 and 2007. Eur J Cardiovasc Prev Rehabil. doi: 10.1097/HJR.0b013e32833a9a0c. [DOI] [PubMed] [Google Scholar]
- 61.Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- 62.Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.