Abstract
The nucleus pulposus is an avascular and aneural tissue that has significant influence on the homeostasis and overall function of the intervertebral disc. The nucleus pulposus is comprised of a heterogeneous population of cells including large notochord cells and smaller chondrocyte-like cells. Loss of notochord cells has been correlated with the pathogenesis of disc degeneration and consequently, it has been hypothesized that regeneration of the disc could be mediated by notochord cells. Attempts to grow and expand notochord cells in vitro have thus far been limited by cell availability and ineffective culturing methodologies. As a result, co-culturing techniques have been developed in order to exploit notochord-derived signals for the differentiation of proliferative mesenchymal stem cells. A recent study by Korecki et al. has demonstrated that notochord cell conditioned medium has the ability to differentiate mesenchymal stem cells toward a nucleus pulposus-like fate, producing high levels of glycosaminoglycans and type III collagen. These findings suggest that growth factors and other soluble proteins may be able to stimulate endogenous IVD tissue maintenance in vivo. While this study advances our understanding of intervertebral disc cell-cell interactions, limitations remain in our ability to determine the phenotype of terminally differentiated cells within the nucleus pulposus (ie mature notochord cells) and therefore assess the relevance of differentiated mesenchymal stem cells for disc regeneration. In order for the field to progress, elucidation of the notochord phenotype remains of utmost importance.
Keywords: Cell differentiation, Conditioned media, Intervertebral disc, Mesenchymal stem cells, Notochord cells, Nucleus pulposus
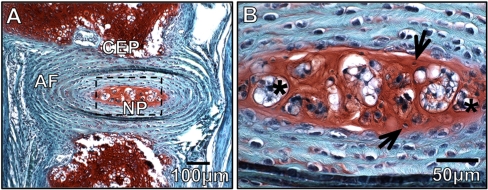
The intervertebral disc (IVD) is an intricate structure consisting of three functionally distinct, yet interdependent tissues: the outer fibro-cartilagenous annulus fibrosus (AF), the central gelatinous nucleus pulposus (NP), and the cartilage end-plates (CEP) that anchor the discs to the adjacent vertebral body bones. The NP is formed through deposition of a proteoglycan rich extracellular matrix, leading to water influx providing the osmotic properties needed to resist compression (Fig. 1). Early in life, the NP contains two distinct cell types within this matrix: clusters of large vacuolated notochord cells (the cell type that forms the NP during embryonic development) and smaller more numerous cartilage-like cells. Until recently, there has been considerable debate as to the origins of the cartilage-like cells, whether they migrate from the surrounding tissues or are derived from the notochord cells (Choi et al. 2008; Kim et al. 2009; Vujovic et al. 2006). Interestingly, the loss of notochord cells has been correlated with disc degeneration, a process marked by decreased proteoglycan content which results in decreased physiological function, specifically the IVD’s role in load bearing and flexibility.
Fig. 1.
Morphology and Composition of the Intervertebral disc. a Histological sections of the IVD from a 4 week old mouse stained with safranin-O/fast green demonstrates IVD morphology and regionalization of the nucleus pulposus (NP), annulus fibrosis (AF), and cartilaginous end-plate (CEP). Proteoglycan content is indicated by red stain (scale bar represents 100 uM). b Enlarged view of the nucleus pulposus region in A. Arrows indicate large aggregations of vacuolated notochord cells and * indicate the smaller more disperse cartilage-like cells (scale bar represents 50 uM)
The nucleus pulposus is an avascular tissue from the onset of development and as a result, the nutrient supply to the disc is limited to the diffusion capability of neighboring tissues. It has been previously established that the addition of growth factors in vitro, including Transforming Growth Factor-β1 (TGF-β1) and Basic Fibroblastic Growth Factor (bFGF) increases proteoglycan content within the disc, specifically within the NP (Thompson et al. 1991). However, when considering the physiological environment of the IVD, the source of cytokines and their relative contribution to IVD maintenance and homeostasis in vivo remains unclear. It would be reasonable to consider notochord cells as a potential source of growth factors given the role of the notochord as a signaling center during development and the localization of notochord cell clusters within the NP of fully formed IVDs. This role for the notochord was determined in a classical experiment that demonstrated that co-culture of purified notochord cells with mature nucleus pulposus cells could significantly increase proteoglycan content from the latter (Aguiar et al. 1999). Additionally, this study established this to be cell contact independent since the culture of NP cells in notochord conditioned media (NCCM) was capable of recapitulating the anabolic effects.
Given the effect of NCCM on nucleus pulposus cell metabolism, notochord cells are broadly considered to be an excellent source of signals for potential cell-based IVD therapies. However the development of strategies to exploit this potential is limited by the fact that these cells are no longer present in human IVDs as early as the age of three (Liebscher et al. 2010). The loss of notochord cells therefore markedly precedes the development of disc degeneration and the resulting need for medical intervention. This limitation prevents the direct therapeutic application of notochord cells and consequently highlights the need for an alternate source of cells for IVD therapy. Mesenchymal stem cells (MSC) provide an obvious source for stem cell therapy due to their ease of availability (Castro-Malaspina et al. 1980; Majumdar et al. 1998; Van et al. 1976), overall plasticity (Jiang et al. 2002) and ability to undergo chondrogenic differentiation (Pittenger et al. 1999). In fact, many studies have focused on the use of co-culture systems using NP cells to drive MSC differentiation towards a NP-like phenotype (Allon et al. 2010; Chen et al. 2009; Niu et al. 2009; Vadala et al. 2008). However, the recent publication of conditioned medium-based approaches presents a more elegant and practically applicable approach that serves as proof-of-principle that growth factors and other soluble proteins might be able to stimulate IVD tissue maintenance from endogenous cells in vivo.
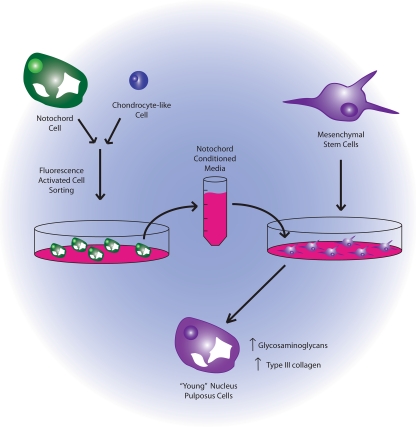
Korecki et al. (2010) demonstrated the ability of NCCM to stimulate the differentiation of MSCs towards to a NP-like phenotype, using as direct comparison MSC treated with TGF-β (used extensively for chondrogenic differentiation) (Barry et al. 2001; Jiang et al. 2002; Worster et al. 2001). In these studies, the NCCM was produced using NC cells isolated by size from porcine IVDs and cultured in alginate to maintain cell viability and mimic the three-dimensional environment of the NP (Fig. 2). MSCs were isolated from the bone marrow of human patients. The authors observed that culture of MSCs in the presence of NCCM produced differentiated cells secreting higher levels of glycosaminoglycans (GAG) and type III collagen when compared to TGF-β treatment. Further suggesting that NCCM induced the differentiation of potential notochord cells rather that chondrocyte-like cells, MSCs demonstrated increased expression of laminin β-1 and tissue inhibitor of metaloproteinases-1 (TIMP-1), genes previously shown to be upregulated in the subset of notochord cells within the NP when compared to adjacent IVD cell types (Hayes et al. 2001).
Fig. 2.
Schematic representation of notochord conditioned media experiments undertaken by Korecki et al.. Nucleus pulposus tissue was isolated and the cellular contents were sorted according to size to obtain the large notochord cells. Notochord cells were grown in vitro to generate conditioned media which was then applied to isolated bone marrow derived mesenchymal stem cells. Differentiated mesencymal stem cells were analyzed and compared to transforming growth factor-β (TGF-β) treatments
This study is limited by two main factors: the origin of the stem cell source used for differentiation and the ability to assess cell fate following differentiation. While we are only recently beginning to understand the phenotype of NP cells (Lee et al. 2007; Minogue et al. 2010), the phenotype of notochord cells has yet to be characterized either during early IVD development or as cells persist in the mature IVD. Without knowing the phenotype of these cells within the NP, our ability to properly assess the success of cell differentiation experiments is limited. However, in their study Korecki et al. (2010) recognize this potential limitation by examining both known chondrogenic markers (SOX9 and type II collagen) and some of the limited known markers of the NP, specifically laminin β-1 and TIMP-1. The authors state that NCCM has the potential to differentiate MSCs towards a “young” NP phenotype based on the expression of type III collagen which is the first collagen to be expressed during early IVD development in rats (Hayes et al. 2001) and is also detected during human IVD development (McAlinden et al. 2002). However, type III collagen is also expressed during disc repair and disc degeneration (Adam and Deyl 1984; Kaapa et al. 1994; Roberts et al. 1991). The upregulation of type III collagen reported during MSC differentiation may be attributed to the advanced age of the sample population from which the MSCs were obtained (mean of 64.3) and consequently the cellular changes that may have occurred (Stolzing et al. 2008). As such, these “mature” MSCs may be inducing a fibrotic repair rather than tissue regeneration.
An additional confounding variable is the use of porcine IVDs as a source of notochord cells. This approach is experimentally feasible given that pigs retain their notochord cells throughout life and therefore cells can be easily isolated (Hunter et al. 2004). However, the inherent difference between the persistence of notochord cells and resulting difference in cellular composition of the NP between porcine and human IVDs (Liebscher et al. 2010) further complicates the interpretation of the reported findings. Since notochord cells are not present in mature human IVDs, is there a species-related difference in the phenotype of the notochord cell, in the profile of growth factors being produced by these cells, and importantly could these studies be recapitulated using a human source of NCCM? Furthermore, to obtain sufficient notochord cells, samples were pooled from throughout the entire spine of five animals. It has been shown that distinctive anatomical regions of the spine contain notochord cells with specific gene expression profiles during development (Yamanaka et al. 2007). This is bound to have an effect on conditioning experiment that obtain a pooled disc sample, given that it is now known that all cells in NP are derived from the notochord during development (Choi et al. 2008). In addition, using mature cell types to direct cellular differentiation might have unknown molecular and physiological effects (Barry et al. 2001) especially when the molecular phenotype of notochord cells are currently unknown both in development and degeneration.
This study clearly demonstrated the ability of notochord-conditioned media to direct the differentiation of MSCs. While the potential of NCCM is greatly recognized, the generation of so-called notochord cells in vitro, is limited by the lack of specific markers to assess the notochord phenotype (Fig. 3). Currently, the phenotype of notochord cells during the stages of embryonic patterning, intervertebral disc formation, and in mature/senescent notochord cells remains to be conclusively established. Differentiation of MSCs can lead to any of the aforementioned stages of notochord cell progression, and unless we know the molecular phenotype of the desired cell type we cannot conclusively determine what cells have been generated. Consequently, the characterization of the notochord cell phenotype throughout development should be undertaken to enable the advancement of the field of intervertebral disc biology.
Fig. 3.
Lack of specific phenotypic markers limits strategies for notochord cell generation. Protocols have been established for the differentiation of mesenchymal stem cells down specific lineages producing specialized cell types with known markers. This has been achieved via internal regulation (transcription factor-mediated differentation), as well as external stimulation such as three-dimensional scaffolding and the use of growth factors and small molecules. These methods have been validated based on well-characterized cell type-specific markers, a limiting factor for the production of notochord cells
Acknowledgements
The authors would like to thank Nicole Watts and Emily LeBlanc for their editing and constructive feedback.
Conflict of Interest The authors declare that they have no competing interests.
Funding M.M. is supported by the Joint Motion Program (JuMP) - A CIHR Training Program in Musculoskeletal Health Research and Leadership. C.S. is supported by NSERC and the Canadian Arthritis Network.
Abbreviations
- AF
Annulus Fibrosus
- CEP
Cartilage End Plate
- GAG
Glycosaminoglycans
- IVD
Intervertebral Disc
- MSC
Mesenchymal Stem Cells
- NCCM
Notochord Cell Conditioned Medium
- NP
Nucleus Pulposus
- SOX9
Sry-related HMG Box 9
- TIMP1
Tissue Inhibitor of Metaloproteinases-1
- TGF-β
Transforming Growth Factor β-1
Footnotes
Concise 2-3 sentence summary
This commentary provides an overview of the current use of cell-conditioned media to differentiate mesenchymal stem cells for intervertebral disc regeneration. We outline how this approach effectively exploits endogenous cell-cell communication to direct stem cell differentiation but is not without specific limitations. The current findings serve as proof-of-concept for cell-based strategies for disc regeneration and importantly highlight the need for further characterization of the notochord cell phenotype to facilitate the application of stem cell therapy.
References
- Adam M, Deyl Z. Degenerated annulus fibrosus of the intervertebral disc contains collagen type II. Ann Rheum Dis. 1984;43:258–263. doi: 10.1136/ard.43.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129–137. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- Allon AA, Aurouer N, Yoo BB, Liebenberg EC, Buser Z, Lotz JC. Structured coculture of stem cells and disc cells prevent disc degeneration in a rat model. Spine J. 2010;10:1089–1097. doi: 10.1016/j.spinee.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D, McKenzie S, Broxmeyer HE, Moore MA. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- Chen S, Emery SE, Pei M. Coculture of synovium-derived stem cells and nucleus pulposus cells in serum-free defined medium with supplementation of transforming growth factor-beta1: a potential application of tissue-specific stem cells in disc regeneration. Spine. 2009;34:1272–1280. doi: 10.1097/BRS.0b013e3181a2b347. [DOI] [PubMed] [Google Scholar]
- Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237:3953–3958. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20:107–121. doi: 10.1016/S0945-053X(01)00125-1. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–362. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Kaapa E, Zhang LQ, Muona P, Holm S, Vanharanta H, Peltonen J. Expression of type I, III, and VI collagen mRNAs in experimentally injured porcine intervertebral disc. Connect Tissue Res. 1994;30:203–214. doi: 10.3109/03008209409061972. [DOI] [PubMed] [Google Scholar]
- Kim KW, Ha KY, Lee JS, Nam SW, Woo YK, Lim TH, An HS. Notochordal cells stimulate migration of cartilage end plate chondrocytes of the intervertebral disc in in vitro cell migration assays. Spine J. 2009;9:323–329. doi: 10.1016/j.spinee.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Korecki CL, Taboas JM, Tuan RS, Iatridis JC. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1:18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, Grad S. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16:2174–2185. doi: 10.1007/s00586-007-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher, T, Haefeli, M, Wuertz, K, Nerlich, AG, and Boos, N (2010) Age-related variation in cell density of human lumbar intervertebral disc. Spine Epub ahead of print. [DOI] [PubMed]
- Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- McAlinden A, Zhu Y, Sandell LJ. Expression of type II procollagens during development of the human intervertebral disc. Biochem Soc Trans. 2002;30:831–838. doi: 10.1042/BST0300831. [DOI] [PubMed] [Google Scholar]
- Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum. 2010;62:3695–3705. doi: 10.1002/art.27710. [DOI] [PubMed] [Google Scholar]
- Niu CC, Yuan LJ, Lin SS, Chen LH, Chen WJ. Mesenchymal stem cell and nucleus pulposus cell coculture modulates cell profile. Clin Orthop Relat Res. 2009;467:3263–3272. doi: 10.1007/s11999-008-0623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Roberts S, Menage J, Duance V, Wotton SF. Type III collagen in the intervertebral disc. Histochem J. 1991;23:503–508. doi: 10.1007/BF01041176. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Thompson JP, Oegema TR, Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Vadala G, Studer RK, Sowa G, Spiezia F, Iucu C, Denaro V, Gilbertson LG, Kang JD. Coculture of bone marrow mesenchymal stem cells and nucleus pulposus cells modulate gene expression profile without cell fusion. Spine. 2008;33:870–876. doi: 10.1097/BRS.0b013e31816b4619. [DOI] [PubMed] [Google Scholar]
- Van RL, Bayliss CE, Roncari DA. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest. 1976;58:699–704. doi: 10.1172/JCI108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujovic S, Henderson S, Presneau N, Odell E, Jacques TS, Tirabosco R, Boshoff C, Flanagan AM. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209:157–165. doi: 10.1002/path.1969. [DOI] [PubMed] [Google Scholar]
- Worster AA, Brower-Toland BD, Fortier LA, Bent SJ, Williams J, Nixon AJ. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19:738–749. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Tamplin OJ, Beckers A, Gossler A, Rossant J. Live imaging and genetic analysis of mouse notochord formation reveals regional morphogenetic mechanisms. Dev Cell. 2007;13:884–896. doi: 10.1016/j.devcel.2007.10.016. [DOI] [PubMed] [Google Scholar]