Abstract
The lysophospholipase D enzyme, autotaxin (ATX), has been linked to numerous human diseases including cancer, neurophatic pain, obesity, and Alzheimer’s disease. Although the ATX protein was initially purified and characterized in 1992, a link to bioactive lipid metabolism was not made until 2002. In the past decade, metal chelators, lysophospholipid product analogs, and more recently small non-lipid inhibitors of the enzyme were successfully identified. The majority of these inhibitors have been characterized using recombinant purified ATX in vitro, with very few examples studied in more complex systems. Translation of ATX inhibitors from the hands of medicinal chemists to clinical use will require substantially expanded characterization of ATX inhibitors in vivo.
Keywords: Autotaxin, lysophosphatidic acid, lysophospholipase D, NPP2, cancer
1. Introduction
The ectoenzyme autotaxin (ATX), possesses lysophospholipase D phosphodiesterase and to a lesser extent nucleotide pyrophosphatase activities. [1] In 1992 the ATX protein was purified and characterized from melanoma cell conditioned medium, [2] after which it was assigned to the nucleotide pyrophosphatase phosphodiesterase subfamily of alkaline phosphatases based on sequence homology. [3] However, it was not until 2002 that two independent groups[4, 5] showed that ATX was the enzyme responsible for a long studied plasma lysophospholipase D activity that had been shown to produce the bioactive lipid lysophosphatidic acid (LPA). [6] Over the past decade, interest in ATX as a pharmacological target has increased as more has been learned about the role of ATX and its hydrolytic product LPA in human health and disease. ATX is required for normal development of the vasculature, and its genetic deletion[7–9] or conversion to a loss-of-function mutant[10] in mice produces lethal defects in blood vessel formation, as well as growth retardation and head cavity formation during embryonic development. [7–9] Likewise, ATX has been shown to play significant roles in several human diseases including obesity, [11] rheumatoid arthritis, [12] chronic pain, [13, 14] and cancers[15, 16] among others. ATX expression is elevated in insulin-resistant diabetes[17] and in highly invasive cancers, [18, 19] where LPA production stimulates cell motility, formation of invadopodia, [20] and exhibits an anti-apoptotic effect that counteracts the effectiveness of chemotherapeutic treatments. [21] Recent studies demonstrate that ATX expression in cancer cells promotes bone metastasis through LPA action at the LPA1 receptor, and silencing of ATX expression in these cells reduces bone metastasis without concomitant reduction in tumor volume. [22]
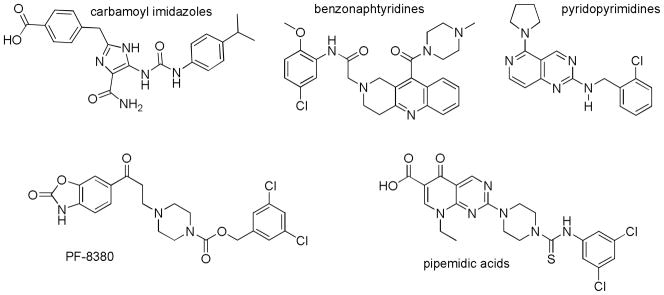
The potential benefits to human health by blocking ATX activity have stimulated efforts by numerous research groups in academia and in the pharmaceutical industry to identify and characterize ATX inhibitors. Inhibition of ATX was first demonstrated using high concentrations of compounds known to chelate metals, [23, 24] a line of investigation stimulated by the membership of ATX in the alkaline phosphatase superfamily of metalloenzymes. [25] Demonstration that LPA exhibited feedback inhibition of ATX[26] spurred a new wave of ATX inhibitors that included a variety of phospholipid analogs (as recently reviewed[18] and additional reports[27, 28]). A more recent achievement has been the identification of small, non-lipid molecules that inhibit ATX (examples shown in Figure 1 as recently reviewed[18] and additional reports[29–38]
Figure 1.
Examples of small molecule, non-lipid ATX inhibitors.
This progress in the discovery and development of inhibitors has occurred in the face of limited details regarding the three-dimensional structure of the enzyme. ATX is expressed and then processed by a furin-type protease, [39] resulting in a free extracellular glycosylated enzyme of approximately 125 kDa. ATX/NPP2 is the only isoform of the nucleotide pyrophosphatase/phosphodiesterase (NPP) family that is not anchored at the surface of the cells in which it is expressed. ATX shares a common catalytic domain with other NPP family members, as well as a carboxy-terminal domain observed in the NPP1-3 isoforms that exhibits limited sequence homology to endonucleases, although without key functional residue conservation. Crystallographic structures of ATX have been solved by two independent two academic groups and are currently in the peer review process, additionally, Proteros Biostructures offers crystallography services with their solved ATX structure. Unfortunately, none of these structures are currently available to the public. The impending availability of ATX crystallographic structure data is likely to stimulate more rapid identification and optimization of small molecule inhibitors.
This perspective will focus on the first medicinal chemistry efforts centered on ATX inhibition as the primary therapeutic target. In particular, the evidence regarding the suitability of ATX inhibitors for cancer treatment will be discussed. While more limited, progress in applying ATX inhibition as a strategy to treat other indications will also be described. Finally, directions essential for the realization of ATX inhibitors as clinical agents will be suggested.
2. Perspective on ATX as a Therapeutic Target for Cancer Treatment
Patents describing the synthesis, discovery, and/or characterization of ATX inhibitors for use in cancer treatments began to appear in 2006. [31, 32, 34, 35, 40–42] These patents describe molecules ranging from lipid-like analogs of the enzymatic product (lysophosphatidic acid) to a variety of non-lipid small molecules. The majority of these molecules were characterized using purified recombinant ATX in combination with either direct detection of products generated from synthetic substrates or indirect detection of products generated from natural substrates. Both of these options are suitable for high-throughput screening of large numbers of candidates, but exhibit different limitations. The indirect detection of products from ATX-mediated hydrolysis of the natural substrate, lysophosphatidyl choline (LPC), using the Amplex Red assay system requires two additional enzymes. Choline oxidase oxidizes the choline produced by ATX to betaine and hydrogen peroxide. Horseradish peroxidase uses hydrogen peroxide to convert the Amplex Red reagent to resorufin, a fluorescent product. The added enzymes offer potential for candidate compounds to show apparent inhibition by interfering in the added enzymatic steps, rather than with the enzymatic function of ATX, as noted in the literature. [38] Direct product detection suffers from the use of non natural substrates (for example absorbance-based reagents such as para-nitrophenyl containing compounds, [26] and FRET-based reagents such as CPF-4[43] and FS-3. [44] Substrate-dependent inhibition potency has been noted for some ATX inhibitors, as well as differences in apparent mechanism of inhibition. [37, 45] These differences are most likely due to the widely varying sizes of synthetic ATX substrates that have been used producing different degrees of overlap with inhibitor binding subsites within the active site.
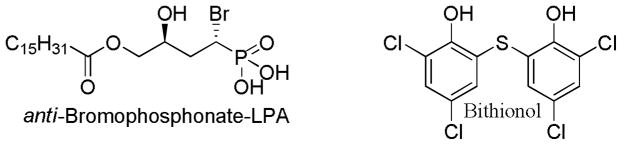
The therapeutic relevance of ATX inhibitors can only be partially understood from in vitro assays using recombinant enzyme. Promising compounds must be further evaluated using in vivo models before clinical relevance can be determined. Only two patents on the anticancer applications of ATX inhibitors have included such in vivo data. The first of these patents described in vivo studies on the anti-bromophosphonate derivative of LPA (Figure 2) and its syn diastereomer. [41] Both of these stereoisomers additionally show activity at the LPA receptors, ranging from full antagonism of LPA1–3 to partial antagonism of LPA4 and opposing partial agonism and full antagonism of LPA5, [46] therefore the in vivo effects cannot be attributed solely to ATX inhibition. The anti-bromophosphonate derivative of LPA was demonstrated to reduce tumor volume in a breast cancer xenograft model and to inhibit tumor growth after injection of colon cancer cells into the livers of nude mice. In the second of these patents, bithionol (Figure 2) was demonstrated to decrease tumor weight in a breast cancer carcinoma model and to reduce metastasis of tumors initiated with A2058 melanoma cells. [34] The selectivity of bithionol for ATX over the LPA receptors has not been reported. The inhibition of melanoma metastasis might appear to provide the largest potential benefit to human health, as metastatic melanoma remains a devastating disease with poor prognosis. While impact on melanoma metastasis in a mouse model certainly provides encouragement to continue developing and evaluating ATX inhibitors for cancer treatment, substantial obstacles remain between the current state of the field and clinical implementation. In particular, demonstration of anti-metastatic effects in a clinical trial is challenging. To demonstrate such an effect, treatment should begin when tumors are localized. However, current treatments for localized melanoma result in a 95% five-year survival rate, with low incidence of metastasis. The sample size that would be required to demonstrate statistically significant improvement over current standards of care is therefore staggeringly large. It is quite fortunate, therefore, that the ATX inhibitors tested in mouse models also proved to inhibit tumor growth.
Figure 2.
Patented ATX inhibitors demonstrated in animal models to inhibit tumor growth (both compounds: breast cancer models, anti-bromophosphonate-LPA: colon cancer cells in liver) and metastasis (bithionol: melanoma).
The localization of ATX suggests a potentially high-impact therapeutic direction that future studies should investigate. ATX is synthesized as a pre-pro-enzyme that is exported by cells and found in the circulation. [39] Inhibitors of ATX dramatically and rapidly decrease circulating levels of ATX-derived lysophosphatidic acid. [47] This extracellular location of ATX suggests that the distribution aspect of ADMET (absorbtion, distribution, metabolism, excretion, and toxicology) may not have the same influence as that for drugs acting on intracellular targets. In particular, ATX should be investigated as a potential target in the treatment of multi-drug resistant (MDR) cancers, particularly those in which drug efflux mechanisms are the largest contributor to the drug resistance. This indication sets up the potential for the MDR efflux machinery to help maintain the concentration of drug molecules outside cancer cells, where ATX inhibition may provide the greatest benefit in disease treatment. Indirect support for the diminished relevance of distribution considerations is provided by the efficacy of bithionol and the alpha-bromo-phosphonate analog of LPA (Figure 2), both of which are highly hydrophobic (log Po/w > 5) and are likely to show poor distribution across cellular membranes.
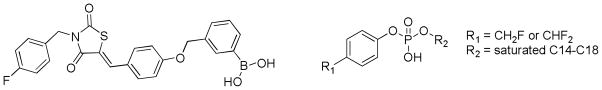
Two types of inhibitors with the potential to covalently bind to ATX have been reported. A boronic acid resulted from the optimization of thiazolidinediones identified by small-molecule library screening (Figure 3). [29, 47] The compound showed mixed-type inhibition, and inhibition was eliminated in wash-out experiments. This suggests that if covalent modification of ATX occurs, it is reversible. Difluoromethylphenyl and fluoromethylphenyl alkyl phosphates (Figure 3) were shown to display time-dependent inhibition of ATX. [42] The in vivo potential of ATX inhibitors that form covalent bonds to the enzyme depends on a number of factors, perhaps the most compelling of which is the lifetime of any individual ATX enzyme molecule. The Bollen lab has demonstrated that exogenously added ATX is rapidly cleared from the circulation (in minutes). [48] This finding could indicate either that all ATX molecules are rapidly cleared and replaced or that ATX levels are tightly regulated and clearance is initiated by increased concentrations of ATX. In either case, the added benefit of covalent modifiers as ATX inhibitors for therapeutic applications may be limited.
Figure 3.
ATX inhibitors described to covalently bind to ATX.
3. Perspective on ATX as a Therapeutic Target for Other Indications
ATX has been implicated in a variety of human diseases beyond cancer as recently reviewed. [18] These diseases include obesity, multiple sclerosis, neuropathic pain, arthritis and Alzheimer’s disease. The majority of these has yet to receive substantial attention in the patent literature. However, one patent describes the use of anti-sense oligodeoxynucleotides in the treatment of generalized pain syndrome in several mouse models including intermittent cold and mechanical stress. [49] In contrast to the application of ATX inhibitors in the treatment of cancer, particularly multi-drug resistant cancers, the distribution of drug to the site of action is considerably more challenging. ATX inhibitors in this case must reach the central nervous system. Anti-sense oligodeoxynucleotides were able to effectively treat generalized pain due to their intraventricular delivery route directly into the brain. An ideal clinical agent will benefit from optimization of distribution properties to allow oral dosing.
4. Expert Opinion
Substantial progress has been made toward the realization of ATX as a clinical target in the treatment of cancer and neuropathic pain in a relatively short amount of time. This progress has been supported by assays amenable to high-throughput formats, demonstration of efficacy in animal models, and discovery of lipid, non-lipid and anti-sense classes of ATX inhibitors. Nevertheless, there are both challenges remaining and promising unexplored directions for the field. First, the fluorescence-based non natural substrate analogs used in direct product detection assays and also the natural LPC used in indirect product detection assays require proper controls to definitively identify false negative and false positive results, which have been lacking in many previous reports. This issue should also be minimized by using secondary validation of primary screening assays. In all cases mechanism of inhibition (and resulting Ki) should be determined for the most promising hits identified through primary screens. Intermediate cell based assays should follow primary screens using purified, recombinant enzyme in vitro. Proliferation/toxicity assays, as well as migration/invasion screens provide the first glimpse of potential toxicity issues and/or disconnects between in vitro and in vivo potencies. A limited subset of promising compounds has been transitioned into animal models to date, therefore further in vivo testing including initial pharmacokinetic/pharmacodynamic analysis will be important components of translating ATX inhibitors into the clinic. The availability of structural information on ATX, via solved crystal structures and other biophysical characterizations, will open the field to additional computational methods that will undoubtedly lead to an expansion of rational approaches to the design and optimization of future ATX inhibitors. The field has yet to take full advantage of the extracellular location of ATX, through consideration of indications such as multiple drug-resistant cancer. Such resistant cancers may provide a showcase where ATX inhibition can provide drastically improved outcomes when contrasted against standard current treatments aimed at intracellular targets. Drugs aimed at extracellular targets should show similar therapeutic effects in the face of such efflux mechanisms. Numerous diseases for which ATX inhibition may prove beneficial, such as obesity and Alzheimer’s disease, also remain largely unexplored.
Acknowledgments
The authors acknowledge assistance in patent translation, from T Fujiwara (University of Memphis).
Footnotes
Declaration of Interest
A Parrill is supported by an NIH grant, R01 HL 084007.
D Baker declares no conflict of interest and has received no payment in preparation of this manuscript.
Contributor Information
Abby L. Parrill, Email: aparrill@memphis.edu, Professor and Chair, Department of Chemistry, The University of Memphis, Memphis, TN 38152, Phone: 1-901-678-2638, FAX: 1-901-678-3447.
Daniel L. Baker, Email: dlbaker@memphis.edu, Assistant Professor, Department of Chemistry, The University of Memphis, Memphis, TN 38152, Phone: 1-901-678-4178, FAX: 1-901-678-3447.
Annotated Bibliography
- 1.Bollen M, Gijsbers R, Ceulemans H, et al. Nucleotide pyrophosphatases/phosphodiesterases on the move. Crit Rev Biochem Mol Biol. 2000;35(6):393–432. doi: 10.1080/10409230091169249. [DOI] [PubMed] [Google Scholar]
- 2.Stracke ML, Krutzsch HC, Unsworth EJ, et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267(4):2524–9. [PubMed] [Google Scholar]
- 3.Murata J, Lee HY, Clair T, et al. cDNA cloning of the human tumor motility-stimulating protein, autotaxin, reveals a homology with phosphodiesterases. J Biol Chem. 1994;269(48):30479–84. [PubMed] [Google Scholar]
- 4.Tokumura A, Majima E, Kariya Y, et al. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277(42):39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- 5.Umezu-Goto M, Kishi Y, Taira A, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158(2):227–33. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokumura A, Yamano S, Aono T, et al. Lysophosphatidic Acids Produced by Lysophospholipase D in Mammalian Serum and Body Fluid. In: Goetzl EJ, Lynch KR, editors. Lysophospholipids and Eicosanoids in Biology and Pathophysiology. New York Academy of Sciences; New York: 2000. pp. 347–350. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Okudaira S, Kishi Y, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281(35):25822–30. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 8.van Meeteren LA, Ruurs P, Stortelers C, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26(13):5015–22. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koike S, Keino-Masu K, Masu M. Deficiency of autotaxin/lysophospholipase D results in head cavity formation in mouse embryos through the LPA receptor-Rho-ROCK pathway. Biochem Biophys Res Commun. 2010;400(1):66–71. doi: 10.1016/j.bbrc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Ferry G, Giganti A, Coge F, et al. Functional invalidation of the autotaxin gene by a single amino acid mutation in mouse is lethal. FEBS Lett. 2007;581(18):3572–8. doi: 10.1016/j.febslet.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 11.Ferry G, Tellier E, Try A, et al. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J Biol Chem. 2003;278(20):18162–9. doi: 10.1074/jbc.M301158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourgoin SG, Zhao C. Autotaxin and lysophospholipids in rheumatoid arthritis. Curr Opin Investig Drugs. 2010;11(5):515–26. [PubMed] [Google Scholar]
- 13.Inoue M, Ma L, Aoki J, et al. Autotaxin, a synthetic enzyme of lysophosphatidic acid (LPA), mediates the induction of nerve-injured neuropathic pain. Mol Pain. 2008;4:6. doi: 10.1186/1744-8069-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue M, Xie W, Matsushita Y, et al. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience. 2008;152(2):296–8. doi: 10.1016/j.neuroscience.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Okudaira S, Yukiura H, Aoki J. Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie. 2010;92(6):698–706. doi: 10.1016/j.biochi.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Samadi N, Bekele R, Capatos D, et al. Regulation of lysophosphatidate signaling by autotaxin and lipid phosphate phosphatases with respect to tumor progression, angiogenesis, metastasis and chemo-resistance. Biochimie. doi: 10.1016/j.biochi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Boucher J, Quilliot D, Praderes JP, et al. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia. 2005;48(3):569–77. doi: 10.1007/s00125-004-1660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parrill AL, Baker DL. Autotaxin inhibition: challenges and progress toward novel anti-cancer agents. Anticancer Agents Med Chem. 2008;8(8):917–23. doi: 10.2174/187152008786847765. [DOI] [PubMed] [Google Scholar]
- 19.Federico L, Pamuklar Z, Smyth SS, et al. Therapeutic potential of autotaxin/lysophospholipase d inhibitors. Curr Drug Targets. 2008;9(8):698–708. doi: 10.2174/138945008785132439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harper K, Arsenault D, Boulay-Jean S, et al. Autotaxin promotes cancer invasion via the lysophosphatidic acid receptor 4: participation of the cyclic AMP/EPAC/Rac1 signaling pathway in invadopodia formation. Cancer Res. 2010;70(11):4634–43. doi: 10.1158/0008-5472.CAN-09-3813. [DOI] [PubMed] [Google Scholar]
- 21.Vidot S, Witham J, Agarwal R, et al. Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells. Cell Signal. 2010;22(6):926–35. doi: 10.1016/j.cellsig.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 22.David M, Wannecq E, Descotes F, et al. Cancer cell expression of autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent activation of osteoclasts. PLoS One. 2010;5(3):e9741. doi: 10.1371/journal.pone.0009741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clair T, Koh E, Ptaszynska M, et al. L-histidine inhibits production of lysophosphatidic acid by the tumor-associated cytokine, autotaxin. Lipids Health Dis. 2005;4(1):5. doi: 10.1186/1476-511X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tokumura A, Miyake M, Yoshimoto O, et al. Metal-ion stimulation and inhibition of lysophospholipase D which generates bioactive lysophosphatidic acid in rat plasma. Lipids. 1998;33(10):1009–15. doi: 10.1007/s11745-998-0299-2. [DOI] [PubMed] [Google Scholar]
- 25.Lee HY, Clair T, Mulvaney PT, et al. Stimulation of tumor cell motility linked to phosphodiesterase catalytic site of autotaxin. J Biol Chem. 1996;271(40):24408–12. doi: 10.1074/jbc.271.40.24408. [DOI] [PubMed] [Google Scholar]
- 26.van Meeteren LA, Ruurs P, Christodoulou E, et al. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem. 2005;280(22):21155–21161. doi: 10.1074/jbc.M413183200. [DOI] [PubMed] [Google Scholar]
- 27.Ferry G, Moulharat N, Pradere JP, et al. S32826, a nanomolar inhibitor of autotaxin: discovery, synthesis and applications as a pharmacological tool. J Pharmacol Exp Ther. 2008;327(3):809–19. doi: 10.1124/jpet.108.141911. [DOI] [PubMed] [Google Scholar]
- 28.Altman MK, Gopal V, Jia W, et al. Targeting melanoma growth and viability reveals dualistic functionality of the phosphonothionate analogue of carba cyclic phosphatidic acid. Mol Cancer. 2010;9:140. doi: 10.1186/1476-4598-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albers HM, van Meeteren LA, Egan DA, et al. Discovery and optimization of boronic acid based inhibitors of autotaxin. J Med Chem. 2010;53(13):4958–67. doi: 10.1021/jm1005012. [DOI] [PubMed] [Google Scholar]
- 30.Gierse JK, Thorarensen A, Beltey K, et al. A Novel Autotaxin Inhibitor Reduces Lysophosphatidic Acid Levels in Plasma and the Site of Inflammation. J Pharmacol Exp Ther. 2010;334(1):310–7. doi: 10.1124/jpet.110.165845. [DOI] [PubMed] [Google Scholar]
- 31.Schiemann K, Schultz M, Staehle W, et al. Application: WO WO. Merck Patent GmbH; Germany: 2010. Preparation of 2,5-diamino-substituted pyrido[4,3-d]pyrimidines as autotaxin inhibitors useful in treating cancer; p. 177. [Google Scholar]
- 32.Staehle W, Kober I, Schiemann K, et al. Application: WO WO. Merck Patent GmbH; Germany: 2010. Preparation of benzo[b][1,6]naphthyridines as inhibitors of autotaxin for the treatment of tumors; p. 142. [Google Scholar]
- 33.Saunders LP, Ouellette A, Bandle R, et al. Identification of small-molecule inhibitors of autotaxin that inhibit melanoma cell migration and invasion. Mol Cancer Ther. 2008;7(10):3352–62. doi: 10.1158/1535-7163.MCT-08-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Braddock D. Application: WO WO. Yale University; USA: 2009. Small molecule inhibitors of autotaxin, and methods of use for the treatment of cancer; p. 82. This patent is notable as one of only two that include demonstration in vivo of anti- cancer activity of an ATX inhibitor. [Google Scholar]
- 35.Schiemann K, Schultz M, Blaukat A, et al. Application: WO WO. Merck Patent GmbH; Germany: 2009. Preparation of carbamoylthiazoles as anticancer agents; p. 102. [Google Scholar]
- 36.Hoeglund AB, Bostic HE, Howard AL, et al. Optimization of a Pipemidic Acid Autotaxin Inhibitor. J Med Chem. 2010;53(3):1056–66. doi: 10.1021/jm9012328. [DOI] [PubMed] [Google Scholar]
- 37.Hoeglund AB, Howard AL, Wanjala IW, et al. Characterization of non-lipid autotaxin inhibitors. Bioorg Med Chem. 2010;18(2):769–76. doi: 10.1016/j.bmc.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 38.North EJ, Howard AL, Wanjala IW, et al. Pharmacophore development and application toward the identification of novel, small-molecule autotaxin inhibitors. J Med Chem. 2010;53(8):3095–105. doi: 10.1021/jm901718z. [DOI] [PubMed] [Google Scholar]
- 39.Jansen S, Stefan C, Creemers JW, et al. Proteolytic maturation and activation of autotaxin (NPP2), a secreted metastasis-enhancing lysophospholipase D. J Cell Sci. 2005;118(Pt 14):3081–9. doi: 10.1242/jcs.02438. [DOI] [PubMed] [Google Scholar]
- 40.Miller DD, Tigyi GJ, Gududuru V, et al. Application: US US. USA: 2006. Acetal phosphate-derived LPA mimics, PPARgamma activators, and autotaxin inhibitors; p. 21. [Google Scholar]
- 41**.Prestwich G, Tigyi G, Jiang G, et al. Application: WO WO. University of Utah Research Foundation; USA: University of Tennessee Research Foundation; 2008. Antitumor alpha -Chloro AND alpha -Bromo phosphonate analogs of lysophosphatidic acid; p. 65. This patent is notable as one of only two that include demonstration in vivo of anti- cancer activity of an ATX inhibitor. [Google Scholar]
- 42.Parrill-Baker AL, Baker DL, Montedonico LE. Application: WO WO. USA: Preparation of fluoromethylphenyl phosphodiesters as mechanism-based inactivators of autotaxin; p. 38. [Google Scholar]
- 43.Takakusa H, Kikuchi K, Urano Y, et al. Design and synthesis of an enzyme-cleavable sensor molecule for phosphodiesterase activity based on fluorescence resonance energy transfer. J Am Chem Soc. 2002;124(8):1653–7. doi: 10.1021/ja011251q. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson CG, Bigman CS, Richardson RD, et al. Fluorogenic phospholipid substrate to detect lysophospholipase D/autotaxin activity. Org Lett. 2006;8(10):2023–6. doi: 10.1021/ol060414i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moulharat N, Fould B, Giganti A, et al. Molecular pharmacology of adipocyte- secreted autotaxin. Chem Biol Interact. 2008;172(2):115–24. doi: 10.1016/j.cbi.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Xu X, Gajewiak J, et al. Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 2009;69(13):5441–9. doi: 10.1158/0008-5472.CAN-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albers HM, Dong A, van Meeteren LA, et al. Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc Natl Acad Sci U S A. 2010;107(16):7257–62. doi: 10.1073/pnas.1001529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jansen S, Andries M, Vekemans K, et al. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 2009;284(2):216–21. doi: 10.1016/j.canlet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 49**.Ueda H. Application: WO WO. Nagasaki University; Japan: Argenes, Inc; Therapeutic or prophylactic agent for generalized pain syndrome; p. 48. This patent is notable as evaluating ATX inhibition in vivo as a treatment for pain, rather than cancer. [Google Scholar]