Abstract
The source of pain and the background to the pain mechanisms associated with mid-portion Achilles tendinopathy have not yet been clarified. Intratendinous degenerative changes are most often addressed when present. However, it is questionable if degeneration of the tendon itself is the main cause of pain. Pain is often most prominent on the medial side, 2–7 cm from the insertion onto the calcaneus. The medial location of the pain has been explained to be caused by enhanced stress on the calcaneal tendon due to hyperpronation. However, on this medial side the plantaris tendon is also located. It has been postulated that the plantaris tendon might play a role in these medially located symptoms. To our knowledge, the exact anatomy and relationship between the plantaris- and calcaneal tendon at the level of complaints have not been anatomically assessed. This was the purpose of our study. One-hundred and seven lower extremities were dissected. After opening the superficial fascia and paratendon, the plantaris tendon was bluntly released from the calcaneal tendon moving distally. The incidence of the plantaris tendon, its course, site of insertion and possible connections were documented. When with manual force the plantaris tendon could not be released, it was defined as a ‘connection’ with the calcaneal tendon. In all specimens a plantaris tendon was identified. Nine different sites of insertion were found, mostly medial and fan-shaped onto the calcaneus. In 11 specimens (10%) firm connections were found at the level of the calcaneal tendon mid-portion. Clinical and histological studies are needed to confirm the role of the plantaris tendon in mid-portion Achilles tendinopathy.
Keywords: calcaneal tendon, connection, insertion, mid-portion Achilles tendinopathy, plantaris tendon
Introduction
Mid-portion Achilles tendinopathy is an entity that is generally difficult to treat. Peritendinous and intratendinous changes seem to co-exist in the majority of patients (Tan & Chan, 2008). The source of pain and the background to the pain mechanisms associated with mid-portion Achilles tendinopathy have not yet been clarified (Maffulli et al. 2004). Therefore, a wide range of conservative and surgical treatments is available, addressing different possible contributing properties of the tendon and its surrounding tissues. Why surgery promotes healing of the Achilles tendon is still not understood (Sandmeier & Renstrom, 1997). Intratendinous degenerative changes are most often addressed when present. However, it is questionable if degeneration of the tendon itself is the main cause of pain, as intratendinous changes are found in up to 34% of people ‘without’ complaints (Kannus & Jozsa, 1991; Haims et al. 2000; Khan et al. 2003; Emerson et al. 2009). Recently, a long-term follow-up study was published revealing persistent structural abnormalities and thickening of the tendon 13 years after intra-tendon surgery for mid-portion Achilles tendinopathy, whereas all patients were satisfied with the results and went back to calcaneal tendon-loading activities without restrictions (Alfredson et al. 2008).
Pain is the main symptom that leads a patient to seek medical help. It is often most prominent at 2–7 cm from the insertion onto the calcaneus on the medial side (Segesser et al. 1995). Most ultrasonographic mid-portion disorders (91–100%) are found in this medial segment of the tendon (Gibbon et al. 1999; de Vos et al. 2009). It has been proposed that the medial pain is caused by enhanced stress on the calcaneal tendon due to hyperpronation. However, on this medial side the plantaris tendon is also located. It is enclosed in a paratendon collectively with the calcaneal tendon. Steenstra and van Dijk described that during Achilles tendoscopy for patients with symptomatic Achilles tendinopathy, the plantaris tendon was fixed to the Achilles tendon at the level of complaints. Where in a normal situation the plantaris tendon can glide in relation to the Achilles tendon, it was postulated that the plantaris tendon plays a role in these medially located symptoms (Steenstra & van Dijk, 2006).
To our knowledge, the exact anatomy and relationship between the anatomical structures at the level of mid-portion calcaneal tendon complaints have not been assessed. This was the purpose of our study.
Materials and methods
Specimens
One-hundred and seven lower extremities were obtained from donors. During their lives the donors signed informed consent for the use of their bodies for scientific or educational purposes. Twenty-six were fresh-frozen (−20 °C) and thawed at room temperature for dissection. Eighty-one had been fixated in formalin. Sixty-two were male legs, 37 female, and of eight this information was not available. History was unknown. The mean age was 84 years (SD ± 9.3 years). Fifty-three left (49.5%) and 54 (50.5%) right lower legs were dissected. Of 22 specimens both legs were available (n = 44), 63 were unilateral.
Anatomical dissection
Dissection was done by two observers. A longitudinal incision though the skin and subcutaneous tissue was made from the condylus medialis femoris to the medial distal third of the calcaneus, after which it was lengthened perpendicularly to the lateral side of the calcaneus. After opening the superficial fascia and paratendon, the mid-portion of the calcaneal tendon was inspected. Distally the plantaris tendon was not always visible; when searched for proximally after bluntly releasing the fascia between the medial belly of the gastrocnemius muscle and the soleus muscle, it could always be identified. After this it was released by hand from the calcaneal tendon moving distally (Fig. 1).
Fig. 1.
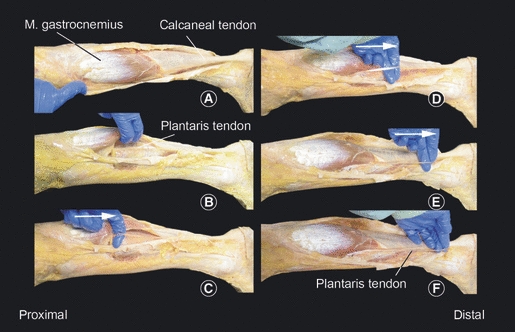
Technique of identification of plantaris tendon. (A) The skin, subcutaneous layers, superficial fascia and paratendon are dissected. (B) The plantaris tendon is identified proximally between the medial head of the gastrocnemius muscle and the soleus muscle. (C–F) With the index finger the tendon is released to its distal insertion. Then the insertion is carefully exposed.
When with maximum manual pulling force the plantaris tendon could not be released from the calcaneal tendon, it was defined as an attachment to the calcaneal tendon. Location of the attachment in relation to the insertion of the calcaneal tendon was measured with a millimetre-precise ruler.
The calcaneal tendon, incidence of the plantaris tendon, their relationships and the site of the mid-portion calcaneal tendon and the insertion of the plantaris tendon onto the calcaneus were documented.
Results
One-hundred and seven lower legs were dissected. In 96 specimens the calcaneal tendon and plantaris tendon run separately and can glide in relation with each other. In the other 11 specimens we found a firm attachment of the plantaris tendon to the calcaneal tendon; in six of 26 fresh-frozen specimens (23%), and in five of 81 formalin-fixated specimens. In three specimens the plantaris- and calcaneal tendon were held together by a retinaculum-like structure, transversally constricting the calcaneal- and plantaris tendon 32–88 mm proximal to the insertion (Fig. 2); in three specimens the plantaris tendon inserted on the mid-portion of the calcaneal tendon at 20–65 mm proximal to the calcaneal insertion of the calcaneal tendon; two inserted into the deep fascia; two adhered onto the anteromedial- and one onto the anterior side of the calcaneal tendon by cords of solid tissue but inserted into the calcaneus. The macroscopic image of a retinaculum-like structure made us consider this as being prone to pathology (Fig. 2). Four of 11 attachments were in legs of which the other side was also available; however, none of the connections was bilateral.
Fig. 2.
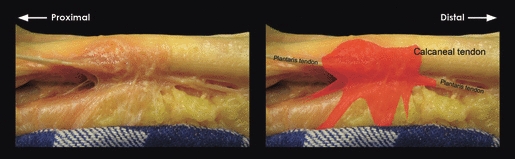
A firm attachment between the calcaneal- and plantaris tendons at the mid-portion level of the Achilles tendon. This type was named ‘retinaculum-like’.
In all specimens a plantaris tendon was identified (100%). We found nine different sites of insertion of the plantaris tendon, which are described and depicted in Fig. 3. In only 14% of 22 paired legs (three pairs) the site of insertion on the left leg was identical to that of the right.
Fig. 3.
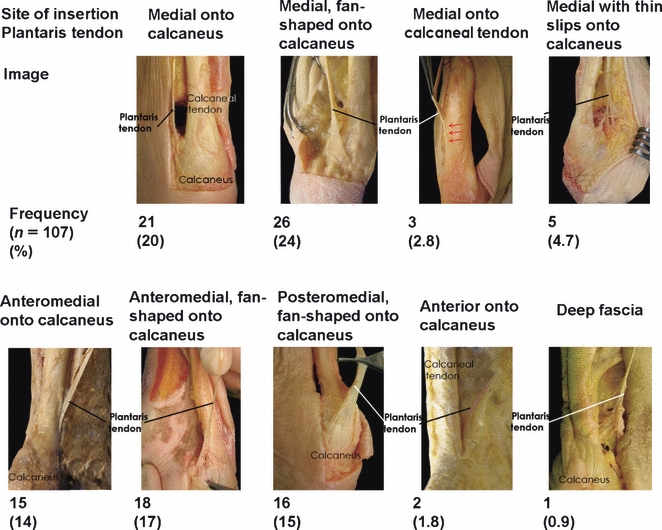
Sites of insertion of the plantaris tendon.
Discussion
In 11 specimens a firm connection between the calcaneal- and plantaris tendon was found, at the level of the mid-portion of the calcaneal tendon. This corresponds to the level at which complaints in patients with mid-portion Achilles tendinopathy are present. In three other specimens (2.8%), the plantaris tendon inserted into the calcaneal tendon. This variance has been described before (Daseler, 1943). On the contrary, the firm connections (10%) between the plantaris- and calcaneal tendon have not been described before. We, however, found connections in 10% of our specimens. How then can we explain these findings? One way could be that these connections are congenital or an anatomical variation. Another explanation could be that these connections have developed later in life. We do not have a history of our specimens, and thus we do not know whether they were symptomatic or not. Degenerative changes in the calcaneal tendon have been found in up to 34% of subjects without complaints (Kannus & Jozsa, 1991; Haims et al. 2000; Khan et al. 2003; Alfredson et al. 2008; Emerson et al. 2009). If peritendinous adhesions occur secondary to intratendinous changes, this can offer an explanation for the connections and adhesions that we found. Development later on in life seems to be the most likely explanation of this high percentage of a firm connection between the plantaris- and calcaneal tendon at the mid-portion level.
Now could these connections between calcaneal- and plantaris tendon explain the medial pain in mid-portion Achilles tendinopathy? We aimed to theorize this by applying anatomical and pathophysiological evidence.
The calcaneal tendon consists of the fibers of two muscle units in the superficial compartment of the posterior leg: the gastrocnemius muscle (medial and lateral head); and the soleus muscle. The gastrocnemius muscle crosses the knee and ankle joints (ankle and subtalar); originating from the posterior surface of condylus medialis and lateralis femoris and inserting onto the calcaneus. The soleus muscle lies anterior to the gastrocnemius muscle and originates from each side of the anterior aponeurosis attached to the tibia and fibula, and from the posterior surfaces of the head of the fibula and its proximal quarter, as well as the middle third of the medial border of the tibia. The soleus muscle only crosses the two ankle joints. Distally, both the gastrocnemius and soleus muscles form an aponeurosis, from each of which a tendon originates. At about the level where the soleus contributes fibers to the calcaneal tendon, rotation of the tendon begins and becomes more marked in the distal 5–6 cm. The gastrocnemius fibers rotate to lateral and the soleus fibers are positioned medial to the insertion (O'Brien, 2005). This anatomical observation means that the medial portion of the calcaneal tendon, where pain is often most prominent, is bi-articular as it consists of fibers originating from the soleus muscle (Fig. 4). The calcaneal tendon is involved in plantarflexion whereas, according to its anatomical course, the tri-articular plantaris tendon also contributes to ankle inversion. These opposite forces result in an intermittent small change of position between calcaneal- and plantaris tendon, which occurs with every step.
Fig. 4.
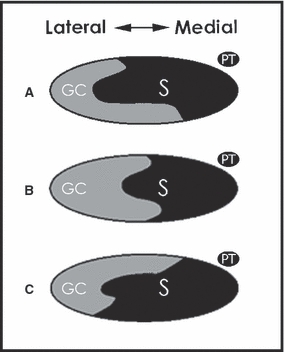
Degree of rotation and position of the gastrocnemius–soleus complex at the mid-portion of the calcaneal tendon. (GC, gastrocnemius; S, soleus.) The most common (52%) position is depicted in (A), (B) occurs in 35% and (C) in 13%. In all situations the soleus fibers are positioned medially. The location of the plantaris tendon is shown (PT). [Illustration as adapted from Cummins et al. (1946).]
Unlike other tendons in the leg, the Achilles tendon lacks a synovial sheath. Instead, it has a paratendon, which is an array of thin, fibrous tissue containing blood vessels (Saxena & Bareither, 2001). In patients with symptomatic Achilles tendinopathy, enhanced neurovascular growth from the paratendon into the calcaneal tendon in an attempt to repair the tendon proper is seen. Also myofibroblasts responsible for the formation of permanent scarring and the shrinkage of peritendinous tissue have been shown. The plantaris tendon runs with the calcaneal tendon inside a collective paratendon. With the development of scarring and shrinkage of peritendinous tissue, the calcaneal- and plantaris tendon will adhere. The opposite movements between plantaris- and calcaneal tendon will result in traction to the firm peritendinous tissue that connects both tendons and the level of the tendinopathy. This tissue has recently been described to be richly innervated by neonerves, which may be involved in causing pain (Schubert et al. 2005; Andersson et al. 2007). Pain then aggravates with movement, given the fact that the traction forces occur with every step with an average of 5000–12 500 steps per day (Tudor-Locke & Bassett, 2004; Tudor-Locke et al. 2008).
There are two drawbacks to our study. Histological examination would have provided us with more information on the nature of the connections, and is necessary to gain more relevant data for conclusions concerning a potential role in mid-portion Achilles tendinopathy. Another drawback is the fact that age and former lifestyle of our specimens did not match with the group of active 30–50-year-olds in which mid-portion Achilles tendinopathy most often occurs.
The consequences of formalin fixation are yet unknown. It may alter the properties of soft tissue structures, making blunt division of calcaneal- and plantaris tendons easier, consequently missing possible connections. We found 23% of connections in fresh-frozen, but only 6% in formalin-fixated specimens. Larger numbers of specimens may be needed to substantiate this finding. Dissection of exclusively fresh-frozen specimens might have been beneficial as this may be the closest we can get to in vivo tissue texture.
A striking finding was that, as opposed to the widely accepted rule that in a large sample of patients a number of plantaris muscles/tendons is missing, in all our 107 specimens a plantaris tendon was identified. According to previous anatomical studies with large numbers of specimens, the plantaris tendon is absent in a number of cases (7–20%; Gruber, 1879; Schwalbe & Pfitzner, 1894; Danforth, 1923; Daseler, 1943; Moss, 1988; Freeman et al. 2008). Daseler found in 542 pairs of legs that in nine the right plantaris tendon was absent, and in 21 the left. Moreover, in 31 pairs the plantaris muscle could not be identified. Harvey et al. (1983) noted the absence of plantaris in 18.2% of 658 lower limb dissections.
These earlier findings of absence in anatomical studies could be attributable to the technique of dissection. In our five cases where the tendon inserted into the calcaneal tendon, anterior to the tendon on the calcaneus and in the crural fascia (4.6%), the plantaris tendon could not be identified distally (Fig. 3). We started our dissection between the gastrocnemius and soleus muscles (Fig. 1), and identified a plantaris tendon in all our specimens. Unfortunately only two studies specified their dissection techniques. Freeman et al. (13% absence) specifically aim for the muscle belly dissecting the proximal dorsal compartment of the lower leg; Harvey (18.2% absence) mentions not dissecting the lower legs all the way to proximal (Harvey et al. 1983). With our relatively large group of specimens, we not only contradict earlier findings of its absence, but also raise implications for daily practise. The plantaris tendon is often used for grafting because it can be sacrificed without markable deficit, and is of adequate length and thickness for most applications (Lynn, 1966; Morrison & Schlicht, 1992; Shuhaiber & Shuhaiber, 2003; Bertelli et al. 2007). It is often excised from distally. In this case it will be missed in a substantial number of cases. We advise to retrieve it more proximally (e.g. with a tendon stripper). Using ultrasound or magnetic resonance imaging prior to surgery to pre-assess its course may be helpful.
Another discrepancy with the existing knowledge was the mid-course of the plantaris tendon in the posterior compartment of the lower leg.
According to Sobotta Anatomy (Putz & Pabst, 1994), the plantaris tendon instantly crosses the posterior side of the calf, to run medial with the calcaneal tendon until its insertion (Fig. 5). In our observations the plantaris tendon along its course gradually crosses the calf between the gastrocnemius and the soleus muscle.
Fig. 5.
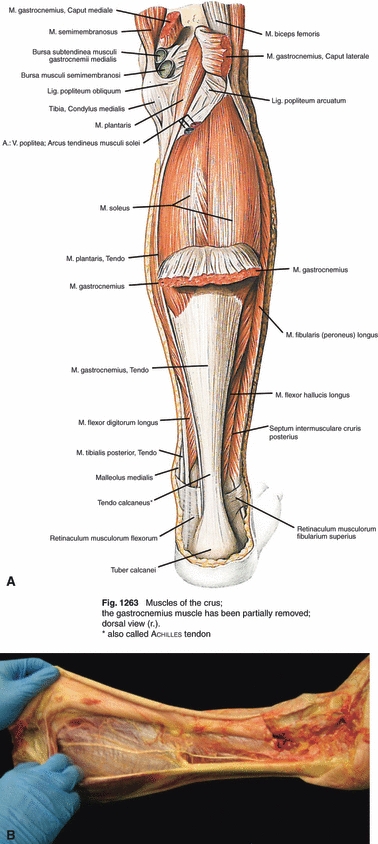
(A) Anatomical course of the plantaris tendon according to Sobotta Anatomy. (B) Course found during 107 dissections.
With this study we can theorize but not confirm that the plantaris tendon plays a role in complaints in patients with mid-portion Achilles tendinopathy. However, we do know more about the course and insertion of the plantaris tendon. An interesting and unexpected finding was the firm connections between plantaris- and calcaneal tendon at the level of the mid-portion of the calcaneal tendon in 10% of specimens. Clinical studies on the outcome of surgically removing plantaris tendons and thereby severing adhesions and accompanying enhanced neurovascular growth from the paratendon at the medial side of the calcaneal tendon may provide us with more evidence on the relation between the plantaris tendon and symptomatic mid-portion Achilles tendinopathy.
Acknowledgments
The authors would like to thank the Department of Anatomy and Embryology, particularly Mrs C.G.J. Cleypool, and the Department of Pathology for facilitating the dissections.
References
- Alfredson H, Zeisig E, Fahlstrom M. No normalisation of the tendon structure and thickness after intratendinous surgery for chronic painful midportion Achilles tendinosis. Br J Sports Med. 2009;43:948–949. doi: 10.1136/bjsm.2008.050955. [DOI] [PubMed] [Google Scholar]
- Andersson G, Danielson P, Alfredson H, et al. Nerve-related characteristics of ventral paratendinous tissue in chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2007;15:1272–1279. doi: 10.1007/s00167-007-0364-2. [DOI] [PubMed] [Google Scholar]
- Bertelli JA, Santos MA, Kechele PR, et al. Flexor tendon grafting using a plantaris tendon with a fragment of attached bone for fixation to the distal phalanx: a preliminary cohort study. J Hand Surg Am. 2007;32:1543–1548. doi: 10.1016/j.jhsa.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Cummins EJ, Anson JB, Carr WB. The structure of the calcaneal tendon [of Achilles] in relation to orthopedic surgery with additional observations on the plantaris muscle. Surg Gynecol Obstet. 1946;83:107–110. [PubMed] [Google Scholar]
- Danforth CH. The heredity of unilateral variations in man. Genetics. 1923;9:199–210. doi: 10.1093/genetics/9.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daseler EH. The plantaris muscle: an anatomical study of 750 specimens. J Bone Joint Surg. 1943;25:822–827. [Google Scholar]
- Emerson C, Morrissey D, Perry M, et al. Ultra-sonographically detected changes in Achilles tendons and self reported symptoms in elite gymnasts compared with controls – an observational study. Man Ther. 2010;15:37–42. doi: 10.1016/j.math.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Freeman AJ, Jacobson NA, Fogg QA. Anatomical variations of the plantaris muscle and a potential role in patellofemoral pain syndrome. Clin Anat. 2008;21:178–181. doi: 10.1002/ca.20594. [DOI] [PubMed] [Google Scholar]
- Gibbon WW, Cooper JR, Radcliffe GS. Sonographic incidence of tendon microtears in athletes with chronic Achilles tendinosis. Br J Sports Med. 1999;33:129–130. doi: 10.1136/bjsm.33.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber W. Beobachtungen aus der Menschlichen und Vergleichenden Anatomie. Berlin: A. Hirschwald; 1879. [Google Scholar]
- Haims AH, Schweitzer ME, Patel RS, et al. MR imaging of the Achilles tendon: overlap of findings in symptomatic and asymptomatic individuals. Skeletal Radiol. 2000;29:640–645. doi: 10.1007/s002560000273. [DOI] [PubMed] [Google Scholar]
- Harvey FJ, Chu G, Harvey PM. Surgical availability of the plantaris tendon. J Hand Surg Am. 1983;8:243–247. doi: 10.1016/s0363-5023(83)80151-8. [DOI] [PubMed] [Google Scholar]
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- Khan KM, Forster BB, Robinson J, et al. Are ultrasound and magnetic resonance imaging of value in assessment of Achilles tendon disorders? A two year prospective study. Br J Sports Med. 2003;37:149–153. doi: 10.1136/bjsm.37.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn TA. Repair of the torn achilles tendon, using the plantaris tendon as a reinforcing membrane. J Bone Joint Surg Am. 1966;48:268–272. [PubMed] [Google Scholar]
- Maffulli N, Sharma P, Luscombe KL. Achilles tendinopathy: aetiology and management. J R Soc Med. 2004;97:472–476. doi: 10.1258/jrsm.97.10.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison WA, Schlicht SM. The plantaris tendon as a tendo-osseous graft. Part II. Clinical studies. J Hand Surg Br. 1992;17:471–475. doi: 10.1016/s0266-7681(05)80277-3. [DOI] [PubMed] [Google Scholar]
- Moss ALH. Is there an association between an absence of palmaris longus tendon and an absence of plantaris tendon? Eur J Plast Surg. 1988;11:32–34. [Google Scholar]
- O'Brien M. The anatomy of the Achilles tendon. Foot Ankle Clin. 2005;10:225–238. doi: 10.1016/j.fcl.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Putz R, Pabst R. Sobotta Atlas of Human Anatomy. 12 (English) edn. Urban and Schwarzenberg: Munich; 1994. [Google Scholar]
- Sandmeier R, Renstrom PA. Diagnosis and treatment of chronic tendon disorders in sports. Scand J Med Sci Sports. 1997;7:96–106. doi: 10.1111/j.1600-0838.1997.tb00125.x. [DOI] [PubMed] [Google Scholar]
- Saxena A, Bareither D. Magnetic resonance and cadaveric findings of the ‘watershed band’ of the achilles tendon. J Foot Ankle Surg. 2001;40:132–136. doi: 10.1016/s1067-2516(01)80078-8. [DOI] [PubMed] [Google Scholar]
- Schubert TE, Weidler C, Lerch K, et al. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64:1083–1086. doi: 10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbe G, Pfitzner W. Varietäten-Statistik und Anthropologie. Deutsche Med Wchnschr. 1894;25 [Google Scholar]
- Segesser B, Goesele A, Renggli P. [The Achilles tendon in sports] Orthopade. 1995;24:252–267. [PubMed] [Google Scholar]
- Shuhaiber JH, Shuhaiber HH. Plantaris tendon graft for atrioventricular valve repair: a novel hypothetical technique. Tex Heart Inst J. 2003;30:42–44. [PMC free article] [PubMed] [Google Scholar]
- Steenstra F, van Dijk CN. Achilles tendoscopy. Foot Ankle Clin. 2006;11:429–438. doi: 10.1016/j.fcl.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Tan SC, Chan O. Achilles and patellar tendinopathy: current understanding of pathophysiology and management. Disabil Rehabil. 2008;30:1608–1615. doi: 10.1080/09638280701792268. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Hatano Y, Pangrazi RP, et al. Revisiting ‘how many steps are enough?’. Med Sci Sports Exerc. 2008;40:S537–S543. doi: 10.1249/MSS.0b013e31817c7133. [DOI] [PubMed] [Google Scholar]
- de Vos RJ, Heijboer MP, Weinans H, et al. Tendon structure is not related to clinical outcome following eccentric exercises in chronic mid-portion Achilles tendinopathy. 2009. [DOI] [PubMed]


