Abstract
BACKGROUND
We examined prescription adherence rates by contraceptive method among women who used oral contraceptive pills (OCP), transdermal patch, or vaginal ring.
STUDY DESIGN
Women in the St. Louis area were provided their choice of OCP, patch, or ring at no cost and followed for 18 months. Time between monthly refills was obtained from pharmacy data and analyzed as a marker of adherence. Risk factors for initial nonadherence were estimated using Cox proportional hazards; predictors for repeated nonadherence were analyzed using Poisson regression with robust error variance.
RESULTS
Overall, 619 participants filled 6,435 contraceptive prescriptions with a median of 10 refills per participant. Only 30% of women (n=187) obtained all refills on time. In the time-to-failure analysis, use of vaginal ring and increased parity were predictors of early nonadherence (p<0.05). In the multivariable analysis, use of the vaginal ring and history of abortion were risk factors for repeated nonadherence (p<0.01).
CONCLUSIONS
Even with financial barriers removed, pharmacy data show that many women inconsistently refill their contraception and may be at risk for unintended pregnancy.
Keywords: prescription adherence, vaginal ring, pharmacy claims data, unintended pregnancy
1. Introduction
Long-term pharmaceutical therapy is associated with medication nonadherence [1]. Studies have shown that patients with a variety of chronic conditions have difficulty taking their medications as prescribed over time [2–4]. Medication inconsistencies may be due to obstacles patients experience over time with either (1) continuation, the overall time that an individual persists with therapy, or (2) adherence, the timely observance of a prescribed treatment regimen [5, 6]. While extended therapy increases the likelihood of inconsistent medication use, both continuation and adherence are further decreased in conditions that are asymptomatic until an index event occurs, such as the first fracture in osteoporosis or the initial pregnancy for a young woman [7, 8].
In the area of reproductive health, the average woman spends a full quarter of her lifetime considering the possibility and consequences of pregnancy [9]. This fact translates into decades of potential reliance on contraceptive medications to prevent unintended pregnancy. Overall, 49% of pregnancies in the United States each year are unintended, and almost half of these pregnancies occur while a woman is using contraception [10]. Nonadherence certainly plays a role in the higher contraceptive failure rates seen in ‘actual’ use versus ‘perfect’ use, with typical failure rates reaching 9% in the first year of use among women who use oral contraceptives [11]. In fact, it is estimated that up to 700,000 unintended pregnancies each year could be prevented by increasing medication adherence with oral contraceptives alone [12].
When attempting to quantify patients’ adherence with medication, studies have employed an assortment of measurement systems, from electronic pill bottles to medication diaries. Each method has observed some success, but each also depends on patient report and creates patient awareness of monitoring, both of which can bias results [13, 14]. Another tool, pharmacy claims data, provides an objective adherence measure that can be used across a wide range of specialties [15]. Although direct measurement of medication use is not possible with this method, observation of timely refills can serve as a surrogate for effective therapy [7]. The study of medication acquisition is a valuable instrument in the attempt to better understand patterns of medication nonadherence [6].
We know that a complex interaction of demographic and behavioral characteristics influence women’s contraceptive adherence [16]. Few studies, however, have relied on an objective measurement system for contraceptive adherence, and even fewer reports have been able to remove the confounding cost of medication [17]. While administrative claims data often lack significant patient-level information or lose patients over time, the Contraceptive CHOICE Project was designed to preclude these limitations. Demographic, behavioral, and pharmacy information are available and linked at the patient level in one database. Our objective was to determine whether selected demographic and behavioral characteristics were associated with contraceptive nonadherence when financial cost has been removed.
2. Materials and methods
2.1. Study population
The Contraceptive CHOICE Project (CHOICE) is a prospective cohort study that seeks to remove the financial barriers to effective methods of contraception and decrease unintended pregnancy at a population level. CHOICE is currently enrolling 10,000 sexually active women who reside in the St. Louis area, range in age from 14–45 years, and represent a wide variety of socioeconomic backgrounds [18]. Participants are provided with the contraceptive method of their choice at no out-of-pocket cost for three years. During the in-person enrollment session, all women undergo standardized contraceptive counseling. Once the participant chooses her method, she receives instructions for use of the contraceptive in accordance with the method’s package insert. Research assistants then administer a structured baseline questionnaire to measure demographic characteristics, sexual and reproductive history, and past contraceptive use. At 3- and 6-months and every 6 months thereafter for a total of 36 months, research assistants administer a similar questionnaire via telephone and assess participants’ continuation and satisfaction with their contraceptive methods. The CHOICE protocol was approved by the Washington University in St. Louis School of Medicine Human Research Protection Office prior to initiation of participant recruitment.
Participants who choose an oral contraceptive pill (OCP), the contraceptive patch, or the contraceptive vaginal ring obtain refills each month according to protocol at a local grocery-pharmacy chain ubiquitous in the St. Louis area. Each participant is given a CHOICE card that serves as her pharmacy benefits card and is instructed how to use the card at the pharmacy. At the time of each refill, multiple variables are collected, including date of fill, type of contraception, number of days dispensed, and store location. The pharmacy data are subsequently merged with demographic and behavioral information collected during the participants’ baseline and follow-up questionnaires.
The partnership between CHOICE and the local pharmacy chain began in February 2008. This study analyzes the refills obtained from February 2008 through August 31, 2009. For inclusion in the study, women must have filled at least two prescriptions during this 18-month period so that time between pharmacy visits could be calculated. The sample was further restricted to the first 2500 CHOICE participants so that all baseline participant data was available for analysis and all participants had the possibility of contributing multiple months of contraceptive use for sufficient follow-up in the study.
We chose to provide CHOICE participants with monthly refills (available every 21 days) in order to duplicate the requirements of the most common insurance plans and to measure monthly refill adherence. We considered women who allowed more than 90 days to lapse between refills as discontinuing the method, which is a distinct phenomenon separate from nonadherence when analyzing pharmacy claims data. Therefore, for each participant, we excluded refill data that occurred subsequent to a greater than 90-day lapse between refills.
2.. Study definitions
Time between refills (i.e., the number of days between pharmacy visits) served as a marker of contraceptive adherence. Thirty-four days was chosen as the cut-off for timely refills. This criterion includes a seven-day grace period after the 28-day supply to allow for some variability in date of fill [19]. Participants who had a gap between refills of 35 days or more were considered nonadherent for that refill. The ratio of 28 days to 34 days also corresponds to a medication possession ratio (MPR) of 82%. An MPR of greater than 80% is generally accepted as one marker of adherence [6, 15].
In a standard time between refills approach to adherence, participants are considered nonadherent at the time of the first late fill [20]. One of the main criticisms of this approach, however, is that a single late prescription refill can put a participant in the ‘nonadherent’ category without her establishing a pattern of nonadherence [1]. For this reason, we estimated nonadherence in two ways. First, women were considered nonadherent at the first late refill, as is customary. In a separate analysis, women were then considered nonadherent at the time of the second late refill, which allowed a participant to establish a pattern of nonadherence and minimized overestimation of one-time nonadherence in our sample.
2.3. Statistical analyses
All statistical analyses were conducted using SAS 9.2 statistical software (Cary, NC, USA). Chi-square tests were performed for categorical data to determine differences between groups. Nine categorical covariates [age (categorized), race (self-described black, white, or other), education, marital status, insurance status, parity (categorized), history of abortion, history of sexually transmitted infection (STI), and contraceptive method] were selected for investigation as predictors of nonadherence based on previously published studies [10, 11, 21–23].
Time to nonadherence, when defined as at least one late refill, was illustrated with Kaplan-Meier survival curves to account for duration of follow-up from the index prescription fill. Statistical significance was assessed with the log-rank test. Participants were censored if they were adherent to the end of their observation period or when they experienced their initial nonadherent event. Independent predictors, with alpha <0.05, and modifiers of covariates were included in a Cox-proportional hazard model to estimate the relative risk of each variable on time to nonadherence. The proportional hazards assumption was evaluated by the Wald test for time-dependent covariates, and the final model was stratified on race.
To analyze the second categorization of nonadherence, defined as two or more late refills, we used Poisson regression with robust error variance. This regression technique allows for a conservative estimation of the relative risk when the outcome of interest occurs more than 10% of the time, as in the case of nonadherence in this analysis [24]. Univariate analysis for each of the nine categorical covariates was performed; independent predictors, with unadjusted alpha of 0.05, or confounders, with greater 10% change in related variable’s beta estimate, were included in this multivariable model to estimate relative risk of nonadherence.
3. Results
Six hundred and nineteen women met the inclusion criteria for this analysis and obtained a total of 6,435 prescriptions for OCP, patch, or ring. The median number of refills per woman was 10 (min = 2, max = 26). Selected demographic and behavioral characteristics are presented in Table 1.
Table 1.
Demographic and behavioral characteristics of women enrolled in the Contraceptive CHOICE Project who use and obtained refills for the pill, patch or ring (n=619)
| Characteristics | Total (%) | OCP (n=297) | Patch (n=49) | Ring (n=273) |
|---|---|---|---|---|
| Age, years | ||||
| 14–19 | 92 (14.9) | 19.5% | 12.2% | 10.3% |
| 20–24 | 319 (51.5) | 50.9 | 38.8 | 54.6 |
| 25–29 | 143 (23.1) | 20.2 | 32.7 | 24.5 |
| ≥30 | 65 (10.5) | 9.4 | 16.3 | 10.6 |
| Race | ||||
| White | 201 (32.6) | 29.6 | 65.3 | 29.9 |
| Black | 354 (57.4) | 58.6 | 26.5 | 61.6 |
| Other | 62 (10.0) | 11.8 | 8.2 | 8.5 |
| Education | ||||
| ≤ High school | 131 (21.2) | 23.2 | 26.5 | 18.0 |
| Some college | 289 (46.7) | 49.2 | 38.8 | 45.4 |
| ≥ College degree | 199 (32.1) | 27.6 | 34.7 | 36.6 |
| Insurance | ||||
| None | 203 (33.4) | 33.7 | 51.0 | 29.8 |
| Medicaid/Medicare | 23 (3.8) | 2.7 | 8.2 | 4.2 |
| Private | 382 (62.8) | 63.6 | 40.8 | 66.0 |
| Marital status | ||||
| Single | 452 (73.0) | 77.4 | 63.3 | 70.0 |
| Married/living with partner | 148 (23.9) | 21.2 | 24.5 | 26.7 |
| Other | 19 (3.1) | 1.4 | 12.2 | 3.3 |
| Parity | ||||
| No children | 448 (72.3) | 75.4 | 55.1 | 72.2 |
| 1 child | 105 (17.0) | 15.2 | 20.4 | 18.3 |
| ≥ 2 children | 66 (10.7) | 9.4 | 24.5 | 9.5 |
| History of abortion | ||||
| Yes | 227 (36.7) | 37.7 | 51.0 | 33.0 |
| History of STI* | ||||
| Yes | 128 (20.9) | 17.6 | 29.2 | 22.9 |
Sexually transmitted infections included chlamydia, gonorrhea, trichomoniasis, genital herpes, human papillomavirus, syphilis and HIV.
On average, participants obtained refills every 31 days (median = 29 days). In our sample, 30% (n=187) of participants obtained every refill on time, 194 women (31.3%) obtained a late refill (e.g., more than 34 days) only one time, and the remaining 238 women (38.5%) obtained a late refill two or more times (Fig. 1). Vaginal ring users were more likely than OCP users to have both one late prescription fill (RR=1.6; 95% CI: 1.2, 2.1) and two or more late refills (RR=1.4; 95% CI: 1.2, 1.6). Patch and OCP users did not differ in nonadherence.
Fig. 1.
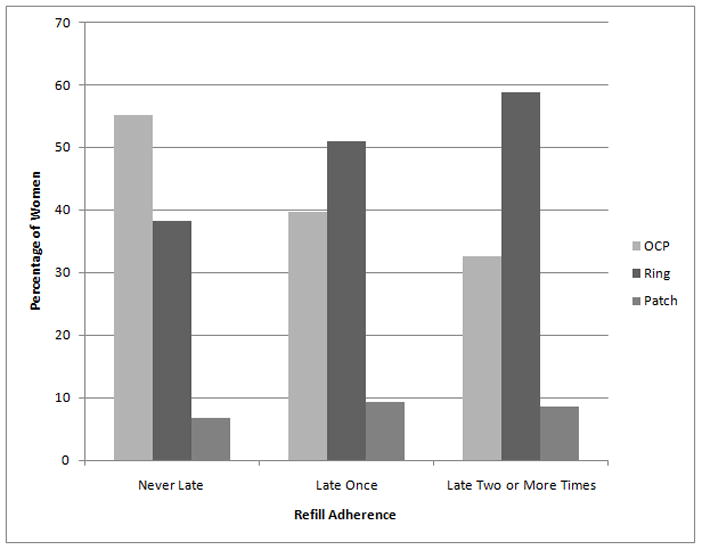
Refill adherence rates by contraceptive method.
We examined two models to determine predictors of nonadherence with refillable methods of contraception. The first model predicted nonadherence defined as the time to first occurrence of a late refill. Using this definition, contraceptive method (HR=1.4; 95% CI: 1.2, 1.7), ‘other’ race (HR=1.5; 95% CI: 1.1, 2.0), and greater parity (HR=1.5; 95% CI 1.1, 2.0) were found to be significant predictors of nonadherence in univariate analyses. Unadjusted time to failure for each significant predictor variable was estimated with Kaplan-Meier curves. Overall, the mean time to initial nonadherence in our sample was six months. OCP users were adherent longer than patch or ring users (6.8 vs. 4.8 and 4.8 months, respectively; p<0.05; Fig. 2). White women experienced significantly longer refill adherence than black women and women of other races (6.3 months vs. 5.2 months and 4.4 months, respectively; p=0.02). Furthermore, nulliparous women experienced significantly longer refill adherence compared to women with one child and women with two or more children (6.2 months vs. 5.6 months and 4.2 months, respectively; p=0.02).
Fig. 2.
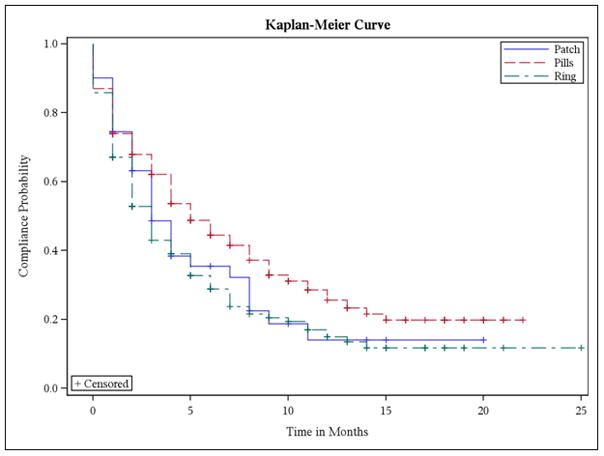
Kaplan-Meier curves representing time to first late refill by contraceptive method.
The multivariable Cox-proportional hazards model included the control variable, time, as well as contraceptive method and parity. These variables were stratified by race to satisfy the proportional hazards assumption. Age, education, insurance status, marital status, parity, and STI history were not significantly associated with time to initial nonadherence and were not included in the final regression model. Women who use the vaginal ring (HR=1.5, 95% CI 1.2, 1.8) and women who have two or more children (HR=1.4, 95% CI 1.0, 2.0) became nonadherent in less time than women who use OCP or the patch or who have fewer children.
In our second model, we examined patterns of nonadherence when we categorized women with two or more late refills as nonadherent users. Using this definition, contraceptive method, race, and history of abortion were significant (p<0.05) predictors of nonadherence in univariate analyses and were included in our multivariable model (Table 2). Women who used the vaginal ring were over 60% more likely than women using OCP or the patch to be repeatedly late obtaining their contraceptive method. Women who identified as neither white nor black and women who had a history of abortion were also more likely to experience two or more late refills during the observation period.
Table 2.
Model 2: Multivariable model of factors associated with consistent nonadherence*
| Variable | Relative risk | (95% CI) | p-value |
|---|---|---|---|
| Contraceptive method | |||
| Oral contraceptive | Reference | -- | -- |
| Vaginal ring | 1.64 | 1.33, 2.02 | <0.01 |
| Transdermal patch | 1.00 | 0.65, 1.54 | 0.99 |
| Race | |||
| White | Reference | -- | -- |
| Black | 1.02 | 0.81, 1.29 | 0.85 |
| Other | 1.48 | 1.14, 1.94 | <0.01 |
| Abortion history | |||
| No | Reference | -- | -- |
| Yes | 1.32 | 1.08, 1.61 | <0.01 |
Adjusted for contraceptive method, race, and abortion history.
4. Discussion
Our data support the well-established fact that patients experience difficulty adhering to long-term medication regimens as prescribed. Less than one-third of our participants were adherent with their contraceptive method regimen when assessed by timely pharmacy visits to obtain monthly refills, which may place them at risk of an unintended pregnancy. Our study required women to return to the pharmacy each month, a common practice mandated by many insurance plans. This practice introduces an impediment to contraceptive adherence and may account for a large proportion of women who did not obtain timely refills, regardless of contraceptive method.
The poor adherence rates demonstrated by our study support the WHO recommendations that clinicians be able to provide up to a year’s supply of contraception at a time and underscore the need to change current insurance coverage and dispensing policies [25]. Although a high percentage of women can discontinue refillable methods within the first 6 months of use, dispensing multiple months of refillable methods at one time increases refill adherence when compared with one month refill cycles [26, 27].
Prior research has reported the difficulty women experience remembering to take oral contraceptives each day [12] and suggests that ring users have increased compliance on a day- to-day basis [13]. In our study, type of contraceptive method was an important predictor of refill adherence; however, women who use the vaginal ring were more likely to (1) experience their first late refill more quickly and (2) be repeatedly late in obtaining their refills. Ring users in our study are instructed to use the ring according to the package insert: Put the ring in for 21 days, remove for 7 days, then insert a new ring and repeat. Alternatively, participants who requested extended or continuous use were instructed to put the ring in the vagina for 28 days, remove, and insert a new ring immediately. It may be that it is more difficult to remember to change the ring with a three weeks in/one week out regimen. The more frequent reminder associated with OCP and patch use may help reinforce the routine and make women more successful in obtaining a new refill at the end of a month. The necessity of thinking about the ring less frequently is one of its attractive attributes, but may make establishment of a routine more difficult. New technology, such as cell phone reminders, may provide useful, monthly cues for women who need to obtain timely refills, however further investigation is required.
Alternatively, the participant’s contraceptive choice may itself be associated with adherence characteristics; women who have previously experienced adherence difficulties with OCP may select the vaginal ring to avoid daily pill-taking. The group of women in our study who use the ring could be predisposed to nonadherence independent of the method used. Regardless of the underlying reason for obtaining late refills, our findings underscore the importance of providing women with practical advice regarding how to use their contraceptive method most effectively [28].
Because women with greater parity experienced nonadherence more quickly than their nulliparous counterparts, parity may signal ambivalence toward future pregnancy. Multiparous women may be contemplating the possibility of more children at the time of refill nonadherence. Another explanation may be that increased parity serves as a marker of additional social barriers that women with children must surmount in order to obtain monthly medication refills. Because women disproportionately bear the burden of family care, finding the time to go to the pharmacy each month for contraception could be a significant obstacle to overcome [29].
Abortion history, an independent predictor of repeated nonadherence, may serve as a proxy for a woman’s pre-existing or unmeasured risk for decreased adherence. Based on our findings, patients who have a history of abortion are more likely to be less likely to be adherent in obtaining timely refills; therefore, providers should assist patients in identifying obstacles to obtaining refills and in formulating an effective plan for using contraception. Long-acting reversible contraception (LARC; intrauterine contraception and subdermal implant) should be considered as a first-line option for women who have difficulty obtaining or remembering to use an OCP, patch, or ring [4, 30].
Studies have shown that out-of-pocket cost directly influences duration of therapy with medication, and that the longer the needed treatment, the more likely cost plays a role in discontinuation or nonadherence [31, 32]. As contraceptive use may be necessary for many years, the expense associated with consistently refilling contraception can be prohibitive for some women. These costs are magnified for contraceptives without a generic alternative, such as the ring. Because cost of contraception is not a barrier to adherence in our study, the true rates of contraceptive nonadherence in the general population are probably higher than we have measured in this study.
Our study has several notable strengths. First, we combined the unique attributes of two data collection systems to formulate a better understanding of the factors associated with obtaining late refills of contraceptive methods. Administrative claims data is objective, longitudinal, and inexpensive. These data also avoid potential adherence bias associated with study participants who may act differently under observation (i.e., Hawthorne effect). The CHOICE data represent a large cohort of women from diverse socioeconomic backgrounds and provide in-depth patient-level data including demographic characteristics, reproductive health behaviors, and contraceptive prescription changes.
Second, we can also be reasonably sure that we did not lose valuable refill information due to participants obtaining methods at non-study affiliated pharmacies. Participants obtain their contraception at no cost when filled at the study-affiliated pharmacy chain. Third, women who enroll in CHOICE desire a new method of contraception. As a result, participants in this study are not previous continuous users of OCP, patch, or ring. Our study protocol reduces the bias caused by combining old and new users of a medication when studying adherence [33].
Our study is not without limitations. Pharmacy claims data only report that the refill was obtained; it does not report medication use. We are therefore only able to examine one half of medication adherence, possession, but are unable to examine compliance once obtained. Although we included nine important factors in our analysis based on the literature, we believe that unmeasured confounders, such as more refined socioeconomic status indicators, frequency of sexual activity, or pregnancy intentions at the time of expected refill, do exist and may cause bias in our estimates.
The generalizability of our methods and our results may be limited by two features of our study design. First, our analyses include detailed patient-level data not always available in other pharmacy claims and administrative databases. Second, our participants do not pay for contraceptive method refills effectively removing the financial barrier that is known to impact medication adherence.
In this population, less than one in three women had perfect refill adherence in a relatively short period of time. High rates of nonadherence further highlight the benefits of LARC: they do not require daily, weekly or monthly action [4]. Although our study provides better insight into important predictors of nonadherence, a fuller understanding of how to improve adherence among women who use an OCP, the patch, or the ring remains. Because CHOICE participants are followed for three years, future analyses will examine whether pregnancy rates differ between refill adherent and nonadherent groups [34].
Acknowledgments
Supported by an Anonymous Foundation. This research was also supported in part by a Midcareer Investigator Award in Women’s Health Research (K24 HD01298), by a Clinical and Translational Science Award (UL1RR024992), and by Grant Number KL2RR024994 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hughes DA, Bagust A, Haycox A, Walley T. The impact of non-compliance on the cost- effectiveness of pharmaceuticals: a review of the literature. Health Econ. 2001;10:601–15. doi: 10.1002/hec.609. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134:968–77. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 3.Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80:856–61. doi: 10.4065/80.7.856. [DOI] [PubMed] [Google Scholar]
- 4.Grimes DA. Forgettable contraception. Contraception. 2009;80:497–9. doi: 10.1016/j.contraception.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Caetano PA, Lam JM, Morgan SG. Toward a standard definition and measurement of persistence with drug therapy: Examples from research on statin and antihypertensive utilization. Clin Ther. 2006;28:1411–24. doi: 10.1016/j.clinthera.2006.09.021. discussion 1410. [DOI] [PubMed] [Google Scholar]
- 6.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 7.Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38:922–8. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Nettleman MD, Chung H, Brewer J, Ayoola A, Reed PL. Reasons for unprotected intercourse: analysis of the PRAMS survey. Contraception. 2007;75:361–6. doi: 10.1016/j.contraception.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Cates W., Jr Contraception, unintended pregnancies, and sexually transmitted diseases: why isn’t a simple solution possible? Am J Epidemiol. 1996;143:311–8. doi: 10.1093/oxfordjournals.aje.a008744. [DOI] [PubMed] [Google Scholar]
- 10.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38:90–6. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- 11.Kost K, Singh S, Vaughan B, Trussell J, Bankole A. Estimates of contraceptive failure from the 2002 National Survey of Family Growth. Contraception. 2008;77:10–21. doi: 10.1016/j.contraception.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg M, Waugh MS. Causes and consequences of oral contraceptive noncompliance. Am J Obstet Gynecol. 1999;180(Pt 2):276–9. doi: 10.1016/s0002-9378(99)70718-0. [DOI] [PubMed] [Google Scholar]
- 13.Gilliam ML, Neustadt A, Kozloski M, Mistretta S, Tilmon S, Godfrey E. Adherence and acceptability of the contraceptive ring compared with the pill among students. Obstet Gynecol. 2010;115:503–10. doi: 10.1097/AOG.0b013e3181cf45dc. [DOI] [PubMed] [Google Scholar]
- 14.Wetzels GE, Nelemans PJ, Schouten JS, van Wijk BL, Prins MH. All that glisters is not gold: a comparison of electronic monitoring versus filled prescriptions--an observational study. BMC Health Serv Res. 2006;6:8. doi: 10.1186/1472-6963-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis JR, Xi J, Westfall AO, et al. Improving the prediction of medication compliance: the example of bisphosphonates for osteoporosis. Med Care. 2009;47:334–41. doi: 10.1097/MLR.0b013e31818afa1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost JJ, Darroch JE. Factors associated with contraceptive choice and inconsistent method use, United States, 2004. Perspect Sex Reprod Health. 2008;40:94–104. doi: 10.1363/4009408. [DOI] [PubMed] [Google Scholar]
- 17.Nelson AL, Westhoff C, Schnare SM. Real-world patterns of prescription refills for branded hormonal contraceptives: a reflection of contraceptive discontinuation. Obstet Gynecol. 2008;112:782–7. doi: 10.1097/AOG.0b013e3181875ec5. [DOI] [PubMed] [Google Scholar]
- 18.Secura GM, Allsworth JE, Madden T, Hladky KJ, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: Reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1–115.e7. doi: 10.1016/j.ajog.2010.04.017. Epub 2010 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11:449–57. [PubMed] [Google Scholar]
- 20.Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82:1493–501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 21.Matteson KA, Peipert JF, Allsworth J, Phipps MG, Redding CA. Unplanned pregnancy: does past experience influence the use of a contraceptive method? Obstet Gynecol. 2006;107:121–7. doi: 10.1097/01.AOG.0000192170.16746.ea. [DOI] [PubMed] [Google Scholar]
- 22.Pilon D, Castilloux AM, LeLorier J. Estrogen replacement therapy: determinants of persistence with treatment. Obstet Gynecol. 2001;97:97–100. doi: 10.1016/s0029-7844(00)01104-2. [DOI] [PubMed] [Google Scholar]
- 23.Ranjit N, Bankole A, Darroch JE, Singh S. Contraceptive failure in the first two years of use: differences across socioeconomic subgroups. Fam Plann Perspect. 2001;33:19–27. [PubMed] [Google Scholar]
- 24.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit. Faculty statement from the CEU on a new publication: WHO Selected Practice Recommendations for Contraceptive Use Update. Missed pills: new recommendations. J Fam Plann Reprod Health Care. 2005;31:153–5. doi: 10.1783/1471189053629572. [DOI] [PubMed] [Google Scholar]
- 26.Foster DG, Parvataneni R, de Bocanegra HT, Lewis C, Bradsberry M, Darney P. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107–14. doi: 10.1097/01.AOG.0000239122.98508.39. [DOI] [PubMed] [Google Scholar]
- 27.Westhoff CL, Heartwell S, Edwards S, et al. Oral contraceptive discontinuation: do side effects matter? Am J Obstet Gynecol. 2007;196:412.e1–412.e7. doi: 10.1016/j.ajog.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frost JJ, Darroch JE, Remez L. Improving contraceptive use in the United States. Issues Brief (Alan Guttmacher Inst) 2008:1–8. [PubMed] [Google Scholar]
- 29.Phillips SD, Imhoff AR. Women and career development: a decade of research. Ann Rev Psychol. 1997;48:31–59. doi: 10.1146/annurev.psych.48.1.31. [DOI] [PubMed] [Google Scholar]
- 30.Homco JB, Peipert JF, Secura GM, Lewis VA, Allsworth JE. Reasons for ineffective pre-pregnancy contraception use in patients seeking abortion services. Contraception. 2009;80:569–74. doi: 10.1016/j.contraception.2009.05.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curkendall S, Patel V, Gleeson M, Campbell RS, Zagari M, Dubois R. Compliance with biologic therapies for rheumatoid arthritis: do patient out-of-pocket payments matter? Arthritis Rheum. 2008;59:1519–26. doi: 10.1002/art.24114. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy J, Coyne J, Sclar D. Drug affordability and prescription noncompliance in the United States: 1997–2002. Clin Ther. 2004;26:607–14. doi: 10.1016/s0149-2918(04)90063-x. [DOI] [PubMed] [Google Scholar]
- 33.Roughead EE, Ramsay E, Priess K, Barratt J, Ryan P, Gilbert AL. Medication adherence, first episode duration, overall duration and time without therapy: the example of bisphosphonates. Pharmacoepidemiol Drug Saf. 2009;18:69–75. doi: 10.1002/pds.1687. [DOI] [PubMed] [Google Scholar]
- 34.Steiner JF. Can we identify clinical predictors of medication adherence….and should we? Med Care. 2010;48:193–5. doi: 10.1097/MLR.0b013e3181d51ddf. [DOI] [PubMed] [Google Scholar]


