Abstract
Carbapenem-resistant K. pneumoniae (CR-Kp) is an emerging multi-drug resistant nosocomial pathogen. This is a retrospective chart review describing the outcomes and treatment of 60 cases of CR-Kp bloodstream infections. All CR-Kp isolated from blood cultures were identified retrospectively from the microbiology laboratory from January 2007 to May 2009. Clinical information was collected from the electronic medical record. Patients with 14 day-hospital mortality were compared to those who survived 14 days. The all-cause in-hospital and 14-day mortality for all 60 CR-Kp bloodstream infections was 58.3% and 41.7%, respectively. In this collection, 98% of tested isolates were susceptible in vitro to tigecycline, compared to 86% to colistimethate, 45% to amikacin and 22% to gentamicin. Nine patients died prior to cultures being finalized, and received no therapy active against CR-Kp. In the remaining 51 patients, those who survived to day14 (n=35) were compared to non-survivor at day 14 (n=16). These patients were characterized by both chronic disease and acute illness. The 90-day readmission rate for hospital survivors was 72%. Time to active therapy was not significantly different between survivors and non-survivors, and hospital mortality was also similar regardless of therapy chosen. Pitt bacteremia score was the only significant factor associated with mortality in Cox regression analysis. In summary, CR-Kp bloodstream infections occur in patients who are chronically and acutely ill. They are associated with high 14-day mortality and poor outcomes regardless of tigecycline or other treatment regimens were selected.
Keywords: Klebsiella pneumoniae, carbapenem resistance, multi-drug resistance, mortality, bacteremia
Since the first reported carbapenem-resistant K. pneumoniae (CR-Kp) was identified in 1996, the incidence of infections due to this multi-drug resistant (MDR) pathogen has increased dramatically [1–4]. Currently available penicillins, cephalosporins, and carbapenems do not demonstrate in vitro activity against this organism. Therefore, very few treatment options remain for CR-Kp bloodstream infections (BSI). Tigecycline and colistimethate (colistin) are two agents with in vitro activity against CR-Kp, which are in current clinical use in the United States [5]. However, the low plasma concentrations of tigecycline and the toxicity associated with colistimethate make these less appealing options for use in BSI. In addition to these concerns, the published outcome data for these two agents in the treatment of CR-Kp BSI are limited. The aim of this study was to describe the treatment and outcomes of CR-Kp BSI in a single large tertiary care center.
Methods
Study Location and Design
The study was conducted at Cleveland Clinic, a 1400 bed academic medical center in Cleveland, Ohio. This was a non-interventional, retrospective chart review of CR-Kp BSI from January 2007 to May 2009. This study was approved by the Cleveland Clinic Institutional Review Board.
Microbiologic Methods
Automated systems were used to process blood cultures (BacTAlert®) and for identification of K. pneumoniae isolates (Vitek 2). Antimicrobial susceptibility testing (AST) was performed using the automated broth microdilution system (Vitek 2). Before April 2009, AST for tigecycline and colistimethate was not routinely performed. When applicable, broth microdilution methods were used for tigecycline and colistimethate AST. Interpretive criteria for colistimethate were extrapolated from data on its in vitro activity against strains of Acinetobacter baumanii, as published by the Clinical Laboratory Standards Institute (CLSI) [6]. Tigecycline susceptibility was determined by the use of minimum inhibitory concentration breakpoints approved by the US Food and Drug Administration [7]. A modified Hodge test was performed on all isolates, as described [8]. Testing for the presence of the blaKPC gene was performed in a subset of isolates, as previously described [9].
Patients and Data Collection
All adult patients with blood cultures positive for CR-Kp from January 2007 to May 2009 were included in this study. Only the first episode of BSI was included. To evaluate the effect of therapy, only patients who received in vitro active therapy for more than 24 hours were included in risk factor analysis. Data, including demographics, co-morbid conditions, source of infection, and treatment were collected retrospectively from the electronic medical records and microbiology databases. The need for mechanical ventilation or dialysis was assessed at the time of bacteremia and 30 days prior. The source of BSI was determined from Infection Control designation, following Centers of Disease Control guidelines. If Infection Control was not involved with the case, the Infectious Diseases consult note was used for source determination. Comorbid conditions and severity of illness were measured using the Charlson comorbidity index and Pitt bacteremia score, as described [10–12]. 14-day mortality was the primary outcome of interest. Secondary outcomes included discharge destination (home vs. other), readmission within 90 days of discharge, and microbiological cure, which was defined as documented sterile blood cultures following CR-Kp BSI. Initial regimen was defined as any therapy with in vitro activity given for more than 24 hours within the first 5 days of positive cultures.
Statistical Analysis
All comparisons were unpaired and all tests of significance were two-tailed. Continuous variables were compared using the Student t test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. The chi-squared or Fisher’s exact test were used to compare categorical variables. A multivariate logistic regression model was used in which all stable variables were included which were significant on univariate analysis (p<0.05). A statistical software program (SPSS, version 11.5 for Windows; SPSS, Inc; Chicago, IL) was used to perform all analyses.
Results
During the study period, a total of 60 patients were identified with a CR-Kp BSI. The all-cause hospital and 14-day mortality was 58% (35/60 patients) and 42% (25/60), respectively. Median time to death from positive culture was 9 days, ranging from 1 to 105 days. Nine patients died before cultures were finalized and did not receive therapy active against CR-Kp. These patients were not included in further analysis. Of the 51 patients who received treatment, hospital and 14-day mortality was 51% and 31%. In this group, 14-day survivors (n=35) were compared to 14-day non-survivors (n=16). In non-survivors, 100% (16 of 16) died while receiving CR-Kp-active therapy. Of the patients, who survived their hospitalization 68% (17 of 25) were discharged to a skilled nursing or long-term care facility and only 32% (8 of 25) to home. Readmission within 90 days occurred in 72% (18 of 25) of the hospital survivors.
No significant differences between survivors and non-survivors were observed in baseline characteristics including age, gender, or race (table 1). Comorbid conditions were also similar in survivors vs. non-survivors. The most frequent co-morbid conditions were diabetes mellitus, heart disease, and kidney disease. The Charlson co-morbidity index was not associated with mortality in this cohort – the mean index in 14-day survivors was 3.5±2.1, compared to 3.1±1.5 in 14-day non-survivors. In contrast, severity of acute illness as measured by the Pitt bacteremia score was significantly higher for 14-day non-survivors compared to 14-day survivors (mean 5.6±3.8 vs. 2.9±2.3, p=0.017 in univariate analysis). More patients in the 14-day non-survivors underwent surgery in the previous 30 days, 44% compared to 14% in the 14-day survivors (p=0.033 in univariate analysis). The presence of a central venous catheter was common overall, and there was no difference in removal rates between 14-day survivors and 14-day non-survivors, 89% vs. 75%. Total hospital length of stay was similar between the groups. More 14-day non-survivors were in the ICU at the time of first positive cultures when compared to 14-day survivors (75% versus 40%, p=0.020 in univariate analysis).
Table 1.
Patient Characteristics
| Total | Survivors* | Non-survivors* | p** | |
|---|---|---|---|---|
| Demographics | n=51 | n=35 | n=16 | |
| Age, years, mean ± SD | 60.4±1.8 | 59.1±12.7 | 63.1±13.8 | |
| Gender, Male, n (%) | 32 (63) | 21 (60) | 11 (69) | |
| Race, Caucasian, n (%) | 31 (61) | 23 (66) | 8 (50) | |
| Hospital length of stay, mean ± SD | 31.6±25.0 | 34.0±27.9 | 26.2±16.6 | |
| ICU admission#, n (%) | 26 (51) | 14 (40) | 12 (75) | 0.020 |
| ICU length of stay, mean ± SD | 20.8±19.1 | 23.2±22.2 | 17.4±13.4 | |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 18 (35) | 12 (34) | 6 (38) | |
| CAD | 13 (26) | 7 (20) | 6 (38) | |
| COPD | 6 (12) | 2 (6) | 4 (25) | .07 |
| Chronic kidney disease | 13 (26) | 10 (29) | 3 (19) | |
| End-stage liver disease | 8 (16) | 5 (14) | 3 (19) | |
| Solid organ transplant | 8 (16) | 5 (14) | 3 (19) | |
| Hematologic disorders | 9 (18) | 6 (17) | 3 (19) | |
| Bone marrow transplant | 3 (6) | 3 (9) | 0 (0) | |
| Charlson comorbidity index, mean ± SD | 3.3±2.0 | 3.5±2.1 | 3.1±1.5 | |
| Pitt Bacteremia score, mean ± SD | 3.7±3.1 | 2.9±2.3 | 5.6±3.8 | 0.017 |
| Risk Factors, n(%) | ||||
| Surgery§ | 12 (24) | 5 (14) | 7 (44) | 0.033 |
| Central venous catheter | 47 (92) | 31 (89) | 16 (100) | |
| Central venous catheter removal | 43 (84) | 31 (89) | 12 (75) | |
| Mechanical ventilation | 21 (41) | 12 (34) | 9 (56) | |
| Dialysis | 16 (31) | 9 (26) | 7 (44) | |
survivors at 14 days, non-survivors at 14 days
univariate analysis (p-values >0.1 are not listed).
ICU admission at the time of bacteremia.
surgery within 30 days prior to bacteremia
SD: standard deviation, ICU: Intensive Care Unit, CAD: coronary artery disease, COPD: Chronic obstructive pulmonary disease
The majority of patients acquired their CR-Kp during their hospitalization, 60% (21/35) of 14-day survivors and 88% (14/16) of 14-day non-survivors (p = 0.050). Of, the remaining patients all had a hospitalization within the previous 90 days and the majority (12/16) were admitted from nursing homes or long-term acute care facilities. More than half of cases (55%) were thought to be primary or intravenous catheter-related (table 2). The other most common sources were urinary (14%), pulmonary (12%) and intra-abdominal (12%). There were no differences between 14-day survivors and non-survivors and source of CR-Kp bloodstream infection.
Table 2.
Source of Blood stream infections
| Total n=51 | Survivors n=35 | Non-survivors n=16 | p* | |
|---|---|---|---|---|
| Source, n (%) | ||||
| Primary/Line-related | 28 (55) | 19 (54) | 9 (56) | |
| Secondary | 23 (45) | 16 (46) | 7 (44) | |
| Urinary | 7 (14) | 7 (20) | 0 | |
| Pulmonary | 6 (12) | 3 (9) | 3 (19) | |
| Intra-abdominal | 6 (12) | 4 (11) | 2 (13) | |
| Surgical site | 2 (4) | 1 (3) | 1 (6) | |
| Bone & joint | 1 (2) | 0 (0) | 1 (6) | |
| Multiple | 1 (2) | 1 (3) | 0 (0) | |
univariate analysis.
Antimicrobial susceptibilities of the CR-Kp blood isolates are shown in figure 1. The majority of isolates were susceptible in vitro to tigecycline (98%). In one isolate the tigecycline MIC increased from a baseline of 0.25 mg/L to 1 mg/L on therapy. Out of 21 CR-Kp isolates tested, 3 were resistant to colistimethate (MIC of 8 mg/L). In vitro susceptibility to aminoglycosides was found in only 45% of isolates for amikacin and 22% for gentamicin. All CR-Kp isolates were modified Hodge test positive, and a random sample of CR-Kp isolates from 10 patients were tested for presence of the blaKPC gene, which was found in all isolates tested.
Figure 1.
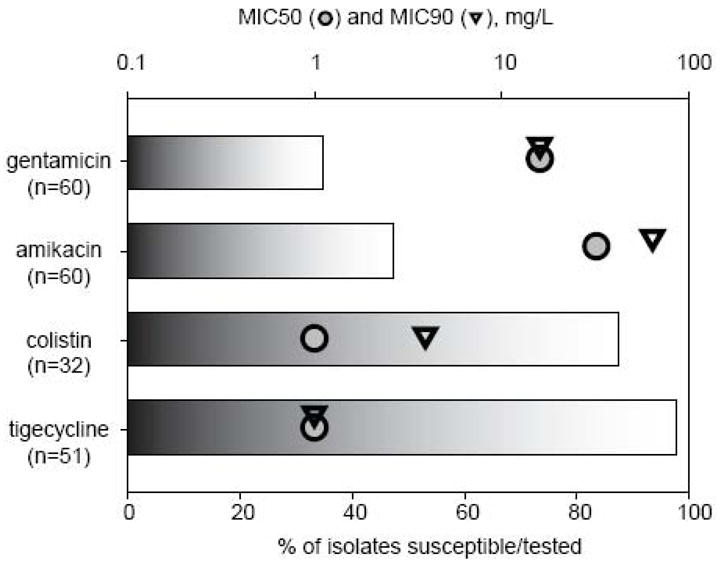
In vitro susceptibility of carbapenem-resistant Klebsiella pneumoniae isolates.
The median number of documented blood cultures positive for CR-Kp per patient was 2 cultures (range 1–11 cultures). Microbiological cure occurred in 74.5% (38 of 51) patients overall, including 7 patients in the 14-day non-survivor group (44%). In the 38 patients with microbiological cure, the median number of days to negative blood culture was 4 days (range 1–18 days). Recurrent CR-Kp BSI (defined as positive CR-Kp blood culture greater than 7 days after documented negative blood culture) was observed in 9 patients.
Treatment in this patient population was characterized by frequent changes and additions to the antibiotic regimen. The mean number of days from time of first positive blood culture to CR-Kp active therapy was 2.5 days (table 3). No difference in time to treatment was seen between 14-day survivors and non-survivors. In this study, 92% of patients received tigecycline at some time during their hospitalization. The total duration of tigecycline therapy was longer in 14-day survivors compared to non-survivors. However, in time-to-event analysis, no difference was seen between patients who received only tigecycline as initial therapy, and all others (figure 2). Initial regimens consisted of tigecycline-based therapies in 71% of 14-day survivors and 56% non-survivors, colistimethate-based for 17% 14-day survivors and 6% non-survivors and tigecycline-colistimethate combinations in 6% 14-day survivors and 25% non-survivors. In univariate survival analysis, differences were not observed between patients who received tigecycline mono-therapy as initial therapy when compared to all others (figure 2). Also, a similar proportion of 14-day survivors and non-survivors were treated with a combination regimen initially (31% versus 56%, p=0.126). In multivariate Cox regression analysis (table 4), a higher Pitt bacteremia score was strongly associated with mortality (HR 1.33 per unit increase, p=0.039).
Table 3.
Treatment**
| Total n=51 | Survivors n=35 | Non-survivors n=16 | p* | |
|---|---|---|---|---|
| Time to active therapy, days, mean ± SD | 2.5 ± 2.2 | 2.6 ± 2.2 | 2.1 ± 2.1 | |
| Tigecycline, n (%) | 47 (92) | 33 (94) | 14 (88) | |
| -Duration, days, mean ± SD | 14.8±11.1 | 19.0±10.7 | 5.6±10.6 | .001 |
| Colistin, n (%) | 19 (37) | 13 (39) | 6 (38) | |
| -Duration, days, mean ± SD | 3.14±5.1 | 3.5±5.6 | 2.3±4.0 | |
| Amikacin, n (%) | 24 (47) | 14 (40) | 10 (63) | |
| -Duration, days, mean ± SD | 3.7±7.0 | 4.2±8.2 | 2.5±3.3 | |
| Gentamicin, n (%) | 2 (4) | 2 (6) | 0 (0) | |
| -Duration, days, mean ± SD | 0.35±2.3 | 0.35±2.3 | - |
Includes antibiotics given at any time after first positive blood culture.
univariate analysis.
SD: standard deviation.
Figure 2.
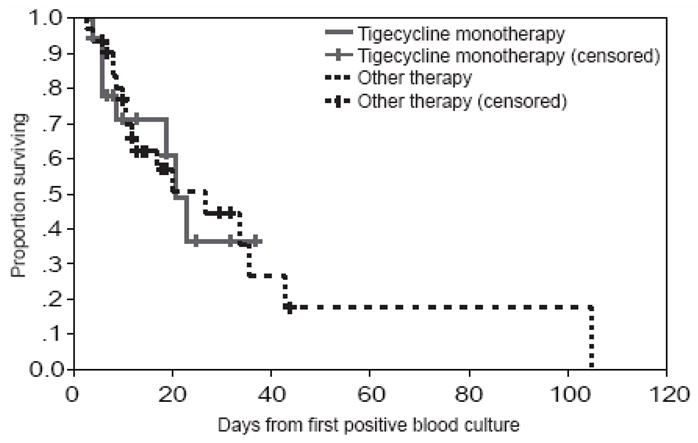
Survival after positive blood culture for carbapenem-resistant Klebsiella pneumoniae (n=51). Nine patients who died prior to cultures being finalized and received no CR-Kp-active therapy were excluded. Data is censored at the time of hospital discharge.
Table 4.
Cox Regression Analysis
| HR (95% CI) | p | |
|---|---|---|
| ICU admission# | 1.46 (0.26–8.10) | .665 |
| Hospital acquired | 2.33 (0.46–11.7) | .305 |
| Mechanical ventilation | 0.16 (0.02–1.19) | .073 |
| Dialysis | 1.02 (0.25–4.13) | .975 |
| COPD | 2.02 (0.53–7.64) | .301 |
| Surgery* | 4.2 (0.77–22.9) | .097 |
| Pitt Bacteremia score** | 1.33 (1.02–1.75) | .039 |
| Primary/line related | 1.79 (0.41–7.90) | .441 |
| Tigecycline monotherapy | 0.76 (0.19–3.08) | .701 |
ICU admission at the time of bacteremia,
surgery within 30 days prior to bacteremia,
HR per unit change.
HR: hazard ratio, ICU: Intensive Care Unit
Discussion
Carbapenem-resistant K. pneumoniae (CR-Kp) isolates are emerging as a cause of MDR gram-negative infections in healthcare settings [2, 3]. Clinically, a limited number of antimicrobial options remain for the treatment of CR-Kp infections, especially BSI. Here, we have summarized the largest reported collection of CR-Kp BSI to date.
The optimal treatment of bacteremia with CR-Kp remains unknown [4]. Randomized trials are not available to compare tigecycline versus colistimethate, which represent the two main treatment options currently available in the US. Colistimethate (also known as polymyxin E) has been available for over 50 years. However, colistimethate use became infrequent after the introduction of aminoglycosides, which had a more favorable side effect profile in comparison. Nephrotoxicity is the most common adverse reaction, occurring in an estimated 45% of patients, although recent data on large patient populations are not available. Neurotoxicity is another concerning side effect [13]. The use of tigecycline for the treatment of BSI remains controversial. The low serum concentrations (mean Cmax 0.63–0.87 mcg/ml) may be suboptimal in this context. This is especially concerning for organisms with higher MICs at or near the breakpoint [7]. Of note, a breakthrough bloodstream infection with carbapenem-resistant Acinetobacter baumanii while on tigecycline therapy has been reported [14]. However, clearance of persistent Cr-KP bacteremia on tigecycline monotherapy has also been reported [15]. Similarly, we observed several patients in whom tigecycline monotherapy resulted in a clinical cure. There are additional reports of outcomes with tigecycline for the treatment of BSI. Eight phase III clinical trials were pooled and analyzed to evaluate tigecycline in the treatment of secondary bacteremias due to skin and skin-structure infections, intra-abdominal infections, and community-acquired pneumonia. Cure rates were similar in patients with BSI with various Gram-negative bacteria in the tigecycline (n=21, cure rate 81%) and the comparator (n=22, cure rate 90.9%) [16]. In addition, there are a few reports on tigecycline for the treatment of BSI due to multi-drug resistant organisms (MDR-O). In a retrospective case series of 12 patients with MDR-O BSI (10 K. pneumoniae and 2 Acinetobacter baumanii) treated with tigecycline, an overall clinical and microbiological success rate of 80% was reported. However, many patients received combination therapy and one patient was persistently bacteremic for 2 weeks prior to responding [17]. In another series of 7 BSI due to MDR A. baumanii, treatment with tigecycline resulted in a positive microbiological outcome in 4 cases. Patients in this series also received concomitant antimicrobials [18]. However, in these small non-randomized studies, as well as in our study, reported outcomes may be unrelated to tigecycline treatment.
Reported risk factors for infection with CR-Kp are similar to risk factors associated with other MDR-O including extended spectrum β-lactamase producing K. pneumoniae [19–21]. These risk factors include higher severity of illness, recent solid organ or stem-cell transplant, mechanical ventilation, longer length of stay and prior antimicrobial exposure to cephalosporins, fluoroquinolones and carbapenems While risk factor analysis for infection with MDR-O was not the focus of our study, we did observe that patients with CR-Kp BSI in our cohort were both chronically ill, as measured by mean Charlson comorbidity index of 3.3±2.0, and acutely ill, as evident by high Pitt bacteremia scores. The Pitt bacteremia score is a marker of severity of illness in patients with gram-negative BSI and patients with scores > 4 are considered critically ill [11, 12]. Similar to previous studies, in our cohort the Pitt bacteremia score was strongly correlated with mortality. Awareness of risk factors for MDR infections, including CR-Kp BSIs, is important in order to reduce time to appropriate treatment. While there was no significant difference in time to active therapy between groups in our study, the delay in appropriate therapy may, in part, explain the high mortality rate.
The in vitro susceptibility profile of CR-Kp isolates has been previously reported in the literature. A report from Brooklyn hospitals on 96 clinical isolates of carbapenemase-producing K. pneumoniae and a series of 60 carbapenemase-producing Klebsiella species from the SENTRY Antimicrobial Surveillance Program showed a susceptibilities of 100% and 100% for tigecycline, 91% and 93% for polymyxin B, and 45% and 53% for amikacin, respectively [5, 22]. In contrast, in our isolates, as well as in other more recent studies, the reported percentage of isolates with in vitro susceptibility to tigecycline and colistimethate is between 15% to 98%, and 80% to 86%, respectively [23–25]. In addition, reports are emerging on CR-Kp isolates which display in vitro resistance to both tigecycline and colistimethate [26]. Per recent reports, around 50% of CR-Kp isolates remain susceptible to fosfomycin, which is not currently available for intravenous use in the United States [23, 24]. Recently reported In vitro results for combination therapy which includes doripenem, rifampin, and polymyxin B are promising, but this combination has not been evaluated in clinical settings [27].
CR-Kp infections are associated with both high morbidity and mortality. The overall hospital mortality of 58% reported in our study is consistent with previous reports. The largest series of CR-Kp BSI (n=32) to date is from Borer et al., who reported a crude and attributable mortality rate of 71.9% and 50%, respectively. No comments on treatment were made in that study [25]. In a New York City outbreak of CR-Kp infections, 19 patients with BSI were reported. The14-day mortality in this cohort was 47% [28]. In reports of KPC-2 carbapenemase-producing K. pneumoniae BSI only 2 of 6 patients, and 4 of 14 patients survived [23, 29]. In addition to a high mortality rate, we noted a high readmission rate in those patients who were discharged after CR-Kp BSI. This was likely a consequence of severe underlying disease.
The limitations of our study are inherent to its retrospective design and the relatively limited number of occurrences of this disease. Evaluation the relative efficacy of the various regimens is extremely limited, since the choice of treatment in our cohort was not randomly assigned. In addition, the care of these critically ill patients is often characterized by frequent alterations in the antibiotic regimen. Therefore, choice of antibiotic regimen is closely associated with clinical status. Similarly, the observed difference in tigecycline duration between survivors and non-survivors is likely being driven by patients dying prior to completing their course, rather than it being an indication of efficacy of prolonged tigecycline therapy. Consistent with this notion, no significant differences between treatment regimens were observed in time-to-event analyses.
In summary, CR-Kp BSI are an increasingly important threat for severely and chronically ill hospitalized patients. Few antibiotic options remain for these organisms. Overall, regardless of chosen treatment, CR-Kp BSI were associated with high hospital mortality, and high readmission rates in those who survive the initial hospitalization.
Acknowledgments
Financial Support: RAB is supported by the following:
Veterans Affairs Merit Review Program
VISN 10 Geriatric Research Education and Clinical Care Center
National Institutes of Health (RO1 AI063517-01 and 1R01AI072219-01A1).
Footnotes
Potential Conflicts of Interest: DvD is a member of the speakers’ bureaus for Pfizer Inc. and Astellas Pharmaceuticals, and has served on the advisory board for Pfizer Inc.
RAB has received research funding from Pfizer Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45:1151–61. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–36. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 3.Schwaber MJ, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: a potential threat. Jama. 2008;300:2911–3. doi: 10.1001/jama.2008.896. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother. 2010 doi: 10.1093/jac/dkq108. Epub April 8, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Bratu S, Tolaney P, Karumudi U, et al. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J Antimicrob Chemother. 2005;56:128–32. doi: 10.1093/jac/dki175. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Nineteenth Informational Supplement. 2009 CLSI document M100–S19. [Google Scholar]
- 7.Tigecycline package insert. Wyeth Pharmaceuticals; Madison, NJ: [Accessed 2/24/2010]. available at: http://www.wyeth.com/content/showlabeling.asp?id=491. [Google Scholar]
- 8.Lee K, Chong Y, Shin HB, et al. Modified Hodge and EDTA-disk synergy tests to screen metallo-beta-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001;7:88–91. doi: 10.1046/j.1469-0691.2001.00204.x. [DOI] [PubMed] [Google Scholar]
- 9.Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother. 2009;63:427–37. doi: 10.1093/jac/dkn547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents. 1999;11:7–12. doi: 10.1016/s0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 12.Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–90. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 13.Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22:535–43. doi: 10.1097/QCO.0b013e328332e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peleg AY, Potoski BA, Rea R, et al. Acinetobacter baumannii bloodstream infection while receiving tigecycline: a cautionary report. J Antimicrob Chemother. 2007;59:128–31. doi: 10.1093/jac/dkl441. [DOI] [PubMed] [Google Scholar]
- 15.Cobo J, Morosini MI, Pintado V, et al. Use of tigecycline for the treatment of prolonged bacteremia due to a multiresistant VIM-1 and SHV-12 beta--lactamase-producing Klebsiella pneumoniae epidemic clone. Diagn Microbiol Infect Dis. 2008;60:319–22. doi: 10.1016/j.diagmicrobio.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Gardiner D, Dukart G, Cooper A, et al. Safety and efficacy of intravenous tigecycline in subjects with secondary bacteremia: pooled results from 8 phase III clinical trials. Clin Infect Dis. 2010;50:229–38. doi: 10.1086/648720. [DOI] [PubMed] [Google Scholar]
- 17.Poulakou G, Kontopidou FV, Paramythiotou E, et al. Tigecycline in the treatment of infections from multi-drug resistant gram-negative pathogens. J Infect. 2009;58:273–84. doi: 10.1016/j.jinf.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Gordon NC, Wareham DW. A review of clinical and microbiological outcomes following treatment of infections involving multidrug-resistant Acinetobacter baumannii with tigecycline. J Antimicrob Chemother. 2009;63:775–80. doi: 10.1093/jac/dkn555. [DOI] [PubMed] [Google Scholar]
- 19.Patel G, Huprikar S, Factor SH, et al. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 20.Gasink LB, Edelstein PH, Lautenbach E, et al. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae Infect Control Hosp Epidemiol. 2009;30:1180–5. doi: 10.1086/648451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Ami R, Rodriguez-Bano J, Arslan H, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–90. doi: 10.1086/604713. [DOI] [PubMed] [Google Scholar]
- 22.Castanheira M, Sader HS, Deshpande LM, et al. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-beta-lactamase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2008;52:570–3. doi: 10.1128/AAC.01114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souli M, Galani I, Antoniadou A, et al. An outbreak of infection due to beta-Lactamase Klebsiella pneumoniae Carbapenemase 2-producing K. pneumoniae in a Greek University Hospital: molecular characterization, epidemiology, and outcomes. Clin Infect Dis. 2010;50:364–73. doi: 10.1086/649865. [DOI] [PubMed] [Google Scholar]
- 24.Falagas ME, Maraki S, Karageorgopoulos DE, et al. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents. 2010;35:240–243. doi: 10.1016/j.ijantimicag.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30:972–6. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 26.Elemam A, Rahimian J, Mandell W. Infection with panresistant Klebsiella pneumoniae: a report of 2 cases and a brief review of the literature. Clin Infect Dis. 2009;49:271–4. doi: 10.1086/600042. [DOI] [PubMed] [Google Scholar]
- 27.Urban C, Mariano N, Rahal JJ. In Vitro Double and Triple Bactericidal Activities of Doripenem, Polymyxin B and Rifampin against Multidrug-Resistant Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother. 2010 doi: 10.1128/AAC.01768-09. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med. 2005;165:1430–5. doi: 10.1001/archinte.165.12.1430. [DOI] [PubMed] [Google Scholar]
- 29.Nadkarni AS, Schliep T, Khan L, et al. Cluster of bloodstream infections caused by KPC-2 carbapenemase-producing Klebsiella pneumoniae in Manhattan. Am J Infect Control. 2009;37:121–6. doi: 10.1016/j.ajic.2007.10.013. [DOI] [PubMed] [Google Scholar]


