Abstract
BACKGROUND
It is well established that the pathogenesis of diabetic nephropathy is associated with abnormalities of renal nitric oxide (NO) generation. Many of the biological actions of NO are mediated by cGMP, which is rapidly degraded by phosphodiesterases. In this study, we evaluated the renoprotective effects of sildenafil (SIL), an inhibitor of phosphodiesterase-5, in type 2 diabetic rats.
METHODS
Male Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a non-insulin-dependent diabetes model, and Long-Evans Tokushima Otsuka rats, a non-diabetic control, were treated with either SIL (2.5 mg·kg−1 in drinking water) or undosed water for 28 weeks, starting at 30 weeks of age.
RESULTS
Sildenafil treatment significantly decreased albuminuria, attenuated glomerular hyperfiltration and resulted in a decrease in glomerular hypertrophy, in addition to a reduced glomerulosclerosis score and a dramatic decrease in the number of glomerular and tubulointerstitial proliferating cell nuclear antigen-positive cells in OLETF rats. This was accompanied by a significant reduction in renal cortical mRNA levels of collagen types I and III. The increased mRNA levels of matrix metalloproteinase (MMP)-2, MMP-9, tissue inhibitors of MMPs (TIMP)-1 and TIMP-2 in the OLETF rats were significantly or partially attenuated by SIL treatment.
CONCLUSIONS
This study suggests that SIL attenuated diabetic nephropathy due to its potent antiproliferative effects and its regulatory effects on extracellular matrix. This latter effect is thought to be a result of its ability to affect the balance between MMPs and their inhibitors.
Keywords: sildenafil, nitric oxide (NO), cGMP, diabetic nephropathy, matrix metalloproteinases (MMPs)
Introduction
Nitric oxide (NO) is an important mediator in a variety of physiological and pathophysiological processes. Many of the biological actions of NO are mediated by cGMP, which is rapidly degraded by phosphodiesterases (PDEs). Sildenafil is an inhibitor of PDE type 5, the enzyme that selectively hydrolyses cGMP, and it has been widely used to treat erectile dysfunction (Jeremy et al., 1997; Rosen and Kostis, 2003). Its pharmacological action is due to its ability to prolong the signalling actions of NO by raising the available cGMP pool in penile smooth muscle. The safety and high tolerability of sildenafil make it an attractive tool to investigate diverse therapeutic effects beyond erectile dysfunction, even for indications in which chronic administration is necessary. The approval of sildenafil for the treatment of pulmonary hypertension in 2005 was a notable success in this area of research (Galie et al., 2005). In the heart, sildenafil has cardioprotective effects against ischaemia-reperfusion and anthracycline toxicity, blunts acute adrenergic contractile stimulation, and can suppress chronic hypertrophy and dysfunction attributable to pressure overload (Kass et al., 2007). A number of other potential new indications are currently in various phases of preclinical research and development (Kass et al., 2007; Sandner et al., 2007)
It is well established that the pathogenesis of diabetic nephropathy is associated with abnormalities of renal NO generation (Komers and Anderson, 2003; Prabhakar, 2004). Several studies have suggested that diabetic nephropathy is associated with a defect in NO production (Reyes et al., 1993; Pieper et al., 1997). The administration of L-arginine, a precursor of NO, has been found to prevent hyperfiltration and ameliorate proteinuria in streptozotocin (STZ)-induced diabetic rats (Pieper et al., 1997). In addition, long-term dietary supplementation of arginine, which increases NO production, prevented the changes in renal function and structure normally found in STZ-induced diabetic rats (Reyes et al., 1993). On the other hand, Komers et al. (1994) demonstrated that, in STZ-induced diabetic rats, urinary NO metabolites (NO2+ NO3) were increased, and inhibition of NO production by N-nitro-L-arginine methyl ester (L-NAME), a NOS inhibitor, prevented the increase in glomerular filtration rate that normally characterizes diabetes in these models. Also, Kamijo et al. (2006) demonstrated that administration of L-arginine exacerbated proteinuria in type 2 diabetic rats. Thus, conflicting evidence continues to exist in this field. Cell culture studies using glomerular endothelial cells or mouse kidney slices have also shown that high glucose levels rapidly up-regulate eNOS protein expression but decrease NO synthesis in glomerular endothelial cells through superoxide overproduction and protein kinase C activation (Hoshiyama et al., 2003; Chu and Bohlen, 2004). Furthermore, it has been shown that NO release and NO-dependent cGMP production declines progressively in glomeruli isolated from diabetic rats (Craven et al., 1994; 1995;).
All these results indicate that pharmacological inhibition of the cGMP-specific PDE-5 has the potential to ameliorate diabetic nephropathy. Therefore, the present study was designed to determine whether sildenafil, a selective PDE type 5 inhibitor, might have renoprotective effects in type 2 diabetic rats.
Methods
Experimental protocol
The experimental protocol for this study was reviewed and approved by the Animal Care Committee of Showa University in Tokyo. Male Otsuka Long-Evans Tokushima Fatty (OLETF) rats (n= 14) at 30 weeks of age, a type 2 (non-insulin-dependent) diabetic model, and age-matched Long-Evans Tokushima Otsuka (LETO) rats (LETO group) (n= 6), a non-diabetic control, were obtained from Otsuka Pharmaceutical Tokushima Research Institute (Tokushima, Japan) (Kawano et al., 1994; Hirashima et al., 1995). These inbred rats were isolated from an outbred colony of Long-Evans rats that had been purchased in 1982 from Charles River Canada (St Constant, Canada). The rats were given free access to tap water and standard rat chow. OLETF rats were administered either sildenafil (OLETF-SIL group) (n= 7) (2.5 mg·kg−1 in drinking water) or undosed water (OLETF-CON group) (n= 7) from 30 weeks of age for 28 weeks. At the end of the study, the rats were anaesthetized, blood was collected by cardiac puncture and the kidneys were removed. Renal tissue was divided; some portions were snap-frozen in liquid nitrogen, and some portions were fixed in 2% paraformaldehyde/PBS for later use.
Metabolic data
Body weight (BW) was recorded periodically for each rat. Systolic and diastolic blood pressure was measured every month by the tail cuff method; at least three readings for each rat were averaged. Plasma glucose levels were measured every month using a glucose oxidase-based method. Serum creatinine (Cr), blood urea nitrogen (BUN), total cholesterol, triglyceride and urinary Cr were measured by standard methods using an automatic analyser (Hitachi 7170, Hitachi, Tokyo, Japan). For the analysis of albuminuria, rats were housed individually in metabolic cages for 24 h urine collection. Urine samples were collected once a month. Urinary albumin was determined using a Nephrat kit (Exocell, Philadelphia, PA, USA). The creatinine clearance (Ccr) was calculated using a standard formula. Urinary cGMP excretion was measured using commercially available assay kits following the manufacturer's instructions (Direct cGMP Enzyme Immunoassay Kit, Sigma-Aldrich, St. Louis, MO, USA).
Light microscopic study
Tissues fixed in 2% paraformaldehyde/PBS were embedded in paraffin using routine protocols. Paraffin-embedded materials were sectioned at 4 µm for routine staining with periodic acid Schiff and Masson trichrome. Two-micrometer-thick sections were used for periodic acid-methenamine silver stains (silver). The glomerular tuft area was quantified in 50 full-sized glomeruli (periodic acid Schiff stain) using WinROOF image processing software (Mitani Corp., Tokyo, Japan). Glomerulosclerosis was assessed in 50 glomeruli on silver-stained sections using a semiquantitative score from 0 to 4: 0, no sclerosis; 1, sclerosis up to 25% of glomeruli; 2, sclerosis from 25% to 50% of glomeruli; 3, sclerosis from 50% to 75% of glomeruli; and 4, sclerosis more than 75% of glomeruli. For evaluating tubulointerstitial fibrosis, 10 fields for each section (Masson trichrome stain) were evaluated at 200× magnification using WinROOF image processing software (Mitani Corp.). The extent of tubulointerstitial damage was evaluated by counting the percentage of areas with tubular dilatation, interstitial infiltration and fibrosis per field of cortex. Scores from 0 to 5 were used: 0, normal interstitium; 1, <10% of areas injured; 2, 11–25% of areas injured; 3, 26–50% of areas injured; 4, 51–75% of areas injured; and 5, >76% of areas injured. All histological analyses were performed by two investigators without knowledge of the origin of the slides, and the mean values were calculated.
Immunohistochemistry
The monoclonal antibody against mouse anti-proliferating cell nuclear antigen (PCNA) was purchased from Progen (Heidelberg, Germany), and polyclonal antibody against goat anti-human collagen type IV was purchased from Southern Biotechnology (Birmingham, AL, USA). Biotinylated rabbit anti-mouse IgG, biotinylated rabbit anti-goat IgG and peroxidase-conjugated streptavidin (LSAB 2 kit/HRP) were purchased from Dako (Glostrup, Denmark). Immunohistochemical staining for PCNA and collagen type IV was performed as follows. The paraffin sections of renal tissues were dewaxed, washed in PBS, drained and incubated with the anti-PCNA and collagen type IV antibodies as the primary antibodies overnight at 4°C. In collagen type IV staining, the sections were autoclave-heated at 121°C for 10 min before using primary antibody. They were then washed three times in PBS and incubated with a secondary antibody. After endogenous peroxidase was inactivated by incubation with 0.3% H2O2 in methanol, the sections were incubated with LSAB 2 kit/HRP and developed using diaminobenzedine (DAB) (Dako) as the substrate to produce a brown stain. The number of PCNA-positive cells in glomeruli was assessed by counting stained cell nuclei in 50 random glomerular cross-sections. The quantification of PCNA-positive cells in the tubulointerstitium was performed by counting stained cell nuclei in 10 consecutive renal cortical fields at 200× magnification and expressed as number of cells per 0.5 mm2. The percentage of glomerular tuft area occupied by collagen type IV-stained matrix was quantified in 20 full-sized glomeruli using WinROOF image processing software (Mitani Corp.).
Real-time reverse transcriptase polymerase chain reaction
Gene expressions of rat collagen type I, collagen type III, fibronectin, MMP-2, MMP-9, TIMP-1, TIMP-2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were analysed using real-time reverse transcriptase polymerase chain reaction (RT-PCR). Briefly, kidney tissues (cortex) were homogenized using a TissueLyser (QIAGEN, Hilden, Germany), and total RNA was isolated using an RNeasy Fibrous Tissue Mini kit (QIAGEN), in accordance with the manufacturer's instructions. cDNA synthesis was carried out using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, USA). Predesigned TaqMan probe sets for the targets indicated above were purchased from Applied Biosystems (Foster City, CA, USA). Each probe had a fluorescent reporter dye (FAM) linked to its 5′ end and a downstream quencher dye (TAMRA) linked to its 3′ end. Each reaction consisted of 25 µL containing 2 × Universal Master Mix (Applied Biosystems), primers, labelled probes and cDNA. The amplification conditions consisted of 40 cycles at 95°C for 15 s and 60°C for 1 min after incubation at 95°C for 10 min. Amplification and fluorescence measurements were performed using the MicroAmp Optical 96-Well Reaction Plate on the ABI PRISM 7700 Sequence Detection System (Applied Biosystems). mRNA expressions were normalized using GAPDH as an endogenous control to correct for the differences in the amount of total RNA added to each reaction.
Homogenization of kidney tissues
Kidney tissues (cortex) were homogenized with T-PER Mammalian Protein Extraction Reagent (20 mL·g−1 renal tissues) (Pierce Biotechnology, Rockford, IL, USA) containing 1% (v/v) protease inhibitor cocktail (Sigma-Aldrich) using a TissueLyser (QIAGEN, Hilden, Germany). Harvested lysates were then centrifuged for 10 min at 4°C to remove the cellular debris. The supernatants were collected and stored at −80°C. Protein concentration was measured using the BCA protein assay reagent kit (Pierce Biotechnology).
Western blot analysis
Ten micrograms of protein in kidney tissue homogenate from each sample was separated on a 4–20% gradient gel (Invitrogen) using SDS-PAGE and transferred to PVDF membrane. The blots were blocked with TBST buffer [20 mM Tris-HCl (pH 7.4), 140 mM NaCl and 0.05% Tween 20] containing 5% skimmed milk at room temperature for 1 h, washed three times in TBST buffer and incubated with primary antibody [MMP-2 and MMP-9 (Daiichi Fine Chemical Co., Ltd, Toyama, Japan, 1:100)] overnight at 4°C. The membranes were then incubated with secondary antibody [HRP-conjugated anti-mouse IgG antibody (Cell Signaling Technology, 1:3000)] at room temperature for 1 h. The reaction products were detected using the enhanced chemiluminescence detection system. After stripping, GAPDH was used as the loading control.
Zymography
Renal cortical tissue samples were homogenized, and protein concentration was measured using the BCA protein assay reagent kit (Pierce Biotechnology). The homogenized samples were loaded onto a 10% SDS acrylamide gel containing 1 mg·mL−1 gelatin (Bio-Rad). After electrophoresis, the gel was incubated in renaturing buffer (Invitrogen) and activated in developing buffer (Invitrogen), and gelatinase activity was visualized by staining with Coomassie blue.
Measurement of transforming growth factor-β1 protein level in kidney tissue homogenate
Transforming growth factor (TGF)-β1 protein level was measured in kidney tissue homogenate from each sample by a commercially available TGF-β1 elisa kit (R&D Systems, Abingdon, Oxfordshire, UK) following the manufacturer's instructions. To control for the difference between samples, the concentration was corrected based on the amount of total tissue protein.
Statistical analysis
Data were recorded as means ± SEM. The Mann–Whitney test was performed, and values of P < 0.05 were considered statistically significant.
Results
Effects of sildenafil on biochemical parameters in OLETF rats
The OLETF-CON and OLETF-SIL rats had higher plasma glucose concentrations, BW, and systolic and diastolic blood pressure than the LETO rats throughout the study period. There were no significant changes in plasma glucose concentrations and BW in the OLETF-CON and OLETF-SIL rats throughout the study period. Sildenafil treatment induced significant changes in systolic and diastolic blood pressure at 8 weeks of treatment (Table 1). Table 2 shows the biochemical results for each group. Kidney weight was significantly higher in OLETF-CON rats than in LETO rats (P < 0.001). Sildenafil treatment did not affect kidney weight in OLETF rats. Serum Cr (P < 0.001) and BUN (P < 0.001) levels were significantly lower, and Ccr (P < 0.05) was significantly higher in OLETF-CON rats than in LETO rats, reflecting glomerular hyperfiltration in diabetic kidneys. Sildenafil treatment significantly attenuated these changes in serum Cr (P < 0.05) and BUN (P < 0.001) levels in OLETF rats. The increase of Ccr in OLETF rats tended to be less with sildenafil treatment, but this trend was not statistically significant. Total cholesterol (P < 0.001) and triglyceride (P < 0.05) levels were significantly higher in OLETF-CON rats than in LETO rats, and sildenafil treatment did not affect these parameters. To verify the effect of sildenafil treatment on NO production, urinary cGMP excretion was measured. Sildenafil treatment significantly increased urinary cGMP excretion in OLETF rats (P < 0.05).
Table 1.
Effect of sildenafil treatment on blood glucose, body weight and blood pressure in the study groups
| Duration of treatment | ||||
|---|---|---|---|---|
| 0 week | 8 weeks | 20 weeks | 28 weeks | |
| Blood glucose (mg·L−1) | ||||
| LETO | 1168.30 ± 28.30 | 1333.30 ± 61.20 | 1075.00 ± 51.00 | 1105.00 ± 63.10 |
| OLETF-CON | 1388.70 ± 101.60 | 1726.20 ± 169.60 | 1571.20 ± 80.40*** | 1445.00 ± 75.70** |
| OLETF-SIL | 1318.80 ± 60.20 | 1722.50 ± 89.30 | 1465.00 ± 44.80 | 1567.50 ± 155.80 |
| Body weight (g) | ||||
| LETO | 493.13 ± 3.05 | 493.01 ± 4.79 | 522.91 ± 6.12 | 536.26 ± 8.29 |
| OLETF-CON | 594.03 ± 12.64*** | 578.41 ± 24.27* | 639.85 ± 22.92** | 653.37 ± 26.69** |
| OLETF-SIL | 605.78 ± 15.09 | 590.75 ± 16.62 | 660.23 ± 16.53 | 657.18 ± 22.68 |
| Systolic blood pressure (mmHg) | ||||
| LETO | 110.38 ± 6.37 | 118.61 ± 6.84 | 115.45 ± 5.76 | 115.16 ± 7.35 |
| OLETF-CON | 138.14 ± 9.71* | 168.51 ± 6.12*** | 146.91 ± 8.51* | 159.77 ± 6.68*** |
| OLETF-SIL | 134.51 ± 3.47 | 148.50 ± 6.43# | 141.46 ± 5.36 | 148.87 ± 3.44 |
| Diastolic blood pressure (mmHg) | ||||
| LETO | 84.71 ± 5.59 | 94.76 ± 5.25 | 90.23 ± 4.52 | 91.90 ± 8.28 |
| OLETF-CON | 108.80 ± 4.82** | 132.17 ± 4.51*** | 115.85 ± 7.53* | 126.51 ± 4.43** |
| OLETF-SIL | 103.47 ± 4.97 | 114.17 ± 4.95# | 110.87 ± 6.62 | 116.73. ± 3.34 |
P < 0.05,
P < 0.01,
P < 0.001 vs. LETO.
P < 0.05 vs. OLETF-CON.
LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty.
Table 2.
Clinical parameters of the study groups at the time of death (58 weeks of age)
| LETO | OLETF-CON | OLETF-SIL | |
|---|---|---|---|
| Total kidney weight (g) | 2.61 ± 0.04 | 3.61 ± 0.09*** | 3.71 ± 0.12 |
| Kidney weight/body weight | 4.94 ± 0.07 | 5.58 ± 0.14** | 5.60 ± 0.15 |
| Blood urea nitrogen (mg·L−1) | 302.30 ± 4.20 | 217.90 ± 7.50*** | 283.10 ± 9.20## |
| Serum creatinine (mg·L−1) | 4.10 ± 0.20 | 2.70 ± 0.10*** | 3.10 ± 0.20# |
| Creatinine clearance (mL·min−1) | 2.08 ± 0.17 | 2.79 ± 0.20* | 2.54 ± 0.14 |
| Total cholesterol (mg·L−1) | 1296.70 ± 28.80 | 1562.50 ± 93.60* | 1666.30 ± 104.10 |
| Triglycerides (mg·L−1) | 60.83 ± 10.68 | 293.38 ± 44.00*** | 270.25 ± 66.60 |
| Urine cGMP | |||
| nmol·24 h−1 | 30.45 ± 1.99 | 37.12 ± 1.89** | 50.29 ± 5.56# |
| nmol·mg−1 creatinine | 1.80 ± 1.05 | 2.45 ± 0.65*** | 3.15 ± 0.26# |
Data are mean ± SEM.
P < 0.05,
P < 0.01,
P < 0.001 vs. LETO.
P < 0.05,
P < 0.001 vs. OLETF-CON.
LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty.
Effects of sildenafil on urine volume and urinary albumin excretion in OLETF rats
Urine volume was significantly higher in OLETF-CON rats than in LETO rats at 4 (P < 0.01), 12 (P < 0.05), 16 (P < 0.01), 20 (P < 0.001) and 24 (P < 0.05) weeks, but not at 8 and 28 weeks. Sildenafil treatment significantly increased urine volume in OLETF rats at 12 (P < 0.05), 20 (P < 0.01), 24 (P < 0.05) and 28 (P < 0.05) weeks, but not at 4, 8 and 16 weeks (Figure 1A). Urine albumin excretion was significantly higher in OLETF-CON rats than in LETO rats throughout the study period. The increased urinary albumin excretion in OLETF rats was significantly reduced by sildenafil treatment at the end of the study (P < 0.05) (Figure 1B).
Figure 1.
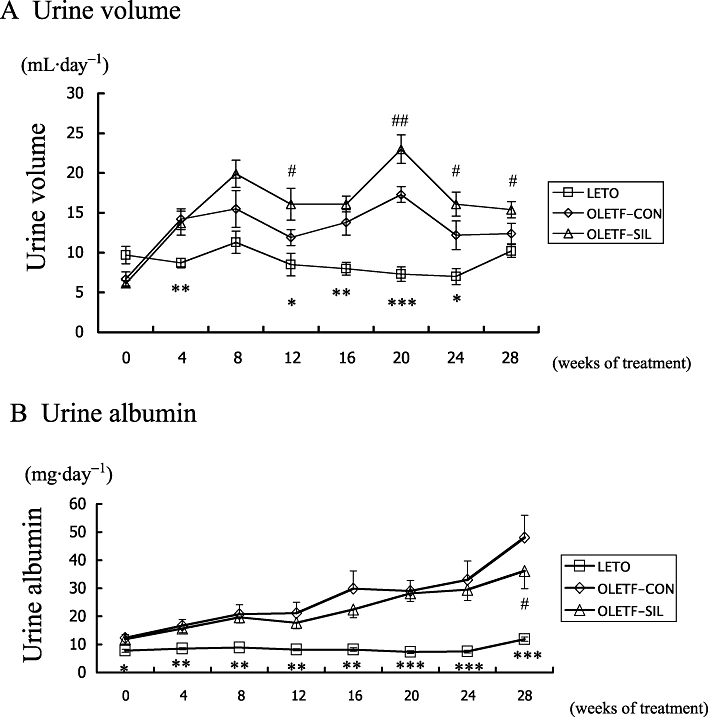
Urine volume and urinary albumin excretion in the study groups. Changes in (A) urine volume and (B) urinary albumin excretion of LETO rats and OLETF rats treated with either sildenafil (OLETF-SIL) or vehicle (OLETF-CON) are shown. Data are means ± SEM. Mann–Whitney test: *P < 0.05, **P < 0.01, ***P < 0.001: LETO vs. OLETF-CON; #P < 0.05, ##P < 0.01: OLETF-SIL vs. OLETF-CON. LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty.
Effects of sildenafil on renal histological findings in OLETF rats
Figure 2 shows the renal histological findings of the study groups. Glomerular lesions in OLETF rats were characterized by hypertrophy, mesangial matrix expansion, sclerotic lesions and hyalinosis. The quantitative analysis of the glomerular tuft area is presented in Table 3. Sildenafil treatment significantly reduced glomerular hypertrophy in OLETF rats (P < 0.05). The glomerular sclerosis score increased significantly in OLETF-CON rats compared with in LETO rats (P < 0.001), and this increase was significantly reduced by sildenafil treatment (P < 0.05) (Table 3). Although the tubulointerstitial damage in OLETF rats was faint, the tubulointerstitial damage score was greater in OLETF-CON rats than in LETO rats (P < 0.001). Sildenafil treatment did not significantly improve the tubulointerstitial damage in OLETF rats (Table 3).
Figure 2.
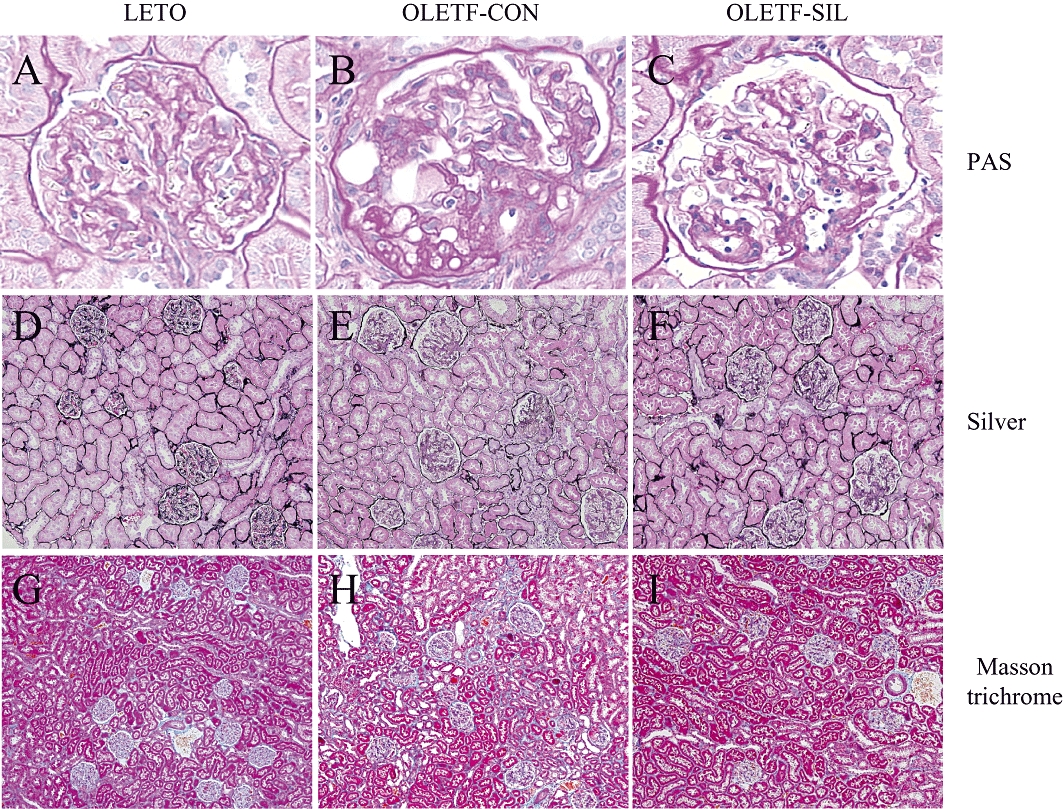
Light microscopic findings in the study groups. Representative pictures stained with periodic acid Schiff (PAS; A–C), silver (D–F) and Masson trichrome (G–I) in a LETO rat (A, D, G), an OLETF-CON rat (B, E, H) and an OLETF-SIL rat (C, F, I). LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty. Original magnifications, ×400 (A–C), ×100 (D–F) and ×40 (G–I).
Table 3.
Morphological evaluation of glomerular size, glomerular sclerosis and tubulointerstitial damage at the end of study (58 weeks of age)
| LETO | OLETF-CON | OLETF-SIL | |
|---|---|---|---|
| Glomerular size (µm2) | 9207.80 ± 199.18 | 10 856.60 ± 206.65* | 9921.71 ± 266.60# |
| Glomerular sclerosis score (0–4) | 0.12 ± 0.02 | 1.42 ± 0.13* | 0.89 ± 0.13# |
| Tubulointerstitial damage score (0–5) | 0 | 0.43 ± 0.19* | 0.29 ± 0.17 |
Morphometric analysis of kidney sections from the LETO rats, OLETF-CON rats and OLETF-SIL rats.
Data are mean ± SEM.
P < 0.001 vs. LETO rats.
P < 0.05 vs. OLETF-CON rats.
LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty.
Effects of sildenafil on cell proliferation in OLETF rats
Quantitative evaluation of glomerular cell proliferation and tubulointerstitial cell proliferation by measurement of PCNA-staining cells (Figure 3) in glomeruli and the tubulointerstitium showed a twofold and a threefold increase in PCNA-positive cells in OLETF-CON rats compared with LETO rats respectively. These increases in PCNA-positive cells in OLETF rats were dramatically reduced to −99% and −93% in glomeruli and in the tubulointerstitium, respectively, by sildenafil treatment (Figure 3).
Figure 3.
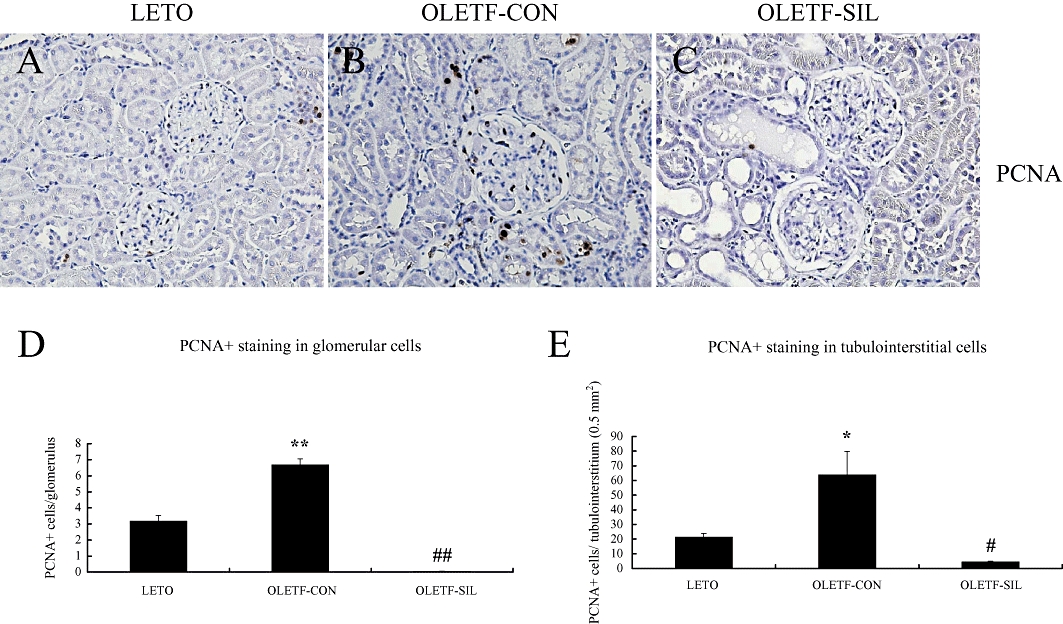
Immunohistochemistry for proliferating cell nuclear antigen (PCNA) in the study groups. (A–C) Representative pictures stained by immunohistochemistry for PCNA of a LETO rat (A), an OLETF-CON rat (B) and an OLETF-SIL rat (C). Original magnifications, ×200. (D, E) Quantitative score of immunohistochemical analysis for PCNA in glomerular cells (D) and tubulointerstitial cells (E) in the study groups. Data are expressed as means ± SEM. Mann–Whitney test: *P < 0.01, **P < 0.001: LETO vs. OLETF-CON; #P < 0.001, ##P < 0.0001: OLETF-SIL vs. OLETF-CON. LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty.
Effects of sildenafil on profibrogenic genes in OLETF rats
The gene expression level of collagen type I was identical in OLETF-CON and LETO rats, as assessed by real-time RT-PCR. Sildenafil treatment significantly decreased collagen type I gene expression in OLETF rats (Figure 4A). There was an increase in collagen type III gene expression in OLETF-CON rats compared with LETO rats. Attenuated collagen type III gene expression was found in the kidneys of OLETF rats treated with sildenafil (Figure 4B). As shown in Figure 4C, fibronectin mRNA was higher in OLETF-CON than in LETO rats. Sildenafil treatment did not affect this parameter in OLETF rats. There was a significant increase in MMP-2 and MMP-9 gene expressions in diabetic kidneys compared with control kidneys and this was significantly reversed by sildenafil treatment (Figure 4D and E). To verify that the changes in mRNA expression are translated to protein and subsequently gelatinolytic activity, we used Western blot analysis and gelatin zymography methods, and showed that an almost similar pattern was obtained. Sildenafil treatment significantly reduced the protein levels of MMP-2 and MMP-9, and activity of MMP-2 but not MMP-9 (Figure 5A–C). A similar pattern was observed when TIMP-1 and TIMP-2 gene expressions were studied. There was a significant increase in the expression of these two inhibitors in diabetic kidneys compared with control kidneys. Sildenafil treatment reversed these changes, although the difference was only significant for TIMP-1 (Figure 4F and G).
Figure 4.
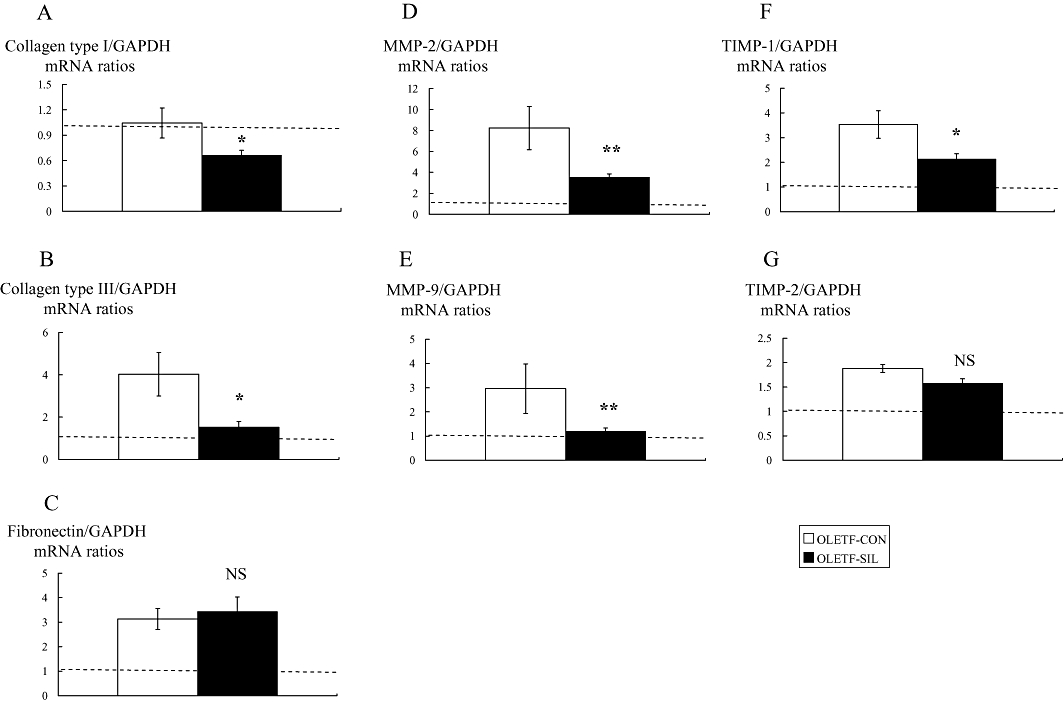
Effects of sildenafil on profibrogenic genes in the study groups. Real-time reverse transcriptase polymerase chain reaction for collagen type I (A), collagen type III (B), fibronectin (C), MMP-2 (D), MMP-9 (E), TIMP-1 (F) and TIMP-2 (G) in each group. The horizontal dotted lines show the expression levels in LETO rats. Data are expressed as means ± SEM. The values were normalized to the GAPDH values and then expressed as relative quantity. Mann–Whitney test: *P < 0.05, **P < 0.01 vs. OLETF-CON. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LETO, Long-Evans Tokushima Otsuka; MMP, matrix metalloproteinase; NS, not significant; OLETF, Otsuka Long-Evans Tokushima Fatty; TIMP, tissue inhibitors of MMPs.
Figure 5.
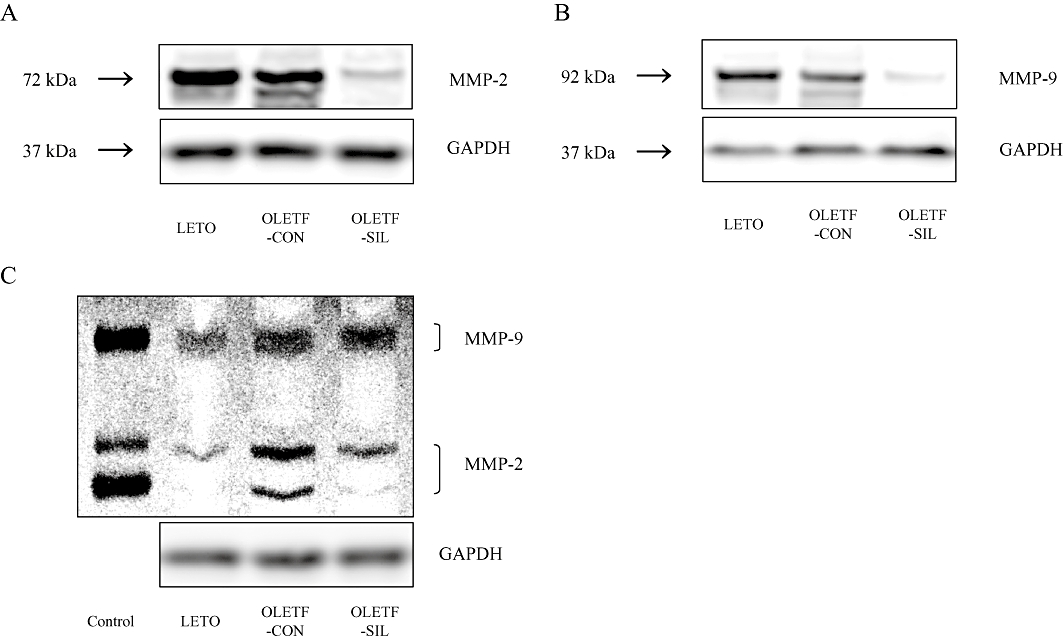
Effects of sildenafil on protein and gelatinolytic activity of MMP-2 and MMP-9 in the study groups. (A, B) Representative Western blot analysis for MMP-2, MMP-9 and GAPDH. (C) Representative gelatinolytic activity of MMP-2 and MMP-9, and Western blot analysis for GAPDH. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LETO, Long-Evans Tokushima Otsuka; MMP, matrix metalloproteinase; OLETF, Otsuka Long-Evans Tokushima Fatty.
Effects of sildenafil on glomerular extracellular matrix in OLETF rats
In order to verify the decrease in collagen gene expressions induced by sildenafil treatment, immunohistochemical staining for collagen type IV was performed. As shown in Figure 6, sildenafil treatment was associated with a significant decrease in the glomerular extracellular matrix as assessed by the percentage of glomerular tuft area occupied by the collagen type IV-stained matrix (P < 0.05).
Figure 6.
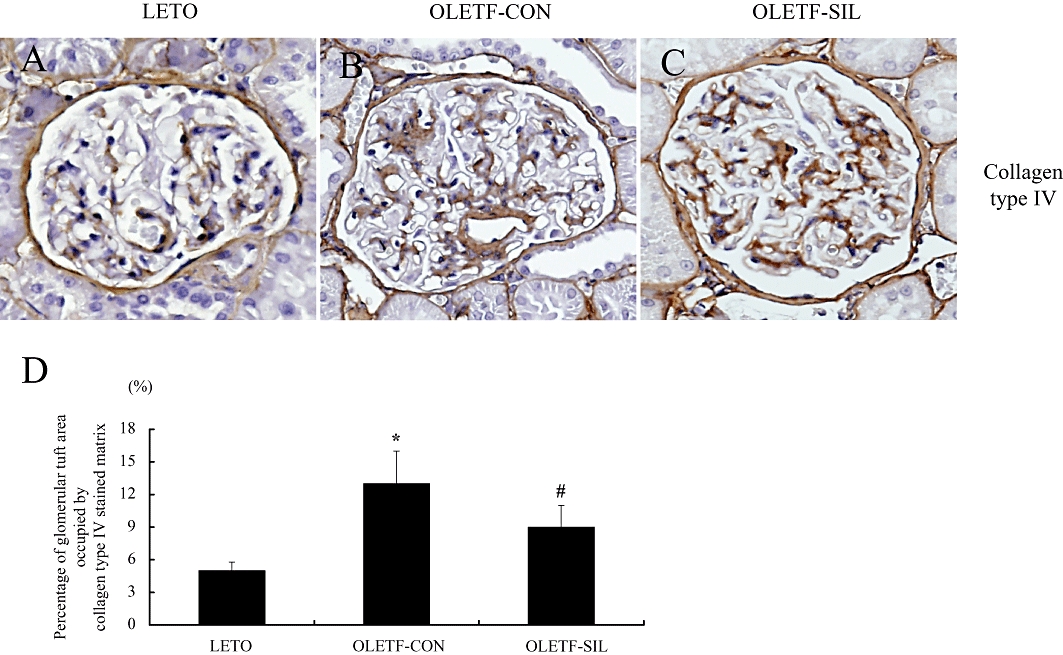
Immunohistochemistry for collagen type IV in the study groups. (A–C) Representative pictures stained with immunohistochemistry for collagen type IV of a LETO rat (A), an OLETF-CON rat (B) and an OLETF-SIL rat (C). Original magnifications, ×200. (D) Percentage of glomerular tuft area occupied by collagen type IV-stained matrix in the study groups. Data are expressed as means ± SEM. Mann–Whitney test: *P < 0.01: LETO vs. OLETF-CON; #P < 0.01: OLETF-SIL vs. OLETF-CON. LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty.
Effects of sildenafil on TGF-β1 protein synthesis in OLETF rats
To examine the effect of sildenafil on TGF-β1 protein synthesis in OLETF rats, the amount of TGF-β1 in kidney tissue homogenates was measured by use of a TGF-β1 elisa kit. The level of TGF-β1 was significantly higher in OLETF-CON rats than in LETO rats. Sildenafil treatment reduced the TGF-β1 level by 15% (P < 0.05) in OLETF rats (Figure 7).
Figure 7.
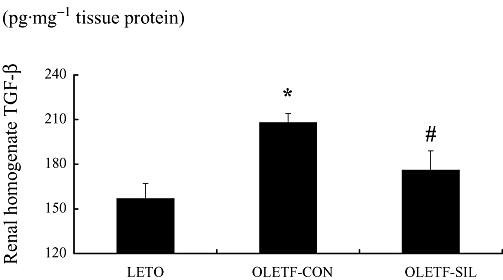
Effects of sildenafil on TGF-β1 protein synthesis in OLETF rats. TGF-β1 protein levels in renal cortical homogenate of each group measured by elisa. Data are expressed as means ± SEM. Mann–Whitney test: *P < 0.01: LETO vs. OLETF-CON; #P < 0.001: OLETF-SIL vs. OLETF-CON. LETO, Long-Evans Tokushima Otsuka; OLETF, Otsuka Long-Evans Tokushima Fatty; TGF, transforming growth factor.
Discussion
The therapeutic benefit of sildenafil in animal models of kidney diseases, such as chronic renal failure (Rodriguez-Iturbe et al., 2005), ischaemia-reperfusion injury (Choi et al., 2009; Oruc et al., 2010), type 1 diabetic nephropathy (Jeong et al., 2009) and cisplatin-induced nephrotoxicity (Lee et al., 2009), has been reported previously. In aggregate, these studies have shown that the beneficial effects of sildenafil treatment are the result of its inhibitory action on apoptosis (Rodriguez-Iturbe et al., 2005; Choi et al., 2009; Lee et al., 2009), inflammation (Rodriguez-Iturbe et al., 2005; Jeong et al., 2009; Oruc et al., 2010) and oxidative stress (Jeong et al., 2009). In the present study, sildenafil treatment ameliorated progression of type 2 diabetic nephropathy in OLETF rats. The attenuation of glomerular hypertrophy, reduced glomerular extracellular matrix expansion and decreased glomerular sclerosis, along with suppression of albuminuria at the end of the study induced by sildenafil clearly show that it has renoprotective effects. Treatment of OLETF rats with sildenafil did not appear to produce adverse effects during the study period. These findings provide the first in vivo evidence that inhibition of PDE type 5 protects against nephropathy in a non-insulin-dependent model of diabetes.
The most outstanding finding in the present study was the potent antiproliferative effect of sildenafil. Sildenafil treatment almost completely suppressed PCNA-positive glomerular cells (−99%), as well as tubulointerstitial cells (−93%). Indeed, the antiproliferative effects of PDE inhibitors have been documented in the acute phase of experimental mesangioproliferative glomerulonephritis (MSGN) (Tsuboi et al., 1996; Hohenstein et al., 2008). Tsuboi et al. (1996) reported that the selective PDE type 3 antagonist lixazinone and the specific PDE type 4 antagonist rolipram decreased the activation and proliferation of mesangial cells, inhibited macrophage accumulation and prevented proteinuria in rat MSGN. Also, recently, Hohenstein et al. (2008) reported that vardenafil, a specific PDE type 5 inhibitor, markedly inhibited glomerular cell proliferation and extracellular matrix accumulation in rat MSGN. Several lines of evidence indicate that cGMP-increasing agents inhibit signal transduction from Ras by preventing Ras-dependent activation of Raf-1, thereby preventing the activity of the downstream kinases, including mitogen-activated protein kinases (MAPK) and MAPK kinase (MEK) (Yu et al., 1997; Li et al., 2007). Thus, sildenafil has the potential to prevent the action of mitogenic stimuli that can activate glomerular or tubulointerstitial cell proliferation by inhibiting the signalling pathway at the Raf-1 step, where different types of mitogenesis-activating signalling pathways converge, in this model of diabetic rats. The antiproliferative effects of sildenafil might, at least in part, contribute to subsequent inhibition of extracellular matrix accumulation and glomerular sclerosis in OLETF rats (Eng et al., 1994; Johnson, 1994). Its potent inhibitory effect against cell proliferation resulted in a significant reduction of PCNA-positive cells in the OLETF-SIL rats compared with LETO rats. We speculate that the high dose of sildenafil used in the present study, which was 2.0- to 3.0-fold higher than the clinical dose, led to these results. However, this dose of sildenafil did not affect BW and other conditions in OLETF rats throughout the study period.
Sildenafil treatment significantly decreased collagen type I (−26%) and type III (−49%) gene expressions in OLETF rats. In the present study, glomerular fibronectin mRNA levels were increased in OLETF rats compared with LETO rats. Nevertheless, the increment of fibronectin mRNA levels was only marginally affected by sildenafil treatment (−9%). Thus, the antifibrotic effect of sildenafil is unlikely to be associated with the suppression of fibronectin. The TGF-β1 protein level in kidney tissue homogenate was significantly higher in OLETF-CON rats than in LETO rats, and this increase was significantly attenuated by sildenafil treatment.
In addition to increased synthesis, diabetes-related glomerulosclerosis is associated with a decrease in ECM degradation by the balance between MMPs and their inhibitors. Variable levels of MMP-2 and MMP-9 have been observed in the diabetic kidney, depending on the duration and model of diabetes. In early STZ-induced diabetes, a type 1 diabetes model, a decrease in MMP-2 and MMP-9 protein expressions and activity has been reported (McLennan et al., 2002; Han et al., 2006; Mankhey et al., 2007); in longer-term STZ-induced diabetes, no changes in MMP-9 were observed (Dong et al., 2004; Rodriguez et al., 2006). On the other hand, MMP-2 protein has been shown to be increased in Goto-Kakizaki rats and MMP-9 mRNA and protein increased in Kkay mice, both type 2 diabetes models (Portik-Dobos et al., 2006; Qing-Hua et al., 2008). In the present study, OLETF-CON rats at 58 weeks of age had increased MMP-2 and MMP-9 gene expressions, and sildenafil treatment significantly reduced these levels by 48% and 60% respectively. The inhibitory effects of sildenafil on MMP-2 and MMP-9 protein levels and MMP-2 activity were confirmed by Western blot analysis and gelatine zymography. To the best of our knowledge, the MMP profiles in OLETF and LETO rats have not been reported previously. We are the first to show that sildenafil treatment exhibited renoprotective effects in diabetic nephropathy by regulating the expressions of MMPs.
One of the mechanisms by which MMP activity is regulated is by altering the expression of its inhibitors, TIMPs. Diabetic nephropathy in humans and experimental models is associated with increased TIMP-1 (McLennan et al., 2002) and TIMP-2 (Han et al., 2006) expressions, consistent with the findings of the present study. Although sildenafil treatment significantly attenuated the increase in TIMP-1 (−27% reduction), no significant effect on TIMP-2 expression (−13% reduction) was observed. Despite the inability of sildenafil to regulate TIMP-2 expression directly, its effects on other ECM regulatory pathways appear to be sufficient to effectively attenuate the progression of glomerulosclerosis in this model of diabetic nephropathy.
However, it should be mentioned here that while sildenafil treatment effectively attenuated glomerular sclerosis, its inhibitory effects on albuminuria or Ccr in OLETF rats were partial. Therefore, sildenafil could be more effective for the treatment of diabetic nephropathy when used in combination with conventional therapies, including renin-angiotensin system inhibitors that possess different modes of action.
In conclusion, sildenafil inhibited extracellular matrix accumulation partially by affecting the balance between MMPs and their inhibitors, exhibited potent antiproliferative effects, and thereby attenuated renal injury in an experimental model of diabetic nephropathy. Our study provides an encouraging example of the therapeutic potential of inhibiting PDE type 5 in diabetic nephropathy and is indicative of the potential success of sildenafil in the treatment of diabetic nephropathy.
Acknowledgments
This work was supported in part by Health and Labour Sciences Research Grants from the Research Group on Progressive Renal Disease in Japan. T.A. is supported by a research grant from Kyowa Hakko Kirin Co., Ltd. and Chugai Pharmaceutical Co., Ltd. The authors would like to thank Ms Tomoko Suzuki, Ms Naoko Ono and Ms Fumiko Kondo for their excellent technical assistance. Sildenafil was a gift from Pfizer (New York, NY, USA), which did not otherwise participate in the design, execution or funding of this study.
Glossary
Abbreviations
- BUN
blood urea nitrogen
- BW
body weight
- Ccr
creatinine clearance
- Cr
creatinine
- DAB
diaminobenzedine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- LETO
Long-Evans Tokushima Otsuka
- L-NAME
N-nitro-L-arginine methyl ester
- MAPK
mitogen-activated protein kinases
- MEK
MAPK kinase
- MMP
matrix metalloproteinase
- NO
nitric oxide
- OLETF
Otsuka Long-Evans Tokushima Fatty
- PAS
periodic acid Schiff
- PDE
phosphodiesterase
- Silver
periodic acid-methenamine silver
- STZ
streptozotocin
- TIMP
tissue inhibitors of MMPs
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary material
Supporting Information: Teaching Materials; Figs 1–7 as PowerPoint slide.
References
- Choi DE, Jeong JY, Lim BJ, Chung S, Chang YK, Lee SJ, et al. Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am J Physiol Renal Physiol. 2009;297:F362–F370. doi: 10.1152/ajprenal.90609.2008. [DOI] [PubMed] [Google Scholar]
- Chu S, Bohlen HG. High concentration of glucose inhibits glomerular endothelial eNOS through a PKC mechanism. Am J Physiol Renal Physiol. 2004;287:F384–F392. doi: 10.1152/ajprenal.00006.2004. [DOI] [PubMed] [Google Scholar]
- Craven PA, Studer RK, DeRubertis FR. Impaired nitric oxide-dependent cyclic guanosine monophosphate generation in glomeruli from diabetic rats. Evidence for protein kinase C-mediated suppression of the cholinergic response. J Clin Invest. 1994;93:311–320. doi: 10.1172/JCI116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven PA, Studer RK, DeRubertis FR. Impaired nitric oxide release by glomeruli from diabetic rats. Metabolism. 1995;44:695–698. doi: 10.1016/0026-0495(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Dong FQ, Li H, Cai WM, Tao J, Li Q, Ruan Y, et al. Effects of pioglitazone on expressions of matrix metalloproteinases 2 and 9 in kidneys of diabetic rats. Chin Med J (Engl) 2004;117:1040–1044. [PubMed] [Google Scholar]
- Eng E, Floege J, Young BA, Couser WG, Johnson RJ. Does extracellular matrix expansion in glomerular disease require mesangial cell proliferation? Kidney Int Suppl. 1994;45:S45–S47. [PubMed] [Google Scholar]
- Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- Han SY, Jee YH, Han KH, Kang YS, Kim HK, Han JY, et al. An imbalance between matrix metalloproteinase-2 and tissue inhibitor of matrix metalloproteinase-2 contributes to the development of early diabetic nephropathy. Nephrol Dial Transplant. 2006;21:2406–2416. doi: 10.1093/ndt/gfl238. [DOI] [PubMed] [Google Scholar]
- Hirashima T, Kawano K, Mori S, Matsumoto K, Natori T. A diabetogenic gene (ODB-1) assigned to the X-chromosome in OLETF rats. Diabetes Res Clin Pract. 1995;27:91–96. doi: 10.1016/0168-8227(95)01028-c. [DOI] [PubMed] [Google Scholar]
- Hohenstein B, Daniel C, Wittmann S, Hugo C. PDE-5 inhibition impedes TSP-1 expression, TGF-beta activation and matrix accumulation in experimental glomerulonephritis. Nephrol Dial Transplant. 2008;23:3427–3436. doi: 10.1093/ndt/gfn319. [DOI] [PubMed] [Google Scholar]
- Hoshiyama M, Li B, Yao J, Harada T, Morioka T, Oite T. Effect of high glucose on nitric oxide production and endothelial nitric oxide synthase protein expression in human glomerular endothelial cells. Nephron Exp Nephrol. 2003;95:e62–e68. doi: 10.1159/000073673. [DOI] [PubMed] [Google Scholar]
- Jeong KH, Lee TW, Ihm CG, Lee SH, Moon JY, Lim SJ. Effects of sildenafil on oxidative and inflammatory injuries of the kidney in streptozotocin-induced diabetic rats. Am J Nephrol. 2009;29:274–282. doi: 10.1159/000158635. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Ballard SA, Naylor AM, Miller MA, Angelini GD. Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br J Urol. 1997;79:958–963. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- Johnson RJ. The glomerular response to injury: progression or resolution? Kidney Int. 1994;45:1769–1782. doi: 10.1038/ki.1994.230. [DOI] [PubMed] [Google Scholar]
- Kamijo H, Higuchi M, Hora K. Chronic inhibition of nitric oxide production aggravates diabetic nephropathy in Otsuka Long-Evans Tokushima Fatty rats. Nephron Physiol. 2006;104:12–22. doi: 10.1159/000093276. [DOI] [PubMed] [Google Scholar]
- Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res. 2007;101:1084–1095. doi: 10.1161/CIRCRESAHA.107.162511. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract. 1994;24(Suppl):S317–S320. doi: 10.1016/0168-8227(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Komers R, Anderson S. Paradoxes of nitric oxide in the diabetic kidney. Am J Physiol Renal Physiol. 2003;284:F1121–F1137. doi: 10.1152/ajprenal.00265.2002. [DOI] [PubMed] [Google Scholar]
- Komers R, Allen TJ, Cooper ME. Role of endothelium-derived nitric oxide in the pathogenesis of the renal hemodynamic changes of experimental diabetes. Diabetes. 1994;43:1190–1197. doi: 10.2337/diab.43.10.1190. [DOI] [PubMed] [Google Scholar]
- Lee KW, Jeong JY, Lim BJ, Chang YK, Lee SJ, Na KR, et al. Sildenafil attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Toxicology. 2009;257:137–143. doi: 10.1016/j.tox.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Li B, Yang L, Shen J, Wang C, Jiang Z. The antiproliferative effect of sildenafil on pulmonary artery smooth muscle cells is mediated via upregulation of mitogen-activated protein kinase phosphatase-1 and degradation of extracellular signal-regulated kinase 1/2 phosphorylation. Anesth Analg. 2007;105:1034–1041. doi: 10.1213/01.ane.0000278736.81133.26. table of contents. [DOI] [PubMed] [Google Scholar]
- McLennan SV, Kelly DJ, Cox AJ, Cao Z, Lyons JG, Yue DK, et al. Decreased matrix degradation in diabetic nephropathy: effects of ACE inhibition on the expression and activities of matrix metalloproteinases. Diabetologia. 2002;45:268–275. doi: 10.1007/s00125-001-0730-4. [DOI] [PubMed] [Google Scholar]
- Mankhey RW, Wells CC, Bhatti F, Maric C. 17beta-Estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. Am J Physiol Regul Integr Comp Physiol. 2007;292:R769–R777. doi: 10.1152/ajpregu.00375.2006. [DOI] [PubMed] [Google Scholar]
- Oruc O, Inci K, Aki FT, Zeybek D, Muftuoglu SF, Kilinc K, et al. Sildenafil attenuates renal ischemia reperfusion injury by decreasing leukocyte infiltration. Acta Histochem. 2010;112:337–344. doi: 10.1016/j.acthis.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Siebeneich W, Moore-Hilton G, Roza AM. Reversal by L-arginine of a dysfunctional arginine/nitric oxide pathway in the endothelium of the genetic diabetic BB rat. Diabetologia. 1997;40:910–915. doi: 10.1007/s001250050767. [DOI] [PubMed] [Google Scholar]
- Portik-Dobos V, Harris AK, Song W, Hutchinson J, Johnson MH, Imig JD, et al. Endothelin antagonism prevents early EGFR transactivation but not increased matrix metalloproteinase activity in diabetes. Am J Physiol Regul Integr Comp Physiol. 2006;290:R435–R441. doi: 10.1152/ajpregu.00300.2005. [DOI] [PubMed] [Google Scholar]
- Prabhakar SS. Role of nitric oxide in diabetic nephropathy. Semin Nephrol. 2004;24:333–344. doi: 10.1016/j.semnephrol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Qing-Hua G, Ju-Ming L, Chang-Yu P, Zhao-Hui L, Xiao-Man Z, Yi-Ming M. The kidney expression of matrix metalloproteinase-9 in the diabetic nephropathy of Kkay mice. J Diabetes Complications. 2008;22:408–412. doi: 10.1016/j.jdiacomp.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Reyes AA, Karl IE, Kissane J, Klahr S. L-arginine administration prevents glomerular hyperfiltration and decreases proteinuria in diabetic rats. J Am Soc Nephrol. 1993;4:1039–1045. doi: 10.1681/ASN.V441039. [DOI] [PubMed] [Google Scholar]
- Rodriguez WE, Tyagi N, Joshua IG, Passmore JC, Fleming JT, Falcone JC, et al. Pioglitazone mitigates renal glomerular vascular changes in high-fat, high-calorie-induced type 2 diabetes mellitus. Am J Physiol Renal Physiol. 2006;291:F694–F701. doi: 10.1152/ajprenal.00398.2005. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Espinoza F, Pons H, et al. Early treatment with cGMP phosphodiesterase inhibitor ameliorates progression of renal damage. Kidney Int. 2005;68:2131–2142. doi: 10.1111/j.1523-1755.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Kostis JB. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am J Cardiol. 2003;92:9M–18M. doi: 10.1016/s0002-9149(03)00824-5. [DOI] [PubMed] [Google Scholar]
- Sandner P, Hutter J, Tinel H, Ziegelbauer K, Bischoff E. PDE5 inhibitors beyond erectile dysfunction. Int J Impot Res. 2007;19:533–543. doi: 10.1038/sj.ijir.3901577. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Shankland SJ, Grande JP, Walker HJ, Johnson RJ, Dousa TP. Suppression of mesangial proliferative glomerulonephritis development in rats by inhibitors of cAMP phosphodiesterase isozymes types III and IV. J Clin Invest. 1996;98:262–270. doi: 10.1172/JCI118788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SM, Hung LM, Lin CC. cGMP-elevating agents suppress proliferation of vascular smooth muscle cells by inhibiting the activation of epidermal growth factor signaling pathway. Circulation. 1997;95:1269–1277. doi: 10.1161/01.cir.95.5.1269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


