Abstract
Coordination of the urinary bladder and the external urethral sphincter (EUS) is controlled by descending projections from the pons, and is also subject to modulation by segmental afferents. We quantified the effects on the micturition reflex of sensory inputs from genital afferents, traveling in the penile component of the somatic pudendal nerve, by electrical stimulation of the dorsal nerve of the penis (DNP) in α-chloralose anesthetized male cats. Depending on the frequency of stimulation (range 1–40 Hz), activation of penile afferents either inhibited contractions of the bladder and promoted urine storage or activated the bladder and produced micturition. Stimulation of the DNP at 5–10 Hz inhibited distension evoked contractions and increased the maximum bladder capacity before incontinence. Conversely, stimulation at 33 and 40 Hz augmented distension evoked contractions. When the bladder was filled above a threshold volume (70% of the volume necessary for distension evoked contractions), stimulation at 20–40 Hz activated de novo the micturition reflex and elicited detrusor contractions that increased voiding efficiency compared to distension evoked voiding. Electrical stimulation of the DNP with a cuff electrode or percutaneous wire electrode produced similar results. The ability to evoke detrusor contractions by activation of the DNP was preserved following acute spinal transection. These results demonstrate a clear role of genital afferents in modulating the micturition reflex and suggest the DNP as a potential target for functional restoration of bladder control using electrical stimulation.
Keywords: electrical stimulation, spinal cord injury, dorsal nerve of the penis, frequency-dependence
INTRODUCTION
The reciprocal coordination of the urinary bladder and the external urethral sphincter (EUS) to maintain continence and produce micturition is controlled by the pontine micturition center (2, 3, 24). The spinobulbospinal micturition reflex is also subject to modulation by peripheral afferent activity (29, 30) that can influence voiding efficiency (41). The objective of the present study was to quantify the contributions of genital afferents, traveling in the penile component of the somatic pudendal nerve, on the micturition reflex in the cat.
Activation of pudendal afferent nerve fibers can engage spinal (5) and spinobulbospinal (2, 3) reflexes that are integral to the regulation of bladder function. Fluid flow through the urethra activates pudendal afferents (56), and this flow-driven activation can elicit detrusor contractions and facilitate micturition (2, 44, 48). Further, electrical stimulation of pudendal urethral afferents in the cat (5, 49) and human (21) elicits detrusor contraction, and these responses are preserved following decerebration and acute and chronic spinal cord transection (5, 7, 49, 52). Conversely, activation of pudendal afferents can also inhibit detrusor contractions (33, 50), and electrical stimulation of genital afferents inhibits the micturition reflex in humans with SCI, multiple sclerosis, Parkinson’s disease, and other conditions resulting in neurogenic detrusor overactivity (19, 22, 28, 31, 39, 58, 62).
Recent experiments in cats revealed that the frequency of electrical stimulation of afferents in the compound pudendal nerve determines their effect on the micturition reflex, with frequencies less than 20 Hz producing inhibition and frequencies above 20 Hz producing activation of the micturition reflex (7, 52). However, the effects of genital afferent inputs on the micturition reflex and whether they exhibit similar frequency dependence is unknown. The present results demonstrate that, similar to effects produced by other pudendal afferents, activation of genital afferents produced either activation or inhibition of the micturition reflex in the α-chloralose anesthetized male cat, depending on the frequency of electrical stimulation of the afferents. The results reveal that genital afferent stimulation can activate both continence and micturition-like neural pathways, challenging the perception that the effect of stimulation of the genital afferents is solely inhibition of the micturition reflex.
METHODS
All animal care and experimental procedures were followed according to the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at Duke University.
Preparation
Twenty sexually intact, adult male cats weighing 2.5–5.5 kg were anesthetized with ketamine HCl (Ketaset, 35 mg/kg i.m.) and anesthesia was maintained with α-chloralose (Sigma-Aldrich, 65 mg/kg i.v., supplemented at 15mg/kg). The animals were intubated and respired artificially to maintain end tidal CO2 between 3.3 and 4.5%. Blood pressure was monitored via a catheter in the carotid artery. Core body temperature was maintained at 38° with a thermostatic heating pad, and IV fluids were administered (saline or lactated Ringer’s solution at 15cc/kg/hr).
The bladder was exposed through a midline abdominal incision and the ureters were isolated, ligated, and cut proximal to the ligation. A suprapubic catheter (3.5 Fr) was inserted into the bladder dome and secured with a purse-string suture, and the abdominal incision was closed in layers. Intravesical pressures were measured with a solid-state pressure transducer (Deltran, Utah Medical) connected to the catheter and recorded (sampling rate=12.5–20 kHz, Astromed8Xe, Astro-Med, Inc).
For isovolumetric experiments, the urethra was occluded with a 3.5 Fr or 5 Fr catheter. In 5 cats, the catheter had three platinum ring electrodes positioned 6, 7, and 8 cm from the tip. The catheter was inserted so that two of the electrodes were within the post-prostatic urethra and bulbourethra (3–5 cm from the urethral meatus (60)) and used to measure the activity of the periurethral musculature. This electromyogram (EMG) recording has been defined as the periurethral EMG (PU EMG) to reflect that it may include contributions from multiple active muscles, including the external urethra sphincter (EUS). Wire electrodes were also inserted into the external anal sphincter (EAS) to record the anal sphincter EMG (EAS EMG). The EMG signals were amplified (gain=1000), filtered (10 Hz-2 kHz), and sampled.
In 2 cats the spinal cord was transected and responses to genital afferent stimulation were measured 8–15 hours after spinal cord transection. The spinal cord was exposed at the T10 vertebral level via laminectomy, the dura was incised, and lidocaine was administered to the exposed cord. The spinal cord was elevated and transected, and Surgicel was packed between the transected ends of the spinal cord.
Nerve Stimulation
The dorsal nerve of the penis (DNP) was stimulated unilaterally either by placing a cuff electrode directly around the nerve (n=13) or by percutaneous insertion of a wire electrode adjacent to the nerve (n=7). With the animal in a supine position, a midline incision was made from the caudal border of the gracilis muscle to a few millimeters cranial to the caudal end of the prepuce. For direct stimulation, the DNP was dissected free from the body of the penis caudal to the bulb of the penis, and a monopolar cuff electrode (platinum contacts embedded in a silicone elastomeric cuff) was placed around the nerve. Indirect stimulation was delivered through an insulated stainless steel wire inserted via a 22G needle between the prepuce and the glans penis and directed along the dorsolateral body of the penis 2.5–3.0 cm from the tip of the glans penis. The percutaneous electrode was inserted so that the DNP was likely activated unilaterally, and no characteristics of the observed responses suggested otherwise. A 20G stainless steel needle was inserted into the ipsilateral leg of the animal and served as the anode during percutaneous and cuff electrode stimulation. Stimuli were 20–30 second trains of monophasic constant current cathodic pulses (100μs pulse width) delivered at varying amplitudes (direct stimulation: 10μA-1mA, percutaneous stimulation: 100μA-8mA) and frequencies (1–40 Hz). The train lengths and frequency range were chosen based on previous data on the effect of stimulus parameters on bladder response (7).
Experimental Design
Experiments were performed either with the urethra occluded (isovolumetric experiments) or unobstructed. All artificial bladder filling was performed with room temperature saline. Isovolumetric experiments were performed on 18 cats, of which 18 cats included stimulation delivered when the detrusor was relaxed (direct stimulation in 11 cats, percutaneous stimulation in 7 cats) and 16 cats included stimulation delivered during distension evoked detrusor contractions (direct stimulation in 9 cats, percutaneous stimulation in 7 cats).
Volume thresholds were investigated systematically in 8 cats. The bladder was filled in discrete 1 ml increments, and stimulation was applied during a 2–3 minute interval in between filling. The minimum volume at which electrical stimulation of the DNP could evoke detrusor contractions was defined as the stimulation threshold volume (STV). The minimum volume at which reflex bladder contractions (>10cmH2O) occurred was defined as the distension threshold volume (DTV). Stimulation for determining thresholds was delivered at 33 Hz and 2 times the amplitude threshold for eliciting an EAS response.
Quantification of Detrusor Responses
For isovolumetric experiments, stimulation evoked detrusor responses were defined as increases in intravesical pressure that exceeded the baseline intravesical pressure by at least 10% and were sustained until the end of stimulation. The baseline intravesical pressure was defined as the mean intravesical pressure over the 2 seconds prior to stimulation onset. The mean pressure of a stimulus evoked contraction was calculated as the mean pressure from the point in which the 10% threshold was crossed until the end of the stimulation. Inhibitory (detrusor relaxation) responses were defined as occurring when stimulation initiated during a distension evoked detrusor contraction resulted in at least a 50% decline from the peak intravesical pressure (the peak pressure after stimulus onset) within the first 5 seconds of stimulation. Relaxation was determined to have failed if the intravesical pressure increased back above 50% of the peak contraction pressure before stimulation ended.
The area under the intravesical pressure curve (the pressure-time product, PTP) was measured to quantify the effects of stimulation delivered during distension evoked contractions (inhibition or augmentation). The onset of a distension evoked contraction was defined by averaging the intravesical pressure in one second increments and subsequently searching for a 10% increase in the intravesical pressure in the absence of stimulation. For a detected distension evoked contraction, the pressure baseline was defined as the two seconds preceding the onset of the distension evoked contractions, and all PTPs were computed from the intravesical pressure (minus baseline) for 20 seconds following onset of the distension evoked contractions. Distension evoked detrusor contractions were only included in the analysis if they lasted at least 10 seconds in the absence of stimulation, and stimulation trials were only included if stimulation began within 10 seconds of the onset of the distension evoked contraction. Trials that occurred in the same animal, at the same volume, and within three minutes of one another were grouped together. Each group of trials included one or more distension evoked contraction during which stimulation did not occur. The PTPs in each group were normalized by dividing by the average PTP of the distension evoked contractions.
Direct DNP stimulation was performed in 4 cats with an unobstructed urethra to investigate the effects of genital afferent activity on bladder storage and voiding. The bladder was filled continuously at 1 ml/min and failure of urine storage was defined as the point when urine leakage was observed or a sustained distension evoked contraction occurred (defined as a contraction lasting at least 20 seconds and having a pressure increase 15 cmH2O). In the urine storage trials, continuous stimulation was applied starting at 50–80% of the previously determined volume at which failure of storage occurred (in absence of stimulation) and the stimulation was stopped when failure of storage occurred. Distension evoked voiding was measured by stopping bladder filling when failure of storage occurred and allowing distension evoked voiding to complete (3–5 minutes after the last volume was voided). Stimulation evoked voiding was measured by stopping filling when failure of urine storage occurred and immediately applying stimulation. Stimulation was applied at a single frequency and at varying amplitudes until stimulus evoked contractions (which resulted in voiding) could no longer be elicited. For each cat, a distension evoked voiding/storage trial was performed first, and subsequent trials for the storage and voiding studies were randomized.
Voiding efficiencies were defined by dividing the difference between the initial bladder volume and the post-void residual volume by the initial bladder volume:
Statistical Analysis
Statistical analysis of the dependence of detrusor responses on frequency was performed using a One-way Kruskal-Wallis test with the null hypothesis that the percent of trials showing a specific response did not vary across stimulus frequencies. Post hoc paired comparisons between individual frequencies were made using Bonferroni inequalities. Comparison of mean contraction amplitudes (across frequencies, across cats, and across frequencies for individual cats) was made using a One-way Kruskal-Wallis test or a Wilcoxon rank sum test (for individual cats if contraction occurred at only 2 frequencies). Statistical analysis of the normalized PTPs computed for distension evoked contractions with and without stimulation was done by a One-way Kruskal-Wallis test and post hoc paired comparisons were made using Bonferroni inequalities. Continent and voided volumes in the presence and absence of stimulation were compared separately for each cat using a Welch two-sample t-test. All reported values are mean ± standard deviation.
RESULTS
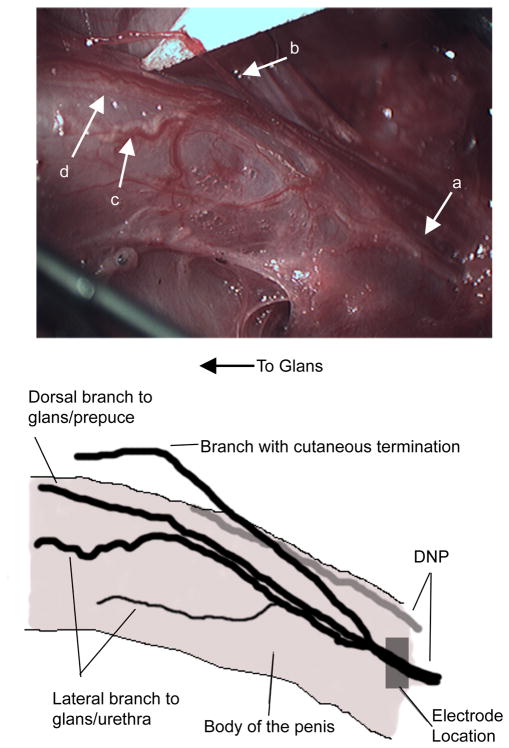
The dorsal nerve of the penis (DNP) originated from the sensory branch of the pudendal nerve, passed superficially along the ventral insertion of the ischiocavernosus muscle into the bulb of the penis, and continued towards the glans penis. As it coursed along the body of the penis, the DNP branched into three discernible fiber populations (FIGURE 1). Superficial fibers traveled along the dorsal aspect of the body of the penis and innervated the glans and the prepuce. Lateral fibers branched off deeper into the penile body and gave off branches directed towards the ventral aspect of the penis, innervating the urethra as well as the glans. Also, a branch of the DNP was directed off of the body of the penis and had cutaneous terminations. Similar DNP anatomy was observed in all 13 cats in which the DNP was isolated for stimulation with a cuff electrode. Some variability was observed in the distance caudal of the bulb of the penis before the branches of the DNP could be distinguished from one another. The cuff electrode was placed on the DNP proximal to any branching unless otherwise specified.
Figure 1. Anatomy of the dorsal nerve of the penis (DNP, a) of the cat.
Dorsolateral view of the penile body from the ventral side of the cat. A branch of the DNP was observed leaving the body of the penis and innervating the skin of the prepuce and perineum (b). Another branch of the DNP coursed along the lateral body of the penis to the glans and gave off nerves towards the urethra (c). A third, more superficial branch traveled along the dorsal aspect of the body of the penis to the glans penis and prepuce (d).
Activation of penile afferents in the DNP elicited stimulation-frequency dependent activation or inhibition of the micturition reflex. Detrusor contraction was elicited by direct or percutaneous stimulation of the DNP in 17 of 20 cats, and inhibition of distension evoked detrusor contractions was elicited in 16 of 18 cats. No response to DNP stimulation was observed in 2 cats, and only activation (detrusor contraction) or only inhibition (detrusor relaxation) was observed in 1 cat each (these cats were omitted from further analysis). Three cats were excluded from quantitative analysis because urethral leakage occurred during detrusor contractions despite the presence of the urethral catheter. Transecting the DNP cranial to the stimulating electrode (3 cats) or transecting the compound pudendal nerve (3 cats) abolished both the detrusor and EMG responses to DNP stimulation.
The ability to elicit detrusor activation by electrical stimulation of the DNP was dependent on the bladder volume. Stimulation threshold volumes (3–10ml, n=8 cats in which thresholds were investigated in 1ml increments) were less than distention threshold volumes (4–13ml), and STVs averaged 70%±7% of DTVs (range = 61–80%).
Stimulation at bladder volumes above the STV elicited frequency dependent activation of the micturition reflex
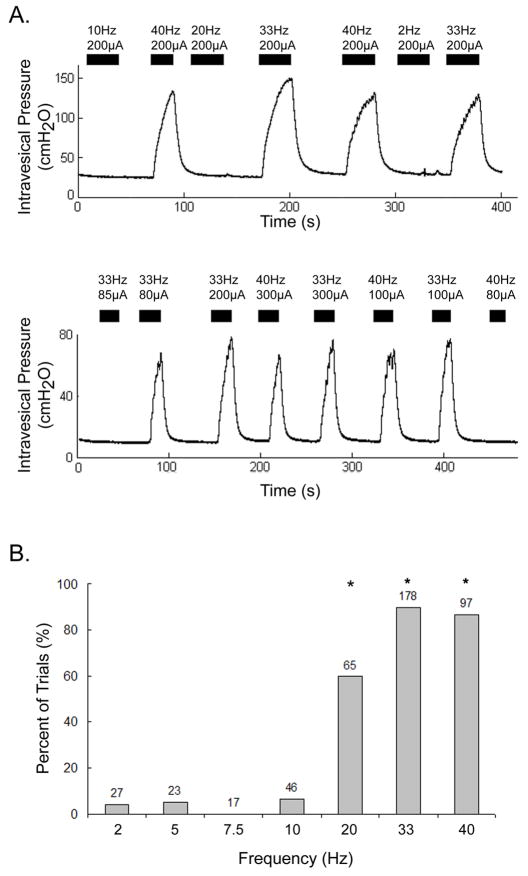
Direct electrical stimulation of the DNP activated the micturition reflex and elicited detrusor contractions in 9 of 11 cats (FIGURE 2A). The effect of stimulation frequency was examined systematically in 7 cats. The ability to elicit sustained detrusor contractions depended on stimulation frequency (FIGURE 2B, p<0.001, Kruskal-Wallis test, n=453 trials across 7 cats, stimulation amplitude at 2–4 times threshold). Stimulation at 20, 33, and 40Hz elicited contractions more consistently than stimulation at any of the lower frequencies (≤10 Hz) (p<0.01, Bonferroni inequalities). However, the response to stimulation at 20 Hz was not consistent across all cats. In 5 of 9 cats, 20 Hz stimulation elicited contractile responses (contraction occurred in >80% of the trials), while in 4 of 9 cats it did not elicit contractile responses (contraction occurred in <15% of the trials). The lower percentage of trials in which 20 Hz stimulation evoked contractions compared to 33 and 40 Hz is a reflection of this interanimal variability and not the effectiveness of 20 Hz stimulation within individual cats. The threshold stimulation amplitudes for eliciting detrusor responses ranged from 50μA-300μA. The ability to elicit contractions and contraction magnitudes increased when stimulation amplitude was increased from threshold to 2 times threshold, but stimulation at 2–4 times threshold evoked similar detrusor contractions.
FIGURE 2. Frequency dependent bladder responses to direct electrical stimulation of the DNP.
(A) Direct stimulation of the DNP is shown evoking destrusor contractions at bladder volumes between the STV and DTV in 2 different cats. Contractions were generated within 5 seconds of the onset of high frequency stimulation (33 and 40 Hz in the upper pressure trace, 20–40 Hz in the lower pressure trace) and ended with the termination of stimulation or shortly thereafter. Stimulation at low frequencies (≤10 Hz) did not elicit contractions. The black bars indicate the duration of stimulation, which consisted of 20 or 30 second trains at the frequency and amplitude above each bar. (B) Percent of trials in which direct stimulation of the DNP elicited detrusor contractions at different stimulus frequencies. The ability to elicit detrusor contraction by stimulation of the DNP was dependent on stimulation frequency (p<0.001, Kruskal-Wallis test, n=453 trials across 7 cats). Stimulation at 33 and 40 Hz consistently evoked detrusor contractions when stimulation was applied at appropriate bladder volumes and stimulus amplitude. Stimulation at 20 Hz evoked detrusor contractions in 4 of the 7 cats represented in the figure. Stimulation at 20, 33, and 40 Hz elicited contractions in a significantly greater percentage of trials than stimulation at 2–10 Hz (*p<0.01, Bonferroni inequalities). Bladder volumes were above STVs and below DTVs. Stimulus amplitudes ranged from 150μA-600μA (all amplitudes were 2–4x the threshold to elicit a bladder response). The number above each bar is the number of trials.
The mean increase in intravesical pressure evoked by stimulation at 20, 33, and 40Hz was 33.5 cmH2O (±15.4 cmH2O, n=283 contractions across 7 cats, stimulation amplitude at 2–4 times threshold), and did not vary significantly across these frequencies (p=0.207, Kruskal-Wallis). While the magnitude of stimulus evoked contractions varied from cat to cat (p<0.01, Kruskal-Wallis), there was no significant difference between contraction amplitudes elicited by stimulation at 20, 33, or 40 Hz in 6 of the 7 cats (p>0.05, Kruskal-Wallis, n=24–67 contractions per cat). In one cat, stimulation at 40 Hz elicited larger contractions than stimulation at 33 Hz (32.5±8.0 vs. 27.1±8.5 cmH2O, p<0.05, Wilcoxon rank sum test, n=60 contractions) while 20 Hz stimulation did not evoke detrusor contraction.
Stimulation at volumes above the DTV elicited frequency dependent detrusor relaxation and contraction
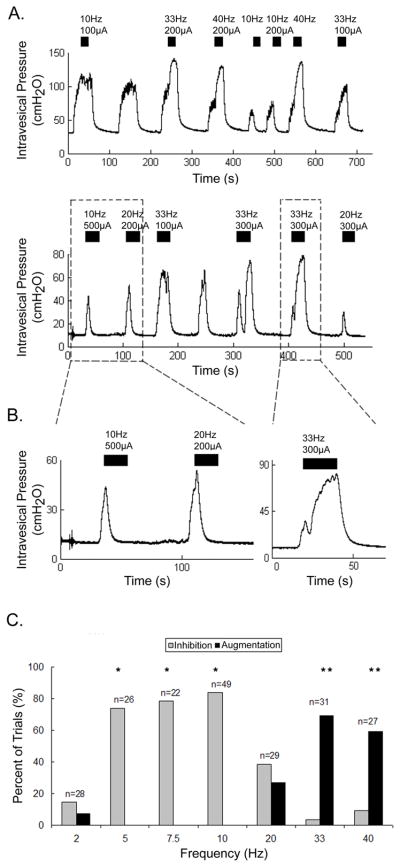
At bladder volumes above the DTV, direct stimulation of the DNP elicited detrusor contraction and relaxation in 8 of 9 cats (FIGURE 3A,B). The effect of stimulation frequency was examined systematically in 7 cats, and the detrusor response depended on stimulation frequency (FIGURE 3C, p<0.001, Kruskal-Wallis, n=212 trials across 7 cats). Stimulation at 5–10 Hz consistently inhibited distension evoked detrusor contractions, and stimulation at these frequencies inhibited distension evoked detrusor contractions more consistently than stimulation at a lower frequency (2 Hz) and higher frequencies (33–40 Hz) (p<0.01, Bonferroni inequalities). Stimulation during distension evoked contractions at 20 Hz inhibited detrusor contractions in 3 of 8 cats, augmented detrusor contractions in 4 cats, and did not elicit either response in 1 cat. Detrusor relaxation was evoked at threshold amplitudes of 80μA-400μA, but was elicited more consistently at 2–4 times the amplitude threshold for relaxation.
FIGURE 3. Direct stimulation of the DNP inhibited or augmented distension evoked detrusor contractions dependent on the stimulation frequency.
(A) Intravesical pressure in 2 cats shows inhibition and augmentation of distension evoked detrusor contractions by stimulation of the DNP. Stimulation at 10 Hz (and 20Hz in the lower trace) inhibited distension evoked contractions, while stimulation at 33 and 40 Hz augmented distension evoked contractions. Bladder volumes were above the DTVs. The black bars indicate the duration of the DNP stimulation, which consisted of 20 or 30 second trains at the frequency and amplitude above each bar. (B) A more detailed view of the detrusor inhibition (left panel) and augmentation (right panel) from the lower trace in (A). Stimulation at 10 and 20 Hz after the onset of distension evoked contractions caused the intravesical pressure to rapidly return to baseline. Stimulation at 33 Hz during a distension evoked contraction caused a rapid rise (after a brief decrease) in the intravesical pressure. (C) Percent of trials in which direct stimulation inhibited or augmented distension evoked detrusor contractions at different stimulus frequencies. Stimulation at 5–10 Hz inhibited detrusor contractions in a greater percentage of trials than stimulation at 2, 20, 33, and 40 Hz (*p<0.01, Bonferroni inequalities, n=7 cats). Stimulation at 33 and 40 Hz augmented contractions in a greater percentage of trials than stimulation at 2–20 Hz (**p<0.05, Bonferroni inequalities, n=7 cats). Bladder volumes were above the DTV and stimulus amplitudes ranged from 160μA-800μA (all amplitudes were 2–4x threshold). Numbers above each bar are the number of trials at each frequency.
At volumes above the DTV, stimulation at higher frequencies elicited detrusor contractions when delivered between distension evoked contractions and augmented detrusor contractions when applied during distension evoked detrusor contractions (FIGURE 3A,B). The ability to augment distension evoked detrusor contractions was dependent on stimulation frequency (FIGURE 3C, p<0.001, Kruskal-Wallis test, n=212 trials across 6 cats). Stimulation at 33 and 40 Hz augmented contractions in a greater percentage of trials than stimulation at 2–20 Hz (p<0.05, Bonferroni inequalities). Augmentation of distension evoked contractions resulted in maximum intravesical pressures similar to maximum stimulation evoked pressures (in absence of distension evoked contractions), but in some cases stimulation elicited a brief period of relaxation prior to contraction (Figure 3B).
The effect of DNP stimulation on distension evoked contractions was investigated systematically in 6 cats. The average pressure-time product (PTP) of detrusor contraction differed significantly across stimulation groups (FIGURE 4, Kruskal-Wallis, p<0.001). Inhibition of a contraction resulted in a mean PTP of 0.47 (±.017), which was significantly less than that of the unstimulated contractions (1.0±0.07) and the augmented contractions (1.47±0.29) (p<0.01, Bonferroni inequalities). Also, the augmented contraction had significantly larger relative PTPs than the unstimulated contractions (p<0.01, Bonferroni inequalities).
FIGURE 4. Effect of direct stimulation of the DNP on distension evoked detrusor contractions.
(A) The pressure-time produce (PTP) was computed as the area under the intravesical pressure trace (minus baseline pressure) for 20 seconds after the onset of a distension evoked contraction. The intravesical pressure trace shows the PTP (shaded area) for 10 Hz stimulation, a distension evoked contraction with no stimulation, and 33 Hz stimulation. (B) The normalized pressure-time products (PTPs) of the first 20 seconds of distension evoked detrusor contractions with or without stimulation are shown (mean ± standard deviation). The relative PTP of the detrusor contraction was dependent on the stimulation frequency (p<0.001, Kruskal-Wallis test, n=112 trials across 6 cats). Inhibitory stimulation (5–10 Hz) within the first 10 seconds of a distension evoked contraction reduced the PTP of distension evoked detrusor contractions significantly, while excitatory stimulation (20–40 Hz) increased detrusor PTP (*p<0.01, Bonferroni inequalities).
The reflex response of periurethral musculature to direct stimulation of the DNP
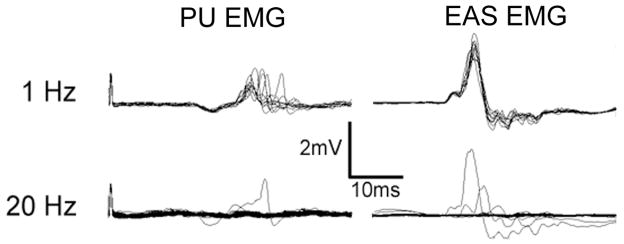
Direct stimulation of the DNP did not directly activate the periurethral musculature but elicited reflex PU EMG response in 4 of 5 intact cats. Whether electrical stimulation of the DNP elicited PU EMG responses depended on stimulation frequency (FIGURE 5). Low frequency stimulation (1–5 Hz) elicited reflex responses in the bulbourethra with reflex latencies of ~ 10–12 ms. The persistence of the reflex response throughout the trial diminished at higher frequencies (7.5–10 Hz) and reflex responses were elicited only by the first 1–4 stimulation pulses at high frequencies (20–40 Hz). The magnitude of reflex responses appeared to decrease with increasing bladder volume and over the course of repeated stimulation during the experiment. Stimulation of the DNP also evoked a reflex response in the EAS in 4 of 5 cats at a latency of 8–9 ms. Similar frequency dependence was observed in the EAS reflex response to DNP stimulation (FIGURE 5).
FIGURE 5. Periurethral (PU) and external anal sphincter (EAS) electromyograms evoked by direct electrical stimulation of the DNP.
Stimulation was delivered at either 1 or 20 Hz (10 second trains, 100μs pulses, 200μA) with the bladder empty. Reflex responses were elicited following each pulse during 1 Hz stimulation, but the response disappeared after the first 2–4 pulses during 20 Hz stimulation.
Stimulation controlled urine storage and voiding
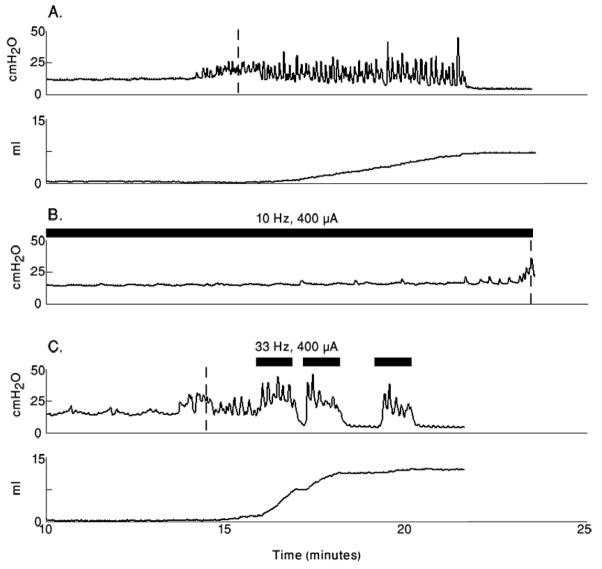
Urine storage and voiding were elicited by direct stimulation of the DNP in 4 cats (FIGURE 6). Bladder volumes at incontinence in the presence of low frequency (5–10 Hz) stimulation were 21±10 ml compared to bladder volumes at incontinence in the absence of stimulation of 15±7 ml (p<0.02 for each cat, n=25 trials across 4 cats with at least 3 trials of each type per cat, Welch Two-Sample t-test). Direct stimulation of the DNP at higher frequencies (33 and 40 Hz), begun at the volume at which continence was lost, resulted in an increase in percent bladder voiding compared to distension evoked percent bladder voiding in 4 of 4 cats (p<0.05, n=27 trials across 4 cats with at least 3 trials of each type per cat, Welch Two-Sample t-test). Distension evoked voiding resulted in 37% (±13.5%) bladder voiding (19–60%), while stimulation evoked voiding resulted in 64% (±12%) voiding (49%–84%).
FIGURE 6. Direct stimulation of the DNP improved continence and voiding.
Cystometrograms are shown for a single cat with no stimulation, inhibitory stimulation, and excitatory stimulation. The bladder was filled at 1ml/min. (A) Intravesical pressure during bladder filling in the absence of stimulation (upper plot) and the volume voided by the distension evoked contractions (lower plot). Continence was lost at 15.3ml (indicated by the dashed vertical line in the upper plot) and the total volume voided was 8.2ml. (B) Intravesical pressure during bladder filling with continuous 10 Hz stimulation of the DNP starting at 8ml infused volume. A sustained detrusor contraction occurred at 23.4ml (indicated by the dashed vertical line). (D) Intravesical pressure during bladder filling in the absence of stimulation but with stimulation evoked bladder voiding. Continence was lost at 14.2ml (indicated by the dashed vertical line in the pressure trace), and the total volume voided was 11.0ml. Stimulation trains are indicated by the black bars above the intravesical pressure traces.
Percutaneous stimulation of the DNP elicited frequency dependent detrusor responses
Electrical stimulation of the DNP with a percutaneous wire electrode evoked activation and inhibition of the micturition reflex in 6 of 7 cats. These responses exhibited the same characteristics as those evoked by direct stimulation of the DNP with a cuff electrode. The threshold amplitude for eliciting responses ranged from 1.5 to 4 mA. Whether stimulation evoked detrusor contraction or relaxation depended on the stimulation frequency (p<0.001 for augmentation and inhibition, Kruskal-Wallis test, n=520 trials across 6 cats). Stimulation at 20–40 Hz elicited detrusor contraction more effectively than stimulation at 2–10 Hz (p<0.01, Bonferroni inequalities). Stimulation at 7.5 and 10 Hz inhibited distension evoked contractions in a greater percentage of trials than stimulation at 33 and 40 Hz ( p<0.01, Bonferroni inequalities).
Selective stimulation of the penile body branches of the DNP elicits detrusor responses
The cutaneous branch of the DNP, observed to branch off of the penile body within a centimeter of the bulb (FIGURE 1), was stimulated selectively, separate from the 2 larger branches of the DNP that continued to course along the penile body. No EAS or detrusor responses were elicited by stimulation of the cutaneous branch in 6 of 6 cats. Conversely, co-stimulation of the 2 penile branches (separately from the cutaneous branch) elicited detrusor contractions and EAS responses comparable to those evoked by stimulation of the DNP in 5 of 6 cats. In 3 of 4 cats in which distension evoked contractions occurred during stimulation of the divisions of the DNP, inhibition of distension evoked contractions was elicited by low frequency co-stimulation of the 2 penile branches.
Responses to DNP stimulation were preserved after acute spinal cord transection (SCT)
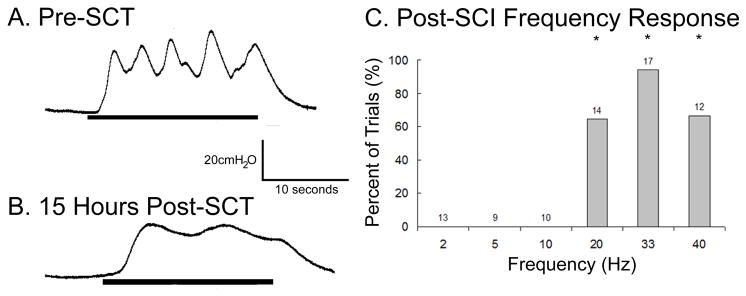
At 8–15 hrs following SCT, direct stimulation of the DNP elicited detrusor contractions in 2 of 2 cats (FIGURE 7B). Detrusor contractions were evoked at stimulation frequencies of 20, 33, and 40 Hz, while stimulation at lower frequencies failed to elicited detrusor contractions (FIGURE 7C). The ability to elicit detrusor contractions depended on stimulation frequency in a manner similar to prior to SCT (p<0.001, Kruskal-Wallis test, n=75 trials across 2 cats), and stimulation at 20, 33, and 40 Hz were significantly more likely to elicit contractions than stimulation at 2, 5, and 10 Hz (p<0.05, Bonferroni inequalities). In one cat the minimum bladder volume at which stimulation evoked bladder contractions occurred was between the STV and DTV observed before the spinal transection, and in the second cat the volume was 20% higher (12ml vs 10ml) than the DTV before the spinal transection.
FIGURE 7. The contractile detrusor response to direct electrical stimulation of the DNP was present following acute SCI.
The intravesical pressure responses to 20 second trains of stimulation at 33 Hz, 200μA before SCT (A) and 15 hours after SCT at T10 (B) in one cat. (C) The percent of trials that elicited detrusor contractions at different frequencies shows that detrusor contraction was dependent on stimulation frequency (p<0.0001, Kruskal-Wallis test, n=75 trials across 2 cats). Stimulation at 20, 33, and 40 Hz elicited contractions significantly more frequently than stimulation at 2, 5, and 10 Hz (*p<0.05, Bonferroni inequalities). Stimulation at 2, 5, or 10 Hz did not elicit any detrusor contractions following SCT.
Discussion
Stimulation of penile afferents in the dorsal nerve of the penis (DNP) activated reflexes that elicited either contraction or relaxation of the detrusor. Detrusor activation and inhibition were evoked differentially by electrical stimulation of the DNP dependent on the stimulation frequency. Low frequency (5–10 Hz) stimulation inhibited distension-evoked detrusor contractions and promoted urine storage, while high frequency stimulation (33–40 Hz) elicited detrusor contractions, augmented distension evoked contractions, and produced bladder voiding. These results demonstrate that a somatic sensory input, heretofore asserted to only cause detrusor inhibition, can also cause detrusor contraction, and that the evoked response is strongly dependent on the frequency of electrical stimulation of the afferent inputs.
Activation of the detrusor by stimulation of the DNP occurred only when there was a sufficient volume of fluid in the bladder, both before and after acute spinal transection, as observed with stimulation of the compound pudendal nerve and urethral and perineal pudendal afferents in cat (5, 7, 49) and human (21, 66), and with contractions evoked by urethral fluid flow (9, 44). The bladder volume dependence of the detrusor response is due to a neural mechanism rather than the length-tension properties of the bladder (5), and the ability of urethral afferent activity to elicit reflex discharges in bladder pelvic nerves is similarly dependent on the level of background facilitation of the micturition reflex (37). That detrusor activation was evoked only if there was a sufficient volume in the bladder implies a convergence between bladder (pelvic) and pudendal afferents in the spinal cord, as the responses were preserved following acute spinal transection. In addition to the volume dependence of the bladder response to DNP stimulation, studies have shown that pudendal stimulation evoked inhibition of bladder activity in chronic spinalized cats and DGN stimulation evoked inhibition of the pelvic C-fiber bladder reflex in acute spinalized cats can be enhanced by increasing the stimulation intensity at inhibitory stimulation frequencies (36, 52). The dependence of DNP stimulation evoked bladder inhibition on stimulus amplitude has also been illustrated in humans with SCI (42).
Detrusor contraction elicited by activation of pudendal afferents occurs via both supraspinal and spinal pathways (2, 5). The presence of a pudendal afferent driven spinal bladder reflex has been confirmed in neonatal and chronic SCI cats (14, 52), but this pathway is masked by supraspinal serotonergic inhibition in the adult cat (55). Contraction of the detrusor evoked by penile afferent activation in the intact cat could reflect activation of a spinal-bulbo-spinal excitatory pathway, a spinal pudendo-vesical reflex, or the simultaneous inhibition of the supraspinal serotonergic inhibition of the spinal pudendo-vesical reflex and activation of the spinal reflex. In the acute SCT cat, the activation is through a spinal reflex, which may only be accessible after removal of supraspinal inhibitory inputs following spinal transection.
Activation of urethral afferents influences the bladder through an apparent convergence with bladder afferents in the spinal cord (5, 54). Pelvic and pudendal afferent projections overlap in the lateral dorsal horn and the dorsal gray commissure of the lumbosacral spinal cord in the rat, cat, and macaque monkey (12, 27, 40, 43, 45, 57). Similarly, afferent fibers from the DNP of the rat project to the dorsal horn, the dorsal gray commissure, and the sacral parasympathetic nucleus (40, 43), and clitoral afferents in the cat terminate in the dorsal gray commissure (27). Activation of penile afferents results in Fos labeling of neurons in the sacral parasympathetic nucleus after spinal transection, consistent with supraspinal inhibition of DNP-activated spinal neurons (43). There is also evidence of supraspinal convergence of pelvic and pudendal afferents in regions associated with control of lower urinary tract function. Penile afferents project to the paragigantocellularis region of the medial reticular formation (20), and afferents activated by DNP stimulation, urethral infusion, and bladder distension converge on neurons in the medullary reticular formation (26). The ventral medullary gigantocellular reticular nuclei (which consists partially of the lateral paragigantocellular nucleus) projects to the intermediate gray and sacral parasympathetic nucleus of the sacral spinal cord (23). The convergence of penile somatic and bladder parasympathetic pathways is not surprising considering that DNP afferents and parasympathetic efferents are involved in erectile function (43). The present results extend the role of DNP afferents to urinary function, as well.
Stimulation of the DNP caused detrusor contraction at high frequencies (33 and 40 Hz) and detrusor relaxation at 5–10 Hz, similar to the frequency dependence for detrusor contraction and relaxation evoked by stimulation of afferents in the compound pudendal nerve (7, 52, 65). No studies have provided evidence to suggest a particular mechanism for this frequency dependence. The frequency dependence of the detrusor response to penile afferent stimulation may be due to frequency-dependent interactions between DNP afferents and parasympathetic bladder afferents and efferents. Low frequency (5–10 Hz) activity in DNP afferents may evoke primary afferent depolarization (PAD) of bladder afferents (1) or post-synaptic inhibition of interneurons activated by bladder afferents. At higher stimulation frequencies a second pathway may be excited by activation of an interneuron via temporal summation of the DNP afferent activity. This pathway would result in the inhibition of the inhibitory PAD in addition to driving further activation of the micturition reflex pathway associated with bladder afferents. This mechanism of frequency dependent pathway activation is similar to that proposed for differential motor responses evoked by different frequencies of epidural spinal cord stimulation (25). Another potential mechanisms for the observed frequency dependence is a synaptic mechanism described previously in the hippocampus (38). A biphasic response to afferent activation depended on stimulation frequency; at 10 Hz disynaptic inhibition predominated, while at frequencies greater than 20 Hz the inhibition was depressed and monosynaptic excitation was dominant. A similar mechanism could exist at the convergence of pudendal afferents and sacral interneurons. Recordings from parasympathetic preganglionic neurons and sacral spinal interneurons reveal the existence of inhibitory and excitatory inputs from pudendal and pelvic afferents (15, 34).
Direct stimulation of the DNP resulted in increases in the continent bladder volume of 35%–77% over no stimulation. A previous study in intact cats with pudendal afferent stimulation found volume increases of 18% over no stimulation (61), and in chronic spinalized cats the increase in continence volume during pudendal nerve stimulation was 147% (53). Similar increases (22–366%) in continent bladder volumes result from genital afferent stimulation in the human (19, 22, 28, 58, 62). Detrusor relaxation was likely caused by inhibition of the Aδ-fiber mediated micturition reflex via reflex activation of sympathetic hypogastric efferents (at low intravesical pressure) or inhibition of pelvic efferents (at high intravesical pressure) (18, 32). The micturition reflex after chronic SCI is mainly mediated by c-fiber bladder afferents (8, 13, 16), but stimulation of the DGN can inhibit both the Aδ and the c-fiber mediated reflexes (36).
Electrical stimulation of the DNP at high frequencies (33 and 40 Hz) improved the voiding efficiency from 37% (±14%, no stimulation) to 64% (±12%, DNP stimulation). Voiding via stimulation of the DNP was achieved with continuous stimulation and without transection of any nerves via reflex activation of parasympathetic pelvic efferents (2, 3). The stimulation evoked voiding efficiencies are comparable with voiding percentages reported from stimulation of perineal afferents of the pudendal nerve (63%±20%) and the compound pudendal nerve (64%±14%) in the α-chloralose anesthetized male cat (5, 6). Incomplete voiding and the consistent post-void residual bladder volume may be due to the effects of the α-chloralose (46) or the reduced drive to the micturition reflex from the bladder afferents in the pelvic nerve when the bladder volume decreases as voiding occurs.
The ability to elicit voiding with continuous DNP stimulation demonstrates an apparent absence of stimulation induced detrusor-sphincter dyssynergia (DSD), which is consistent with the present PU EMG recordings and previous EUS recordings during pudendal afferent stimulation (5, 7, 49). The PU EMG response was strongly dependent on stimulation frequency and did not persist at stimulus frequencies ≥ 20 Hz, consistent with previous findings (7, 10, 11). The lack of direct activation of the urethral sphincteras measured in the PU EMG, combined with the ability to void during continuous stimulation, suggest that stimulation of the DNP in the intact cat does not result in DSD. However, the bulbocavernosus reflex and EUS response to DNP stimulation during detrusor contraction are enhanced in patients with DSD (17, 59), so the effect of high frequency DNP stimulation evoked detrusor contractions on already present DSD is unclear.
While reorganization of the sacral spinal components of the micturition reflex pathway (4, 13) may affect the ability to elicit detrusor contractions by electrical stimulation of the DNP, recent results suggest that pudendal afferent mediated reflex activation of the bladder is preserved in humans with chronic SCI (21, 66). Also, perigenital electrical stimulation in the chronic spinal transected cat can activate and inhibit the bladder at similar frequencies as DNP stimulation (51). This method of stimulation in the female cat is likely activating the dorsal clitoral nerve, the female analog of the DNP, suggesting that the frequency dependent bladder responses to electrical stimulation of the DNP observed in the intact and acute cat are still present in the chronic spinal cord injured cat.
Prespectives and Significance
Stimulation of the DNP at high frequencies (20–40 Hz) elicited detrusor contractions and augmentation of distension evoked detrusor contractions, while stimulation at 5–10Hz consistently inhibited distension evoked detrusor contractions. These findings may find application in electrical restoration of bladder function – both storage and emptying, and electrical stimulation of the DNP is an especially compelling approach because of the ease of access to the dorsal genital nerves in human (63). Access to the DGN for electrical stimulation can be achieved more readily than access to the compound pudendal nerve or other urethral pudendal afferents (35, 47, 64), and the proximity of the DGN to the skin makes it an ideal target for percutaneous stimulation (64). Percutaneous stimulation of the DNP produced bladder responses equivalent to those generated by direct nerve stimulation. In addition, transection or block of the nerve distal to the stimulating electrode is not necessary because the DGN does not innervate the EUS (35). However, DNP stimulation may only be applicable to a limited population as certain individuals may be unable to tolerate stimulation at an adequate intensity (19). Further, the excitatory bladder response to DNP stimulation has only been accessed in cats, and the existence of a similar excitatory bladder pathway from penile afferents in humans is unknown.
Acknowledgments
The authors thank Ms. Gilda Mills for her technical assistance. This work was supported by the National Institutes of Health (R01NS050514) and the National Science Foundation Graduate Research Fellowship Program.
References
- 1.Angel MJ, Fyda D, McCrea DA, Shefchyk SJ. Primary afferent depolarization of cat pudendal afferents during micturition and segmental afferent stimulation. J Physiol. 1994;479:451–461. doi: 10.1113/jphysiol.1994.sp020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrington F. The component reflexes of micturition in the cat. Parts I and II. Brain. 1931;54:177–188. [Google Scholar]
- 3.Barrington F. The component reflexes of micturition in the cat. Part III. Brain. 1941;64:239–243. [Google Scholar]
- 4.Beattie MS, Leedy MG, Bresnahan JC. Evidence for alterations of synaptic inputs to sacral spinal reflex circuits after spinal cord transection in the cat. Exp Neurol. 1993;123:35–50. doi: 10.1006/exnr.1993.1138. [DOI] [PubMed] [Google Scholar]
- 5.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol. 2005;93:2688–2697. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- 6.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. J Neural Eng. 2006;3:43–51. doi: 10.1088/1741-2560/3/1/005. [DOI] [PubMed] [Google Scholar]
- 7.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol. 2006;577:115–126. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am J Physiol. 1999;277:R786–794. doi: 10.1152/ajpregu.1999.277.3.R786. [DOI] [PubMed] [Google Scholar]
- 9.Combrisson H, Allix S, Robain G. Influence of temperature on urethra to bladder micturition reflex in the awake ewe. Neurourol Urodyn. 2007;26:290–295. doi: 10.1002/nau.20311. [DOI] [PubMed] [Google Scholar]
- 10.Cook JR, Jr, Oliver JE, Jr, Purinton PT. Comparison of genitoanal and bulbospongiosus reflexes and measurement of penile nerve conduction velocity in cats. Am J Vet Res. 1991;52:24–28. [PubMed] [Google Scholar]
- 11.Cook JR, Oliver JE, Purinton PT. Pudendal reflexes and effects of conditioning stimuli in cats. Am J Vet Res. 1991;52:18–23. [PubMed] [Google Scholar]
- 12.de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006;147 (Suppl 2):S25–40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 14.de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Sys. 1981:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- 15.de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol. 1969;200:87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- 17.Dyro FM, Yalla SV. Refractoriness of urethral striated sphincter during voiding: studies with afferent pudendal reflex arc stimulation in male subjects. J Urol. 1986;135:732–736. doi: 10.1016/s0022-5347(17)45834-4. [DOI] [PubMed] [Google Scholar]
- 18.Fall M, Erlandson BE, Carlsson CA, Lindstrom S. The effect of intravanginal electrical stimulation on the feline urethra and urinary bladder. Neuronal mechanisms. Scand J Urol Nephrol Suppl. 1978;44:19–30. [PubMed] [Google Scholar]
- 19.Fjorback MV, Rijkhoff N, Petersen T, Nohr M, Sinkjaer T. Event driven electrical stimulation of the dorsal penile/clitoral nerve for management of neurogenic detrusor overactivity in multiple sclerosis. Neurourol Urodyn. 2006;25:349–355. doi: 10.1002/nau.20170. [DOI] [PubMed] [Google Scholar]
- 20.Giuliano F, Rampin O. Central neural regulation of penile erection. Neurosci Biobehav Rev. 2000;24:517–533. doi: 10.1016/s0149-7634(00)00020-8. [DOI] [PubMed] [Google Scholar]
- 21.Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci Lett. 2004;360:9–12. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Hansen J, Media S, Nohr M, Biering-Sorensen F, Sinkjaer T, Rijkhoff NJ. Treatment of neurogenic detrusor overactivity in spinal cord injured patients by conditional electrical stimulation. J Urol. 2005;173:2035–2039. doi: 10.1097/01.ju.0000158160.11083.1b. [DOI] [PubMed] [Google Scholar]
- 23.Hermann GE, Holmes GM, Rogers RC, Beattie MS, Bresnahan JC. Descending spinal projections from the rostral gigantocellular reticular nuclei complex. J Comp Neurol. 2003;455:210–221. doi: 10.1002/cne.10455. [DOI] [PubMed] [Google Scholar]
- 24.Holstege G, Griffiths D, de Wall H, Dalm E. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449–461. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- 25.Jilge B, Minassian K, Rattay F, Dimitrijevic MR. Frequency-dependent selection of alternative spinal pathways with common periodic sensory input. Biol Cybern. 2004;91:359–376. doi: 10.1007/s00422-004-0511-5. [DOI] [PubMed] [Google Scholar]
- 26.Kaddumi EG, Hubscher CH. Convergence of multiple pelvic organ inputs in the rat rostral medulla. J Physiol. 2006;572:393–405. doi: 10.1113/jphysiol.2005.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawatani M, Tanowitz M, de Groat WC. Morphological and electrophysiological analysis of the peripheral and central afferent pathways from the clitoris of the cat. Brain Res. 1994;646:26–36. doi: 10.1016/0006-8993(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 28.Kirkham AP, Shah NC, Knight SL, Shah PJ, Craggs MD. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord. 2001;39:420–428. doi: 10.1038/sj.sc.3101177. [DOI] [PubMed] [Google Scholar]
- 29.Kruse MN, Mallory BS, Noto H, Roppolo JR, de Groat WC. Properties of the descending limb of the spinobulbospinal micturition reflex pathway in the cat. Brain Res. 1991;556:6–12. doi: 10.1016/0006-8993(91)90541-3. [DOI] [PubMed] [Google Scholar]
- 30.Kruse MN, Mallory BS, Noto H, Roppolo JR, de Groat WC. Modulation of the spinobulbospinal micturition reflex pathway in cats. Am J Physiol. 1992;262:R478–484. doi: 10.1152/ajpregu.1992.262.3.R478. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y, Creasey G. Self-controlled dorsal penile nerve stimulation to inhibit bladder hyperreflexia in incomplete spinal cord injury: A case report. Arch Phys Med Rehabil. 2002;83:273–277. doi: 10.1053/apmr.2002.28817. [DOI] [PubMed] [Google Scholar]
- 32.Lindström S, Fall M, Carlsson CA, Erlandson BE. The neurophysiological basis of bladder inhibition in response to intravaginal electrical stimulation. J Urol. 1983;129:405–410. doi: 10.1016/s0022-5347(17)52127-8. [DOI] [PubMed] [Google Scholar]
- 33.Lindström S, Sudsuang R. Functionally specific bladder reflexes from pelvic and pudendal nerve branches; an experimental study in the cat. Neurourol Urodyn. 1989;8:392–394. [Google Scholar]
- 34.Lu Y, Inokuchi H, Tanaka E, Li JS, Higashi H. A spinal cord slice preparation for analyzing synaptic responses to stimulation of pelvic and pudendal nerves in mature rats. J Neurosci Methods. 2000;100:71–78. doi: 10.1016/s0165-0270(00)00232-6. [DOI] [PubMed] [Google Scholar]
- 35.Martin WD, Fletcher TF, Bradley WE. Innervation of feline perineal musculature. Anat Rec. 1974;180:15–29. doi: 10.1002/ar.1091800104. [DOI] [PubMed] [Google Scholar]
- 36.Mazieres L, Jiang C, Lindstrom S. The C fibre reflex of the cat urinary bladder. J Physiol. 1998;513:531–541. doi: 10.1111/j.1469-7793.1998.531bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazières L, Jiang C, Lindtröm S. Bladder parasympathetic response to electrical stimulation of urethral afferents in the cat. Neurourol Urodyn. 1997;16:471–472. [Google Scholar]
- 38.Mori M, Abegg MH, Gahwiler BH, Gerber U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature. 2004;431:453–456. doi: 10.1038/nature02854. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura M, Sakurai T. Bladder inhibition by penile electrical stimulation. Br J Urol. 1984;56:413–415. doi: 10.1111/j.1464-410x.1984.tb05833.x. [DOI] [PubMed] [Google Scholar]
- 40.Nunez R, Gross GH, Sachs BD. Origin and central projections of rat dorsal penile nerve: possible direct projection to autonomic and somatic neurons by primary afferents of nonmuscle origin. J Comp Neurol. 1986;247:417–429. doi: 10.1002/cne.902470402. [DOI] [PubMed] [Google Scholar]
- 41.Peng CW, Chen JJ, Cheng CL, Grill WM. Role of pudendal afferents in voiding efficiency in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R660–672. doi: 10.1152/ajpregu.00270.2007. [DOI] [PubMed] [Google Scholar]
- 42.Previnaire JG, Soler JM, Perrigot M, Boileau G, Delahaye H, Schumacker P, Vanvelcenaher J, Vanhee JL. Short-term effect of pudendal nerve electrical stimulation on detrusor hyperreflexia in spinal cord injury patients: importance of current strength. Paraplegia. 1996;34:95–99. doi: 10.1038/sc.1996.17. [DOI] [PubMed] [Google Scholar]
- 43.Rampin O, Gougis S, Giuliano F, Rousseau JP. Spinal Fos labeling and penile erection elicited by stimulation of dorsal nerve of the rat penis. Am J Physiol. 1997;272:R1425–1431. doi: 10.1152/ajpregu.1997.272.5.R1425. [DOI] [PubMed] [Google Scholar]
- 44.Robain G, Combrisson H, Mazieres L. Bladder response to urethral flow in the awake ewe. Neurourol Urodyn. 2001;20:641–649. doi: 10.1002/nau.1014. [DOI] [PubMed] [Google Scholar]
- 45.Roppolo JR, Nadelhaft I, de Groat WC. The organization of pudendal motoneurons and primary afferent projections in the spinal cord of the rhesus monkey revealed by horseradish peroxidase. J Comp Neurol. 1985;234:475–488. doi: 10.1002/cne.902340406. [DOI] [PubMed] [Google Scholar]
- 46.Rudy D, Downie J, McAndrew J. alpha-Chloralose alters autonomic reflex function of the lower urinary tract. Am J Physiol. 1991;261:R1560–1567. doi: 10.1152/ajpregu.1991.261.6.R1560. [DOI] [PubMed] [Google Scholar]
- 47.Schraffordt SE, Tjandra JJ, Eizenberg N, Dwyer PL. Anatomy of the pudendal nerve and its terminal branches: a cadaver study. ANZ J Surg. 2004;74:23–26. doi: 10.1046/j.1445-1433.2003.02885.x. [DOI] [PubMed] [Google Scholar]
- 48.Shafik A, Shafik AA, El-Sibai O, Ahmed I. Role of positive urethrovesical feedback in vesical evacuation. The concept of a second micturition reflex: the urethrovesical reflex. World J Urol. 2003;21:167–170. doi: 10.1007/s00345-003-0340-5. [DOI] [PubMed] [Google Scholar]
- 49.Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett. 1998;244:137–140. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- 50.Sundin T, Carlsson CA, Kock NG. Detrusor inhibition induced from mechanical stimulation of the anal region and from electrical stimulation of pudendal nerve afferents. An experimental study in cats. Invest Urol. 1974;11:374–378. [PubMed] [Google Scholar]
- 51.Tai C, Shen B, Wang J, Chancellor MB, Roppolo JR, de Groat WC. Inhibitory and Excitatory Perigenital-to-Bladder Spinal Reflexes in the Cat. Am J Physiol Renal Physiol. 2007 doi: 10.1152/ajprenal.00443.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol. 2006;197:225–234. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Tai C, Wang J, Wang X, de Groat WC, Roppolo JR. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn. 2007;26:570–577. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- 54.Tai C, Wang J, Wang X, Roppolo JR, de Groat WC. Voiding reflex in chronic spinal cord injured cats induced by stimulating and blocking pudendal nerves. Neurourol Urodyn. 2007;26:879–886. doi: 10.1002/nau.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thor KB, Hisamitsu T, de Groat WC. Unmasking of a neonatal somatovesical reflex in adult cats by the serotonin autoreceptor agonist 5-methoxy-N,N- dimethyltryptamine. Brain Res Dev Brain Res. 1990;54:35–42. doi: 10.1016/0165-3806(90)90062-4. [DOI] [PubMed] [Google Scholar]
- 56.Todd JK. Afferent Impulses in the Pudendal Nerves of the Cat. Q J Exp Physiol Cogn Med Sci. 1964;49:258–267. doi: 10.1113/expphysiol.1964.sp001730. [DOI] [PubMed] [Google Scholar]
- 57.Ueyama T, Mizuno N, Takahashi O, Nomura S, Arakawa H, Matsushima R. Central distribution of efferent and afferent components of the pudendal nerve in macaque monkeys. J Comp Neurol. 1985;232:548–556. doi: 10.1002/cne.902320411. [DOI] [PubMed] [Google Scholar]
- 58.Vodusek D, Light J, Libby J. Detrusor inhibition induced by stimulation of pudendal nerve afferents. Neurourol Urodyn. 1986:381–389. [Google Scholar]
- 59.Walter JS, Wheeler JS, Jr, Dunn RB. Dynamic bulbocavernosus reflex: dyssynergia evaluation following SCI. J Am Paraplegia Soc. 1994;17:140–145. doi: 10.1080/01952307.1994.11735924. [DOI] [PubMed] [Google Scholar]
- 60.Wang B, Bhadra N, Grill WM. Functional anatomy of the male feline urethra: morphological and physiological correlations. J Urol. 1999;161:654–659. [PubMed] [Google Scholar]
- 61.Wenzel BJ, Boggs JW, Gustafson KJ, Grill WM. Closed loop electrical control of urinary continence. J Urol. 2006;175:1559–1563. doi: 10.1016/S0022-5347(05)00657-9. [DOI] [PubMed] [Google Scholar]
- 62.Wheeler JS, Jr, Walter JS, Zaszczurynski PJ. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol. 1992;147:100–103. doi: 10.1016/s0022-5347(17)37145-8. [DOI] [PubMed] [Google Scholar]
- 63.Yang CC, Bradley WE. Neuroanatomy of the penile portion of the human dorsal nerve of the penis. Br J Urol. 1998;82:109–113. doi: 10.1046/j.1464-410x.1998.00669.x. [DOI] [PubMed] [Google Scholar]
- 64.Yang CC, Bradley WE. Peripheral distribution of the human dorsal nerve of the penis. J Urol. 1998;159:1912–1917. doi: 10.1016/S0022-5347(01)63194-X. [DOI] [PubMed] [Google Scholar]
- 65.Yoo PB, Grill WM. Minimally-invasive electrical stimulation of the pudendal nerve: a pre-clinical study for neural control of the lower urinary tract. Neurourol Urodyn. 2007;26:562–569. doi: 10.1002/nau.20376. [DOI] [PubMed] [Google Scholar]
- 66.Yoo PB, Klein SM, Grafstein NH, Horvath EE, Amundsen CL, Webster GD, Grill WM. Pudendal nerve stimulation evokes reflex bladder contractions in persons with chronic spinal cord injury. Neurourol Urodyn. 2007;26:1020–1023. doi: 10.1002/nau.20441. [DOI] [PubMed] [Google Scholar]