Abstract
Introduction
Bisphosphonates are currently the medical treatment most often used in children with osteogenesis imperfecta (OI). The purpose of this retrospective pre–post study was to evaluate the efficacy of treatment with bisphosphonates. We measured the effect by evaluating the number of outpatient department consultations and operative interventions before and after treatment with bisphosphonates in children with OI.
Methods and materials
Outpatient department consultation and operative intervention frequencies before and after treatment with bisphosphonates were registered. Children who had at least 2 years of medical records before treatment and at least 2 years after treatment were used in this study.
Results
Of 118 children who were treated with bisphosphonates, 51 (23 boys and 28 girls) fulfilled the inclusion criteria. Statistical analysis revealed a significant decrease in outpatient department consultations (P < 0.000) and operative intervention (P < 0.003) before and after bisphosphonate treatment.
Conclusion
The pre-post design of our study shows a significant reduction of the number of outpatient department consultations and operative interventions in patients with OI after treatment with bisphosphonates.
Keywords: Bisphosphonates, Osteogenesis imperfecta, Children, Outpatient department, Operative intervention
Introduction
Osteogenesis imperfecta (OI) is an inherited connective tissue disorder that has been classified into four major subtypes by Sillence [1] based on genetic, radiographic and clinical considerations. Mutations in the COL1A1 and COL1A2 genes, that codify for type I collagen, are responsible for more than 90% of all cases of OI. Type I collagen provides structure and strength in bone, skin and other connective tissues [2]. One of the outcomes of the changes in bones of patients with osteogenesis imperfecta is the presence of osteopenia, that in itself is a factor in the increased fracture risk.
The aims of treatment for patients with OI are to reduce pathologic fracture rates, minimize chronic pain, prevent long bone deformities and scoliosis, and maximize mobility. Surgical placement of intramedullary rods may be an essential contribution to prevent or correct long bone deformities [3–5].
Bisphosphonates, a group of stable analogs of pyrophosphates, are potent inhibitors of bone resorption and bone turnover and are used in the treatment of osteopenia [6]. Bisphosphonates are currently the medical treatment most often used in children with moderate to severe OI. In previous studies, increased bone mineral density (BMD) and a decreased incidence of fractures due to bisphosphonate therapy were observed in children with OI [7–10]. Treated children reported improved mobility, ambulation and relief from chronic pain [11–13].
The purpose of this retrospective pre–post study is to evaluate the clinical efficacy of treatment with bisphosphonates.
Materials and methods
Outcomes in children with OI before and after treatment with bisphosphonates were studied in a pre–post study design. Therefore, retrospective chart review was performed on all the children by the Department of Paediatric Orthopaedic Surgery, Wilhelmina Children’s Hospital (WKZ) of the University Medical Center in Utrecht, The Netherlands, between February 1988 and December 2009.
Because patient data were charted over different time periods per patient, annual mean pre- and post-intervention rates were created and compared. Furthermore, bisphosphonate treatment was started for many patients shortly after referral to our center. Hence, it was impossible to compare all parameters before and after treatment. In order to compare these mean pre- and post intervention rates correctly, the inclusion of children was allowed on statistical grounds if they were treated a minimum of 2 years before and after starting the bisphosphonates. Outcome parameters were the number of outpatient department consultations and operative interventions before and after treatment with bisphosphonates.
Statistical analysis
Differences between results before and after bisphosphonate treatment were tested for significance using the Wilcoxon signed-rank test as we could not assume that the population was normally distributed. To justify our statements about a possible spontaneous age-related decline in outpatient department consultations and operative interventions in children with OI before treatment with bisphosphonates, a trend analysis was performed. Furthermore, aggregated data was used in a multiple linear regression including interaction terms in order to estimate the effect of bisphosphonate treatment. Finally, the potential influences of disease severity and initiation of treatment were evaluated using multiple linear regression analysis. P < 0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package of Social Sciences (SPSS), version 16.0.
Results
Between February 1988 and December 2009, 201 patients were diagnosed with OI, among whom 118 were treated with bisphosphonates, starting in 1998. Of these 118 children, 51 (23 boys and 28 girls) fulfilled the inclusion criteria and were used in this study (Fig. 1). In these patients, a lumbar spine BMD and a protocol check-up were obtained at the beginning of bisphosphonate treatment and at intervals of approximately 1 year afterwards. Immobilization of children with OI often results in vitamin D and calcium deficiency. Accordingly, in the protocol, all patients received a daily supplement of 1,200 IU vitamin D and 500 mg calcium during bisphosphonate treatment.
Fig. 1.
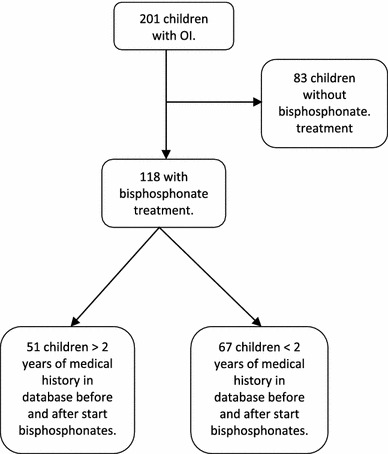
Trial profile
Twenty-four children were diagnosed as having OI type I, thirteen had type III, twelve had type IV, and the OI type of two patients was unknown. Twenty-six patients were treated with risedronate, fifteen with pamidronate, two with alendronate, and eight with pamidronate (who switched later to risedronate) (Table 1).
Table 1.
General characteristics of our study population
| Agea | 10 (SD 4,7) |
| Male/female | 23/28 |
| Total | 51 |
| Type OI | |
| I | 24 |
| III | 13 |
| IV | 12 |
| Unknown | 2 |
| Bisphosphonates | |
| Risedronateb | 34 |
| Pamidronatec | 15 |
| Alendronated | 2 |
aMean in years
bAdministered 1dd 5 mg orally
cAdministered intravenously at a dose of 2 mg/kg given every 3 months
dAdministered 70 mg orally once a week
Statistical analysis revealed changes in the numbers of outpatient department consultations and operative interventions after treatment.
Wilcoxon signed-rank test
Outpatient department consultations
The number of outpatient department consultations decreased from 3.35 (SD 2.07) per year during the period before bisphosphonate treatment to 2.14 (SD 2.12) per year in the period after treatment (P < 0.000) (Table 2).
Table 2.
Outpatient department consultation: statistical analysis by Wilcoxon signed-rank test
| N | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Without bisph | 51 | 3.35 | 2.12 | 0.44 | 9.50 |
| With bisph | 51 | 2.14 | 2.08 | 0 | 9.33 |
| Without bisph—with bisph | |||||
|---|---|---|---|---|---|
| Test statisticsb | |||||
| Z | −4.312a | ||||
| Asymp. sig. (two-tailed) | .000 | ||||
aBased on negative ranks
bWilcoxon signed-rank test
Operative interventions
The number of operative interventions decreased from 0.73 (SD 0.47) per year during the period before bisphosphonate treatment to 0.38 (SD 0.45) per year in the period after treatment (P < 0.003) (Table 3).
Table 3.
Operative intervention: statistical analysis by Wilcoxon signed-rank test
| N | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Without bisph | 32 | 0.73 | 0.47 | 0.1 | 1.67 |
| With bisph | 32 | 0.38 | 0.45 | 0 | 1.75 |
| With bisph—without bisph | |||||
|---|---|---|---|---|---|
| Test statisticsb | |||||
| Z | −3.018a | ||||
| Asymp. sig. (two-tailed) | .003 | ||||
aBased on positive ranks
bWilcoxon signed-rank test
Trend analysis
In the trend analysis, we observed an increase (RC = 0.27) in outpatient department consultations every year in the group before bisphosphonate treatment.
Unfortunately, trend analysis was impossible to apply to the number of operative interventions before treatment with bisphosphonates due to a poor distribution of measurements.
Multiple linear regression analysis
In the multiple linear regression analysis, age and bisphosphonates represented 70% of the effect on the number of outpatient department consultations. This interaction effect was significant (P > 0.000). The amount of outpatient department visits declined with increasing age. No significant differences in outpatient department visits between the patients with and without bisphosphonate treatment were found when analyzed for the whole age range. Before the age of seven, patients with bisphosphonate use, had relatively more outpatient department visits as compared to patients that where not on bisphosphonate medication. With increasing age however, the amount of outpatient department visits with bisphosphonate use was increasingly less as compared to the patients that did not use bisphosphonates at that same age. There was no relevant or significant influence of disease severity on the number of outpatient department consultations or operations.
Discussion
In the pre-post study design, bisphosphonate therapy was found to decrease the frequency of outpatient visits and surgical intervention.
Due to a low prevalence, many previous evaluations of bisphosphonate therapy in OI used a retrospective study design. Limiting factors of these studies are considered to be the selection bias and the dependency on the availability and accuracy of the medical records. It is often hard to obtain a homogeneous control group since OI is associated with wide variations between individuals and variations over time within one person. Because our study is based on an examination of existing data, a pre–post retrospective chart review was chosen.
Other factors besides the bisphosphonate therapy might contribute to the decrease in treatment frequency in OI patients. These factors include the age-related acquisition of motor skills and an increase in BMD due to entrance into puberty [14, 15].
In our population, however, the number of outpatient visits increased before bisphosphonate treatment. Therefore, the correction due to age-related decline was considered insignificant.
The effect of the bisphosphonate treatment on the decrease of the number of outpatient department visits seemed to increase with increasing age (Fig. 2). At a young age children with bisphosphonates had more outpatient department visits as compared to children that were still without bisphosphonate use at that same age, probably because of extra hospital visits associated with intravenous bisphosphonate treatment (4–5 infusions per year). At an older age, most children used oral bisphosphonates, and less outpatient department visits were seen as compared to patients at that same age that were still without bisphosphonate use.
Fig. 2.
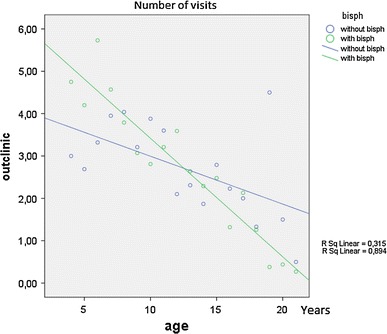
Number of outpatient department visits in children with OI
Other factors that influence the response to bisphosphonates in OI remain unclear. Previous studies have shown differences in effects on DEXA parameters between patients with OI types III and IV [15].
Also, a relationship between start of treatment at an early age and a better outcome has been reported, suggesting the need for early diagnosis and initiation of treatment [8]. In the pre-post analysis in our study, we could not find a significant relationship between differences in response to bisphosphonates and disease severity or age at initiation of treatment. As for the influence of the use of fixed or extendable rods we assumed no difference since, for the surgical placement of intramedullary rods, regular replacement is needed with fixed rods, and more complications are seen with the use of extendable rods.
The observed increasing decrease in outpatient department visits with increasing age is believed to reflect a decrease in bone-related disease such as low BMD, increased fracture risk, chronic pain and decreased mobility [7–13]. We believe that the decrease in outpatient department visits is comparable to the fracture rate declines presented by others when using bisphosphonates as a medical treatment for OI.
However, we have not encountered literature in which the clinical parameters were compared before and after bisphosphonate treatment. Thus, a critical comparison with results presented by others is not possible due to a lack of reference material.
In conclusion, the results of our study show that bisphosphonates can significantly reduce the number of outpatient department consultations and operative interventions in patients with OI.
Conflicts of interest
None declared.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prockop DJ, Kivirikko KI. Heritable diseases of collagen. N Engl J Med. 1984;311:376. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- 3.Engelbert RH, Pruijs HE, Beemer FA, Helders PJ. Osteogenesis imperfecta in childhood: treatment strategies. Arch Phys Med Rehabil. 1998;79:1590. doi: 10.1016/S0003-9993(98)90426-9. [DOI] [PubMed] [Google Scholar]
- 4.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 5.Engelbert RH, Beemer FA, van der Graaf Y, Helders PJ. Osteogenesis imperfecta in childhood: impairment and disability–a follow-up study. Arch Phys Med Rehabil. 1999;80:896. doi: 10.1016/S0003-9993(99)90080-1. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay R. Modeling the benefits of pamidronate in children with osteogenesis imperfecta. J Clin Invest. 2002;110:9. doi: 10.1172/JCI0215999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakkers R, Kok D, Engelbert R, van Dongen A, Jansen M, Pruijs H, Verbout A, Schweitzer D, Uiterwaal C. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomised placebo-controlled study. Lancet. 2004;363(9419):1427–1431. doi: 10.1016/S0140-6736(04)16101-1. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin H, Rauch F, Bishop NJ, et al. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab. 2000;85:1846. doi: 10.1210/jcem.85.5.6584. [DOI] [PubMed] [Google Scholar]
- 9.DiMeglio LA, Ford L, McClintock C, Peacock M. Intravenous pamidronate treatment of children under 36 months of age with osteogenesis imperfecta. Bone. 2004;35:1038. doi: 10.1016/j.bone.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Rauch F, Plotkin H, Zeitlin L, Glorieux FH. Bone mass, size, and density in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate therapy. J Bone Miner Res. 2003;18:610–614. doi: 10.1359/jbmr.2003.18.4.610. [DOI] [PubMed] [Google Scholar]
- 11.Kok DH, Sakkers RJ, Janse AJ, Pruijs HE, Verbout AJ, Castelein RM, Engelbert RH (2007) Quality of life in children with osteogenesis imperfecta treated with oral bisphosphonates (Olpadronate): a 2-year randomized placebo-controlled trial. Eur J Pediatr 166:1155 [DOI] [PubMed]
- 12.Munns CF, Rauch F, Travers R, Glorieux FH. Effects of intravenous pamidronate treatment in infants with osteogenesis imperfecta: clinical, histomorphometric outcome. J Bone Miner Res. 2005;20:1235. doi: 10.1359/JBMR.050213. [DOI] [PubMed] [Google Scholar]
- 13.Montpetit K, Plotkin H, Rauch F, Bilodeau N, Cloutier S, Rabzel M, et al. Rapid increase in grip force after start of pamidronate therapy in children and adolescents with severe osteogenesis imperfecta. Pediatrics. 2003;111:601–603. doi: 10.1542/peds.111.5.e601. [DOI] [PubMed] [Google Scholar]
- 14.Glorieux FH. The use of bisphosphonates in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2001;14:1491–1495. [PubMed] [Google Scholar]
- 15.Arikoski P, Silverwood B, Tillmann V, Bishop NJ. Intravenous pamidronate treatment in children with moderate to severe osteogenesis imperfecta: assessment of indices of dual-energy X-ray absorptiometry and bone metabolic markers during the first year of therapy. Bone. 2004;34(3):539–546. doi: 10.1016/j.bone.2003.11.019. [DOI] [PubMed] [Google Scholar]


