Abstract
BACKGROUND AND PURPOSE
The P2Y1 receptor promotes chloride secretion in epithelial cells, a process critical for regulation of extracellular ion and fluid levels. Here we have examined the role of phosphorylation in agonist-induced internalization of P2Y1 receptors.
EXPERIMENTAL APPROACH
A high-affinity radiolabelled antagonist, MRS2500, was used to quantify cell surface-binding sites of P2Y1 receptors in Madin-Darby canine kidney (MDCK) epithelial cells, following exposure to agonists. The regions in the carboxyl terminus involved in both agonist-induced internalization of the receptor and its phosphorylation were identified by mutational analysis.
KEY RESULTS
Endogenous and stably expressed recombinant P2Y1 receptors rapidly internalized with similar time courses in response to agonist in MDCK cells, ensuring that the levels of recombinant receptor achieved by retroviral infection did not adversely affect function of the internalization machinery. Four protein kinase C inhibitors of varying specificity did not affect internalization of recombinant receptors. Agonist-promoted internalization of a series of truncated P2Y1 receptors identified a region between residues 349 and 359 in the carboxyl terminus as critical for regulation. Two amino acids within this region, Ser352 and Ser354, were shown to be both necessary and sufficient for agonist-promoted receptor phosphorylation and internalization.
CONCLUSIONS AND IMPLICATIONS
Our results firmly establish Ser352 and Ser354 in the carboxyl terminus of P2Y1 receptors as critical residues for agonist-induced receptor internalization in MDCK cells. As the mechanism mediating this regulation requires phosphorylation of these key residues, the relevant receptor-regulated protein kinase can now be identified.
Keywords: P2Y1 receptor, agonist-promoted internalization, agonist-promoted phosphorylation, MDCK cells, MRS2500, protein kinase C, radioligand binding
Introduction
A clear understanding of the mechanisms governing agonist-promoted regulation of G protein-coupled receptors (GPCRs) is critical for understanding the actions of current and potential therapeutic agents. Cells have evolved a number of ways to regulate agonist-promoted signalling of these receptors, including a system of receptor desensitization and internalization, which prevents further coupling to and activation of G proteins. The current model for GPCR desensitization and internalization, involving phosphorylation by GPCR kinases, arrestin binding and internalization into clathrin-coated pits, is largely based on studies of the β2-adrenoceptor. However, many other GPCRs desensitize and internalize by other mechanisms (Waugh et al., 1999; Bhattacharyya et al., 2002; Paing et al., 2002; Pierce et al., 2002).
The P2Y family of GPCRs is activated by extracellular adenine and uridine nucleoside di- and triphosphates and nucleotide sugars. The eight recognized mammalian P2Y receptors are subclassified based on sequence homology and G protein coupling. P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptors (receptor nomenclature follows Alexander et al., 2009) are members of the P2Y1 subfamily of receptors that promote inositol lipid signalling through Gαq-dependent activation of phospholipase C-β. P2Y12, P2Y13 and P2Y14 receptors are members of the P2Y12 subfamily of receptors that couple to the Gi family of Gα subunits to inhibit adenylyl cyclase activity (Costanzi et al., 2004; Abbracchio et al., 2006).
The ADP-activated P2Y1 and P2Y12 receptors are expressed in platelets and mediate ADP-promoted platelet aggregation (Hechler et al., 1998; Jin et al., 1998; Jantzen et al., 1999; Hollopeter et al., 2001). We showed recently that the P2Y1 receptor in platelets desensitizes very rapidly (t1/2∼18 s) in response to the selective agonist (N)-methanocarba-2-methylthioadenosine-diphosphate (MRS2365) (Bourdon et al., 2006). Others have reported that the P2Y1 receptor is phosphorylated in an agonist-dependent manner and undergoes rapid, reversible internalization in human platelets and 1321N1 astrocytoma cells. Both conventional and novel isoforms of protein kinase C (PKC), but not GPCR kinases, have been implicated (Baurand et al., 2000; Hardy et al., 2005; Mundell et al., 2006).
We have described the synthesis and utility of two high-affinity radioligands ([32P]MRS2500 and [125I]MRS2500) with identical binding properties for quantification of P2Y1 receptor-binding sites in mammalian tissues (Houston et al., 2006; Ohlmann et al., 2010). Here, we apply radiolabelled MRS2500 as a probe in studies designed to determine the molecular determinants of agonist-induced internalization of the P2Y1 receptor. Agonist-promoted loss of surface-binding sites of both endogenous and stably expressed receptors was quantified directly, and Ser352 and Ser354 in the carboxyl terminus were identified as important sites for both agonist-promoted phosphorylation and consequent internalization of P2Y1 receptors. A preliminary account of these data has been published elsewhere (Morris-Glast et al., 2008).
Methods
Construction of mutant P2Y1 receptor cDNAs
The human P2Y1 receptor was cloned into the pLXSN retroviral expression vector with an amino-terminal HA-epitope tag (YPYDVPDYA) following the initiating methionine residue as described by Wolff et al. (2005). Unpublished studies have demonstrated that incorporation of an amino-terminal HA-epitope tag does not interfere with P2Y1 receptor function. Truncation mutants were constructed by PCR amplification using Pfu polymerase (Stratagene, La Jolla, CA, USA) with a 5′ primer containing an EcoRI restriction site and 3′ primers containing a stop codon following Thr339 (P2Y1-339Z), Asn349 (P2Y1-349Z), Asn359 (P2Y1-359Z) or Gly369 (P2Y1-369Z) and an XhoI restriction site to facilitate cloning into similarly digested pLXSN.
The P2Y1-340/0P mutant receptor was constructed using 5′ and 3′ primers up to 60 bases in length in which all Ser and Thr codons were mutated to Ala. The sense and antisense primers contained XhoI and BamHI restriction sites, respectively, at their 3′ ends. The primers, which overlapped by 18 bases, were annealed, filled-in with the Klenow fragment of DNA polymerase, and then digested and ligated into the P2Y1-339Z truncation mutant containing a silent mutation that incorporated a XhoI site at codons 336–338. Mutation of individual residues S343A, S346A, S352A, S354A and T354A, the double residue mutants S352A/S354A and S343A/S346A, and the triple residue mutant S352A/S354A/T358A were constructed using the Stratagene QuikChange Mutagenesis Kit (Stratagene) with HA-P2Y1 in pLXSN as the template and reverse complementary primers between 30 and 45 bases in length containing alanine codon substitutions at the indicated sites. Constructs were confirmed by DNA sequencing at the UNC DNA Sequencing facility and purified using a Purelink DNA purification kit (Invitrogen, Carlsbad, CA, USA).
Cell culture and expression of receptor constructs
Madin-Darby canine kidney (MDCK) epithelial cells were maintained in 50/50 Dulbecco's modified Eagle medium (DMEM)/F12 medium (Invitrogen) supplemented with 5% fetal bovine serum (Sigma, St. Louis, MO, USA). PA317 retroviral packaging cells were maintained in DMEM supplemented with 10% fetal bovine serum. Recombinant receptor constructs were stably expressed in MDCK cells by retroviral infection of target cells using the method of Comstock et al. (1997) as described previously (Qi et al., 2001). Virally infected cells were selected in medium containing 1 mg·mL−1 G418 antibiotic (Invitrogen). After approximately 7 days in culture, the concentration of G418 was reduced to 0.2 mg·mL−1, and cells were cultured as above and used for experiments.
Confocal microscopy of P2Y1 receptors in MDCK cells
MDCK-P2Y1 cells expressing the HA-P2Y1 receptor were plated on glass cover slips at 20–30% confluence and allowed to grow for 24 h. The cells were cooled to 4°C, labelled with mouse HA.11 antibody (Covance, Princeton, NJ, USA) and washed to remove excess antibody. The temperature was then increased to 37°C and the cells were treated with either phosphate-buffered saline (PBS) or 100 µM ADP for 30 min. The cells were then fixed with 4% paraformaldehyde, permeabilized with ice-cold MeOH for 30 s, and then incubated with AlexaFluor 488 goat anti-mouse antibody (Invitrogen/Molecular Probes, Eugene, OR, USA). Cells were examined by confocal microscopy on an Olympus Fluoview 300 laser scanning confocal imaging system (Melville, NY, USA) configured with an IX70 fluorescence microscope fitted with a PlanApo 60X oil objective. Quantification of confocal images was accomplished with MetaMorph software as described (Wolff et al., 2010).
Intact cell binding assays with radiolabelled MRS2500
MRS2500 is a selective high affinity antagonist of the P2Y1 receptor (Kim et al., 2003), and we recently developed 32P- (Houston et al., 2006) and 125I- (Ohlmann et al., 2010) labelled forms of this molecule for quantification of the P2Y1 receptor in radioligand binding assays. The binding properties of these two radioligands are identical; therefore, [32P]MRS2500 (6000 Ci·mmol−1) and [125I]MRS2500 (2200 Ci·mmol−1) have been used interchangeably in the current study, and to avoid confusion, we do not usually designate whether individual binding assays were carried out with the 32P- or 125I-labelled radioligand. [32P]MRS2500 (Houston et al., 2006) and [125I]MRS2500 (Ohlmann et al., 2010) were prepared as previously described. Intact cell binding assays were carried out with wild-type MDCK cells plated in 12-well plates. Intact cell binding assays with the various MDCK cell lines generated after infection with pLXSN vectors for wild-type or mutant P2Y1 receptors were carried out in 24-well plates, except when directly comparing wild-type and recombinant receptor levels, in which case 12-well plates were used. Assays were carried out approximately 24 h after plating, when cell densities were approximately 80–90% of confluence. The medium was replaced with 1 mL of assay buffer (DMEM/F12 medium supplemented with 20 mM HEPES, pH 7.4), and the culture plates were placed in a 37°C water bath for 15 min prior to experiments. Receptor internalization was initiated by addition of maximally effective concentrations of agonist [usually 10 µM 2-methyl thioadenosine 5′-diphosphate (2MeSADP) or 100 µM ADP], and incubations were terminated by placing the culture plates on an ice-water slurry and adding 8 mL of ice-cold wash buffer (1% BSA in PBS, pH 7.4). Cells were washed two additional times with 8 mL of ice-cold buffer. Intact cell binding assays were carried out by adding 0.1–0.5 nM of radiolabelled MRS2500 in 200 µL (24-well plates) or 500 µL (12-well plates) of ice-cold assay buffer. Non-specific binding was determined for each condition by including 100 µM MRS2179 (Tocris-Cookson, Ellisville, MO, USA). Steady-state binding is attained under these assay conditions within 10 min, and therefore control and agonist-preincubated cells were incubated with radiolabelled MRS2500 for 10 min prior to removal of the medium and addition and rapid aspiration of 8 mL of ice-cold wash buffer. Cells were removed from the cell surface by addition of a solution of 1% SDS in PBS, and radioactivity was quantified in either a scintillation or gamma counter.
Experiments quantifying the reappearance of P2Y1 receptors at the cell surface following incubation with ADP for various times were carried out as described above but with modifications. Briefly, cells were incubated with ADP for up to 60 min with 100 µM ADP to initiate internalization. At the end of the incubation, 0.1 U of potato apyrase (Sigma-Aldrich, St. Louis, MO, USA) was added directly to the cell medium to hydrolyse ADP (this amount of apyrase is enough to completely hydrolyse 100 µM ADP to AMP within 1 min). The cells were incubated for a further 30 or 60 min, and cell surface P2Y1 receptors were quantified as described above.
Phosphorylation of HA-P2Y1 receptors
MDCK cells were seeded at a density of 2.5 × 106 cells per 10 cm dish. After 48 h, cells were incubated first in phosphate-free DMEM for 1 h at 37°C and then with 1 mCi [32P]-orthophosphate for 3 h at 37°C. The cells were challenged with either buffer or 100 µM ADP for 5 min and immediately placed on ice. Cells were lysed in RIPA buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with protease and phosphatase inhibitors. Receptor protein was immunoprecipitated with anti-HA.11 monoclonal antibody and Protein A/G beads, and then combined with SDS gel loading buffer. A portion (80%) of the sample was subjected to SDS-PAGE on a 10% gel, and incorporation of 32P into the receptor was detected by exposing the fixed and dried gel to X-OMAT film for 6–18 h at −80°C. The remaining 20% of the sample was analysed by electrophoresis on a 10% gel and receptor protein was detected by Western blotting following transfer of proteins from the gel to a PVDF support.
Data analysis
Data are presented as the mean ± SEM from a data set from a typical experiment repeated at least three times with similar results, except for the experiments quantifying the reappearance of P2Y, receptors at the cell surface, which were repeated twice. Data were analysed using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Results
Our laboratory and others have utilized MDCK cells as a model system to study the membrane targeting and cell signalling properties of P2Y receptors. These cells endogenously express low levels of the P2Y1 receptor (Wolff et al., 2005), and therefore provide a system in which agonist-induced trafficking of recombinant wild-type and mutant receptors can be compared to P2Y1 receptors constitutively expressed in a native environment.
MRS2500 is a selective, high-affinity P2Y1 receptor antagonist (Kim et al., 2003) that we recently labelled with 32P (Houston et al., 2006) or 125I (Ohlmann et al., 2010) for application as a radioligand for quantification of P2Y1 receptors in a variety of mammalian cells and tissues. In this study, we have utilized labelled MRS2500 to investigate agonist-promoted trafficking of the P2Y1 receptor in MDCK cells. As illustrated in Table 1A, the level of specific MRS2500 binding observed with intact wild-type MDCK cells was approximately eightfold less than in MDCK cells in which the P2Y1 receptor was overexpressed by retroviral infection (MDCK-P2Y1 cells). The P2Y1 receptor antagonist, MRS2179, and the P2Y1 receptor agonist, 2MeSADP, inhibited MRS2500 binding to both wild-type or MDCK-P2Y1 cells with potencies similar to those we previously reported for a variety of P2Y1 receptor-expressing tissues (data not shown). Given the high hydrophilicity of this bisphosphate radioligand, we expect that essentially all MRS2500 binding detected in these assays is from proteins on the cell surface.
Table 1.
Binding of MRS2500 to wild-type and P2Y1 receptor-expressing MDCK cells
| Cell line | Total binding (cpm) | Non-specific binding (cpm) | Specific binding (fmol) |
|---|---|---|---|
| A | |||
| MDCK | 565 | 300 | 0.056 |
| MDCK-P2Y1 | 2455 | 390 | 0.43 |
| B | |||
| MDCK-P2Y1 | 4325 | 1390 | 0.61 |
| MDCK-P2Y1-339Z | 5370 | 1415 | 0.82 |
| MDCK-P2Y1-S352A/S354A | 4745 | 1355 | 0.70 |
Binding reactions were carried out in a 12-well culture dish (∼0.9 × 106 cells) with 0.1 nM [125I]MRS2500 [a concentration well below saturating levels (Ohlmann et al., 2010)]. Non-specific binding was determined in the presence of 100 µM 2MeSADP. The data are representative of results obtained in many experiments with each of the cell lines studied. MDCK, Madin-Darby canine kidney.
Agonist-promoted changes in MRS2500-binding sites were quantified in intact cell-binding assays with wild-type (Figure 1A) and P2Y1 receptor-overexpressing (Figure 1B) MDCK cells. Cells were incubated for various times at 37°C with 10 µM 2MeSADP, washed with ice-cold buffer to remove agonist, and incubated with radiolabelled MRS2500 on ice under conditions in which steady-state binding was obtained. Incubation with 2MeSADP caused a rapid decrease (t1/2∼8 min) in MRS2500-binding sites, and a similar time course and extent of receptor loss was observed with wild-type and recombinant receptors. No decrease in MRS2500-binding sites occurred in cells preincubated with agonist on ice instead of 37°C (data not shown). The potency of 2MeSADP (EC50= 46 nM) for promoting loss of MRS2500-binding sites (data not shown) was similar to the potency of this agonist (EC50= 13 nM) for activation of the P2Y1 receptor (Schachter et al., 1996) and to its Ki (50 nM) determined in radioligand-binding experiments (Houston et al., 2006). As illustrated in Figure 1C, P2Y1 receptors were predominantly localized at the plasma membrane in MDCK cells (98% by image quantification) stably expressing HA-tagged P2Y1 receptors. However, a 30 min incubation with 100 µM ADP resulted in the marked redistribution of receptors (47% by image quantification) to intracellular vesicles.
Figure 1.
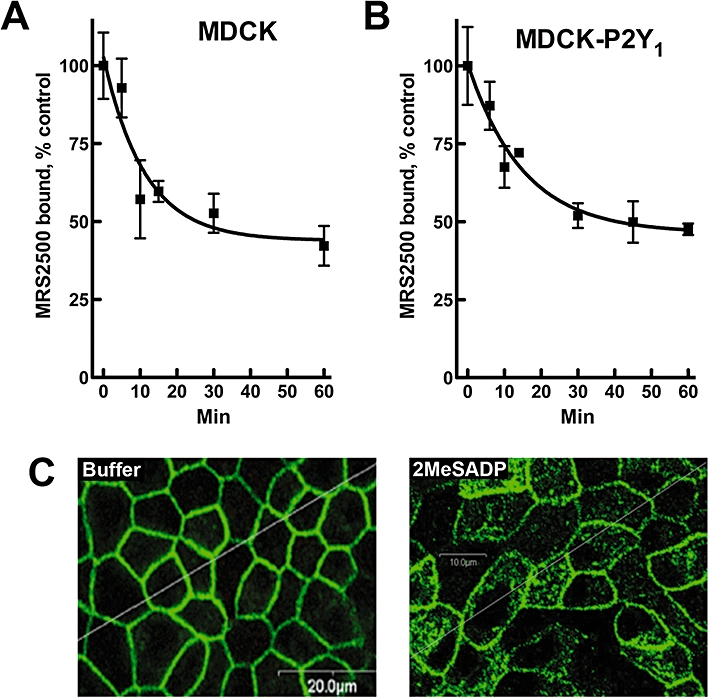
Agonist-promoted loss of radiolabelled MRS2500 surface-binding sites in Madin-Darby canine kidney (MDCK) cells. MDCK cells expressing endogenous (A) or recombinant (B) P2Y1 receptors were incubated with 10 µM 2MeSADP for the indicated times. Cells were chilled to 4°C, washed thoroughly and surface-binding sites were quantified with MRS2500. All values are normalized to the total amount of MRS2500 binding in the absence of agonist treatment. Data shown are means of triplicate samples ± SEM from a representative experiment (n= 3). (C) Internalization of the HA-tagged P2Y1 receptor in MDCK-P2Y1 cells was determined by confocal microscopy. Cells were treated with buffer (left panel) or 100 µM ADP (right panel) for 30 min before fixing and immunostaining. Quantitative image analysis revealed a surface : internalized ratio of staining of 98:2 in the absence of agonist and 53:47 in the presence of agonist.
The reversibility of the agonist-induced loss of MRS2500-binding sites also was examined (Figure 2). MDCK-P2Y1 cells were preincubated with either vehicle (0 min) or 100 µM ADP for various times prior to addition of potato apyrase, a soluble nucleotidase that rapidly converts ADP to AMP under the conditions of these assays. Preincubation of cells with agonist for 60 min resulted in an ∼80% decrease in MRS2500-binding sites, and addition of apyrase resulted in recovery of approximately one-half of these receptors over the ensuing 60 min. Importantly, apyrase did not affect the number of MRS2500-binding sites when added to cells in the absence of agonist pretreatment, and the extent of recovery of binding sites was dependent on the time of preincubation with agonist. Thus, binding recovered to essentially control levels after addition of apyrase to cells pretreated for only 5 min with ADP, but this clearly was not the case with longer periods of preincubation with agonist.
Figure 2.
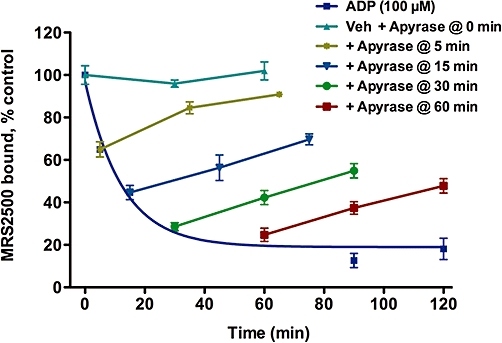
Reversibility of agonist-promoted loss of cell surface P2Y1 receptors. Madin-Darby canine kidney cells expressing recombinant P2Y1 receptors were incubated with buffer (0 min) or 100 µM ADP for 5, 15, 30, 60, 90 or 120 min. Potato apyrase (0.1 U) then was added to rapidly hydrolyse the ADP (including buffer-treated cells), the cells were allowed to recover for 30 or 60 min, and the levels of cell surface P2Y1 receptor-binding sites were quantified by MRS2500 binding. All values were normalized to the total amount of MRS2500 bound in the absence of agonist treatment. Data shown are means of triplicate samples ± SEM from a representative experiment (n= 2).
Taken together these results are consistent with the conclusion that radiolabelled MRS2500 detects cell surface P2Y1 receptors, and that both native and overexpressed P2Y1 receptors undergo agonist-promoted internalization to an intracellular compartment of MDCK cells. Importantly, agonist-promoted trafficking of recombinant P2Y1 receptors is similar to that of endogenous P2Y1 receptors, which validates this system for the evaluation of receptor mutants. The fact that the agonist-induced loss of MRS2500-binding sites observed after 5 min of incubation with agonist is reversible upon removal of ADP from the medium also is consistent with a model in which agonists promote trafficking of P2Y1 receptors to an intracellular compartment that is inaccessible to radioligand. Upon removal of agonist, these receptors are trafficked back to the cell surface.
Clathrin-mediated endocytosis has been suggested to occur for many GPCRs including P2Y1 receptors in human platelets (Baurand et al., 2005; Mundell et al., 2008). To determine if internalization of the P2Y1 receptor in MDCK cells occurs through a clathrin-dependent mechanism, we examined the effects of monodansylcadaverine (MDC) and dynasore, two drugs that inhibit clathrin-mediated processes (Schutze et al., 1999; Paing et al., 2002; Kirchhausen et al., 2008). The presence of either compound decreased agonist-induced loss of cell surface receptors by 30–40% (Figure 3). Incubation with a high concentration of sucrose, which also inhibits the formation of clathrin-coated pits (Heuser and Anderson, 1989), completely prevented agonist induced loss of MRS2500-binding sites in MDCK-P2Y1 cells (data not shown). These data are consistent with the idea that clathrin-coated pit formation contributes to internalization of P2Y1 receptors in MDCK cells.
Figure 3.
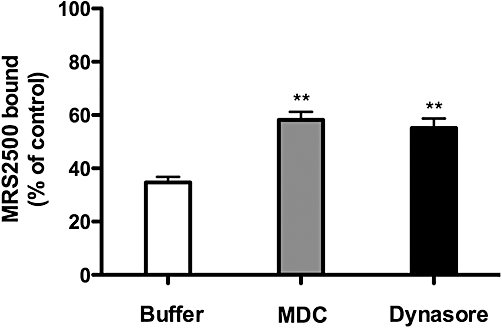
Internalization of P2Y1 receptors in Madin-Darby canine kidney (MDCK) cells is dynamin- and clathrin-dependent. MDCK cells stably expressing recombinant P2Y1 receptors were incubated with 10 µM 2MeSADP for 30 min in the absence (Buffer) or presence of monodansylcadaverine (MDC; an inhibitor of clathrin endocytosis) or dynasore (an inhibitor of the dynamin GTPase). MRS2500-binding sites were then quantified. All values are normalized to the total amount of radiolabelled MRS2500 binding in the absence of agonist treatment. Data shown are means of triplicate samples ± SEM from a representative experiment (n= 3). **P < 0.01.
Agonist-promoted internalization of GPCR predictably occurs as a consequence of phosphorylation of activated receptors, and conventional and novel isoforms of PKC were reported to promote agonist-dependent phosphorylation and internalization of the P2Y1 receptor in human platelets and 1321N1 astrocytoma cells (Mundell et al., 2006). To determine if PKC isozymes are involved in P2Y1 receptor internalization in MDCK cells, we examined the capacities of four different PKC inhibitors to block agonist-induced internalization. MDCK-P2Y1 cells were preincubated for 10 min with vehicle or with 1 µM GF109203X (a pan-PKC inhibitor), 10 µM Gö6983 (inhibits PKC-α, -β, -γ, -δ and -ζ isoforms), 10 µM Ro31-8245 (inhibits PKC-α, -βI, -βII, -γ and -ε) or 10 µM rottlerin (selective for PKC-δ), and the incubation then was continued in the presence or absence of 100 µM ADP for an additional 30 min. ADP promoted an approximately 65% decrease in MRS2500-binding sites on intact cells under these conditions, and this level of agonist-induced internalization was not altered by any of the PKC inhibitors (Figure 4A). In contrast, three of the four inhibitors markedly inhibited the capacity of phorbol 12-myristate 13-acetate (PMA) to promote phosphorylation of ERK1/2, indicating that a considerable inhibition of PKC was achieved under the conditions of this experiment (Figure 4B). These data suggest that PKC does not play a major role in agonist-induced internalization of the P2Y1 receptor in MDCK cells.
Figure 4.
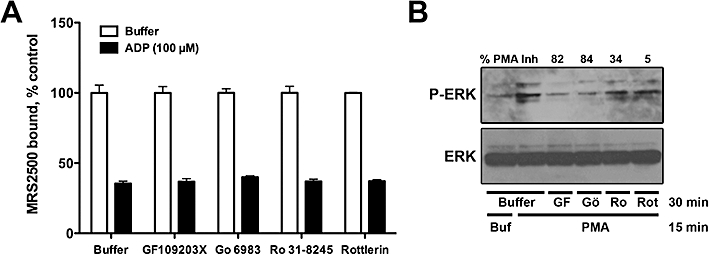
Internalization of P2Y1 receptors in Madin-Darby canine kidney (MDCK) cells is independent of protein kinase C (PKC). MDCK cells stably expressing recombinant P2Y1 receptors were preincubated for 10 min with the PKC inhibitors GF109203X (1 µM), Gö6983 (10 µM), Ro31-8245 (10 µM) or rottlerin (10 µM), following which either buffer or 100 µM ADP was added and the cells were further incubated for 30 min. MRS2500-binding sites were then quantified. All values are normalized to the total amount of radiolabelled MRS2500 binding in the absence of agonist treatment. Data shown are means of triplicate samples ± SEM from a representative experiment (n= 3). (B) The capacity of the PKC inhibitors to block phorbol 12-myristate 13-acetate (PMA)-promoted ERK phosphorylation in MDCK cells is shown. The inhibitors were used at the same concentrations as in (A). Per cent inhibition of PMA-promoted ERK phosphorylation is shown for each inhibitor (n= 3).
We hypothesized that agonist-induced phosphorylation of a residue(s) in the carboxyl terminus plays a key role in agonist-promoted internalization of the P2Y1 receptor, and a potential role was investigated for the nine Ser and Thr residues in this region. A series of mutants was constructed that progressively truncated the carboxyl terminus (Figure 5), and these P2Y1 receptor mutants were stably expressed in MDCK cells by retroviral infection. The amount of specific MRS2500 binding determined for each cell line was similar to that observed for MDCK cells stably expressing the wild-type P2Y1 receptor (data not shown), and the EC50 values of 2MeSADP for stimulation of inositol lipid signalling also were similar for each of these truncation mutants (data not shown).
Figure 5.
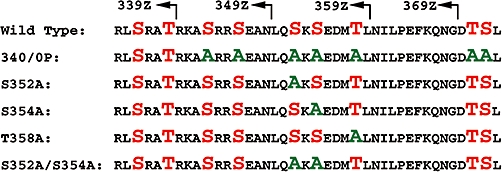
P2Y1 mutant constructs expressed in Madin-Darby canine kidney cells. Ser and Thr residues in the carboxyl tail of the wild-type receptor are highlighted in red, and those residues mutated to alanine are highlighted in green. Numbered residues indicate the last residue before insertion of a stop codon in the truncation mutants (arrows).
Intact cell MRS2500-binding assays were utilized to determine whether these truncation mutants differed in their capacity to undergo agonist-promoted internalization during a 30 min incubation with 30 µM 2MeSADP (Figure 6A). P2Y1-369Z retains all Ser and Thr residues but lacks the Class I PDZ-binding motif, Asp-Thr-Ser-Leu (-DTSL), at the extreme C-terminus. The Na+/H+ exchanger regulatory protein, NHERF, binds to this region and was proposed to act as a scaffold for the receptor and its downstream effector, phospholipase C-β1 (Fam et al., 2005). PDZ ligands in GPCRs also have been suggested to play a role in surface retention time and targeting to subsets of clathrin-coated pits (Puthenveedu and von Zastrow, 2006). However, the degree of agonist-promoted internalization of P2Y1-369Z was similar to that observed with the wild-type receptor, and therefore, the PDZ-binding motif is not directly involved in agonist-promoted internalization of the P2Y1 receptor in MDCK cells (Figure 6A). Truncation of an additional 10 residues (P2Y1-359Z) also had no effect on 2MeSADP-induced internalization. In contrast, the extent of agonist-promoted internalization of P2Y1-349Z truncated receptor was markedly reduced, and a similarly low level of internalization was observed with a receptor missing 10 additional residues (P2Y1-339Z). Thus, the region between residues 349 and 359 appears to be critical in the mechanism of agonist-promoted internalization of the P2Y1 receptor.
Figure 6.
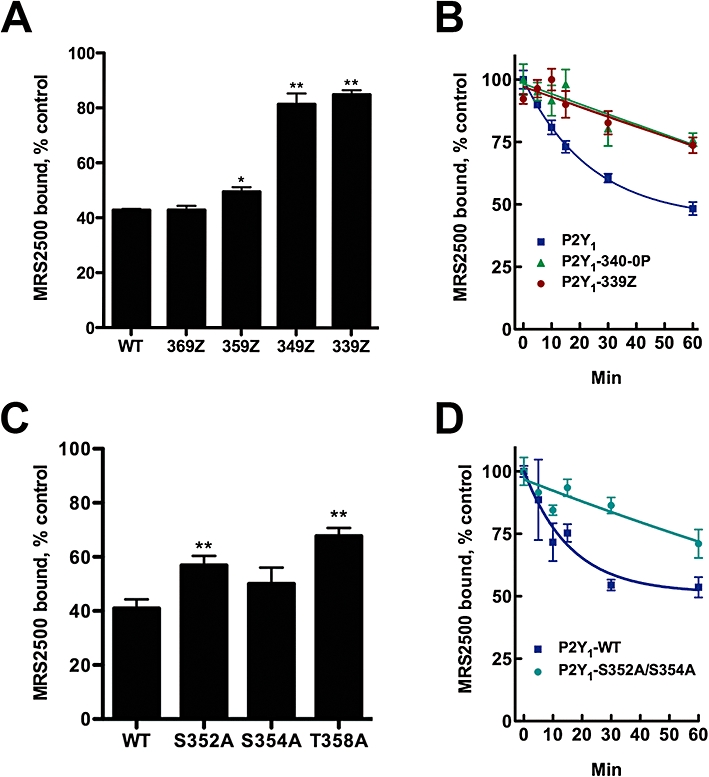
Ser and Thr residues in the carboxyl terminus of the P2Y1 receptor are required for agonist-promoted internalization. (A) Madin-Darby canine kidney (MDCK) cells stably expressing the indicated P2Y1 receptor truncation mutants were incubated with 10 µM 2MeSADP for 0 or 30 min and cell surface MRS2500-binding sites were quantified. (B) MDCK cells stably expressing P2Y1, P2Y1-340/0P or P2Y1-339Z receptors were incubated with 10 µM 2MeSADP for the indicated times and surface MRS2500-binding sites were quantified. (C) MDCK cells stably expressing the indicated P2Y1 receptor single point mutants were treated with 10 µM 2MeSADP for 0 or 30 min and cell surface MRS2500-binding sites were quantified. (D) MDCK cells stably expressing P2Y1 or P2Y1-S352/354A receptors were incubated with 10 µM 2MeSADP for the indicated times and cell surface MRS2500-binding sites were quantified. All values are normalized to the total amount of radiolabelled MRS2500 binding in the absence of agonist treatment. Data shown are means of triplicate samples ± SEM from a representative experiment (n= 3). *P < 0.05; **P < 0.01 relative to wild-type receptor. In (C), none of the single point mutants were significantly different from one another. WT, wild type.
Time courses of agonist-induced loss of MRS2500-binding sites were generated for the wild-type receptor, the P2Y1-339Z truncation mutant and a mutant lacking the last seven Ser and Thr residues of C-terminus (P2Y1-340/0P, see Figure 5). As shown in Figure 6B, agonist-promoted loss of surface MRS2500-binding sites in the P2Y1-339Z receptor mutant occurred with much slower kinetics and to a lesser extent than that of the wild-type receptor. Lack of rapid, agonist-promoted internalization of the truncated P2Y1-339Z receptor was mimicked almost identically by the P2Y1-340/0P receptor mutant, indicating a clear role for carboxyl terminal Ser and Thr residues, and by inference, phosphorylation, in agonist-promoted internalization.
Two Ser residues (S352 and S354) and one Thr residue (T358) are located between amino acids 349 and 359 in the region crucial for agonist-induced internalization of the P2Y1 receptor, and each of these residues was mutated individually to Ala with the goal of identifying putative phosphorylation sites involved in internalization. However, the degree of 2MeSADP-induced internalization of P2Y1 receptors harbouring single mutations at residues Ser352, Ser354 or Thr358 was only marginally decreased relative to that observed with wild-type receptor (Figure 6C). As serine clusters in the carboxyl terminus of several GPCRs, including the P2Y4 receptor, were shown to be important for agonist-induced internalization (Brinson and Harden, 2001; Oakley et al., 2001), we generated a double mutant receptor, P2Y1-S352A/S354A, and measured the rate and extent of agonist-induced loss of surface MRS2500-binding sites. As observed with the P2Y1-339Z and P2Y1-340/0P receptor mutants, the P2Y1-S352A/S354A double mutant exhibited a marked impairment in agonist-induced internalization (Figure 6D), and additional mutation of T358A (i.e. a triple mutant) resulted in no further loss of agonist-promoted internalization (data not shown). In contrast, mutation of the two upstream Ser residues, Ser343 and Ser346, to Ala resulted in no impairment of agonist-promoted internalization (data not shown), indicating that these residues are not involved in internalization. Both the P2Y1-339Z and P2Y1-S352A/S354A mutant receptors were expressed at similar levels as wild-type P2Y1 receptors (Table 1B). These results illustrate that residues Ser352 and Ser354 are required for agonist-promoted internalization of the P2Y1 receptor in MDCK cells.
To assess directly whether Ser352 and Ser354 are subject to agonist-promoted phosphorylation, we examined the capacity of wild-type, P2Y1-340/0P and P2Y1-S352A/S354A receptors to be phosphorylated in an agonist-dependent manner. MDCK cells expressing HA-tagged receptors were preincubated with [32P]phosphate to label the ATP pool, and then incubated with 100 µM ADP for 5 min. ADP promoted a marked incorporation (3.3-fold over basal) of 32P into wild-type P2Y1 receptors (Figure 7). In contrast, no agonist-promoted incorporation of 32P occurred with the P2Y1-340/0P or P2Y1-S352A/S354A receptors, which is consistent with the marked decrease in agonist-promoted internalization observed with these mutant receptors (Figure 6B,D). These data indicate that agonist-induced phosphorylation occurs at two residues, Ser352 and Ser354, and suggest that phosphorylation of these two residues is critical in the mechanism of agonist-promoted internalization of the P2Y1 receptor.
Figure 7.
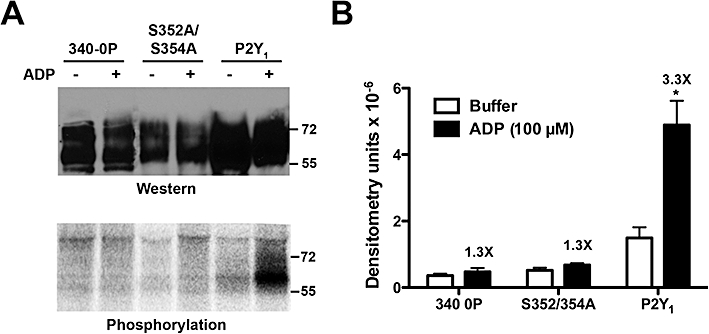
Agonist-dependent phosphorylation of the P2Y1 receptor in Madin-Darby canine kidney (MDCK) cells. (A) MDCK cells stably expressing recombinant HA-tagged P2Y1-wild type, P2Y1-340/0P and P2Y1-S352A/S354A receptors were pre-labelled for 3 h with 1 mCi [32P]Pi, followed by incubation with buffer or 100 µM ADP for 5 min. P2Y1 receptors were immunoprecipitated and 20% of the sample was used for Western blotting (top panel) and the remaining 80% of the sample was resolved by SDS-PAGE and exposed to film following fixation and drying of the gel. Data shown is from a typical experiment (n= 3). (B) Densitometric quantification of agonist-promoted phosphorylation. Numbers above the black bars represent the mean fold-increase in densitometry for each receptor construct for all three experiments. *P < 0.05.
Discussion and conclusions
In the studies presented here, we describe the use of a selective, high-affinity radioligand to examine agonist-induced changes in cell surface P2Y1 receptors. Agonists promote the loss of cell surface receptors in both wild-type MDCK cells and in MDCK cells stably overexpressing P2Y1 receptors with similar rates. Loss of surface receptors is promoted with a concentration dependence for agonist that closely coincides with that for P2Y1 receptor binding and activation, is independent of PKC and is dependent on the formation of clathrin-coated pits. Taken together, our data strongly suggest that these changes occur as a consequence of an agonist-dependent increase in the rate of internalization, but we do not formally rule out the occurrence of additional changes in other processes of receptor trafficking, for example recycling. Two Ser residues in the carboxyl terminus of the P2Y1 receptor, Ser352 and Ser354, were shown to be critical for agonist-promoted internalization. Moreover, these two Ser residues are phosphorylated in an agonist-dependent manner, and their mutation results in marked inhibition of both agonist-induced phosphorylation and internalization of the receptor. Thus, our studies strongly suggest an obligatory role for agonist-induced phosphorylation in the process of agonist-dependent internalization of the P2Y1 receptor, and the two key residues involved in this activity have been identified.
The development of the selective radioligands [32P]MRS2500 and [125I]MRS2500 is the result of a series of structure–activity relationships and molecular modelling studies of the P2Y1 receptor that in collaboration with Jacobson and his colleagues have yielded a number of high-affinity antagonists and radioligands, including MRS2500, a potent antiplatelet agent in vivo, and [3H]MRS2279, the first non-nucleotide antagonist radioligand for a P2Y receptor (Waldo et al., 2002; Hechler et al., 2006). These compounds have proved invaluable for studying the activity of P2Y1 receptors in various physiological systems and mammalian cell lines. The development of high-specific-radioactivity radioligands of MRS2500 introduces important new methodology, as their high-specific radioactivity (i.e. 6000 and 2200 Ci·mmol−1 for [32P] and [125I] forms of MRS2500, respectively) provides the sensitivity needed to quantify P2Y1 receptors in all tissues as shown by detection of P2Y1 receptors in rat tissues at receptor densities less than 10 fmol·mg−1 of protein, as well as in human platelets (Houston et al., 2006; Ohlmann et al., 2010). Therefore, these radioligands were applied to quantify agonist-promoted internalization of endogenous P2Y1 receptors of MDCK cells as well as the trafficking of recombinant wild-type and mutant receptors. Observation of agonist-induced internalization of the endogenous receptor indicates that MDCK cells express the native cell machinery for agonist-induced trafficking of P2Y1 receptors. Moreover, the similarity of the kinetics of internalization for the endogenous receptor versus the recombinant receptor suggests that this system is appropriate for evaluation of the physiologically relevant behaviour of overexpressed mutant receptors.
P2Y1 receptors in platelets internalize rapidly (>1 min) in response to agonist treatment and reside in the open canalicular system (Baurand et al., 2005). The kinetics of this response were much more rapid than those reported here and in other studies (Tulapurkar et al., 2004), suggesting that platelets perhaps utilize a distinct mechanism of internalization. However, these experiments depended on the use of antibodies whose sensitivity in detecting functional P2Y1 receptor-binding sites is not clear. MRS2500 provides a useful radioligand for quantification of active receptors on the surface of platelets (Ohlmann et al., 2010), and future experiments will compare the properties of agonist-induced internalization of the platelet receptor with those described here.
A role for PKC in P2Y1 receptor desensitization, phosphorylation and internalization has been reported for both platelets and 1321N1 human astrocytoma cells. Thr339 in the C-terminus of the P2Y1 receptor is located within a PKC consensus motif and was required for desensitization (Fam et al., 2003; Hardy et al., 2005; Mundell et al., 2006). Our data using inhibitors of various PKC isoforms (Figure 4) suggests that PKC was not required for agonist-promoted internalization of P2Y1 receptor in MDCK cells. Moreover, our data indicated that Thr339 is neither phosphorylated in response to agonist nor required for P2Y1 receptor internalization (Figures 6 and 7) in MDCK cells. The reason(s) for the differences between earlier results and those described here are unclear, but may be a function of the cell line used, that is, 1321N1 astrocytoma cells instead of MDCK cells. Direct observation of agonist-promoted phosphorylation of the P2Y1 receptor and the relative absence of both phosphorylation and internalization of receptors bearing mutations of Ser352 and Ser354 strongly suggests that phosphorylation plays a key role in agonist-promoted trafficking of the P2Y1 receptor. Identification of the involved protein kinase(s) remains unclear, although our data suggest that PKC is not involved. GPCR kinases and Ca2+/calmodulin-dependent protein kinases remain obvious possibilities.
A study of P2Y1 receptor internalization in 1321N1 and HEK293 cells was reported while the current manuscript was in preparation (Reiner et al., 2009). Interestingly, Ser352 and Thr358 were identified as crucial residues involved in agonist-promoted phosphorylation and internalization of the P2Y1 receptor in these cells, which differs from our results identifying Ser352 and Ser354 as the most important residues in agonist-promoted internalization in MDCK cells. An explanation for our differing conclusions, in addition to the obvious difference in cell lines, is that phosphorylation of Ser352 and either Ser354 or Thr358 may be sufficient to promote internalization. We show here that the markedly reduced rate of agonist-promoted internalization of the P2Y1-S352A/S354A receptor is identical to that observed with both the P2Y1-349Z truncated receptor and the P2Y1-S352A/S354A/T358A triple mutant receptor, and that no agonist-promoted phosphorylation occurs in the double mutant. These results demonstrate that in MDCK cells, Ser352 and Ser354 are necessary and sufficient to promote agonist-induced internalization of P2Y1 receptors.
Acknowledgments
The authors are indebted to Steve Cotten and Sam Wolff for technical assistance and to Joann Trejo and Gary Waldo for helpful discussions. This work was supported by National Institutes of Health grants HL54889 (RAN) and GM38213 (TKH), and Grant-In-Aid 0755493U from the American Heart Association Midwest Affiliate (RAN). DCH acknowledges past support by a Howard Hughes Predoctoral Fellowship.
Glossary
Abbreviations
- 2MeSADP
2-methyl thioadenosine 5′-diphosphate
- GPCR
G protein-coupled receptor
- HEK293
human embryonic kidney 293
- MDC
monodansylcadaverine
- MDCK
Madin-Darby canine kidney
- MRS2179
2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate
- MRS2279
2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS2500
2-iodo-N6-methyl-(N)-methanocarba-2-deoxyadenosine-3,5-bisphosphate
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
Conflicts of interest
None of the authors have any conflicts of interest.
Supplementary material
Supporting Information: Teaching Materials; Figs 1–7 as PowerPoint slide.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurand A, Eckly A, Bari N, Leon C, Hechler B, Cazenave JP, et al. Desensitization of the platelet aggregation response to ADP: differential down-regulation of the P2Y1 and P2cyc receptors. Thromb Haemost. 2000;84:484–491. [PubMed] [Google Scholar]
- Baurand A, Eckly A, Hechler B, Kauffenstein G, Galzi JL, Cazenave JP, et al. Differential regulation and relocalization of the platelet P2Y receptors after activation: a way to avoid loss of hemostatic properties? Mol Pharmacol. 2005;67:721–733. doi: 10.1124/mol.104.004846. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Puri S, Miledi R, Panicker MM. Internalization and recycling of 5-HT2A receptors activated by serotonin and protein kinase C-mediated mechanisms. Proc Natl Acad Sci USA. 2002;99:14470–14475. doi: 10.1073/pnas.212517999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon DM, Mahanty SK, Jacobson KA, Boyer JL, Harden TK. (N)-methanocarba-2MeSADP (MRS2365) is a subtype-specific agonist that induces rapid desensitization of the P2Y1 receptor of human platelets. J Thromb Haemost. 2006;4:861–868. doi: 10.1111/j.1538-7836.2006.01866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinson AE, Harden TK. Differential regulation of the uridine nucleotide-activated P2Y4 and P2Y6 receptors. Ser-333 and Ser-334 in the carboxyl terminus are involved in agonist-dependent phosphorylation desensitization and internalization of the P2Y4 receptor. J Biol Chem. 2001;276:11939–11948. doi: 10.1074/jbc.M009909200. [DOI] [PubMed] [Google Scholar]
- Comstock KE, Watson NF, Olsen JC. Design of retroviral expression vectors. In: Tuan R, editor. Methods in Molecular Biology: Recombinant Gene Expression Protocols. Totowa: Humana Press; 1997. pp. 207–222. [DOI] [PubMed] [Google Scholar]
- Costanzi S, Mamedova L, Gao ZG, Jacobson KA. Architecture of P2Y nucleotide receptors: structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem. 2004;47:5393–5404. doi: 10.1021/jm049914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam SR, Gallagher CJ, Kalia LV, Salter MW. Differential frequency dependence of P2Y1- and P2Y2-mediated Ca2+ signaling in astrocytes. J Neurosci. 2003;23:4437–4444. doi: 10.1523/JNEUROSCI.23-11-04437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam SR, Paquet M, Castleberry AM, Oller H, Lee CJ, Traynelis SF, et al. P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc Natl Acad Sci USA. 2005;102:8042–8047. doi: 10.1073/pnas.0408818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood. 2005;105:3552–3560. doi: 10.1182/blood-2004-07-2893. [DOI] [PubMed] [Google Scholar]
- Hechler B, Léon C, Vial C, Vigne P, Frelin C, Cazenave JP, et al. The P2Y1 receptor is necessary for adenosine 5′-diphosphate-induced platelet aggregation. Blood. 1998;92:152–159. [PubMed] [Google Scholar]
- Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, et al. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J Pharmacol Exp Ther. 2006;316:556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- Houston D, Ohno M, Nicholas RA, Jacobson KA, Harden TK. [32P]2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate ([32P]MRS2500), a novel radioligand for quantification of native P2Y1 receptors. Br J Pharmacol. 2006;147:459–467. doi: 10.1038/sj.bjp.0706453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen HM, Gousset L, Bhaskar V, Vincent D, Tai A, Reynolds EE, et al. Evidence for two distinct G-protein-coupled ADP receptors mediating platelet activation. Thromb Haemost. 1999;81:111–117. [PubMed] [Google Scholar]
- Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, et al. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a northern conformation: enhanced potency as P2Y1 receptor antagonists. J Med Chem. 2003;46:4974–4987. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T, Macia E, Pelish HE. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 2008;438:77–93. doi: 10.1016/S0076-6879(07)38006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Glast D, Houston D, Qi AD, Harden TK, Nicholas RA. Regulation of P2Y1 receptors. Purinergic Signal. 2008;4(Suppl 1):S79. [Google Scholar]
- Mundell SJ, Jones ML, Hardy AR, Barton JF, Beaucourt SM, Conley PB, et al. Distinct roles for protein kinase C isoforms in regulating platelet purinergic receptor function. Mol Pharmacol. 2006;70:1132–1142. doi: 10.1124/mol.106.023549. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Barton JF, Mayo-Martin MB, Hardy AR, Poole AW. Rapid resensitization of purinergic receptor function in human platelets. J Thromb Haemost. 2008;6:1393–1404. doi: 10.1111/j.1538-7836.2008.03039.x. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis. J Biol Chem. 2001;276:19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- Ohlmann P, de Castro S, Brown GG, Jr, Gachet C, Jacobson KA, Harden TK. Quantification of recombinant and platelet P2Y1 receptors utilizing a [125I]-labeled high-affinity antagonist 2-iodo-N(6)-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate ([125I]MRS2500) Pharmacol Res. 2010;62:344–351. doi: 10.1016/j.phrs.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J. β-Arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem. 2002;277:1292–1300. doi: 10.1074/jbc.M109160200. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Qi AD, Kennedy C, Harden TK, Nicholas RA. Differential coupling of the human P2Y11 receptor to phospholipase C and adenylyl cyclase. Br J Pharmacol. 2001;132:318–326. doi: 10.1038/sj.bjp.0703788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner S, Ziegler N, Leon C, Lorenz K, von Hayn K, Gachet C, et al. β-Arrestin-2 interaction and internalization of the human P2Y1 receptor are dependent on C-terminal phosphorylation sites. Mol Pharmacol. 2009;76:1162–1171. doi: 10.1124/mol.109.060467. [DOI] [PubMed] [Google Scholar]
- Schachter JB, Li Q, Boyer JL, Nicholas RA, Harden TK. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinergic receptor. Br J Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze S, Machleidt T, Adam D, Schwandner R, Wiegmann K, Kruse ML, et al. Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling. J Biol Chem. 1999;274:10203–10212. doi: 10.1074/jbc.274.15.10203. [DOI] [PubMed] [Google Scholar]
- Tulapurkar ME, Laubinger W, Nahum V, Fischer B, Reiser G. Subtype specific internalization of P2Y1 and P2Y2 receptors induced by novel adenosine 5′-O-(1-boranotriphosphate) derivatives. Br J Pharmacol. 2004;142:869–878. doi: 10.1038/sj.bjp.0705859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD, et al. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–1257. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh MG, Challiss RA, Berstein G, Nahorski SR, Tobin AB. Agonist-induced desensitization and phosphorylation of m1-muscarinic receptors. Biochem J. 1999;338:175–183. [PMC free article] [PubMed] [Google Scholar]
- Wolff SC, Qi AD, Harden TK, Nicholas RA. Polarized expression of human P2Y receptors in epithelial cells from kidney, lung, and colon. Am J Physiol Cell Physiol. 2005;288:C624–C632. doi: 10.1152/ajpcell.00338.2004. [DOI] [PubMed] [Google Scholar]
- Wolff SC, Qi AD, Harden TK, Nicholas RA. Charged residues in the C-terminus of the P2Y1 receptor constitute a basolateral-sorting signal. J Cell Sci. 2010;123:2512–2520. doi: 10.1242/jcs.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


