Abstract
Microtubule polymerization is initiated from the microtubule organizing centre (MTOC), which contains the γ-tubulin complex. We have identified fission yeast Alp4 and Alp6, which are homologues of the γ-tubulin-interacting proteins Sc.Spc97/Hs.Gcp2 and Sc.Spc98/Hs.Gcp3, respectively. The size of the fission yeast γ-tubulin complex is large (>2000 kDa), comparable to that in metazoans. Both Alp4 and Alp6 localize to the spindle pole body (SPB) and also to the equatorial MTOC. Temperature-sensitive (ts) alp4 and alp6 mutants show two types of microtubular defects. First, monopolar mitotic spindles form. Secondly, abnormally long cytoplasmic microtubules appear that do not stop at the cell tips and are still associated with the SPB. Alp4 function is required in G1 phase and ts mutants become lethal before S-phase. alp4 and alp6 mutants are hypersensitive to the microtubule- destabilizing drug thiabendazole (TBZ) and show a lethal ‘cut’ phenotype in its presence. Furthermore, alp4mad2 double mutants show an exaggerated multiple septation phenotype in TBZ. These results indicate that Alp4 and Alp6 may play a crucial role in the spindle pole-mediated checkpoint pathway.
Keywords: fission yeast/microtubules/MTOC/spindle checkpoint/γ-tubulin
Introduction
In many eukaryotic cells, microtubule nucleation occurs at a specific structure called the microtubule organizing centre (MTOC; Pickett-Heaps, 1969). The MTOC plays a vital role not only in nucleating microtubules, but also in determining their polarity. The slow growing minus end is embedded within the MTOC, while the fast growing plus end radiates out into either the cytoplasm or the nucleus (Heidemann and McIntosh, 1980). MTOC structure varies considerably between different species. In animal cells, the centrosome, in particular the pericentriolar material (PCM), functions as the MTOC (Gould and Borisy, 1978; Kellogg et al., 1994). In yeast, the equivalent structure is the spindle pole body (SPB).
Central to MTOC function is the γ-tubulin complex. γ-tubulin, a member of the tubulin superfamily, was originally identified genetically as an intergenic suppressor of a β-tubulin mutation in Aspergillus nidulans (Oakley and Oakley, 1989). Subsequent work in various systems both in vivo and in vitro has established that γ-tubulin is a universal component of the MTOC, and plays a central role in microtubule nucleation (Oakley et al., 1990; Horio et al., 1991; Stearns et al., 1991; Zheng et al., 1991; Joshi et al., 1992, 1993; Oakley, 1992; Felix et al., 1994; Stearns and Kirschner, 1994; Schnackenberg et al., 1998). γ-tubulin does not appear to exist in the cell on its own; instead it is part of a large complex (Stearns and Kirschner, 1994). This γ-tubulin complex in animal cells comprises an open ring structure of 25 nm diameter, called the γ-tubulin ring complex (γTuRC), which exists in both the cytoplasm and the PCM (Moritz et al., 1995, 1998; Zheng et al., 1995) and functions as a minus end capping factor for microtubule nucleation (Keating and Borisy, 2000; Leguy et al., 2000; Moritz et al., 2000; Wiese and Zheng, 2000).
Genetic studies in budding yeast have been instrumental in identifying other components of the γTuRC. Spc97 and Spc98 were identified as γ-tubulin-interacting proteins and shown to constitute the major components of the Saccharomyces cerevisiae γ-tubulin complex (Geissler et al., 1996; Knop and Schiebel, 1997; Knop et al., 1997). Homologues of Spc97 and Spc98 have subsequently been found in humans and are called Gcp2 and Gcp3, respectively (Martin et al., 1998; Murphy et al., 1998; Tassin et al., 1998). The budding yeast counterpart of γ-tubulin, Tub4, which also plays an important role in microtubule organization in this organism, is more divergent from γ-tubulins in other organisms (<40% amino acid identity with metazoan γ-tubulins as opposed to 70% identity between them), and metazoan γ-tubulin is not capable of substituting for Tub4 function (Burns, 1995; Sobel and Snyder, 1995; Marschall et al., 1996). Furthermore, the Tub4-containing complex is much smaller than the mammalian γTuRC (200–250 kDa versus >2000 kDa; Stearns and Kirschner, 1994; Zheng et al., 1995; Knop et al., 1997).
The fission yeast γ-tubulin homologue Gtb1/Tug1 shares >70% identity with the vertebrate protein (Horio et al., 1991; Stearns et al., 1991) and, more importantly, human γ-tubulin can rescue the lethal gtb1 deletion (Horio and Oakley, 1994). Gtb1 localizes to the SPB throughout the cell cycle and to the equatorial MTOC in post-anaphase (Horio et al., 1991; Masuda et al., 1992). Like most other eukaryotes, fission yeast microtubule organization alters dynamically during cell cycle progression, where interphase cytoplasmic arrays give way for the mitotic bipolar spindle (Hagan and Hyams, 1988). Despite the attractions of this system, no conditional mutants that are defective in the components of the γ-tubulin complex have been available until recently (Paluh et al., 2000).
The spindle assembly checkpoint is a surveillance mechanism that ensures that paired chromatids do not segregate until the chromosomes are aligned properly along the mitotic spindle, and it plays a pivotal role in the maintenance of genome integrity and fidelity of chromosome separation (Hoyt et al., 1991; Li and Murray, 1991; Lengauer et al., 1998). Recent analysis has shed more light on the complexities of this regulatory mechanism. In yeast, two distinct pathways are operational, one is Mad2 dependent whilst the other is Bub2 (Cdc16 in fission yeast) dependent (Burke 2000; Cerutti and Simanis, 2000). The Mad2 pathway is believed to be involved in the regulation of kinetochore function, such that its structural component plays a role in the checkpoint system (e.g. budding yeast Ndc10; Tavormina and Burke, 1998). In contrast, how microtubule/spindle integrity is monitored by the Bub2 pathway remains elusive, although recent analysis indicates that it regulates mitotic exit via nuclear positioning in budding yeast (Bardin et al., 2000; Bloecher et al., 2000; Pereira et al., 2000) and septation/cytokinesis in fission yeast (Cerutti and Simanis, 2000).
We previously isolated a number of mutants that display temperature-sensitive (ts) defects in the maintenance of growth polarity control (alp loci, altered polarity; Radcliffe et al., 1998). In line with the fact that fission yeast microtubules play a crucial role in the determination of growth polarity, many of the alp+ genes encode conserved proteins that are required for microtubule function, including tubulins and cofactor homologues (Hirata et al., 1998; Radcliffe et al., 1998, 1999). In this study, we describe the characterization of Alp4 and Alp6, which are the fission yeast homologues of Spc97/Gcp2 and Spc98/Gcp3, respectively. We show that Alp4 and Alp6 are required for the regulation of both interphase microtubules and mitotic bipolar spindles. Importantly, these components execute their essential role in G1 phase. Furthermore, we show that Alp4 and Alp6 are essential components of the spindle assembly checkpoint.
Results
ts alp4 and alp6 mutants are defective in microtubule organization and show growth polarity defects
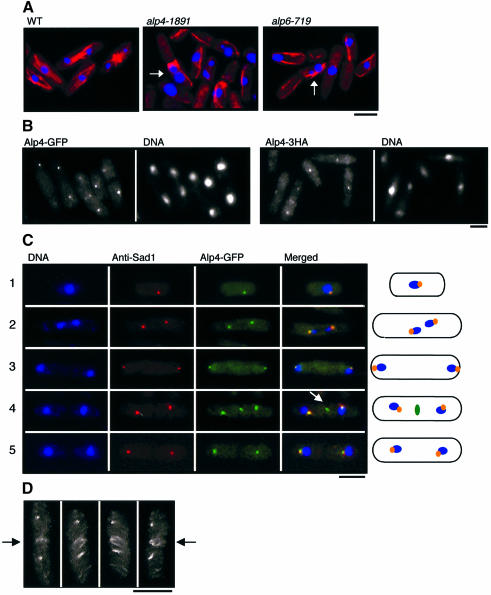
ts alp4 and alp6 mutants were isolated from a visual screen for growth polarity mutants (Radcliffe et al., 1998). Morphological characterization showed that these three strains showed similar, if not identical, phenotypes at the restrictive temperature, i.e. a bent shape associated with ‘cut’ phenotypes (9–10%). Furthermore, immunofluorescence microscopy using anti-α-tubulin antibody showed that mutant cells incubated for 6 h at 36°C displayed abnormal microtubules, and nuclear DNA often became displaced from the centre of the cell and segregated aberrantly into two or three masses (Figure 1A). These observations indicate that Alp4 and Alp6 are required for microtubule organization and proper chromosome separation.
Fig. 1. Defective phenotypes of ts alp4 and alp6 mutants and the cellular localization of Alp4 and Alp6 at the MTOC. (A) Wild-type (left, HM123, Table II), alp4-1891 (middle, DH1891) or alp6-719 (right, DH719) cells were incubated at 36°C for 6 h and processed for immunofluorescence microscopy. Merged images of anti-tubulin staining (TAT-1, red) and nuclear staining (DAPI, blue) are shown. Abnormally segregated mitotic chromosomes are marked with arrows. (B) Localization of Alp4. Fluorescence from GFP (two left panels; Alp4–GFP, LV11) or immunofluorescence microscopy using anti-HA antibody (two right panels; Alp4-3HA, LV15) are shown. (C) Localization of Alp4–GFP during the cell cycle. Triple staining using DAPI (left), anti-Sad1 (the second panels), GFP (the third panels) and merged pictures (right) during the cell cycle are shown. Representative images from interphase (row 1), metaphase (row 2), anaphase (row 3), post-anaphase (row 4) and septated cells (row 5) are presented. In the rightmost corner, combined images are depicted, in which blue corresponds to chromosomal DNA, orange shows the merged images between Sad1 and Alp4, and green presents Alp4 at the equatorial MTOC (marked with an arrow in row 4). (D) ‘Ring’ structures of the equatorial MTOC (shown by arrows, Alp4-3HA). Anti-HA signals from a post-anaphase cell (corresponding to row 4) have been rotating around a vertical axis after observation under a confocal microscope. The bar indicates 10 µm.
alp4+ and alp6+ encode conserved components of the γ-tubulin complex
A fission yeast genomic library was used to isolate genes that complemented the ts alp4 and alp6 mutations. Two different plasmids (pLV4-1 and pLV6-1) were isolated that complemented alp4-1891 and alp6-719, respectively. Genetic linkage analysis indicated that the gene in pLV4-1 is alp4+, whilst that in pLV6-1 is alp6+ (see Materials and methods). Nucleotide sequencing showed that alp4+ and alp6+ encode fission yeast homologues of universal components of the γ-tubulin complex, Gcp2 (human)/Spc97 (budding yeast) and Gcp3/Spc98, respectively (Schiebel, 2000). The identity between Alp4 and Gcp2 or Spc97 was 22% (32% if conservative changes are considered) and 11% (22%), whilst that between Alp6 and Gcp3 or Spc98 was 25% (39%) and 18% (32%), respectively, indicating that the fission yeast proteins are evolutionarily closer to vertebrates than budding yeast (Table I). In a similar manner to the vertebrate proteins (Martin et al., 1998; Murphy et al., 1998; Tassin et al., 1998), Alp4 and Alp6 show a distant evolutionary relatedness to each other (data not shown), and probably evolved from a common ancestor.
Table I. Homology of γ-tubulin-interacting proteins between fission yeast and other eukaryotes.
| Gcp2 | Dgrip84 | Spc97 | |
|---|---|---|---|
| Alp4 | 22 (32) | 18 (32) | 11 (22) |
| Gcp2 | 27 (42) | 11 (24) | |
| Dgrip84 |
|
|
10 (22) |
| |
Gcp3 |
Dgrip91 |
Spc98 |
| Alp6 | 25 (39) | 19 (32) | 18 (32) |
| Gcp3 | 29 (44) | 15 (28) | |
| Dgrip91 | 15 (28) |
The percentage identity or similarity (in parentheses) in amino acid sequence between γ-tubulin-interacting proteins from fission yeast (Alp4 and Alp6), human (Gcp2 and Gcp3), fly (Dgrip84 and Dgrip91) and budding yeast (Spc97 and Spc98) is shown.
The cellular localization of Alp4 and Alp6 at the MTOC
In order to examine the cellular localization of Alp4 and Alp6, the green fluorescent protein (GFP) gene was fused to the C-terminus of the chromosomal alp4+ and alp6+ genes. GFP tagging did not interfere with protein function as strains containing Alp4–GFP or Alp6–GFP grew as well as wild-type strains, and no morphological or mitotic defects were apparent. Signals from Alp4–GFP and Alp6–GFP were essentially the same (although the signal from Alp4–GFP was stronger than that from Alp6–GFP). Signals were seen either as a single or double spot around the nuclear periphery and/or as dot-like structures in the cell centre in exponentially growing Alp4–GFP cells (the left panels in Figure 1B). As this localization appeared similar to that of the SPB, double staining with both Alp4–GFP and anti-Sad1 antibodies was performed (Sad1 is an SPB component; Hagan and Yanagida, 1995). As shown in Figure 1C, in both interphase (rows 1) and mitotic cells (rows 2 and 3), Alp4–GFP co-localized precisely with Sad1 either as a single (row 1) or double spot (rows 2 and 3). Of particular interest were post-anaphase cells (row 4), in which the localization of Alp4–GFP differed from that of Sad1. At this stage, in addition to the SPBs, which are located on the side of each nucleus, an equatorial dot(s) of Alp4–GFP but not anti-Sad1 was clearly visible (arrow in row 4). After the completion of septum formation, this central dot(s) disappeared (row 5). These dots appeared to be identical to those seen with anti-Gtb1, which have been termed the equatorial MTOC and which generate the post-anaphase array during cytokinesis (Hagan and Hyams, 1988; Horio et al., 1991; Hagan, 1998).
In order to observe the three-dimensional structure of the equatorial MTOC, confocal images were rotated around a vertical axis. It was found that these central signals are in fact not ‘dots’, but instead formed a ‘ring’ structure during the early stages of cytokinesis (arrow, Figure 1D). It has been reported previously that cytoplasmic microtubules also exist as ‘equatorial rings’ in the centre of the cell at the end of anaphase and during telophase (Pichová et al., 1995). These results show that Alp4 and Alp6 are not only homologous to components of the MTOC in their amino acid sequence, but are also an integral part of the two fission yeast MTOCs that are functional during the mitotic cell cycle.
Physical and genetic interactions between Alp4, Alp6 and γ-tubulin
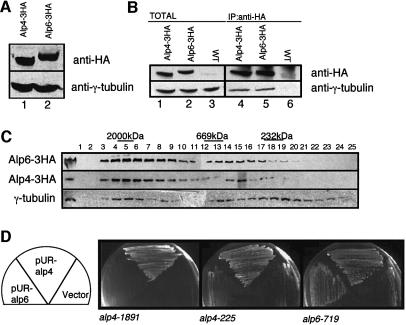
Immunoprecipitation experiments were performed to explore the physical interactions between Alp4, Alp6 and Gtb1. Haemagglutinin (HA) epitope-tagged strains (Alp4-3HA and Alp6-3HA) were constructed in a manner similar to GFP tagging. Immunoblotting using anti-HA antibody identified Alp4-3HA and Alp6-3HA on an SDS–polyacrylamide gel (Figure 2A). These gene fusions were also used to confirm that Alp4 and Alp6 localize to the MTOC. Immunofluorescence microscopy using anti-HA antibody showed the same localization patterns as those of GFP-tagged proteins (see the right two panels in Figure 1B). Immunoprecipitation experiments showed that Alp4-3HA and Alp6-3HA co-precipitated with Gtb1 (Figure 2B, lanes 4 and 5). It appeared that the amount of Gtb1 that was co-precipitated was not as much as the total protein (compare lanes 1 and 2 with lanes 3 and 4). It is possible that Alp4-3HA and Alp6-3HA, which form a complex with Gtb1, are less efficient than free proteins for precipitation with anti-HA antibody, or that a subpopulation of Gtb1 may exist independently of Alp4 and Alp6.
Fig. 2. Physical and genetic interactions between Alp4/Alp6 and γ-tubulin. (A) Identification of the alp4+ and alp6+ gene products. Immunoblotting was performed with anti-HA antibody against cell extracts prepared from an Alp4-3HA (lane 1, LV15) or Alp6-3HA strain (lane 2, LV16). Gtb1 (γ-tubulin) was used as a loading control. (B) Physical interaction between Alp4/Alp6 and γ-tubulin. Cell extracts were prepared from an Alp4-3HA strain (lanes 1 and 4, LV15), an Alp6-3HA strain (lanes 2 and 5, LV16) or an untagged wild type (lanes 3 and 6), and immunoprecipitation performed with anti-HA antibody (lanes 4–6). Precipitated proteins were detected with anti-HA or anti-Gtb1 antibody. Total cell extracts (corresponding to 1/50 amount used for immunoprecipitation) were also run (lanes 1–3). (C) Gel filtration chromatography. Soluble cell extracts were analysed by immunoblotting with anti-HA or anti-Gtb1 antibody. Total extract (10 µg) was run in the far-left panel. Protein extracts from two strains (Alp4-3HA and Alp6-3HA) were loaded on separate columns, and separation profiles were superimposed according to a control pattern using anti-Gtb1 antibody. The positions of size markers (2000, 669 and 232 kDa) are also shown. (D) Suppression analysis by multicopy plasmids. ts alp4 or alp6 mutants were transformed with an empty vector or multicopy plasmids containing alp4+ or alp6+ (pUR-Alp4 or pUR-Alp6, respectively), and transformants were streaked on rich plates and incubated at 36°C for 3 days.
In order to address the size of the γ-tubulin complex in fission yeast, gel filtration analysis was performed. As shown in Figure 2C, Alp4, Alp6 and Gtb1 co-fractionate predominantly in a large complex (>2000 kDa). It should be noted that this large size is comparable to that in higher eukaryotes such as Drosophila and mammalian cells (Martin et al., 1998; Murphy et al., 1998). We have observed that a small amount of Gtb1 is also found in the smaller fractions (fractions 13–22), some of which still overlap with Alp4 and Alp6 (13–16). It is possible that, as in other eukaryotes, multiple forms of the complex may exist in fission yeast, which vary in size (Akashi et al., 1997; Moritz et al., 1998).
We sought genetic interactions to substantiate the biochemical data that Alp4, Alp6 and Gtb1 physically interact. As shown in Figure 2D, multicopy plasmids containing the alp4+ gene suppressed the defects of the ts alp6-719 mutant. Taken together, these results show that Alp4 and Alp6 localize to the MTOC and constitute part of the γ-tubulin complex, and that the alp4+ and alp6+ genes interact genetically with gtb1+ and also with each other.
alp4 mutants exhibit defects in the formation of bipolar mitotic spindles and maintenance of the length of cytoplasmic microtubules
In order to characterize the ts alp4 phenotypes more carefully, a culture of alp4 cells was synchronized with respect to cell cycle progression by centrifugal elutriation and shifted to the restrictive temperature. Small early G2 alp4-1891 cells grown at 26°C were collected by elutriation and the culture was divided into two halves. One half was incubated at 26°C to monitor the degree of synchrony of cell cycle progression, whilst the other half was shifted up to 36°C to examine the phenotypes arising from incubation under the restrictive conditions. Samples were collected at 20 min intervals in order to measure viability and to use for immunofluorescence analysis of microtubules and the SPB.
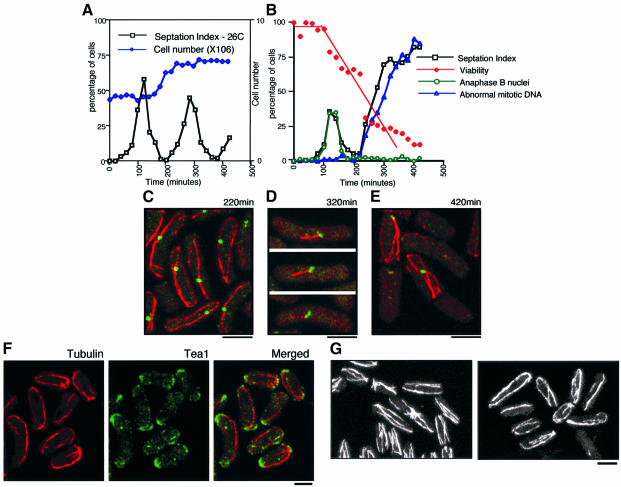
Synchrony was high as the septation index was <0.5% at 0 min, reached 62% at 120 min (the first mitosis), dropped to 1% at 200 min and peaked again up to 47% at 300 min (the second mitosis) at 26°C (Figure 3A). At the restrictive temperature, defective phenotypes, including abnormal mitotic DNA (Figure 1A), became evident only during the second mitosis; the first mitosis appeared to occur normally (Figure 3B; note the normal mitotic pattern until 200 min). Cell viability remained high during the first 100 min when the septation index (equivalent to the population of cells that have passed mitosis) peaked, and then started to drop as the second cycle proceeded. It is of note that in fission yeast, G1 phase is very short and S phase occurs almost simultaneously with nuclear division and septation (Moreno et al., 1991). This result, therefore, suggested that alp4 mutants are committed to death before the second M phase.
Fig. 3. Alp4 is required for both the formation of mitotic bipolar spindles and the integrity of interphase cytoplasmic microtubules. (A and B) Centrifugal elutriation. Small early G2 cells of an alp4-1891 strain (DH1891) were collected by centrifugal elutriation and the cultures were divided into two parts, one part incubated at 26°C (A) and the other at 36°C (B). Samples were collected at 20 min intervals, and cell number (open diamonds in dark blue), septation index (open squares in black) and viability (open diamonds in red, the percentage of viable colonies) were measured. Immunofluorescence microscopy with anti-tubulin, anti-Sad1 antibody and DAPI was performed to examine the percentage of cells containing anaphase B nuclei (open circles in green) and abnormal mitotic DNA (open triangles in blue). (C) Abnormally long interphase microtubules in the alp4-1891 mutant. Confocal microscopy was performed using synchronous interphase samples (220 min at 36°C) with anti-tubulin (red) and anti-Sad1 (green) antibodies. Merged images are shown. (D) Aberrant mitotic spindles. Characteristic cells showing monopolar-like spindles (320 min) are presented. (E) Displaced long interphase microtubules. Interphase-like cells with longer cytoplasmic microtubules after an aberrant mitosis (420 min) are shown. (F) Tea1 localization in the alp4-1891 mutant. alp4 mutants were incubated at 26°C for 3 h in the presence of 10 mM HU and shifted up to 36°C after washout of HU. After 2.5 h incubation, cells were fixed and processed for immunofluorescence microscopy using anti-tubulin (left) and anti-Tea1 (middle) antibodies. Merged images are shown on the right. (G) Defects in microtubules by overexpression of alp4+. Cells containing integrated nmt1-alp4+ were grown in the absence of thiamine, and processed for immunofluorescence microscopy using anti-tubulin antibody. Images are from confocal microscopy. Cells from a wild-type control (left) and alp4+ overexpression after 16 h (right) are shown. The bar indicates 10 µm.
Immunofluorescence microscopy at each time point enabled us to follow temporally the defective phenotypes of the alp4 mutant and delineate the kinetics of alterations in microtubules. The first phenotype to be seen was the appearance of abnormally long cytoplasmic microtubules. In wild-type cells, the interphase microtubule cytoskeleton consists of several filamentous forms that extend along the long axis of the cell with varied lengths but never curl around the cell tip (Hagan, 1998). In contrast, in the alp4 mutant, the majority of interphase cells had longer microtubules, which curved around the cell end (220 min, Figure 3C). Whilst most of the microtubules in wild-type cells appear to be independent of the SPB (Hagan, 1998), these longer microtubules generally associated with or passed through the SPB. Upon entry into the second mitosis, chromosome separation often occurred, albeit only partially, and the SPB separated into two bodies. However, bipolar spindles were rarely observed, and, instead, aberrant ‘monopolar’-like spindles were formed (320 min, Figure 3D). In these cells, spindles generally emanated from only one of the two SPBs.
The cell cycle continued despite these mitotic defects and cells exited mitosis as monopolar spindles disappeared and the septum cleaved the undivided nuclei to produce a ‘cut’ phenotype. At this stage, the interphase microtubules reappeared (420 min, Figure 3E). Again, like the earlier interphase time point (Figure 3C), the length of cytoplasmic microtubules was abnormal and they grew right around the end of the cell. These cytoplasmic microtubules were often formed only in one half of the cell, which corresponded to the side with the displaced nucleus (see Figure 1A). Taken together, this analysis established the notion that Alp4 is required for the formation of cell cycle-dependent microtubule structures. In interphase, it is required to maintain the length of cytoplasmic microtubules and, in mitosis, it is essential for the formation of bipolar spindles.
Localization of the end marker Tea1 is not defective in alp4 mutants
Abnormally long interphase microtubules have been reported previously in tea1 mutants, which are defective in the cell end marker for growth polarity control (Mata and Nurse, 1997). In the absence of Tea1, which usually localizes to the cell end, as well as along microtubules and at their tips, cytoplasmic microtubules do not terminate at the cell end; instead they curve around the cell cortex. In order to examine whether or not the long microtubules observed in alp4 mutants are attributable to a disfunction of Tea1, double immunofluorescence microscopy was performed with anti-Tea1 and anti-tubulin antibodies. A synchronous culture was prepared using a hydroxyurea (HU) block and release method. Upon washout of HU and release to the restrictive temperature, elongated and curved microtubules were observed (Figure 3F). Co-staining with anti-Tea1 antibody revealed that Tea1 localization is not disturbed. Tea1 localized to both the cell ends and the tips of microtubules. Therefore, the longer cytoplasmic microtubules are not ascribable to Tea1 mislocalization, rather they might be due to defects in SPB function.
Longer interphase microtubules were also seen in cells in which alp4+ or alp6+ were overexpressed ectopically. The thiamine-repressible strong nmt1+ promoter was integrated into the chromosomal alp4+ and alp6+ loci just prior to the initiation codon. It was found that ectopic overexpression of alp4+ was toxic and inhibits colony formation on plates in the absence of thiamine (data not shown). Very few mitotic spindles (<0.5%, in contrast to 2–4% in exponentially growing wild-type culture) were observed after 24 h of overinduction. Instead, as in ts mutants, elongated interphase microtubules were seen (16 h after induction, Figure 3G). These results indicate that the protein levels or activities of Alp4 and Alp6 have to be regulated correctly during the cell cycle to ensure the formation of functional interphase microtubules and mitotic spindles.
Alp4 function is executed prior to S phase regardless of the fact that the mutant shows mitotic abnormalities in the subsequent M phase
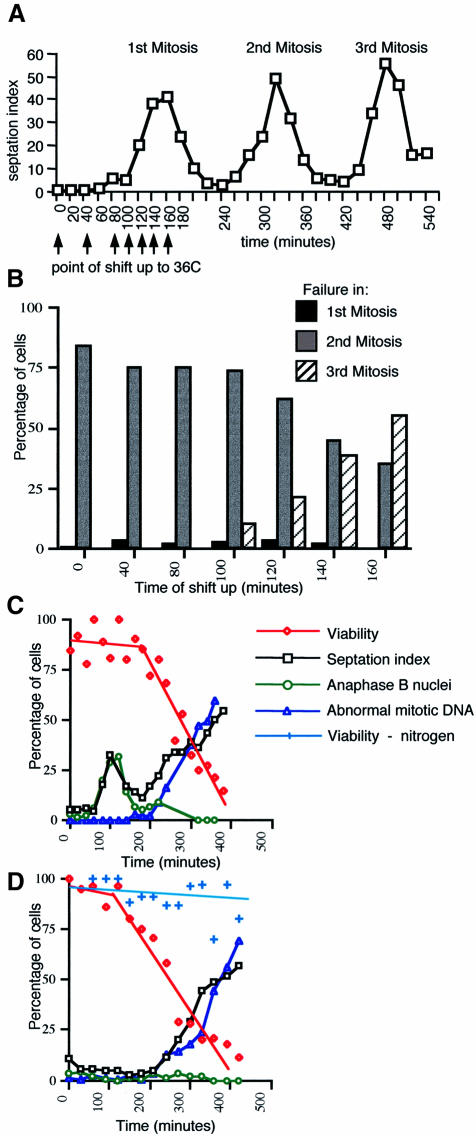
We were interested in the cell cycle stage at which Alp4 executes its essential function. The results of the previous elutriation analysis were somewhat unexpected, as, although the alp4 mutant showed a variety of mitotic defects, these phenotypes appeared only in the second mitosis. In order to address the question of when Alp4 executes its essential role, centrifugal elutriation experiments were repeated with the following modifications; after elutriation, the cultures were kept at 26°C for various times (0, 40, 80, 100, 120, 140 and 160 min; Figure 4A), then shifted up to 36°C and the appearance of abnormal mitosis in each sample was followed. The results are summarized schematically in Figure 4B. It transpired from these manipulations that there is a critical time when Alp4 function is executed. Until 100 min when the mutant was finishing the first mitosis (Figure 4A), the defects appeared in the second mitosis, as in the case when the culture was shifted up at 0 min (Figure 4B). On the other hand, if the culture was shifted up at 140 min or later after the first mitosis was complete, the defective phenotypes started to appear upon entry into the third mitosis. Thus, it appeared that the crucial stage at which Alp4 function is executed occurs between 120 and 140 min, which is likely to correspond to G1 phase. These results suggest that an Alp4 function has already been accomplished before S, G2 and M phases, irrespective of the fact that alp4 mutants show defective phenotypes only in the subsequent mitosis.
Fig. 4. Essential Alp4 function is executed in G1 phase. (A) Elutriation centrifugation and shift-up at different times. Centrifugal elutriation was performed to collect small G2 cells from an exponentially growing alp4 mutant at 26°C, and the synchronized cells continued to grow at 26°C. After various incubation times at 26°C (shown by arrows, 0, 40, 80, 100, 120, 140 and 160 min), the cultures were shifted up to 36°C. The septation index at 26°C is shown (up to the third mitosis). (B) Appearance of cells showing the defective mitosis at different time points. The percentage of cells that show aberrant mitosis after the first (black column), second (grey) or third mitosis (hatched) is plotted. (C) HU block and release experiments. The cultures of alp4 mutant grown at 26°C in rich medium containing 10 mM HU for 3 h were shifted up to 36°C after washing out the HU. The septation index (open squares in black), viability (open diamonds in red), anaphase B nuclei (open circles in green) and abnormal mitotic DNA (open triangles in blue) at 36°C were examined as in Figure 3A and B. (D) Nitrogen deprivation and addition experiments. alp4 (LV6) cells starved of nitrogen for 7 h at 26°C were divided into two parts: to one half, fresh rich medium was added and shifted up at 36°C, whilst the other half was kept in nitrogen-deprived medium and shifted up at 36°C. Symbols are the same as in (C) except that viability under nitrogen deprivation at 36°C (crosses in light blue) is also shown.
To examine more precisely the cell cycle timing of the requirement for Alp4 function, alp4 mutant cells were synchronized at 26°C at different stages by methods other than elutriation. These included HU block and release (early S), and nitrogen deprivation and addition (G1) experiments. Following synchronization, cultures were shifted up to 36°C and the appearance of defective mitoses and cell viability were monitored. Following release from an HU block at the restrictive temperature, alp4 cells divided once normally and then died in the second mitosis (Figure 4C), with kinetics very similar to those of the previous elutriation experiments when cells were shifted to 36°C at early G2 (Figure 3B). On the other hand, following nitrogen deprivation and addition, cells died in the first mitosis accompanied by the appearance of abnormal chromosome separation (Figure 4D). In addition, the alp4 mutant became lethal only when the cell cycle was allowed to progress, as cells maintained viability under continued incubation in nitrogen-deprived medium at 36°C for 7 h (Figure 4D). From this, we conclude that the execution point of Alp4 occurs during the G1 phase of the cell cycle.
alp4 mutants lose viability before entry into M phase
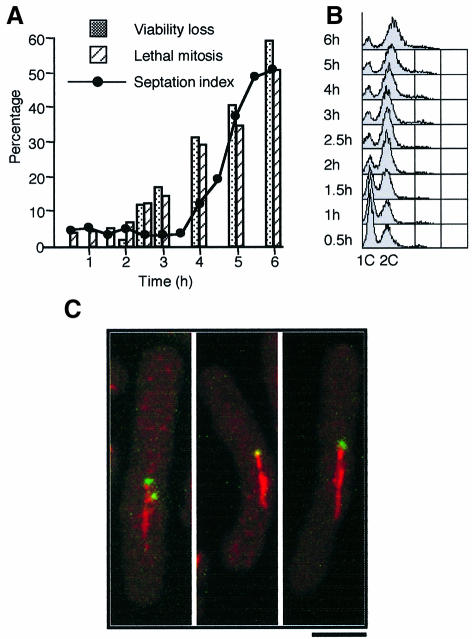
We were interested in which stage alp4 mutants become irreversibly committed to mitotic defects and viability loss under restrictive conditions. The issue addressed was whether or not continued incubation after G1 phase until mitotic entry at the restrictive temperature was necessary for abnormal mitotic phenotypes and loss of viability. To answer this question, synchronous culture analysis was repeated using nitrogen starvation at the permissive temperature (26°C) and released to 36°C upon addition of nitrogen. Aliquots of the culture were removed at each time point and divided into four parts: the first part was used to determine DNA content by flow cytometry (FACS); the second was used to observe mitotic phenotypes by 4′,6-diamidino 2-phenylindole (DAPI) and calcofluor; the third was spread directly on rich plates at 26°C to measure viability; and the fourth was shifted down to 26°C in liquid culture in order to monitor mitotic progression after transfer from restrictive to permissive conditions.
As shown in Figure 5A and B, this analysis indicated that viability loss already started after 2.5 h at 36°C (Figure 5A, closed boxes), at which point S phase had finished (Figure 5B). Entry into the subsequent mitosis occurred only after 4 h (Figure 5A, closed circles). Thus, there was a 1.5 h period in which viability started to decrease before entry into M phase. This result indicated that alp4 mutants are committed to lethality before entry into M phase at the restrictive temperature. Furthermore, observation of the consequent lethal phenotypes of these cells following incubation in permissive conditions showed that mutant cells displayed an abnormal mitosis at 26°C, as in the case of continued incubation at 36°C (Figure 5A and see below).
Fig. 5. alp4 mutants become irreversibly lethal before entry into mitosis. (A) The timing of lethality. Nitrogen-starved, G1-synchronized alp4 mutants at 26°C were shifted to 36°C upon addition of nitrogen. Samples were taken every 30 min, and viability loss (shaded boxes) and percentage of septated cells (closed circles) were measured. At each time point, aliquots were shifted down to 26°C and the percentage of cells that progressed through an abnormal mitosis (hatched) was counted. (B) DNA content upon nitrogen addition. Samples used in (A) were fixed and processed for FACS analysis. (C) Appearance of monopolar spindles under permissive conditions. alp4 mutant cells grown at 26°C were shifted to 36°C and HU (10 mM) was added simultaneously. After 4 h incubation, HU was washed out, and the cultures were returned to 26°C. Samples were then taken at different time points for immunofluorescence microscopy with anti-tubulin antibody (red) and Cut12–GFP (green, an integral SPB protein; Bridge et al., 1998). The figure (confocal microscopy) shows representative abnormal mitotic cells that have monopolar spindles (2 h at 26°C). The bar indicates 5 µm.
Further support for the requirement for Alp4 function during G1 came from the experiment using HU. In this case, HU was added to alp4 mutants at the same time as cultures were shifted from the permissive to the restrictive temperature. After 4 h at 36°C, HU was washed out and the cultures were returned to 26°C. If the essential Alp4 function is executed during G1, abnormal mitosis should be observed under permissive conditions at 26°C, once alp4 mutants pass through G1 at 36°C in the presence of HU. As expected, after 2 h incubation at 26°C upon washout of HU, abnormal mitotic cells with monopolar spindles were observed (40% among mitotic cells, Figure 5C). These results established the notion that in the alp4 mutant, when the essential function in G1 is not accomplished, cells proceed irreversibly through a lethal mitosis with monopolar spindles even if they are shifted down to permissive conditions prior to entry into mitosis.
The alp4 mutation does not affect the formation of the γ-tubulin complex but is deficient in its cellular localization to the SPB
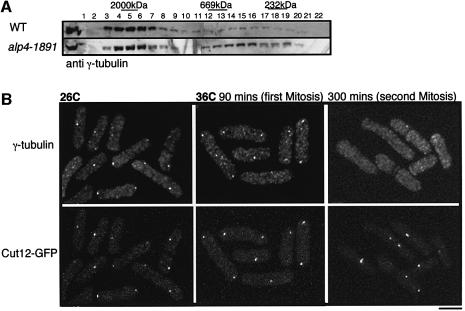
We sought to clarify what is defective in the alp4 mutant. As a first step in addressing this question, the size of the γ-tubulin complex was examined in the alp4-1891 mutant. Soluble extracts were prepared from wild type and the alp4-1891 mutant, which had been incubated at the restrictive temperature for 6 h, and gel filtration was performed. As shown in Figure 6A, the majority of γ-tubulin still exists in a large complex, although, compared with wild type, there appears to be more γ-tubulin in the smaller fractions. This result suggests that the γ-tubulin complex is not significantly compromised in the alp4 mutant.
Fig. 6. alp4 mutants are defective in γ-tubulin localization to the SPB without affecting the capability of complex formation with γ-tubulin. (A) Gel filtration chromatography in alp4 mutants. Soluble cell extracts were prepared from wild type and alp4-1891 mutants that were incubated at 36°C for 6 h, separated through a Superose-6 column and immunoblotted as in Figure 2C. (B) Cellular localization of SPB (Cut12–GFP) and γ-tubulin. alp4 mutants in which cut12+ was tagged with GFP (LV25, Table II) were grown at 26°C in the presence of HU and shifted up to 36°C upon washout of HU. Immunofluorescence microscopy using affinity-purified anti-Gtb1 antibody was performed. At the same time, the location of Cut12 was determined by GFP. The bar indicates 10 µm.
To address further the possible defects in the alp4 mutant, the cellular localization of γ-tubulin was examined. A synchronous culture was prepared using an HU block and the cultures were released to 36°C upon washout of HU. Affinity-purified anti-Gtb1 antibody stained both SPBs and the equatorial MTOC at 26°C (Figure 6B, left), as reported previously (Horio et al., 1991). In contrast, in the alp4 mutants at 36°C, γ-tubulin localized normally in the first mitosis when viability is high (90 min); however, it failed to localize almost completely in the second cycle (300 min). Therefore, the alp4-1891 mutant appears to be able to assemble the γ-tubulin complex but is defective in its localization to the SPB.
Alp4 and Alp6 are required for spindle checkpoint control
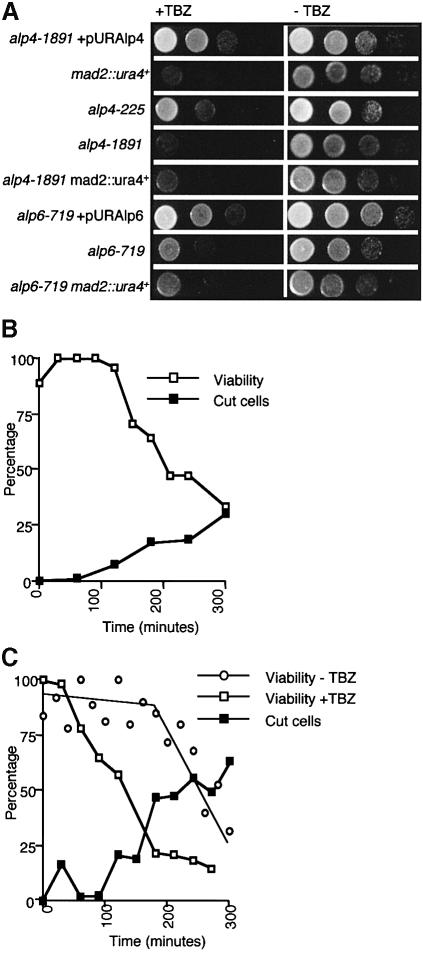
As shown earlier (see Figure 3B–E), alp4 and alp6 mutants incubated at the restrictive temperature, despite undergoing an abnormal mitosis with microtubular defects, did not display a typical ‘cdc’ arrest; instead they passed through the later mitotic stages such as septation and cytokinesis. Is there any functional link between Alp4/Alp6 and the spindle assembly checkpoint pathways? In order to answer this question, we examined the following points in the alp4 mutants: (i) sensitivity to microtubule-destabilizing drugs; (ii) the ability of the alp4 mutant to activate the spindle assembly checkpoint; and (iii) the involvement of Alp4 in the spindle checkpoint pathway itself. As shown in Figure 7A, it was found that both alp4 and alp6 mutants were hypersensitive to TBZ even at the permissive temperature. The hypersensitivity to TBZ appeared not to be attributable to simple structural deficiencies of microtubules, as the addition of TBZ to alp4 mutants resulted in a sharp drop in viability, with cells displaying a ‘cut’ phenotype at 26°C instead of a mitotic arrest (Figure 7B). In addition, following an HU block at 26°C and release to 36°C, the alp4 mutant also lost viability immediately on entry into the first mitosis in the presence of TBZ (Figure 7C). This is in sharp contrast to the situation when this strain was released to 36°C in the absence of this drug (viability loss in the second cycle). Thus, alp4 (and alp6) is (are) deficient in some aspect of the surveillance mechanisms of spindle/microtubule integrity.
Fig. 7. Alp4 and Alp6 are required for the spindle checkpoint pathways. (A) Hypersensitivity of alp4 and alp6 mutants to thiabendazole. The alp4-1891 mutant containing plasmids carrying alp4+ (wild-type equivalent), mad2 deletion, alp4-225, alp4-1891, alp4-1891mad2, the alp6-719 mutant containing plasmids carrying alp6+ (wild-type equivalent), alp6-719 and alp6-719mad2 strains were spotted on to rich plates (–TBZ, right) or plates containing 20 µg/ml thiabendazole (+TBZ, left) as serial dilutions (106 cells in the top row and then diluted 10-fold in each subsequent spot below) and incubated at 30°C. (B) Loss of viability in the presence of TBZ at the permissive temperature. alp4 mutants were grown in rich medium at 26°C and TBZ (50 µg/ml) was added. The percentage of ‘cut’ cells (closed squares) and the viability (open squares) were measured. (C) Loss of viability in the presence of TBZ at the restrictive temperature. alp4 mutants were grown in rich medium in the presence of HU at 26°C for 3 h and shifted to 36°C upon washout of HU and addition of TBZ (50 µg/ml). The percentage viability with (open squares) or without (open circles) addition of TBZ is also shown.
Alp4 functions in spindle checkpoint control independently of the Mad2 pathway
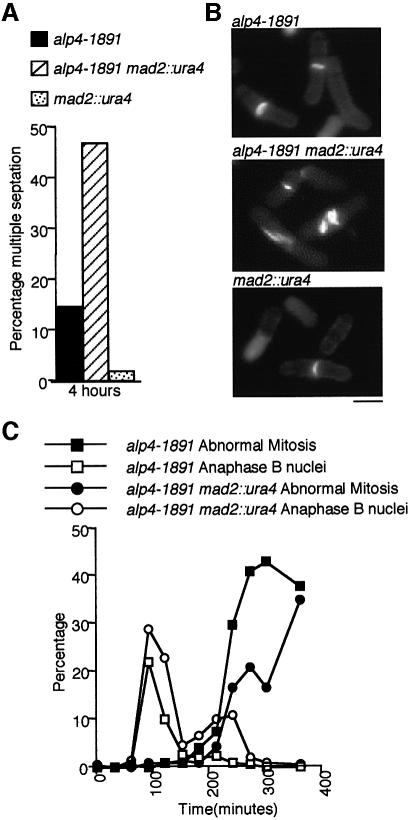
Next, a functional relationship between the Alp4- and Mad2-dependent checkpoints was explored. Sensitivity to TBZ was indistinguishable between alp4 and alp4mad2 mutants or between alp6 and alp6mad2 mutants at the permissive temperature (Figure 7A). None the less, the double mutant with alp4-1891 showed novel phenotypes in the presence of TBZ at 26°C, in which multiply septated cells became greatly increased compared with the single mutants (Figure 8A and B). This indicated that Alp4 functions in the spindle checkpoint independently of Mad2. However, after an HU block (26°C) and release (36°C) in the absence of the drug, the double mutant showed a reduced appearance of the abnormal phenotypes (Figure 8C), suggesting that the Mad2-dependent pathway somehow plays a role in the appearance of the defective phenotypes of the alp4 mutants at 36°C. It should be noted that the alp4mad2 mutant showed neither rapid viability loss nor a ‘cut’ phenotype at 36°C in the first mitosis in the absence of TBZ, which is in clear contrast to the lethal phenotypes in the presence of this drug (compare Figures 8C with 7B and C). This indicated that there is no Mad2-dependent mitotic delay in the alp4 mutant. Taking these results together, we conclude that Alp4 plays a role in the spindle checkpoint pathway independently of, but related to, the Mad2-mediated pathway.
Fig. 8. Alp4 functions independently of but is related to the Mad2 pathway in spindle checkpoint control. (A) Multiple septation phenotypes of the alp4mad2 double mutant in the presence of TBZ. alp4-1891 (black), alp4mad2 (hatched) and mad2 (dotted) mutants were grown in rich medium at 26°C and synchronized with HU (10 mM). After 3 h incubation, HU was washed out and TBZ was added, and the culture was monitored for 4 h. The percentage of septating cells displaying multiple septa is shown. (B) Cell morphology in the presence of TBZ. Typical cell morphology stained with calcofluor is shown (top, alp4-1891; middle, alp4mad2; bottom, mad2). The bar indicates 10 µm. (C) Progression through mitosis of the double mutant at the restrictive temperature. alp4 single (squares) or alp4mad2 double (circles) mutants were blocked with HU for 3 h at 26°C and released into HU-free medium at 36°C. The percentages of abnormal mitotic cells (closed symbols) and anaphase B nuclei (open symbols) were counted.
Discussion
In this study, we describe the identification and characterization of two fission yeast homologues of components of the universally conserved γ-tubulin complex. The results shed light on a crucial role for the γ-tubulin complex in microtubule integrity, in particular, formation of both mitotic bipolar spindles and interphase microtubules, and also in spindle assembly checkpoint control.
A large γ-tubulin complex in fission yeast: the size matters
Gel filtration chromatography showed that the size of the fission yeast complex is large and is almost indistinguishable from that in metazoans, and it is likely that the complex exists in multiple forms with varied sizes (Akashi et al., 1997; Moritz et al., 1998; Oegema et al., 1999). Biochemical work in higher systems has detected several additional proteins comprising the γTuRC besides γ-tubulin, Gcp2 and Gcp3 (Zheng et al., 1995; Martin et al., 1998; Murphy et al., 1998; Fava et al., 1999). Given the similar size of the γ-tubulin complex between fission yeast and animals, fission yeast should be a suitable organism in which to identify such novel factors.
The essential role for Alp4 and Alp6 is accomplished during G1: the timing matters
Careful analysis performed with various synchronous cultures using ts alp4 mutants led us to conclude that Alp4 has already executed its essential role by S phase, and that the loss of viability occurred long before entry into the subsequent mitosis. What is the essential Alp4-dependent event that occurs during G1? One possibility is that the recruitment of the γ-tubulin complex on to the SPB, possibly coupled with the duplication of the SPB, takes place during G1 in wild-type cells. In the ts alp4 mutants, this process may be defective, rather than the inability to play a role in microtubule nucleation per se. The Alp4 mutant protein could remain functional if it is recruited on to the SPB sometime in G1 at the permissive temperature. The fact that assembly of the γ-tubulin complex itself is not impaired in ts alp4 mutants suggests that it may be its recruitment to the SPB that is defective.
Reports on the timing of duplication (not separation) of the fission yeast SPB have been inconclusive (McCully and Robinow, 1971; King and Hyams, 1982; Kanbe et al., 1990; Ding et al., 1997). Recent careful analysis, however, suggests that SPB duplication occurs during G1 (S.Uzawa and W.Z.Cande, personal communication). The fact that the alp4 mutant is still capable of nucleating a mitotic spindle from one SPB could be explained by γ-tubulin complexes having been retained from the previous cycle, albeit that they were not detectable by immunofluorescence microscopy. There is evidence for non-equivalence of the two SPBs, e.g. the appearance of monopolar mitotic spindles in ts cut12 mutants that are defective in an SPB component (Bridge et al., 1998) and the asymmetrical localization of the GTPase-activating proteins (Cdc16 and Byr4) and the effector (Cdc7) for the Spg1–GTP-binding protein to only one of the duplicated SPBs (Cerutti and Simanis, 2000). It should be noted that these proteins are all involved in the SPB-mediated spindle checkpoint pathway (see below).
The role of the γ-tubulin complex in interphase microtubule formation: the length matters
In wild type, cytoplasmic microtubules exist as several distinct filaments, most of which do not associate with the SPB (Hagan and Hyams, 1988). In contrast, alp4 mutant cells exhibit the following two abnormalities. First, one or two greatly elongated microtubule bundles are seen. Secondly, these long microtubules generally associate with or pass through the SPB. We show that the localization of the end marker Tea1 is not abolished in the alp4 mutant. This suggests that the longer cytoplasmic microtubules may arise due to defects in microtubule organization at the SPB rather than a failure in Tea1-dependent growth polarity control.
It is possible that in the alp4 mutant, by interacting with the SPB, these interphase microtubules become more stabilized than wild-type forms. Thus, Alp4 and Alp6 may play an important role in both the maintenance of the length and the orientation of cytoplasmic microtubules. A similar phenotype displaying longer microtubules has been observed in mutations in the γ-tubulin gene in budding yeast (Marschall et al., 1996; Spang et al., 1996) and recently in fission yeast (Paluh et al., 2000). Defective phenotypes arising from ectopic overproduction of Alp4 appear similar to the mutant in terms of interphase microtubule morphology. This supports a role for these proteins in the maintenance of interphase microtubule integrity.
It remains to be determined how interphase microtubules are generated and organized. It is possible that they originate at the MTOC (SPB and/or the equatorial MTOC) and are then released into the cytoplasm. Alternatively, the nucleation of cytoplasmic microtubules could be independent of the MTOC, but still require some interaction with it. Alp4 and Alp6 may be necessary for the liberation of cytoplasmic microtubules from the MTOC by some biochemical processes such as microtubule-severing reactions. Microtubule-nucleating activity is under cell cycle control in fission yeast (Masuda and Shibata, 1996), and it is now important to address the molecular mechanisms underlying cell cycle-dependent regulation of MTOC function.
The role of the γ-tubulin complex in spindle checkpoint control: the place matters
The other significant finding in this study is the involvement of Alp4 and Alp6 in the spindle assembly checkpoint pathways. alp4 and alp6 mutants do not activate the Mad2-dependent checkpoint as other mitotic mutants do (e.g. mutations in β-tubulin encoding nda3 or kinesin-encoding cut7; He et al., 1997; Kim et al. 1998). Instead, Alp4 and Alp6 are required for the activation of the spindle checkpoint pathway per se. This is a unique character because, in addition to checkpoint control, Alp4 and Alp6 play an essential role as structural components involved in microtubule/spindle biogenesis. The reason why alp4 mutants do not activate the Mad2 pathway might be attributable to the location of Alp4 and Alp6. Analysis from several organisms suggests that the Mad2 pathway monitors the state of kinetochores or kinetochore–spindle interactions (Taylor and McKeon, 1997; Burke, 2000). As Alp4 and Alp6 localize to the SPB rather than the kinetochores, kinetochore–spindle interactions might not be compromised, resulting in a failure to activate the Mad2-dependent checkpoint.
It has become clear that spindle assembly checkpoint control bifurcates, and both kinetochore- and the SPB-mediated pathways exist (Burke, 2000). The kinetochore checkpoint is believed to arrest the cells in metaphase by preventing sister chromatid segregation, whilst the SPB-mediated pathway is involved in the inhibition of mitotic exit, which in fission yeast involves septation and cytokinesis (Hwang et al., 1998; Kim et al., 1998; Cerutti and Simanis, 2000). The fact that the alp4mad2 double mutant causes an exaggeration of the TBZ-sensitive phenotype at 26°C suggests that these two proteins may function independently in their response to this drug. Given the localization of Alp4 and Alp6 to the SPB, it is possible that Alp4 is involved in the SPB-mediated pathway. Unlike the kinetochore-mediated pathway, in which most of the components are conserved from yeasts to humans, components involved in the SPB checkpoint pathway so far have only been identified in yeast (e.g. the aforementioned Cdc16/Bub2, Byr4/Bfa1, Spg1/Tem1 and Cdc7/Cdc15). Given the universal conservation of the γ-tubulin complex, we propose that a centrosome- and γ-tubulin complex-dependent checkpoint is operational in vertebrates, in which Gcp2 and Gcp3 may play a crucial role.
Materials and methods
Strains, media and genetic methods
Strains used in this study are listed in Table II. YPD (2% dextrose, 2% polypeptone and 1% yeast extract) and YE5S were used as rich media, and modified synthetic EMM2 was used as minimal medium. The standard methods were followed as described (Moreno et al., 1991).
Table II. Strain list.
| Strains | Genotypes | Derivations |
|---|---|---|
| HM123 | h–leu1 | our stock |
| DH225 | h–leu1alp4-225 | our stock |
| DH719 | h–leu1alp6-719 | our stock |
| DH1891 | h–leu1alp4-1891 | our stock |
| AE148 | h–leu1ura4mad2::ura4+ | from Dr Tomohiro Matsumoto |
| 1356 | h+leu1ura4cut12+–GFP-ura4+ | from Dr Iain M.Hagan |
| LV1 | h–leu1ura4alp4-1891 | this study |
| LV2 | h–leuura41alp4-225 | this study |
| LV3 | h–leu1ura4alp6-719 | this study |
| LV6 | h–alp4-1891 | this study |
| LV7 | h–alp6-719 | this study |
| LV11 | h–leu1alp4+–GFP-kanr | this study |
| LV12 | h–leu1alp6+–GFP-kanr | this study |
| LV15 | h–leu1alp4+-3HA-kanr | this study |
| LV16 | h–leu1alp6+-3HA-kanr | this study |
| LV21 | h–leu1nmt1-alp4+-kanr | this study |
| LV22 | h–leu1nmt1-alp6+-kanr | this study |
| LV23 | h–leu1ura4alp4-1891mad2::ura4+ | this study |
| LV24 | h–leu1ura4alp6-719mad2::ura4+ | this study |
| LV25 | h–leu1ura4alp4-1891cut12+–GFP-ura4+ | this study |
Cloning of the alp4+ and alp6+ genes
A Schizosaccharomyces pombe genomic library in pUR19 (Barbet et al., 1992) was used for the isolation of genes that complemented the ts alp4-1891 and alp6-719 mutants (LV1 and LV3, respectively, Table II). Thirty (for alp4-1891) and seven (for alp6-719) plasmids were isolated independently. Restriction enzyme mapping showed that they were classified into two (pLV4-1 and -2) and three different plasmids (pLV6-1, -2 and -3), respectively. pLV4-1 and pLV4-2 contained overlapping insert DNA, as did pLV6-1, pLV6-2 and pLV6-3. The identity of the two cloned genes as alp4+ and alp6+, respectively, was confirmed by genetic crosses between tagged strains and original mutants as follows. Random spore analysis between an Alp4-3HA strain (LV15, kanamycin resistance as a marker, Table II) and a ts alp4-1891 strain, or between an Alp6-3HA strain (LV16, Table II) and a ts alp6-719 strain, showed that the gene that was isolated in pLV4-1 and pLV6-1 was most likely to be derived from the alp4+ and alp6+ loci, respectively (no recombinants, namely KanrTs– or KansTs+, were obtained from >103 haploid segregants).
Nucleic acid preparation and manipulation
Enzymes were used as recommended by the suppliers (New England Biolabs). Nucleotide sequence data reported herein are in the DDBJ/EMBL/Genbank databases under accession Nos AB026664 (alp4+) and AB040811 (alp6+).
Gene disruption
The alp6+ gene was deleted using PCR-generated fragments (Bähler et al., 1998). Dissection of asci from heterozygous diploid cells showed that the alp6+ gene is essential for cell viability, as two viable and two non-viable spores were obtained from 20 tetrads dissected and viable colonies were Ura–. Microscopic observation of haploid cells germinated from spores deleted for alp6+ showed morphological defects similar to those of ts mutants.
Overexpression and C-terminal epitope tagging by chromosomal integration
The thiamine-repressible strong nmt1 promoter was integrated in the genome in front of the initiator ATG of the alp4+ and alp6+ genes by a PCR-based gene targeting method (LV21 and LV22, Table II; Bähler et al., 1998). C-terminal tagging with GFP or HA epitope was also performed using a PCR-generated fragment.
Synchronous culture
Centrifugal elutriation was performed using an elutriator rotor (JE-5.0, Beckman Instruments) as described previously (Moreno et al., 1991). For HU block–release experiments, HU (10 mM) was added to exponentially growing cells at 26°C, incubated for 3 h, filtered, resuspended in HU-free rich medium and cultured at 36°C. Nitrogen deprivation and release experiments were performed using a prototrophic strain (LV6). EMM2 liquid medium lacking nitrogen (EMM2–N) was used and cells were starved in this medium for 7 h at 26°C. The medium was then washed out and replaced with fresh complete medium and the cultures were shifted up to 36°C. Samples were collected at each time point for immunofluorescence microscopy, the measurement of septation index and nuclear staining.
Gel filtration chromatography
Soluble protein extracts were prepared in buffer A (20 mM Tris–HCl pH 7.5, 20% glycerol, 0.1 mM EDTA, 1 mM mercaptoethanol, 5 mM ATP plus a cocktail of inhibitors; Sigma). Gel filtration chromatography was performed on a Superose-6 column by FPLC (Pharmacia Biotech). The column was equilibrated with two column volumes of buffer A containing 100 mM NaCl. To determine molecular weight, a parallel column was run with standards consisting of dextran (2000 kDa), thyroglobulin (669 kDa) and α-amylase (232 kDa). Fractions (50 µl each) were separated by SDS–PAGE on 10% gels, and fractionated proteins were detected with individual antibodies.
Immunochemical assays
Affinity-purified rabbit polyclonal anti-Gtb1 and anti-Tea1 antibodies were a gift from Drs Hirohisa Masuda (Masuda and Shibata, 1996) and Manuel Arellano (Mata and Nurse, 1997), respectively. Rabbit polyclonal anti-Sad1 antibody was obtained from Dr Iain Hagan (Hagan and Yanagida, 1995). Mouse monoclonal anti-α-tubulin antibody (TAT-1) was provided by Dr Keith Gull. Mouse monoclonal anti-HA antibody (16B12) and anti-γ-tubulin antibody were purchased from BAbCO Ltd and Sigma, respectively. Horseradish peroxidase-conjugated goat anti-rabbit IgG, goat anti-mouse IgG (Bio-Rad Laboratories) and a chemiluminescence system (ECL, Amersham) were used to detect bound antibody. Fission yeast whole-cell extracts were prepared using glass beads to disrupt cells as described before (Radcliffe et al., 1999). For immunoprecipitation, 2 mg of total protein extracts were used.
Indirect immunofluorescence microscopy
Cells were fixed with methanol, and primary antibodies (TAT-1, 1/50; anti-HA, 1/1000; anti-Sad1 antibody, 1/15; or anti-Gtb1 antibody, 1/50) were applied, followed by Cy3-conjugated goat anti-rabbit IgG (Sigma) or fluorescein-linked sheep anti-mouse IgG (Amersham). Immunofluorescence images were viewed with a chilled video-rated CCD camera (model C5985, Hamamatsu) connected to a computer (Apple Power Macintosh G3/400) and processed by use of Adobe® Photoshop (version 4). A confocal microscope LSM510 (Zeiss Co.) was also used.
Acknowledgments
Acknowledgements
We thank Drs Manuel Arellano, Keith Gull, Iain M.Hagan, Hirohisa Masuda and Tomohiro Matsumoto for providing antibodies and strains (anti-Tea1, TAT-1, anti-Sad1 and anti-Gtb1 antibody, and a mad2 deletion and a Cut12–GFP-tagged strain) used in this study. We are indebted to Dr Iain M.Hagan who generously instructed us how to perform elutriation centrifugation, to Dr Gohta Goshima for stimulating discussion, and to W.Zac Cande and Satoru Uzawa for communicating results prior to publication. We thank Drs W.Zac Cande, Iain M.Hagan, Paul Nurse and Janet L.Paluh for critical reading of the manuscript and useful suggestions. This work is supported by the ICRF and an HFSP research grant.
References
- Akashi T., Yoon,Y. and Oakley,B.R. (1997) Characterization of γ-tubulin complexes in Aspergillus nidulans and detection of putative γ-tubulin interacting proteins. Cell Motil. Cytoskeleton, 37, 149–158. [DOI] [PubMed] [Google Scholar]
- Bähler J., Wu,J., Longtine,M.S., Shah,N.G., McKenzie,A.,III, Steever,A.B., Wach,A., Philippsen,P. and Pringle,J.R. (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast, 14, 943–951. [DOI] [PubMed] [Google Scholar]
- Barbet N., Muriel,W.J. and Carr,A.M. (1992) Versatile shuttle vectors and genomic libraries for use with Schizosaccharomyces pombe. Gene, 114, 59–66. [DOI] [PubMed] [Google Scholar]
- Bardin A.J., Visintin,R. and Amon,A. (2000) A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell, 102, 21–31. [DOI] [PubMed] [Google Scholar]
- Bloecher A., Venturi,G.M. and Tatchell,K. (2000) Anaphase spindle position is monitored by the BUB2 checkpoint. Nature Cell Biol., 2, 556–558. [DOI] [PubMed] [Google Scholar]
- Bridge A.J., Morphew,M., Bartlett,R. and Hagan,I.M. (1998) The fission yeast SPB component Cut12 links bipolar spindle formation to mitotic control. Genes Dev., 12, 927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D.J. (2000) Complexity in the spindle checkpoint. Curr. Opin. Genet. Dev., 10, 26–31. [DOI] [PubMed] [Google Scholar]
- Burns R.G. (1995) Identification of two new members of the tubulin family. Cell Motil. Cytoskeleton, 31, 255–258. [DOI] [PubMed] [Google Scholar]
- Cerutti L. and Simanis,V. (2000) Controlling the end of the cell cycle. Curr. Opin. Genet. Dev., 10, 65–69. [DOI] [PubMed] [Google Scholar]
- Ding R., West,R.R., Morphew,M., Oakley,B.R. and McIntosh,J.R. (1997) The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol. Biol. Cell, 8, 1461–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava F. et al. (1999) Human 76p: a new member of the γ-tubulin-associated protein family. J. Cell Biol., 147, 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix M.A., Antony,C., Wright,M. and Maro,B. (1994) Centrosome assembly in vitro: role of γ-tubulin recruitment in Xenopus sperm aster formation. J. Cell Biol., 124, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler S., Pereira,G., Spang,A., Knop,M., Souès,S., Kilmartin,J. and Schiebel,E. (1996) The spindle pole body component Spc98p interacts with the γ-tubulin-like Tub4p of Saccharomyces cerevisiae at the sites of microtubule attachment. EMBO J., 15, 3899–3911. [PMC free article] [PubMed] [Google Scholar]
- Gould R.R. and Borisy,G.G. (1978) The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J. Cell Biol., 73, 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I.M. (1998) The fission yeast microtubule cytoskeleton. J. Cell Sci., 111, 1603–1612. [DOI] [PubMed] [Google Scholar]
- Hagan I.M. and Hyams,J.S. (1988) The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast. J. Cell Sci., 89, 343–357. [DOI] [PubMed] [Google Scholar]
- Hagan I. and Yanagida,M. (1995) The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J. Cell Biol., 129, 1033–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Patterson,T.E. and Sazer,S. (1997) The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc. Natl Acad. Sci. USA, 94, 7965–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann S.R. and McIntosh,J.R. (1980) Visualization of the structural polarity of microtubules. Nature, 286, 517–519. [DOI] [PubMed] [Google Scholar]
- Hirata D., Masuda,H., Eddison,M. and Toda,T. (1998) Essential role of tubulin-folding cofactor D in microtubule assembly and its association with microtubules in fission yeast. EMBO J., 17, 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T. and Oakley,B.R. (1994) Human γ-tubulin functions in fission yeast. J. Cell Biol., 126, 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T., Uzawa,S., Jung,M.K., Oakley,B.R., Tanaka,K. and Yanagida,M. (1991) The fission yeast γ-tubulin is essential for mitosis and is localized at microtubule organizing centers. J. Cell Sci., 99, 693–700. [DOI] [PubMed] [Google Scholar]
- Hoyt M.A., Totis,L. and Roberts,B.T. (1991) S.cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell, 66, 507–117. [DOI] [PubMed] [Google Scholar]
- Hwang L.H., Lau,L.F., Smith,D.L., Mistrot,C.A., Hardwick,K.G., Hwang,E.S., Amon,A. and Murray,A.W. (1998) Budding yeast Cdc20: a target of the spindle checkpoint. Science, 279, 1041–1044. [DOI] [PubMed] [Google Scholar]
- Joshi H.C. (1993) γ-Tubulin: the hub of cellular microtubule assemblies. BioEssays, 15, 637–643. [DOI] [PubMed] [Google Scholar]
- Joshi H.C., Palacios,M.J., McNamara,L. and Cleveland,D.W. (1992) γ-Tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature, 356, 80–83. [DOI] [PubMed] [Google Scholar]
- Kanbe T., Hiraoka,Y., Tanaka,K. and Yanagida,M. (1990) The transition of cells of the fission yeast β-tubulin mutant nda3-311 as seen by freeze-substitution electron microscopy. Requirement of functional tubulin for spindle pole body duplication. J. Cell Sci., 96, 275–282. [DOI] [PubMed] [Google Scholar]
- Keating T.J. and Borisy,G.G. (2000) Immunostructural evidence for the template mechanism of microtubule nucleation. Nature Cell Biol., 2, 352–357. [DOI] [PubMed] [Google Scholar]
- Kellogg D.R., Moritz,M. and Alberts,B.M. (1994) The centrosome and cellular organization. Annu. Rev. Biochem., 63, 639–674. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Lin,D.P., Matsumoto,S., Kitazono,A. and Matsumoto,T. (1998) Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science, 279, 1045–1047. [DOI] [PubMed] [Google Scholar]
- King S.M. and Hyams,J.S. (1982) Interdependence of cell cycle events in Schizosaccharomyces pombe. Terminal phenotypes of cell division cycle mutants arrested during DNA synthesis and nuclear division. Protoplasma, 110, 54–62. [Google Scholar]
- Knop M. and Schiebel,E. (1997) Spc98p and Spc97p of the yeast γ-tubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO J., 16, 6985–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M., Pereira,G., Geissler,S., Grein,K. and Schiebel,E. (1997) The spindle pole body component Spc97p interacts with the γ-tubulin of Saccharomyces cerevisiae and functions in microtubule organization and spindle pole body duplication. EMBO J., 16, 1550–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leguy R., Melki,R., Pantaloni,D. and Carlier,M.-F. (2000) Monomeric γ-tubulin nucleates microtubules. J. Biol. Chem. 275, 21975–21980. [DOI] [PubMed] [Google Scholar]
- Lengauer C., Kinzler,K.W. and Vogelstein,B. (1998) Genetic instabilities in human cancers. Nature, 396, 643–649. [DOI] [PubMed] [Google Scholar]
- Li R. and Murray,A.W. (1991) Feedback control of mitosis in budding yeast. Cell, 66, 519–531. [DOI] [PubMed] [Google Scholar]
- McCully E.K. and Robinow,C.F. (1971) Mitosis in fission yeast Schizosaccharomyces pombe: a comparative study with light and electron microscopy. J. Cell Sci., 9, 475–507. [DOI] [PubMed] [Google Scholar]
- Marschall L.G., Jeng,R.L., Mulholland,J. and Stearns,T. (1996) Analysis of Tub4p, yeast γ-tubulin-like protein: implications for microtubule-organizing center function. J. Cell Biol., 134, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin O.C., Gunawardane,R.N., Iwamatsu,A. and Zheng,Y. (1998) Xgrip109: a γ tubulin-associated protein with an essential role in γ tubulin ring complex (γTuRC) assembly and centrosome function. J. Cell Biol., 141, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H. and Shibata,T. (1996) Role of γ-tubulin in mitosis-specific microtubule nucleation from the Schizosaccharomyces pombe spindle pole body. J. Cell Sci., 109, 165–177. [DOI] [PubMed] [Google Scholar]
- Masuda H., Sevik,M. and Cande,W.Z. (1992) In vitro microtubule-nucleating activity of spindle pole bodies in fission yeast Schizosaccharomyces pombe: cell cycle-dependent activation in Xenopus cell-free extracts. J. Cell Biol., 117, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata F. and Nurse,P. (1997) tea1p and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell, 89, 939–949. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar,A. and Nurse,P. (1991) Molecular genetic analyses of fission yeast Schizosaccharomyces pombe. Methods Enzymol., 194, 773–782. [DOI] [PubMed] [Google Scholar]
- Moritz M., Braufeld,M.B., Dedat,J.W., Alberts,B. and Agard,D.A. (1995) Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature, 378, 638–640. [DOI] [PubMed] [Google Scholar]
- Moritz M., Zheng,Y., Alberts,B.M. and Oegema,K. (1998) Recruitment of the γ-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J. Cell Biol., 142, 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M., Braunfeld,M.B., Guénebaut,V., Heuser,J. and Agard,D.A. (2000) Structure of the γ-tubulin ring complex: a template for microtubule nucleation. Nature Cell Biol., 2, 365–370. [DOI] [PubMed] [Google Scholar]
- Murphy S.M., Urbani,L. and Stearns,T. (1998) The mammalian γ-tubulin complex contains homologues of the yeast spindle pole body components Spc97p and Spc98p. J. Cell Biol., 141, 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B.R. (1992) γ-Tubulin: the microtubule organizer? Trends Cell Biol., 2, 1–5. [DOI] [PubMed] [Google Scholar]
- Oakley B.R., Oakley,C.E., Yoon,Y. and Jung,M.K. (1990) γ-Tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell, 61, 1289–1301. [DOI] [PubMed] [Google Scholar]
- Oakley C.E. and Oakley,B.R. (1989) Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature, 338, 662–664. [DOI] [PubMed] [Google Scholar]
- Oegema K., Wiese,C., Martin,O.C., Milligan,R.A., Iwamatsu,A., Mitchison,T.J. and Zheng,Y. (1999) Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol., 144, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh J.L., Nogales,E., Oakley,B.R., McDonald,K., Pidoux,A.L. and Cande,W.Z. (2000) A mutation in γ-tubulin alters microtubule dynamics and organization and is synthetically lethal with the kinesin-like protein Pkl1p. Mol. Biol. Cell, 11, 1225–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G., Höfken,T., Grindlay,J., Manson,C. and Schiebel,E. (2000) The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol. Cell, 6, 1–10. [PubMed] [Google Scholar]
- Pichová A., Kohlwein,S.D. and Yamamoto,M. (1995) New arrays of cytoplasmic microtubules in the fission yeast Schizosaccharomyces pombe. Protoplasma, 188, 252–257. [Google Scholar]
- Pickett-Heaps J.D. (1969) The evolution of the mitotic apparatus: an attempt at comparative ultrastructural cytology in dividing plant cells. Cytobios, 3, 257–280. [Google Scholar]
- Radcliffe P., Hirata,D., Childs,D., Vardy,L. and Toda,T. (1998) Identification of novel temperature-sensitive lethal alleles in essential β-tubulin and nonessential α2-tubulin genes as fission yeast polarity mutants. Mol. Biol. Cell, 9, 1757–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe P.A., Hirata,D., Vardy,L. and Toda,T. (1999) Functional dissection and hierarchy of tubulin-folding cofactor homologues in fission yeast. Mol. Biol. Cell, 10, 2987–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiebel E. (2000) γ-Tubulin complexes: binding to the centrosome, regulation and microtubule nucleation. Curr. Opin. Cell Biol., 12, 113–118. [DOI] [PubMed] [Google Scholar]
- Schnackenberg B.J., Khodjakov,A., Rieder,C.L. and Palazzo,R.E. (1998) The disassembly and reassembly of functional centrosomes in vitro. Proc. Natl Acad. Sci. USA, 95, 9295–9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel S.G. and Snyder,M. (1995) A highly divergent γ-tubulin gene is essential for cell growth and proper microtubule organization in Saccharomyces cerevisiae. J. Cell Biol., 131, 1775–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A., Geissler,S., Grein,K. and Schiebel,E. (1996) γ-Tubulin-like Tub4p of Saccharomyces cerevisiae is associated with the spindle pole body substructure that organizes microtubules and is required for mitotic spindle formation. J. Cell Biol., 134, 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T. and Kirschner,M. (1994) In vitro reconstitution of centrosome assembly and function: the central role of γ-tubulin. Cell, 76, 623–637. [DOI] [PubMed] [Google Scholar]
- Stearns T., Evans,L. and Kirschner,M. (1991) γ-Tubulin is a highly conserved component of the centrosome. Cell, 65, 825–836. [DOI] [PubMed] [Google Scholar]
- Tassin A.-M., Celati,C., Moudjou,M. and Bornens,M. (1998) Characterization of the human homologue of the yeast Spc98p and its association with γ-tubulin. J. Cell Biol., 141, 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavormina P.A. and Burke,D.J. (1998) Cell cycle arrest in cdc20 mutants of Saccharomyces cerevisiae is independent of Ndc10p and kinetochore function but requires a subset of spindle checkpoint genes. Genetics, 148, 1701–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.S. and McKeon,F. (1997) Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell, 89, 727–735. [DOI] [PubMed] [Google Scholar]
- Wiese C. and Zheng,Y. (2000) A new function for the γ-tubulin ring complex as a microtubule minus-end cap. Nature Cell Biol., 2, 358–364. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Jung,M.K. and Oakley,B.R. (1991) γ-Tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell, 65, 817–823. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wong,M.L., Alberts,B. and Mitchison,T. (1995) Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature, 378, 578–583. [DOI] [PubMed] [Google Scholar]