Abstract
Inside the mammalian nose lies a labyrinth of bony plates covered in epithelium collectively known as turbinates. Respiratory turbinates lie anteriorly and aid in heat and water conservation, while more posterior olfactory turbinates function in olfaction. Previous observations on a few carnivorans revealed that aquatic species have relatively large, complex respiratory turbinates and greatly reduced olfactory turbinates compared with terrestrial species. Body heat is lost more quickly in water than air and increased respiratory surface area likely evolved to minimize heat loss. At the same time, olfactory surface area probably diminished due to a decreased reliance on olfaction when foraging under water. To explore how widespread these adaptations are, we documented scaling of respiratory and olfactory turbinate surface area with body size in a variety of terrestrial, freshwater, and marine carnivorans, including pinnipeds, mustelids, ursids, and procyonids. Surface areas were estimated from high-resolution CT scans of dry skulls, a novel approach that enabled a greater sampling of taxa than is practical with fresh heads. Total turbinate, respiratory, and olfactory surface areas correlate well with body size (r2 ≥ 0.7), and are relatively smaller in larger species. Relative to body mass or skull length, aquatic species have significantly less olfactory surface area than terrestrial species. Furthermore, the ratio of olfactory to respiratory surface area is associated with habitat. Using phylogenetic comparative methods, we found strong support for convergence on 1 : 3 proportions in aquatic taxa and near the inverse in terrestrial taxa, indicating that aquatic mustelids and pinnipeds independently acquired similar proportions of olfactory to respiratory turbinates. Constraints on turbinate surface area in the nasal chamber may result in a trade-off between respiratory and olfactory function in aquatic mammals.
Keywords: Carnivora, nasal turbinates, olfaction, pinniped, respiration
Introduction
The nasal chambers of mammals house three sets of plate-like bones, maxilloturbinates, nasoturbinates, and ethmoturbinates, each of which has a distinct function (Moore, 1981). The maxilloturbinates are located anteriorly within the nasal fossa, and are covered in non-sensory respiratory epithelium, with a small amount of squamous epithelium anteriorly (Negus, 1958; Moore, 1981; Smith et al. 2007a,b;). They function to warm and moisten inspired air, as well as to recover water and heat on exhalation. Above the maxilloturbinates lie the pipe-like nasoturbinates, which likely function in directing air to the more posterior ethmoturbinates (Craven et al. 2007) housed in a blind-ended recess outside the respiratory airflow pathway (Moore, 1981). Ethmoturbinates and nasoturbinates are covered in a combination of sensory and non-sensory epithelia, although by far the largest sensory area is found on the ethmoturbinates (e.g. Negus, 1958; Moore, 1981; Smith et al. 2007a,b; Smith and Rossie, 2008). Until recently, descriptions of the turbinates relied on the sectioning of heads or skulls, making it difficult to visualize them in three dimensions and limiting the number of species that could be studied due to the destructive methods. However, advances in imaging technology, both high-resolution computed tomography (HRCT) and magnetic resonance imaging (MRI), have made it possible to visualize the turbinates and associated cranial sinuses in silico and have opened the door for broader comparative studies of their structure and function (e.g. Joeckel et al. 2002, Van Valkenburgh et al. 2004; Rowe et al. 2005; Craven et al. 2007; Smith et al. 2007a).
As part of a larger, ongoing study of the association between turbinate morphology and ecology (e.g. body size, habitat, diet, activity levels) across the order Carnivora, we used HRCT scans of dry skulls to explore aquatic adaptations in the nose of 16 species of arctoid carnivorans, including the almost fully aquatic pinnipeds, semi-aquatic mustelids and polar bear (Ursus maritimus), as well as terrestrial mustelids, ursids and procyonids. The transition from land to water has occurred to varying degrees multiple times within the order. Pinnipeds and sea otters (Enhydra lutris) are the most aquatic of carnivorans, as they spend relatively little time on land, but must still be considered only semi-aquatic relative to cetaceans. Among other mustelids, river otters (Lontra canadensis) and minks (Neovison vison) evolved semi-aquatic habits, but not to the extent seen in pinnipeds, and probably are better described as semi-terrestrial. Similarly, the polar bear is the most aquatic of ursids but forages largely on land and so is even less aquatic than the otters.
The transition from a purely terrestrial to a more or less aquatic existence in all these lineages required specializations to reduce heat loss while swimming and, in marine species, to reduce respiratory evaporative water loss because the highly saline sea is a poor water source. To better conserve heat, aquatic species evolved larger body size, denser fur, counter-current heat exchangers, and insulating layers of fat (blubber) (Berta & Sumich, 1999). In addition, to conserve water they evolved complex, efficient kidneys and likely modified their maxilloturbinates to better retain both heat and water during lung ventilation. A dramatic expansion of the maxilloturbinates has been documented in a few studies of individual seals and sea lions based on the sectioning of fresh heads (Huntley et al. 1984; Folkow et al. 1988; Lester & Costa, 2006) but similar studies have not been done for other semi-aquatic arctoids, nor has the overall scaling of maxilloturbinate surface area with body size been quantified in any group of mammals. Without this, it is difficult to assess the functional and evolutionary significance of the spectacular maxilloturbinates of the few pinnipeds that have been studied.
Whereas maxilloturbinates are expected to be expanded in aquatic mammals, structures associated with olfactory ability, including the ethmoturbinates, are likely to be reduced to some degree, given that olfaction is of less use in detecting resources (food, mates) or predators under water than on land. Previous studies have shown that semi-aquatic mammals tend to have smaller olfactory bulbs (Gittleman, 1991), reduced cribriform plates (the perforated bony ethmoid plate through which the nasal sensory neurons reach the olfactory bulb; Pihlström et al. 2005), and diminished olfactory epithelial surface areas (Negus, 1958; Pihlström, 2008). This has been suggested to indicate reduced olfactory abilities because species known to have a well-developed sense of smell, such as dogs, have greater olfactory epithelial surface areas than those with a relatively poor sense of smell, such as humans and perhaps other Old World primates (Negus, 1958, Gilad et al. 2004). However, recent experiments have demonstrated that fur seals (Arctocephalus pusillus) are similar to some terrestrial mammals (humans, squirrel monkeys) in their ability to discriminate among a set of structurally similar odorants (Laska et al. 2010), and harbor seals (Phoca vitulina) are able to detect a chemical signature of marine productivity at very low concentrations (Kowalewsky et al. 2006). Here we use combined ethmoturbinate and nasoturbinate surface area as a proxy for olfactory epithelial surface area and compare this parameter across our sample of arctoids to document patterns of allometry and to test whether olfactory surface area is negatively correlated with degree of aquatic adaptation.
Materials and methods
Sixteen species, representing six families of arctoid carnivorans, were chosen for comparison, ranging in body mass from < 1 kg (long-tailed weasel, Mustela frenata) to > 500 kg (polar bear, Ursus maritimus) (Table 1). They were classified as terrestrial, semi-terrestrial, or aquatic based on their habits as reported in the literature. Species defined here as ‘aquatic’ are not fully aquatic in the sense that they never leave the water, as is true of cetaceans. However, they do not move about on land to a great extent, and thus are labeled as aquatic to distinguish them from the three much less aquatic species in our sample, mink (N. vison), river otter (L. canadensis) and polar bear (U. maritimus). These three semi-terrestrial species vary in degree of aquatic habits, with river otters being more aquatic than either polar bears or mink. Whenever possible, two adult skulls were scanned for each species, preferably male and female, of individuals caught in the wild. Skulls were chosen for scanning if the maxilloturbinates appeared complete and undamaged when viewed from the external nares. Specimen numbers and museum provenance are listed in the Supporting Information. All skulls were scanned twice at the University of Texas (UT) High Resolution X-Ray Computed Tomography Facility. The initial scan was a low-resolution scan of the entire skull where coronal slice thickness ranged from 0.1 to 1 mm depending on the size of the skull. A second higher resolution scan focused on the maxilloturbinate and ethmoturbinate regions, with slice thickness ranging from 0.01 to 0.015 mm. We recognize that a sample size of one or two individuals per species is not ideal, but the costs of scanning are high and data collection from the many scans is labor intensive. All of the scans are available on the UT Digital Morphology Group website (http://www.digimorph.org/).
Table 1.
Sampled species with associated body size, turbinate chamber volume and surface area measurements. Code refers to abbreviation used to identify points in Figs 5 and 6; habitat is A, aquatic, S-T, semi-terrestrial, and T, terrestrial. Olf SA and Resp SA are the estimated surface areas available for olfactory epithelia and respiratory epithelia, respectively (see text). Estimated body masses are from the literature; skull lengths are condylobasal and were taken from digital images of the skulls using ImageJ. This was not possible for the male Ursus americanus as the scale bar was not visible
| Code | Species | Sex | Habitat | Body mass (kg) | Skull length (mm) | Chamber volume (mm3) | Total surface area (mm2) | Resp SA (mm2) | Olf SA (mm2) | Olf SA/Resp SA |
|---|---|---|---|---|---|---|---|---|---|---|
| MME | Mephitis mephitis | M | T | 3.5 | 91.14 | 8615.20 | 9763.40 | 3076.00 | 6687.40 | 2.2 |
| F | 3.1 | 75.83 | 5645.00 | 5123.99 | 1547.00 | 3576.99 | 2.3 | |||
| ELU | Enhydra lutris | M | A | 34.5 | 158.823 | 37 867.00 | 36 496.35 | 30 314.11 | 6182.24 | 0.2 |
| F | 19.6 | 157.91 | 38 845.90 | 37 621.55 | 30 098.29 | 7523.26 | 0.3 | |||
| GGU | Gulo gulo | M | T | 22.4 | 173.36 | 39 380.85 | 43 863.39 | 8042.25 | 35 821.14 | 4.5 |
| F | 16.6 | 152.5 | 32 360.85 | 32 143.40 | 5868.21 | 26 275.19 | 4.8 | |||
| LCA | Lontra canadensis | F | S-T | 8.1 | 121.02 | 8818.42 | 10 723.40 | 6573.51 | 4149.89 | 0.6 |
| M | 6.7 | 121.93 | 9713.72 | 14 831.65 | 9334.03 | 5497.62 | 0.6 | |||
| MFR | Mustela frenata | F | T | 0.17 | 40.27 | 431.84 | 1027.22 | 179.68 | 847.54 | 4.7 |
| M | 0.31 | 49.78 | 798.24 | 2008.95 | 499.83 | 1509.12 | 3 | |||
| NVI | Neovison vison | M | S-T | 1.2 | 75.15 | 1954.93 | 3528.51 | 1171.53 | 2356.98 | 2 |
| TTA | Taxidea taxus | M | T | 8 | 121.79 | 15 723.59 | 15 723.59 | 4100.11 | 11 623.48 | 2.8 |
| F | 6 | 128.99 | 19 595.52 | 16 022.78 | 4545.21 | 11 477.57 | 2.5 | |||
| ZCA | Zalophus californianus | M | A | 275 | 376.03 | 164 064.00 | 79 931.00 | 62 403.80 | 17 527.70 | 0.3 |
| F | 91 | 250.49 | 62 140.20 | 36 641.83 | 27 318.07 | 9323.76 | 0.3 | |||
| HLE | Hydrurga leptonyx | F | A | 500 | 462.13 | 366 250.00 | 233 811.00 | 191 735.50 | 42 075.50 | 0.2 |
| M | 500 | 439.57 | 448 186.00 | 330 213.50 | 265 132.28 | 65 081.22 | 0.3 | |||
| MAN | Mirounga angustirostris | F | A | 450 | 305.85 | 164 213.00 | 124 057.00 | 114 867.80 | 9189.19 | 0.1 |
| MTR | Monachus tropicalis | F | A | 135 | 283.98 | 111 319.60 | 34 093.70 | 16 045.50 | 18 048.20 | 1.1 |
| M | 135 | 316.03 | 156 227.70 | 41 688.70 | 20 458.19 | 21 230.51 | 1 | |||
| PFL | Potos flavus | F | T | 2.5 | 87.45 | 7146.08 | 7560.55 | 1867.99 | 5692.56 | 3.1 |
| M | 2.5 | 88.71 | 7998.20 | 8280.92 | 1853.77 | 6427.15 | 3.5 | |||
| PLO | Procyon lotor | M | T | 6.3 | 120.95 | 16 686.67 | 14 250.11 | 4550.45 | 9699.66 | 2.1 |
| F | 5.7 | 122.62 | 17 768.79 | 17 477.02 | 6298.44 | 11 178.58 | 1.8 | |||
| UAM | Ursus americanus | F | T | 90 | 261.7 | 162 129.40 | 60 702.40 | 25 410.30 | 35 292.10 | 1.4 |
| M | 155 | n/a | 301 335.40 | 68 425.84 | 32 084.40 | 36 341.44 | 1.1 | |||
| UAR | Ursus arctos | M | T | 240 | 565.62 | 735 532.00 | 164 965.45 | 82 645.10 | 82 320.35 | 1 |
| F | 120 | 416.28 | 481 331.00 | 132 228.86 | 60 364.10 | 71 964.76 | 1.2 | |||
| UMA | Ursus maritimus | M | S-T | 550 | 414.15 | 614 321.00 | 200 805.64 | 73 410.10 | 127 395.54 | 1.7 |
| U | 550 | 518.895 | 807 961.56 | 238 015.40 | 101 833.34 | 136 182.06 | 1.3 |
The quantification of surface area of the turbinates from HRCT scan data first required that the turbinate bones be cleanly separated from the remainder of the image. This process of separating a region or structure of interest from the surrounding structures or air is referred to as segmentation. It is usually accomplished by adjusting threshold values for image brightness and contrast such that the relevant structure is easily seen as distinct from the background. In the case of the turbinates, this is a challenge because the turbinate bones are very thin compared with the nasal, maxilla, and frontal bones that surround them, and are often imaged as structures that are just a few pixels wide with image brightness values that are near the level of noise produced by the scan. Thus a threshold that reveals the turbinates also tends to pick up background ‘noise’ or ‘snow’ (Fig. 1).
Fig. 1.
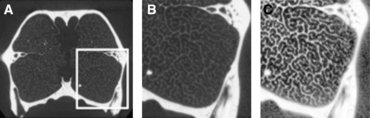
(A) Coronal HRCT slice of the rostrum of a river otter, Lontra canadensis, prior to processing with CLAHE. (B) Enlargement of boxed in area indicated in (A). (C) Same image after processing with CLAHE.
To solve this problem we experimented with contrast limited adaptive histogram equalization (CLAHE) (Jain, 1989), anisotropic diffusion (Perona & Malik, 1990), and a combination of CLAHE followed by anisotropic diffusion. CLAHE is a contrast stretching technique that adapts the distribution of intensity values in each piece of the image to match a desired distribution (e.g. a uniform distribution). Instead of uniformly increasing the contrast in the image, the technique improves contrast in areas that need it. If there is a strong bone response in one part of the image, the method does not increase the intensity values of the surrounding noise. Meanwhile, the contrast is increased elsewhere in the image where there are primarily low contrast turbinates and no strong bone response. In principle, following CLAHE with anisotropic diffusion (which boosts intensity values which are along the boundary between a bright and dark region) should further improve the ability to segment the turbinates. However, we found that while it does improve the connectedness of the structures, it also tends to uniformly increase the thickness. Since this would potentially cause a bias in the resulting surface area and volume measurements, we elected to use CLAHE alone. For all of our 1024 × 1024 pixel images, we used 16 tiles across by eight tiles down, set our desired distribution to be exponential with alpha = 1 and 256 bins, and limited the contrast stretching to 0.01. A processed image and the resulting segmentation are shown in Fig. 1.
After processing with CLAHE, the scans were imported into amira, a three-dimensional visualization software package (VSG, Visualization Sciences Group; http://www.vsg3d.com/) and the three sets of turbinates were segmented. The delineation of the maxilloturbinates and nasoturbinates was relatively straightforward, as the former can be seen to include all turbinate bones extending from a single origin on the maxilla, and nasoturbinates are those attached to the nasals (Figs 2 and 3). However, the delineation of the ethmoturbinates was complicated by the fact that they have multiple bony attachments, including the frontal, ethmoid, and sphenoid. In addition, there were small branches (‘ectoturbinals’sensuMoore, 1981) that extended into the paranasal sinuses in some species. All of these were included in our estimates of olfactory epithelial area, although they may not be covered with sensory tissue in all species.
Fig. 2.
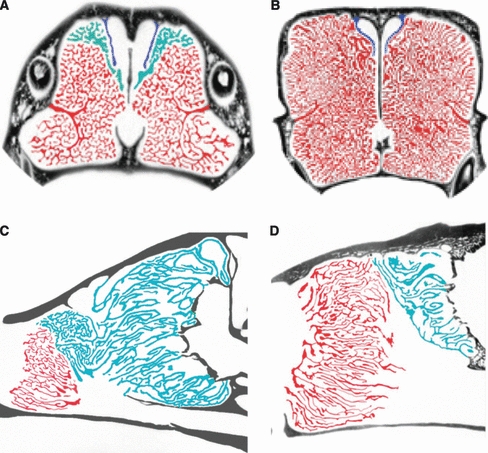
Coronal (top) and sagittal (bottom) HRCT slices of the nasal cavities of wolverine, Gulo gulo (A,C), sea otter, Enhydra lutris (B,D), Turbinates are highlighted with color as follows: ethmoturbinates (turquoise), maxilloturbinates (red), and nasoturbinates (blue). In the sea otter, only maxilloturbinates and nasoturbinates are present in the anterior of the nasal cavity. The slices are not to scale but are shown as approximately the same dorsoventral height.
Fig. 3.
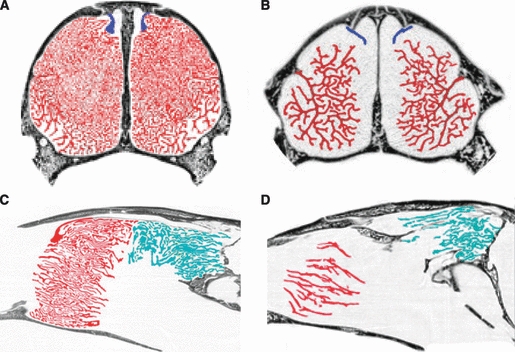
Coronal (top) and sagittal (bottom) HRCT slices of the nasal cavities of leopard seal, Hydrurga leptonyx (A,C), tropical monk seal (Monachus tropicalis) (B,D). Turbinates are highlighted with color as in Fig. 2. In both pinnipeds, only maxilloturbinates and nasoturbinates are present in the anterior of the nasal cavity.
After segmentation, the total surface area of all turbinates was calculated as well as the total volume of the chambers in which they were housed (nasal chamber, olfactory recess, and all portions of the paranasal sinuses that included ethmoturbinates). This combined volume is hereafter referred to as the ‘turbinate chamber’. For our purposes, we estimated olfactory area as the combined total of ethmoturbinate and nasoturbinate surface area. This overestimates sensory tissue area as it assumes both turbinates are fully covered by sensory epithelia, but this is mitigated somewhat because we excluded the septum, which also bears some sensory epithelium (Negus, 1958). Respiratory surface area was estimated as that of the maxilloturbinates alone. Because data on the distribution of sensory and respiratory epithelia on the turbinates of the skulls we scanned were not available, our results should be viewed as proxies for sensory and non-sensory epithelial surface areas. Because all skulls were treated similarly, our results are valid for comparisons of relative rather than absolute turbinate area. As mentioned in the Introduction, previous studies of sensory vs. respiratory epithelia distribution in carnivoran noses indicate that the majority of olfactory epithelium is distributed on the ethmoturbinates and that the maxilloturbinates are covered nearly entirely with respiratory epithelium, with a minor amount of squamous epithelium. Our assumptions concerning epithelial distribution have been confirmed by ongoing collaborative histological studies of a few carnivorans (sea otter, domestic cat, mink, raccoon) (N. Rawson, personal communication). The addition of data on the actual tissue distribution would likely reveal more subtle differences among species than are apparent based on the bony turbinates alone.
The relationships between surface areas and chamber volume, as well as with estimates of body size (skull length, body mass) were explored with least squares linear regression using spss Statistics 17.0 for the Mac (http://www.spss.com), and regression slopes and intercepts were compared among habitat groups with analysis of covariance using r 2.11.0 (R Development Core Team, 2010).
Comparative analysis
We constructed a time-calibrated phylogeny specifically for use in this project using Bayesian inference and a relaxed molecular clock implemented in beast v1.5.4 (Drummond & Rambaut, 2007). We obtained six nuclear gene segments spanning all taxa plus an outgroup (the gray wolf Canis lupus) from GenBank. We also identified nine fossils representing dates for the most recent common ancestors of crown clades from the literature and used them as calibration points in our dating analyses. The tropical monk seal, Monachus tropicalis, has been extinct since the mid-20th century (Adam, 2004) and no molecular data are available for this species. We therefore substituted sequences for the Hawaiian monk seal, Monachus schauinslandi. Full details of phylogenetic analyses are available in the Supporting Information and the resulting time-calibrated phylogeny is shown in Fig. 4.
Fig. 4.
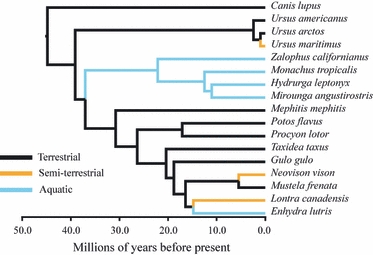
Time-calibrated phylogeny of taxa included in this study, derived from Bayesian analysis of six nuclear loci and nine fossil calibrations. Colors indicate habitat preferences. The gray wolf Canis lupus is an outgroup and was pruned from the tree for comparative analyses.
We used phylogenetic independent contrasts (PICs, Felsenstein, 1985) to reappraise scaling relationships among turbinate surface areas and body and skull size measures in light of phylogenetic covariance among taxa. Contrasts were computed from natural log-transformed (ln) species mean values using functions from the APE library (Paradis et al. 2004) for r 2.11.0 (R Development Core Team, 2010) and least-squares regressions performed without fitting intercepts (Felsenstein, 1985; Garland et al. 1992).
We tested whether the degree of aquatic adaptation influences the relative proportion of turbinate surface area given over to respiratory and olfactory function by comparing the fit of Brownian motion (BM) and Orstein-Uhlenbeck (OU) models of trait evolution to our comparative data. BM is a random-walk process where the only model parameters are the phylogenetic mean (i.e. the root state) and the rate of trait evolution σ2. Under BM, variance in trait values among a group of species is related to the age of the clade, the phylogenetic covariance among species, and the rate of trait evolution (e.g. O'Meara et al. 2006). OU differs from BM in being a central tendency process; traits evolve under BM but are ‘pulled back’ towards a central, optimal value if they stray too far from it (Hansen, 1997; Butler & King, 2004). The stronger the pull, denoted by the parameter α and equivalent to selection, the smaller the expected trait variance resulting from the OU process, regardless of the rate of evolution. Conversely, as α degenerates to zero, OU becomes BM (Butler & King, 2004). It is important to stress that although the central peak of an OU process is often referred to as an ‘optimum’, this is not a physiological optimum and not all taxa are expected to exhibit that value. Rather, a range of values are expected, with the ‘optimal’ value falling at the center of the distribution. If we suspect that there might be different OU optima for a continuous trait, such as turbinate surface area, depending on membership in some categorical state, such as habitat, we can additionally fit models where taxa in different habitats are allowed to evolve under regimes with different central values. We fit these models to data describing the proportional contribution of olfactory and respiratory turbinate surface area to total turbinate surface area using the OUCH package (Butler & King, 2004) in r. We initially compared the fit of three different models of turbinate evolution; Brownian motion (BM), a single optimum OU model (OU1), and a 3-optimum OU model where aquatic, terrestrial, and semi-terrestrial taxa took different optima (OU2). We subsequently added two additional models allowing the semi-terrestrial taxa to evolve under the aquatic (OU3) or terrestrial regimes (OU4). For analyses incorporating multiple trait optima, we treated all internal branches of our phylogenetic tree as terrestrial, except for those associated with Pinnipedia (Fig. 4). Model fits were assessed using small-sample corrected Akaike information criterion (AICc) values, with an AICc difference of four or more indicating strong support for one model over others (Burnham & Anderson, 2002).
Results
In addition to using estimates of body size, such as estimated mass or skull length, as the independent variable for comparisons of relative turbinate surface area, we also used turbinate chamber volume. In some ways, chamber volume has advantages over either mass or skull length in that it is more closely associated with the turbinates than the other variables. When comparing across a broad range of species that vary widely in size and ecology, such as weasels, kinkajous, and seals, differences in skull shape and amount of body fat might be expected to confound results. Turbinate chamber volume is likely to be directly related to turbinate surface area and shape, and thus is a relatively comparable measure across taxa. However, it cannot provide insights into the physiological scaling of surface areas with body size, and so both types of analyses are worthwhile.
For all regressions discussed below, results were similar between analyses of raw data and PICs. We present results from analysis of raw data to highlight outliers. PIC results are presented in full in the Supporting Information.
Surface area relative to body size
The combined surface area of all turbinates is well correlated with body mass (r2 = 0.93), with larger species having relatively less surface area for their size (Fig. 5A). The slope of the regression is 0.61 (Table 2) and does not differ significantly from 0.66, which is what would be expected if they followed geometric similarity. A similar relationship is found when turbinate surface area is regressed on skull length (Fig. 5B), a metric often used as a proxy for body mass. The correlation is equally strong (r2 = 0.94) and the slope of the line (b = 1.96) does not differ significantly from two, the value expected for geometric similarity. In both plots, semi-aquatic species are not well separated from terrestrial or semi-terrestrial species.
Fig. 5.
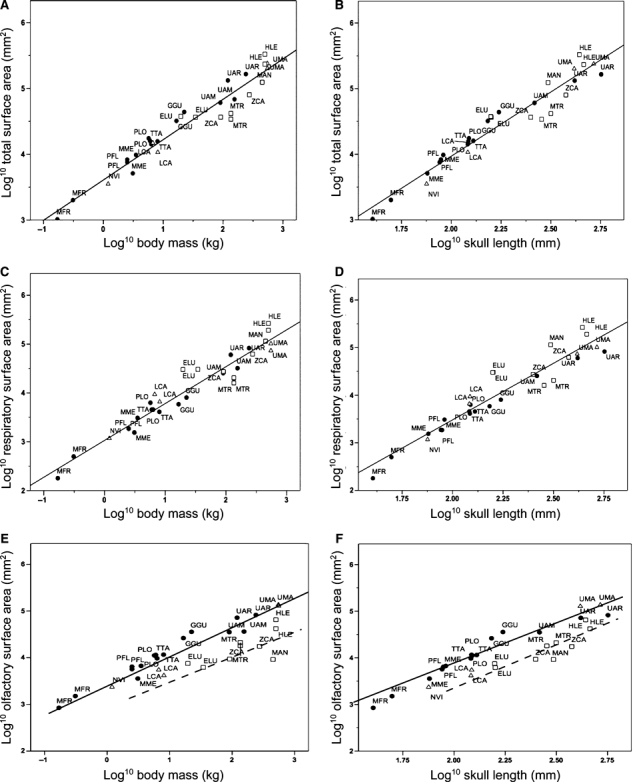
Log10/log10 plots of surface area parameters against estimates of body size. Open squares, aquatic species; open triangles, semi-terrestrial species; solid circles, terrestrial species. Least-squares regression lines are shown based on the total sample in (A–D), and separately for aquatic (dashed line) and terrestrial species (solid line) in (E, F). See Table 1 for species abbreviations and Table 2 for line equations and statistics.
Table 2.
Summary data for log10/log10 linear regressions of surface area (SA) parameters against body size estimates and chamber volume. ‘Group’ indicates whether the entire sample was used (all) or one of the habitat groups; A, aquatic, T, terrestrial. For species assignments, see Table 1. All regressions were significant at the 0.05 level. A * indicates that the y-intercepts for the aquatic and terrestrial species were significantly different at the 0.05 level
| Linear regression | n | Group | Slope (b) | y-intercept | r2 | 95% CI for slope |
|---|---|---|---|---|---|---|
| Against body mass | ||||||
| Total surface area | 30 | All | 0.61 | 3.61 | 0.93 | 0.55–0.68 |
| Respiratory SA | 30 | All | 0.75 | 3.03 | 0.93 | 0.67–0.83 |
| Olfactory SA | 30 | All | 0.47 | 3.48 | 0.72 | 0.36–0.58 |
| 9 | A | 0.49 | 3.13* | 0.45 | 0.08–0.91 | |
| 16 | T | 0.6 | 3.5* | 0.94 | 0.52–0.69 | |
| Against skull length | ||||||
| Total surface area | 28 | All | 1.96 | 0.06 | 0.94 | 1.75–2.16 |
| Respiratory SA | 28 | All | 2.38 | −1.27 | 0.90 | 2.07–2.68 |
| Olfactory SA | 28 | All | 1.56 | 0.61 | 0.80 | 1.25–1.87 |
| 9 | A | 1.77 | −0.14 | 0.72 | 0.87–2.68 | |
| 16 | T | 1.77 | 0.31 | 0.93 | 1.48–2.05 | |
| Against chamber volume | ||||||
| Total surface area | 30 | All | 0.71 | 1.213 | 0.95 | 0.65–0.77 |
| 9 | A | 0.86 | 0.48 | 0.75 | 0.41–1.31 | |
| 16 | T | 0.67 | 1.34 | 0.97 | 0.61–0.73 | |
| Respiratory SA | 9 | A | 0.86 | 0.33 | 0.56 | 0.18–1.54 |
| 16 | T | 0.79 | 0.3 | 0.99 | 0.74–0.84 | |
| Olfactory SA | 9 | A | 0.82 | 0.03 | 0.83 | 0.49–1.15 |
| 16 | T | 0.61 | 1.44 | 0.95 | 0.53–0.69 | |
Respiratory turbinate surface area (= maxilloturbinate surface area) scales slightly more positively than total turbinate surface area with body mass (b = 0.75, r2 = 0.93) or skull length (b = 2.38, r2 = 0.90) (Fig. 5C,D). In both plots, there is a tendency for aquatic species to fall above the line, with relatively greater respiratory surface areas. The differences in density of the maxilloturbinates in aquatic vs. terrestrial species can be quite striking, as shown in individual slices of HRCT scans of the wolverine, sea otter, and leopard seal (Figs 2 and 3). Notably, the tropical monk seal (MTR) falls well below the line, with perhaps the least respiratory surface area for its size in the entire sample (Figs 3 and 5C,D).
Olfactory surface area (= ethmoturbinates plus nasoturbinates) differs from total turbinate or respiratory turbinate surface areas in being less well correlated with estimates of body size (Fig. 5E,F; r2 ≤ 0.8). The scatter of points reflects habitat differences, with aquatic species exhibiting reduced olfactory surface areas as expected (Figs 2 and 3). For example, wolverines (GGU) and sea otters (ELU) are similar in body mass but the former has a much larger olfactory turbinate surface area. The regression lines fit to the terrestrial and aquatic species have similar slopes but distinct y-intercepts (P < 0.05), with the line for the aquatic species falling below that for the terrestrial species (Fig. 5E,F, Table 2). The three semi-terrestrial species vary in their placement; relative to body mass, both the mink (NVI) and the polar bear (UMA) are aligned with the terrestrial species, but the river otter (LCA) is closer to the aquatic species, with a considerably reduced olfactory turbinate surface area compared with the similar-sized, terrestrial raccoon (PLO). Relative to skull length, both otter and mink appear to have slightly reduced olfactory surface areas, whereas the polar bear has an enlarged olfactory surface area (Fig. 5F).
Surface area relative to chamber volume
Because pinnipeds do not have paranasal sinuses and we included paranasal sinuses in the estimate of chamber volume, this might produce greater calculated surface areas to chamber volume among pinnipeds than terrestrial species. However, the correlation between total turbinate surface area and chamber volume is high (r2 = 0.95) across the entire sample and there is no separation among the habitat groups (Fig. 6A). In plots of both respiratory and olfactory surface areas, respectively, against chamber volume, there is a clear separation between terrestrial and aquatic species that seems little affected by the presence or absence of paranasal sinuses. Despite having sinuses, terrestrial species have a larger olfactory surface area relative to chamber volume than do aquatic species (Fig. 6B). On the other hand, all aquatic species, except the tropical monk seal (MTR) have a larger respiratory surface area relative to chamber volume than do terrestrial species (Fig. 6C). Among the semi-terrestrial species, the river otter (LCA) is similar to the aquatic species in having a large respiratory surface area relative to chamber volume, but differs from them in having a relative olfactory surface area that is greater and more similar to that of terrestrial species. The mink (NVI) and polar bear (UMA) are similar to the terrestrial species in both relative olfactory and respiratory surface areas.
Fig. 6.
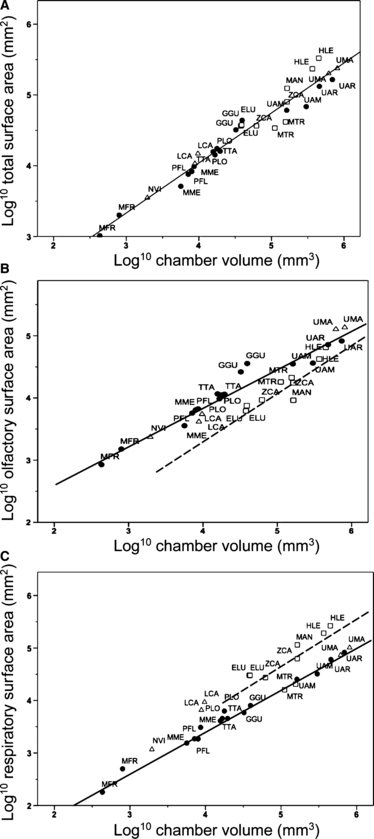
Log10/log10 plots of estimated surface areas against estimated nasal chamber volume. Symbols as in Fig. 4. Least-squares regression lines are indicated for all species in (A), terrestrial (solid line) and semi-aquatic species (dashed line) in (B,C). See Table 1 for species abbreviations and Table 2 for line equations and statistics.
Proportion of respiratory and olfactory turbinate areas
Examination of the proportion of total turbinate area devoted to either olfaction or respiration produces comparable results. Among terrestrial species, olfactory surface area always exceeds or equals that of respiratory surface area, whereas the inverse is true of aquatic species. For example, among terrestrial species the ratio of olfactory to respiratory surface areas averages 3 : 1, but ranges from a high of nearly 5 : 1 in the wolverine (G. gulo) and long-tailed weasel (M. frenata) to 1 : 1 in the ursids. By contrast, among the aquatic pinnipeds and mustelids, the same ratio averages 0.4 : 1, and ranges from a low of 1 : 9 in the elephant seal (M. angustirostris) to 1 : 1 in the tropical monk seal (M. tropicalis) (Table 1). Among the semi-terrestrial species, the river otter (L. canadensis) is similar to the aquatic species in showing a reduction in olfactory surface area (38% olfactory). By contrast, the polar bear (U. maritimus) and mink (N. vison) are similar to the terrestrial species in having about twice as much olfactory surface area as respiratory surface area.
Comparative analysis of turbinate proportions
Habitat-based OU models (OU2-4) showed substantially better (ΔAICc > 8) fit to turbinate surface area proportions than either single optimum OU (OU1) or BM models (Table 3). The third model (OU3) with semi-terrestrial taxa treated as aquatic performed best, followed by the model with semi-terrestrial taxa treated as terrestrial (OU4) and finally the model with separate optima for each group (OU2). ΔAICc between the best and worst habitat models was < 4, indicating that we are unable to choose among them. This is confirmed by comparison of Akaike weights, which are evenly spread among the three habitat models (Table 3).
Table 3.
Scores and weights for BM and OU models fit to turbinate proportion data. ΔAICc > 4 indicates strong support for the best model over the candidate model. See text for model descriptions
| Model | AICc | ΔAICc | Weights |
|---|---|---|---|
| OU3 | −10.679 | 0 | 0.599 |
| OU4 | −8.587 | 2.092 | 0.21 |
| OU2 | −8.349 | 2.33 | 0.187 |
| BM | −0.491 | 10.189 | 0.004 |
| OU1 | 2.609 | 13.288 | 0.001 |
Comparison of parameter estimates from the OU models confirms qualitative observations from the raw data. Optimal olfactory/respiratory proportions are approximately 2.3/1 for terrestrial taxa and 1/3 for aquatic taxa. Optimal proportions for semi-terrestrial taxa are, as expected, intermediate (Table 4). We recovered evidence of moderately strong selection (i.e. a high α) combined with more rapid rates of evolution (σ2) for these optima than would be predicted under a Brownian motion model of evolution.
Table 4.
Model parameters for BM and OU model fitting. Optima are given as proportional contribution to total turbinate surface area of olfactory\respiratory surfaces. – indicates that no value is produced under a given model. α indicates the strength of selection, or rubber-band parameter in OU models, σ2 indicates the rate of trait evolution (see Methods for more details)
| Optima | ||||||
|---|---|---|---|---|---|---|
| Model | All | Aquatic | Semi-terrestrial | Terrestrial | α | σ2 |
| OU3 | – | 0.24\0.76 | – | 0.71\0.29 | 4.02 | 0.13 |
| OU4 | – | 0.24\0.76 | – | 0.66\0.34 | 7.31 | 0.26 |
| OU2 | – | 0.23\0.77 | 0.45/0.55 | 0.71\0.29 | 6.16 | 0.17 |
| BM | – | – | – | – | – | 0.08 |
| OU1 | 0.52/0.48 | – | – | – | 1.36 | 0.16 |
Discussion
When this study began, it was not certain that meaningful estimates of turbinate bone surface area could be derived from HRCT scans of skulls due to the paper-thin quality of the bones and the difficulties of image segmentation. Although Rowe et al. (2005) were able to do so for a single skull of a gray short-tailed possum, Monodelphis domestica, the procedure was time-intensive and would have been difficult to apply to our sample of 30 skulls. However, processing of images using our CLAHE-based software allowed us to use amira to more rapidly delineate and measure turbinate bone surface areas. Our sample size of a maximum of two individuals per species is not ideal, but in all but two species, the individuals within a species exhibited very similar proportions of olfactory and respiratory surface areas (usually within 3% of each other), despite differing in absolute values that probably reflected differences in body mass (Table 1). This same pattern was found in Adams’ (1972) study of intact heads of 14 individual deer mice, in which the total estimated areas of respiratory and olfactory epithelium varied according to body size, but the ratio of these areas was relatively constant. In our study, the long-tailed weasel (M. frenata) and the California sea lion (Zalophus californianus) were unusual in showing a larger difference between the sexes (> 5%) in the percentage of olfactory vs. respiratory turbinate surface areas. The scans for one of the individuals in both of these species was fairly ‘noisy’ and it is possible that this may have produced ambiguous results. In addition, the marked sexual dimorphism in size in the sea lion may contribute to the differences we observed. Additional individuals need to be sampled to either confirm or refute this.
Total turbinate and respiratory surface area, respectively, tend to be well correlated with both body mass and skull length, with larger species having relatively less total surface area for their body mass than smaller species, as expected under geometric similarity (0.66) (Table 2). The same is true for respiratory surface area, although the slope is slightly greater (0.75). This decrease in turbinate surface relative to body mass probably reflects reduced needs for heat conservation due to a greater body volume to surface area in larger species, and reduced needs for water conservation due to slower ventilation rates in large than in small species. Olfactory surface area is less well-correlated with either body mass or skull length (r2 ≤ 0.8), presumably due to functional differences among species in relative olfactory ability that are independent of body size (see below). It scales significantly more negatively with body mass (slope = 0.47) than does respiratory surface area. In part, this is driven by the fact that many of the largest species in the sample are pinnipeds, but it remains the case when only terrestrial species are analyzed (slope = 0.57). This strong negative allometry of olfactory organ size (as estimated here as olfactory surface area) is paralleled by patterns of negative allometry in other sense organs such as the eyes and ears (Jerison, 1973; Nummela, 1995; Kay & Kirk, 2000). For its size, the wolverine (G. gulo) has an unusually large olfactory turbinate surface area relative to both body mass and skull length. Avid scavengers, wolverines depend on carrion to survive winter and their enlarged olfactory surface area might reflect their ability to detect carrion over distances of several miles (Hornocker, 1982).
On average, olfactory surface area is approximately double that of respiratory surface area in the terrestrial and semi-terrestrial species in our sample. This same relationship was found in Rowe et al.'s (2005) detailed study of the short-tailed possum based on histological analyses of multiple specimens and CT scans of a skull, as well as an MRI study of the intact head of a dog (Craven et al. 2007). However, earlier estimates based on measurements of sectioned heads (e.g. Adams, 1972; Gross et al. 1982) did not find a consistent ratio of olfactory to respiratory surface areas in terrestrial mammals, so it is not yet possible to infer what the primitive mammalian condition might be. The 2 : 1 ratio we observed contrasts markedly with the average 2.5 : 7.5 (1 : 3) ratio of olfactory to respiratory surface areas in the aquatic species. Among the three semi-terrestrial species, only the river otter is comparable to the aquatic species in displaying less olfactory than respiratory surface area. The fact that river otters have diminished olfactory turbinates is somewhat surprising as they spend considerable time on land and scent mark regularly (Kruuk, 2006). Given that both the polar bear and mink are considerably less aquatic than the river otter, it is not surprising that they do not show the same degree of reduction of olfactory turbinates. Polar bears diverged relatively recently (approximately 500 000–800 000 years ago) from brown bears but have undergone rapid and dramatic evolution in their feeding morphology (skull shape, teeth) in response to a specialized diet of seal blubber (Slater et al. 2010). However, this has not been the case for their turbinates as they mostly forage for seals on land using a well-developed sense of smell that allows them to detect prey over distances of 2–3 km as well as below the ice (Kolenosky, 1987).
Our comparative phylogenetic analysis confirms that the degree of aquatic adaptation has influenced the relative proportion of turbinate surface area devoted to respiratory and olfactory function. Based on the OU models, aquatic species tend to exhibit areas that are approximately 25% olfactory and 75% respiratory, whereas it is nearly the opposite for terrestrial species (70% and 30%, respectively). Although aquatic adaptation is most pronounced in the monophyletic pinnipeds, independent evolution of almost complete aquatic habits in the sea otter reveals parallel patterns. Because total turbinate surface area is correlated with skull length and exhibits little variation that can be ascribed to habitat (Fig. 5B), this suggests that there may be upper limits on total turbinate surface area for a given skull length. Consequently, selection for either increased respiratory or olfactory turbinates will necessarily result in a diminution of the other. Perhaps it is not possible to have both greatly expanded olfactory and respiratory turbinates due to the multiple functional demands placed on the cranium, such as feeding and vision as well as conditioning incoming air and olfaction. Consistent with this, Repenning (1976) observed that a reduction in olfactory turbinates was associated with greatly enlarged orbits in extant and extinct pinnipeds, including the oldest known genus, Enaliarctos (ca. 22 million years old, Berta et al. 1989). He argued that selection for enhanced light-gathering abilities and vision under water necessitated a reduction in olfactory bulb and ethmoturbinate size. Unfortunately, the maxilloturbinates of Enaliarctos remain unknown, so we do not know whether they were enhanced as in extant pinnipeds. Notably, both sea and river otters have diminished olfactory turbinates without having enlarged orbits, suggesting that factors other than vision have selected for olfactory turbinate reduction in at least these two species.
Our study confirms previous work demonstrating a reduction in olfactory organ size (defined in various ways) and presumed olfactory ability in semi-terrestrial and aquatic mammals, including some shrews (Sorex palustris; Larochelle & Baron, 1989), fissipeds (Ferron, 1973; Gittleman, 1991), pinnipeds, monotremes, and insectivores (Pihlström et al. 2005; Pihlström, 2008). Some of these studies used osteological proxies for olfactory ability, including cribriform (ethmoid) plate area and olfactory bulb endocast size, and others estimated olfactory epithelial surface area from serial sections of whole heads. Nevertheless, all of these studies and ours came to similar conclusions. Moreover, a reduction in olfactory function in aquatic and semi-aquatic species was further implicated by a recent analysis of olfactory receptor (OR) gene repertoires in 50 mammals that revealed a repeated, convergent loss of functional OR gene families in these species relative to terrestrial species (Hayden et al. 2010).
Olfactory function has at least three components – the ability to detect a wide array of odorants (acuity), the ability to detect them at low concentrations (sensitivity), and the ability to distinguish similar odorants (discrimination). A reduction in olfactory organ size (turbinate or epithelial surface area) does not seem to be strongly correlated with discrimination ability (Laska et al. 2005), and it may not be correlated with olfactory sensitivity. For example, harbor seals (P. vitulina) have an extraordinary ability to detect atmospheric dimethyl sulfide (DMS) at concentrations several orders of magnitude below that detectable by humans (Kowalewsky et al. 2006). DMS is produced by phytoplankton and, as such, is an excellent indicator of high marine productivity that likely serves as a significant foraging cue for seals. Seals probably also use their olfactory system for mother–pup recognition and other social interactions but this has yet to be verified (Insley et al. 2003; Laska et al. 2010). The diminution in the size of OR gene repertoires in aquatic and semi-aquatic taxa suggests that the reduction in organ size might reflect a decrease in the diversity of odorants that can be perceived at either high or low concentrations, but it should not be interpreted as indicating that a sense of smell is not critical in these species.
It is possible that constraints on the availability of turbinate surface area in the nasal chamber result in a trade-off between respiratory and olfactory function in aquatic mammals. Only essential olfactory demands are accommodated in aquatic species due to the increased need for expanded respiratory turbinates to enhance heat and water conservation. In addition, because olfaction is of limited use in detecting food or predators under water, selection pressure on olfactory performance will be reduced. This and the fact that the brain and peripheral sensory systems are among the most energetically costly structures to operate and maintain (Niven & Laughlin, 2008) could rapidly drive the loss of olfactory turbinates. The possibility of a loss due to energetic costs helps explain the diminution of the olfactory turbinates very early in the history of pinnipeds (Repenning 1976), as well as their reduction in semi-terrestrial species, such as the river otter, that still spend considerable time on land.
Respiratory turbinates facilitate both heat and water conservation and it is not clear which of these functions is more important in determining turbinate size and complexity. Heat loss is a greater problem in water than air, and respiratory turbinate surface area relative to skull length or body mass tends to be greater in the aquatic species (pinnipeds, sea otter) and the semi-terrestrial river otter than in the other species. In addition, because marine species have little or no access to fresh water, they need to reduce respiratory evaporative water loss due to ventilation more than either freshwater or terrestrial species. This further favors enlargement of the respiratory turbinates. Some pinnipeds, such as the elephant seal, undergo lengthy fasts (up to 12 weeks) during the breeding season, during which they acquire no water. During these fasts, they minimize respiratory evaporative water loss by using periods of apnea as well as through the action of their extensive respiratory turbinates. With 90% of their turbinate surface area devoted to respiration, elephant seals retain as much as 92% of the water added to each breath (Lester & Costa, 2006). This suggests that water conservation may be equally or more important than heat conservation in driving turbinate evolution in the elephant seal and other pinnipeds, with the interesting exception of the extinct tropical monk seal. This species inhabited warm Caribbean waters, and had respiratory turbinates that were similar in size to those of terrestrial rather than aquatic species, suggesting little specialization for either heat or water conservation. Finally, in the river otter, access to fresh water is not an issue, and so its expanded respiratory turbinates evolved in response to heat conservation needs alone. It would be interesting to examine the relative size of the respiratory turbinates in a more tropical otter, such as the giant otter of South America, Pteronura brasiliensis, to see if it shows a reduction in respiratory turbinates similar to that of the extinct tropical monk seal.
We have assumed that the greater complexity and surface areas of the respiratory turbinates of aquatic species results in a more efficient transfer of water and heat, and there is some experimental evidence to suggest that is the case for the elephant seal (Lester & Costa, 2006). Similarly, we have assumed that the more extensive olfactory surface area of terrestrial species translates into improved detection of odorants. Clearly, we need to test these ideas through studies of airflow dynamics and odorant transport in the nasal cavities of species that vary greatly in turbinate size and complexity. Although it is difficult to acquire data on rates of transport, airflow pathways, and airflow resistance from live animals, recent advances in computational fluid dynamics and imaging (HRCT, MRI) will allow us to model airflow through the turbinates in a variety of species using both preserved heads and dry skulls (e.g. Craven et al. 2007; Craven et al. 2009a,b;). These tools will open new windows into the form and function of turbinates and provide us with a greater understanding of their adaptive and evolutionary significance.
Conclusions
Our study of 16 species of carnivorans has established that an aquatic lifestyle selected for enhanced respiratory and reduced olfactory turbinates independently within pinnipeds and mustelids. It also demonstrated isometric and allometric patterns of scaling of turbinate surface area with body size and turbinate chamber volume, as well as possible constraints on turbinate size that we plan to explore across the entire order Carnivora to establish baselines of expected turbinate dimensions. It is clear that dramatic shifts in habitat, such as from land to sea, result in strong selection on olfactory and respiratory function, and it will be interesting to see whether more subtle changes in habitat or behavior, such as dwelling in arid or mesic environments, or being ambulatory as opposed to cursorial, or having a frugivorous rather than carnivorous diet are also revealed in turbinate dimensions.
Acknowledgments
We thank M.C., R.K., and J.M. of the University of Texas HRCT Digital Morphology group for their dedication and skills in producing the CT scans, and the multiple curators and collection managers that allowed us to borrow skulls for scanning. We also thank N. R. and F. L. for sharing their data on histological analyses of turbinate epithelia distribution in carnivorans. Two anonymous reviewers and A.R.F. provided helpful comments on the manuscript, and A.M. contributed greatly to Figs 2 and 3. Funding was provided by NSF IOB-0517748 to B.V.V.
Author contributions
B.V.V. contributed to study concept and design, data acquisition, analysis, and interpretation, and was primary author of the manuscript. A.C., D.B., J.S., and J.M. were involved in data acquisition, analysis, and interpretation. B.F. wrote the image processing software and G.J.S. performed and interpreted the comparative phylogenetic analyses. All authors participated in the writing of the paper.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. Estimation of respiratory and olfactory turbinate dimensions in aquatic and terrestrial carnivorans.
References
- Adam PJ. Monachus tropicalis. Mammalian Species. 2004;747:1–9. [Google Scholar]
- Adams DR. Olfactory and non-olfactory epithelia in the nasal cavity of the mouse, Peromyscus. Am J Anat. 1972;133:37–49. doi: 10.1002/aja.1001330104. [DOI] [PubMed] [Google Scholar]
- Berta A, Sumich JL. Marine Mammals: Evolutionary Biology. San Diego: Academic Press; 1999. [Google Scholar]
- Berta A, Ray C, Wyss AR. Skeleton of the oldest known pinniped, Enaliarctos mealsi. Science. 1989;244:60–62. doi: 10.1126/science.244.4900.60. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd edn. New York: Springer; 2002. [Google Scholar]
- Butler MA, King AA. Phylogenetic comparative analysis: a modeling approach for adaptive evolution. Am Nat. 2004;164:683–695. doi: 10.1086/426002. [DOI] [PubMed] [Google Scholar]
- Craven B, Neuberger T, Paterson EG, et al. Reconstruction and morphometric analysis of the nasal airway of the dog (Canis familiaris) and implications regarding olfactory airflow. Anat Rec. 2007;290:1325–1340. doi: 10.1002/ar.20592. [DOI] [PubMed] [Google Scholar]
- Craven BA, Paterson EG, Settles GS. Development and verification of a high-fidelity computational fluid dynamics model of canine nasal airflow. J Biomech Eng. 2009a;131:1–11. doi: 10.1115/1.3148202. [DOI] [PubMed] [Google Scholar]
- Craven BA, Paterson EG, Settles GS. The fluid dynamics of canine olfaction, unique nasal airflow patterns as an explanation of macrosmia. J R Soc Interface. 2009b;7:933–943. doi: 10.1098/rsif.2009.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- Ferron J. Morphologie comparée de l'organe de l'odorat chez quelques mammifères carnivores. Nat. Can. 1973;100:525–541. [Google Scholar]
- Folkow LP, Blix AS, Eide TS. Anatomical and functional aspects of the nasal mucosal and ophthalmic retia of phocid seals. J Zool. 1988;216:417–436. [Google Scholar]
- Garland T, Jr, Harvey PH, Ives AR. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol. 1992;41:18–32. [Google Scholar]
- Gilad Y, Weibel V, Prezworski M, et al. Loss of olfactory receptor genes coincides with the acquistion of full trichromatic vision in primates. PLoS Biology. 2004;2:120–125. doi: 10.1371/journal.pbio.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittleman JL. Carnivore olfactory bulb size, allometry, phylogeny, and ecology. J Zool. 1991;225:253–272. [Google Scholar]
- Gross EA, Swenberg JA, Fields A, et al. Comparative morphometry of the nasal cavity in rats and mice. J Anat. 1982;135:83–88. [PMC free article] [PubMed] [Google Scholar]
- Hansen TH. Stabilizing selection and the comparative analysis of adaptation. Evolution. 1997;51:1341–1351. doi: 10.1111/j.1558-5646.1997.tb01457.x. [DOI] [PubMed] [Google Scholar]
- Hayden S, Bekaert M, Crider TA, et al. Ecological adaptation determines functional mammalian olfactory subgenomes. Genome Res. 2010;20:1–9. doi: 10.1101/gr.099416.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornocker M. Ecology of the wolverine in northwestern Montana. NGS Research Reports. 1982;14:341–350. [Google Scholar]
- Huntley AC, Costa DP, Rubin RD. The contribution of nasal counter-current heat exchange to water balance in the Northern elephant seal, Mirounga angustirostris. J Exp Biol. 1984;113:447–454. doi: 10.1242/jeb.113.1.447. [DOI] [PubMed] [Google Scholar]
- Insley SJ, Phillips AV, Charrier I. A review of social recognition in pinnipeds. Aquat Biol. 2003;29:189–201. [Google Scholar]
- Jain AK. Fundamentals of Digital Image Processing. Upper Saddle River, NJ: Prentice-Hall, Inc; 1989. [Google Scholar]
- Jerison HJ. Evolution of the Brain and Intelligence. New York: Academic Press; 1973. [Google Scholar]
- Joeckel RM, Peigne S, Hunt RM, et al. The auditory region and nasal cavity of Oligocene Nimravidae (Mammalia: Carnivora) J Vert Paleo. 2002;22:830–847. [Google Scholar]
- Kay RF, Kirk EC. Osteological evidence for the evolution of activity pattern and visual acuity in primates. Am J Phys Anthropol. 2000;113:235–262. doi: 10.1002/1096-8644(200010)113:2<235::AID-AJPA7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Kolenosky GB. Polar bear. In: Novak M, Baker JA, Obbard ME, Malloch B, editors. Wild Furbearer Management and Conservation in North America. Ontario: Ministry of Natural Resources; 1987. pp. 474–485. [Google Scholar]
- Kowalewsky S, Dambach M, Mauck B, et al. High olfactory sensitivity for dimethyl sulphide in harbour seals. Biol Lett. 2006;2:106–109. doi: 10.1098/rsbl.2005.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk H. Otters: Ecology, Behaviour, and Conservation. New York: Oxford University Press; 2006. [Google Scholar]
- Larochelle R, Baron G. Comparative morphology and morphometry of the nasal fossae of four species of North American shrews (Soricinae) Am J Anat. 1989;186:306–314. doi: 10.1002/aja.1001860307. [DOI] [PubMed] [Google Scholar]
- Laska M, Genzel D, Wieser A. The number of functional olfactory receptor genes and the relative size of olfactory brain structures are poor predictors of olfactory discrimination performance with enantiomers. Chem Senses. 2005;30:171–175. doi: 10.1093/chemse/bji013. [DOI] [PubMed] [Google Scholar]
- Laska L, Lord E, Selin S, et al. Olfactory discrimination of aliphatic odorants in South African fur seals (Arctocephalus pusillus) J Comp Psychol. 2010;124:187–193. doi: 10.1037/a0018189. [DOI] [PubMed] [Google Scholar]
- Lester CW, Costa DP. Water conservation and fasting in northern elephant seals (Mirounga angustirostris) J Exp Biol. 2006;209:4283–4294. doi: 10.1242/jeb.02503. [DOI] [PubMed] [Google Scholar]
- Moore WJ. The Mammalian Skull. Cambridge: Cambridge University Press; 1981. [Google Scholar]
- Negus V. The Comparative Anatomy and Physiology of the Nose and Paranasal Sinuses. Edinburgh: E. & S. Livingstone; 1958. [Google Scholar]
- Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- Nummela S. Scaling of the mammalian middle ear. Hear Res. 1995;85:18–30. doi: 10.1016/0378-5955(95)00030-8. [DOI] [PubMed] [Google Scholar]
- O'Meara BC, Ané C, Sanderson MJ, et al. Testing for different rates of continuous trait evolution using likelihood. Evolution. 2006;60:922–933. [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Perona P, Malik J. Scale-space and edge detection using anisotropic diffusion. IEEE Trans Pattern Anal Mach Intell. 1990;12:629–639. [Google Scholar]
- Pihlström H. Comparative anatomy and physiology of chemical senses in aquatic mammals. In: Thewissen JGM, Nummela S, editors. Sensory Evolution on the Threshold. Berkeley: University of California Press; 2008. pp. 95–109. [Google Scholar]
- Pihlström H, Fortelius M, Hemilä S, et al. Scaling of mammalian ethmoid bones can predict olfactory organ size and performance. Proc R Soc Lond B Biol Sci. 2005;272:957–962. doi: 10.1098/rspb.2004.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2010. ISBN3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- Repenning CA. Adaptive evolution of sea lions and walruses. Amer Zool. 1976;25:375–390. [Google Scholar]
- Rowe T, Eiting TP, Macrini TE, et al. Organization of the olfactory and respiratory skeleton in the nose of the gray short-tailed opossum Monodelphis domestica. J Mamm Evol. 2005;12:303–336. [Google Scholar]
- Slater GJ, Figueirido B, Louis L, et al. Biomechanical consequences of rapid evolution in the polar bear lineage. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0013870. doi: 10.1371/journal.pone.0013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Rossie JB. Nasal fossa of mouse and dwarf lemurs (Primates, Cheirogaleidae) Anat Rec. 2008;291:895–915. doi: 10.1002/ar.20724. [DOI] [PubMed] [Google Scholar]
- Smith T, Bhatnagar K, Rossie J, et al. Scaling of the first ethmoturbinal in nocturnal strepsirrhines, olfactory and respiratory surfaces. Anat Rec. 2007a;290:215–237. doi: 10.1002/ar.20428. [DOI] [PubMed] [Google Scholar]
- Smith T, Rossie JB, Bhatnagar K. Evolution of the nose and nasal skeleton in primates. Evol Anthropol. 2007b;16:132–146. [Google Scholar]
- Van Valkenburgh B, Theodor J, Friscia A, et al. Respiratory turbinates of canids and felids, a quantitative comparison. J Zool. 2004;264:281–293. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


