Summary
Mammalian puberty is initiated by an increasedpulsatile release of the neuropeptide gonadotropin-releasing hormone (GnRH) from hypothalamic neuroendocrine neurons. Although this increase is primarily set in motion by neuronal networks synaptically connected to GnRH neurons, glial cells contribute to the process via at least two mechanisms. One involves production of growth factors acting via receptors endowed with either serine-threonine kinase or tyrosine kinase activity. The other involves plastic rearrangements of glia-GnRH neuron adhesiveness. Growth factors of the epidermal growth factor (EGF) family acting via erbB receptors play a major in glia-to GnRH neuron communication. In turn, neurons facilitate astrocytic erbB signaling via glutamate-dependent cleavage of erbB ligand precursors. Genetic disruption of erbB receptors delays female sexual development due to impaired erbB ligand-induced glial prostaglandin E2 (PGE2) release. The adhesiveness of glial cells to GnRH neurons involves at least two different cell-cell communication systems endowed with both adhesive and intracellular signaling capabilities. One is provided by Synaptic Cell Adhesion Molecule (SynCAM1), which establishes astrocyte-GnRH neuron adhesiveness via homophile interactions; the other involves the heterophilic interaction of neuronal contactin with glial Receptor-like Protein Tyrosine Phosphatase-β (RPTPβ). These finding indicate that the interaction of glial cells with GnRH neurons involves not only secreted bioactive molecules, but also cell-surface adhesive proteins able to set in motion intracellular signaling cascades.
Keywords: glial cells, hypothalamus, neuroendocrine neurons, female sexual development, glial-neuronal interactions, intercellular signaling
Neuroendocrine control of sexual development. General aspects
The mammalian basal forebrain contains only a handful (800–1,000) of GnRH producing neurons. In primates, they are mostly located in the medial basal hypothalamus (Plant and Witchel 06); in rodents, they are located in preoptic area (POA) (Ojeda and Skinner 06). GnRH released into the portal vessels connecting the median eminence (ME) of the hypothalamus to the pituitary gland, reaches the pituitary gland and stimulate release of the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which stimulate the gonads to produce steroids, peptides, and mature germ cells. In turn, gonadal hormones control the release of GnRH and gonadotropins via negative and positive feedback mechanisms. Pulsatile gonadotropin secretion, reflecting changes in GnRH release, increases in a diurnal fashion at the end of juvenile development, signaling the initiation of the pubertal process [reviewed in (Ojeda and Terasawa 02;Plant 02)].
These changes are, in turn, determined by modifications in transsynaptic (Kordon et al 94;Ojeda and Terasawa 02) and glial (Ojeda and Terasawa 02;Ojeda et al 03) inputs to the GnRH neuronal network. While the transsynaptic changes involve an increase in excitatory inputs and a reduction in inhibitory influences (Terasawa and Fernandez 01;Ojeda and Terasawa 02;Plant and Witchel 06), the glial component is predominantly facilitatory, and exerted by growth factors that directly or indirectly stimulate GnRH secretion (Ojeda et al 03;Ojeda and Skinner 06;Lomniczi and Ojeda 09). The excitatory transsynaptic regulation of GnRH secretion is provided by neurons that use glutamate (Brann 95;Ojeda and Skinner 06;Plant and Witchel 06), kisspeptin (Ojeda and Skinner 06;Dungan et al 06), and apparently, neurokinin B (Topaloglu et al 08), as neurotransmitters/neuromodulators. The inhibitory counterpart of this circuitry depends principally on GABAergic neurons, but also on opiatergic neurons that employ different peptides and a variety of different receptors for inhibitory neurotransmission [reviewed in (Terasawa and Fernandez 01)]. Although both the neuronal and glial networks controlling GnRH secretion are responsive to gonadal steroids (Mong and McCarthy 99;Garcia-Segura and McCarthy 04;Ojeda and Skinner 06;Mong and Blutstein 06), the initial changes in activity that ultimately result in the initiation of puberty are thought to be set in motion by gonad-independent mechanisms (Ojeda and Terasawa 02;Ojeda et al 03;Plant and Witchel 06).
The present review will discuss the role of glial-neuronal interactions in the control of GnRH release, emphasizing the contribution of these interactions to the neuroendocrine regulation of female puberty.
Glia-to-GnRH neuron signaling
Growth factors
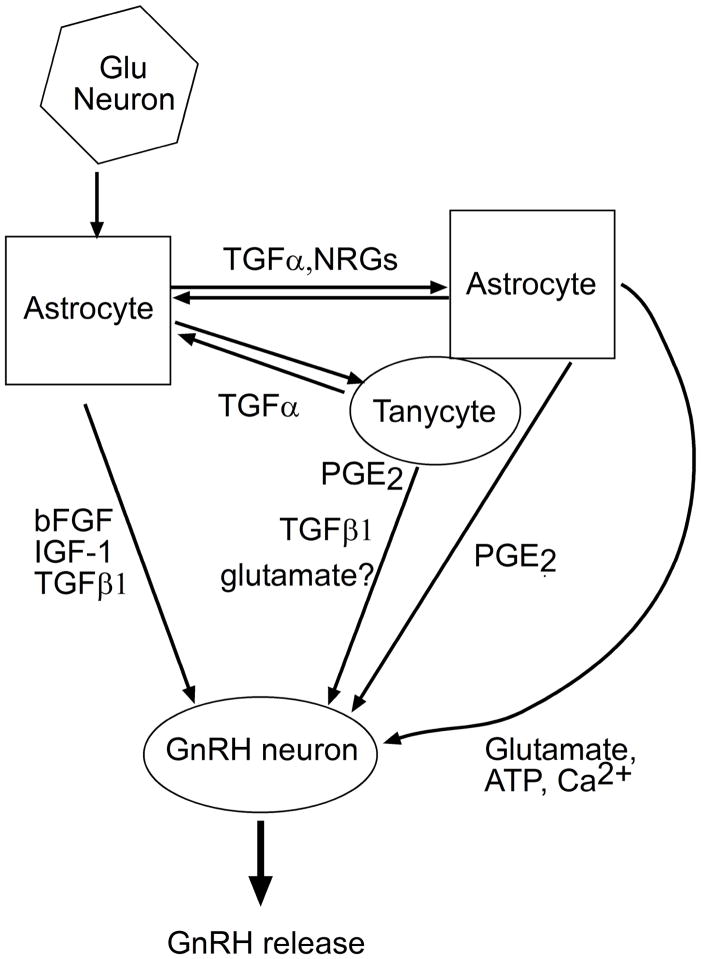
Hypothalamic astrocytes produce several growth factors including transforming growth factor beta (TGFβ), basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1) and EGF-like peptides [reviewed in (Ojeda and Skinner 06;Mahesh et al 06;Lomniczi and Ojeda 09)] (Fig. 1). TGFβ acts on GnRH neurons via receptors endowed with serine-threonine kinase activity to stimulate the synthesis and release of GnRH (Melcangi et al 95;Galbiati et al 96) [reviewed in (Mahesh et al 06)]. Basic bFGF acts via FGF tyrosine kinase receptors type I to promote GnRH neuronal differentiation and survival (Tsai et al 95;Voigt et al 96;Tsai et al 05), and enhance GnRH processing (Wetsel et al 96); IGF-I stimulates GnRH release (Hiney et al 91) by binding to IGF-1 tyrosine kinase receptors also located on GnRH neurons (Olson et al 95). The EGF-like peptides, transforming growth factor alpha (TGFα and neuregulins (NRGs) elicit GnRH secretion indirectly via the activation of erbB receptors located on both astroglial and ependymoglial cells (Voigt et al 96;Ma et al 97;Ma et al 99) (Fig. 1). The TGFα-NRG/erbB receptor signaling system is considered to play a major role in the mechanism by which glial cells regulate GnRH secretion.
Figure 1.
Control of GnRH secretion by growth factors and small molecules produced by glial cells. Some growth factors (bFGF, IGF-1, TGFβ1) act directly on GnRH neurons to stimulate production of GnRH. In contrast, growth factors of the epidermal growth factor family (TGFα and NRGs) act indirectly by stimulating the glial release of bioactive substances which, like PGE2, act on GnRH neurons to stimulate GnRH release. A neuron-to-astrocyte communication pathway is provided by glutamatergic neurons, which facilitate TGFα/NRG-dependent signaling in astrocytes. Tanycytes produce and respond to TGFα by synthesizing PGE2, which elicits TGFβ1 release.
The EGF family
There are ten EGF or EGF-like ligands (Buonanno and Fischbach 01;Falls 03;Rimer 03), of which the NRG family is the most complex. It is composed of four different subfamilies, each containing several isoforms. NRG1s consist of 15 different alternatively derived isoforms, abundantly expressed in neurons and glia. There are twoNRG2s, NRG2α and NRG2β (Chang et al 97;Carraway et al 97), which are mostly expressed in neurons of the central nervous system. NRG3 and NRG4 are divergent members of the NRG family, with only NRG3 being expressed in the nervous system (Falls 03;Rimer 03).
TGFα and NRGs, like all EGF family members, are synthesized as membrane-anchored peptides and bind to their cognate receptors on adjacent cells, upon proteolytic cleavage of the mature peptides from their membrane-bound precursors (Peschon et al 98;Montero et al 00;Sahin et al 04). All EGF-like peptides signal through a family of four transmembrane tyrosine kinase receptors known as erbB receptors. ErbB1 binds at least six different ligands including, EGF, TGFα, amphiregulin, heparin-binding EGF-like growth factor (HB-EGF), epiregulin and betacellulin (Carpenter and Cohen 90;Riese et al 96a;Shelly et al 98). ErbB3 and erbB4 bind NRGs (Chang et al 97;Burden and Yarden 97;Carraway et al 97;Zhang et al 97), as well as epiregulin (Shelly et al 98) and betacellulin (Riese et al 96a). ErbB2 does not have a ligand; instead it is recruited as a coreceptor (Karunagaran et al 96) by each of the other erbB receptors after ligand binding (Beerli et al 95;Riese et al 96b).
The Glial TGFα/erbB1 signaling complex
TGFα and EGF stimulate GnRH release by activating erbB1 receptors (Ojeda et al 90). These receptors are expressed on astroglial cells of the ME and tanycytes of the third ventricle, but not on GnRH neurons (Ma et al 94b;Voigt et al 96). It is now clear that ligand-dependent activation of erbB1 receptors located on astrocytes and tanycytes results in release of bioactive substances able to stimulate GnRH release. One of these substances is prostaglandin E2 (Ma et al 97;Prevot et al 03a); another is TGFβ1 (Prevot et al 03a).
During normal female sexual development in the rat, there is a transient increase of hypothalamic TGFα mRNA levels at two phases of postnatal development when gonadotropin secretion is elevated: the second week of life and puberty (Ma et al 92). Highest levels are detected at the time of the first preovulatory surge of gonadotropins, when GnRH secretion is maximal(Ma et al 92). Glial erbB1 receptor-dependent regulation of GnRH release is prominent in the ME(Ma et al 92), and activation of erbB1 receptors located in this region is necessary for the correct timing of puberty. For instance, blockade of erbB1 receptor tyrosine kinase activity in the ME delays puberty (Ma et al 92), whereas TGFα overexpression induced via either a transgenic approach (Ma et al 94a) or by grafting cells genetically engineered to secrete TGFα into the ME accelerates the onset of puberty in female rats (Rage et al 97).
The pubertal process can be set in motion prematurely by the pathological activation of discrete subsets of astrocytes functionally connected to the GnRH network. For instance, puberty-inducing lesions of the anterior hypothalamic area in rats, result in activation of TGFα and erbB1 receptor expression in astrocytes surrounding the lesion site (Junier et al 91;Junier et al 93). When erbB1 receptors are blocked, the advancing effect of the lesion on sexual maturation is prevented (Junier et al 91). Some hypothalamic hamartomas associated with sexual precocity in humans are endowed with a rich network of astrocytes containing TGFα and erbB1 receptors (Jung et al 99), suggesting that foci of glial activation in the proximity of GnRH neurons, such as these, may be a cause of idiopathic sexual precocity of central origin in human females.
The Glial Neuregulin-erbB2/4 signaling complex
Cultured hypothalamic astrocytes express NRG1, NRG3 as well as erbB2 and erbB4 receptors. When the cells are exposed NRG1β or TGFα there is phosphorylation of erbB4 and erbB1 receptors, respectively, in addition to erbB2 receptor cross-phosphorylation. As a result, production of PGE2 increases (Ma et al 99). ErbB2 receptors play an important role in amplifying intracellular signals initiated by TGFα and NRGs; in vitro blockade of astrocytic erbB2 synthesis prevents both the stimulatory effect of NRG1 on PGE2 release and the increase in GnRH secretion elicited by NRG1-conditioned astrocyte culture medium (Ma et al 99).
Consistent with the presence of erbB2 and erbB4 receptors in cultured astrocytes, immunohistochemistry and in situ hybridization studies demonstrated the presence of erbB2 mRNA and protein in hypothalamic astrocytes and tanycytes of the third ventricle/ME, and erbB4 in astrocytes, but not in tanycytes (Ma et al 99). Hypothalamic erbB2 and erbB4 mRNA abundance increases during juvenile development in female rats, when circulating sex steroid levels are low, and then again at the time of the preovulatory surge of gonadotropins (Ma et al 99). This secondary increase can be reproduced by treating immature female rats with estrogen and progesterone to induce a premature gonadotropin surge. These findings suggest that during female sexual development hypothalamic expression of erbB2 and erbB4 receptors is sequentially regulated by sex steroid- independent and sex steroid-dependent mechanisms.
Both receptors are required for the normal timing of puberty. In vivo disruption of erbB2 synthesis and transgenic overexpression of a dominant negative form of the erbB4 receptor delay female puberty (Ma et al 99;Prevot et al 03b). Because disruption of astrocytic erbB2/4 signaling was accompanied by normal erbB1 function, these findings established the importance of a functional astrocytic NRG/erbB4 signaling system for the normal initiation of puberty in the mouse. Mice carrying this dominant negative form of the erbB4 receptor and an inactivating point mutation of the erbB1 receptor show impaired erbB1 and erbB4 signaling in astrocytes, a further delay in the onset of puberty, and a striking decrease in adult reproductive capacity, in comparison to wild type and single mutant littermates (Prevot et al 05). These studies indicate that the integrity of both erbB1 and erbB4 signaling system in hypothalamic astrocytes is critical for glial cells to facilitate GnRH secretion during female sexual maturation.
Other Glia-Derived Factors
In addition to the growth factors mentioned above, astrocytes produce and release other growth factors such as tumor necrosis factor-alpha and activity-dependent growth factor, and a variety of small molecules including cholesterol, neuropeptides, cytokinins, ATP, prostaglandins and glutamate [reviewed in (Lomniczi and Ojeda 09)]. Although glial PGE2 is a major mediator of the stimulatory actions that TGFα and NRGs exert on GnRH release, astrocytes release additional substances capable of stimulating GnRH release (Ma et al 97). Among these substances, calcium, glutamate and ATP are the most conspicuous (Araque et al 99;Fields and Burnstock 06). Calcium reaches adjacent astrocytes via gap junctions (Haydon 01;Nedergaard et al 03), and stimulates the release of ATP and glutamate, which then affect neuronal function upon binding to specific receptors (Parpura et al 94;Cotrina et al 00;Fields and Stevens 00;Fields and Burnstock 06). In the primate hypothalamus, GnRH neurons respond to extracellular ATP, viaP2X2 and P2X4 receptors, with an immediate increase in intracellular calcium and release of GnRH (Terasawa 02;Terasawa et al 05). ATP and glutamate can also activate calcium mobilization in astrocytes (Fellin et al 06;Fields and Burnstock 06); calcium releases more glutamate, which in turn stimulates PGE2 formation (Zonta et al 03). PGE2 elicits further release of astrocytic glutamate (Bezzi et al 98), which enhances astroglial release of arachidonic acid (Stella et al 94). In turn, arachidonic acid inhibits glutamate uptake into astrocytes (Barbour et al 89), thereby increasing the half life of the neurotransmitter in synapses.
Hypothalamic astrocytes regulate glutamate metabolism
In addition to producing glutamate, astrocytes also regulate glutamate synthesis. This function changes according to the reproductive stage of the animals. For instance, glutamate metabolism changes in the adult mouse hypothalamus in response to preovulatory levels of estradiol (Blutstein et al 06) and in female rats during the normal onset of puberty (Roth et al 06). Shortly after estradiol administration to mice, there is an increased expression of glutamine synthase (GS) in the hypothalamus. GS is almost exclusively expressed in astroglial cells, where it catalyzes the conversion of glutamate to glutamine (Erecinska and Silver 90). Because astrocyte-derived glutamine is converted back to glutamate in neurons, and this glutamate is the principal source of vesicular GABA release at inhibitory synapses (Liang et al 06), an increased synthesis of GS at the time of estrogen negative feedback, may result in increased GABA availability to the synaptic cleft (Liang et al 06), and inhibition of GnRH secretion.
The hypothalamus of female rats at puberty shows opposite changes in the abundance of two enzymes expressed in glial cells: GS, and glutamate dehydrogenase (GDH), which reversibly catalyzes the synthesis of glutamate from oxoglutarate. While GS abundance decreases, GDH content increases, In contrast, the content of the neuron-specific glutamate-synthesizing enzyme phosphate-activated glutaminase (PaG) remains unchanged. These changes in GDH and GS protein expression may result in increased glutamatergic excitatory input to GnRH neurons (Roth et al 98;Roth et al 06), because they are accompanied by increased glutamate release in response to blockade of glutamate re-uptake transporters (Roth et al 06).
Rearrangements in glia-GnRH neuron connectivity
Following the pioneer work of Hatton and colleagues on vasopressin neurons (Hatton et al 84), a wealth of evidence has accumulated showing that astroglial cells are also physically and functionally linked to GnRH neurons [reviewed in (Mong and McCarthy 99;Garcia-Segura and McCarthy 04;Ojeda and Skinner 06)].
GnRH neurons are profusely apposed by astroglial cells; at the ME, tanycytes contribute significantly to this apposition (Kozlowski and Coates 85). Studies in the rat showedthat astrocytes apposition of GnRH neuronal cell bodies varies in a diurnal fashion, with lower apposition seen in the morning of proestrus, when estrogen levels are elevated (Cashion et al 03). This reduction in contact area may reflect a switch inglial engagement from neuronal cell bodies to the dendritic tree. Because astrocytes associate preferentially to postsynaptic dendritic spines (Haber et al 06), which contain almost exclusively glutamatergic synapses, an increased glial apposition of GnRH dendritic spines would result in facilitation of excitatory inputs to GnRH neurons. This interpretation is consistent with the finding that the glutamatergic input to GnRH neurons, determined by the abundance of dendritic spines, increases during sexual development (Cottrell et al 06). A sin the rat, glial apposition to GnRH neuronal perikarya in nonhuman primates is decreased by estrogen. Ovariectomy of adult rhesus monkeys increases this apposition, whereas administration of estradiol reverses the change (Witkin et al 91). Prepubertal female monkeys, which produce only small amounts of estrogen, also exhibit extensive glial apposition of GnRH neuronal perikarya (Witkin et al 95).
Noteworthy, the effects of estrogen on astrocyte morphology are not the same everywhere in the hypothalamus. In the rodent arcuate nucleus, the astrocytic surface apposing nonGnRH neurons increases when estradiol levels are high (Garcia-Segura and McCarthy 04), instead of decreasing. Because this increase is accompanied by a reduced number of axo-somatic synapses (which are mostly GABAergic) (Garcia-Segura and McCarthy 04), it is possible that, at the time of proestrous, astrocytes in this region of the hypothalamus reduce the inhibitory synaptic input to neuronal subsets synaptically connected to GnRH neurons.
During the rat estrous cyclemore GnRH neuronal terminals make physical contact with the pericapillary space of the ME in proestrus (when estrogen levels are high) than in diestrous II(when estrogen levels are low) (King and Rubin 96;Prevot et al 99). This rearrangement results from changes in tanycyte plasticity. Tanycytes, which line the ventral part of the third ventricle (Kozlowski and Coates 85;Witkin et al 91;King and Letourneau 94;Silverman et al 94;Rodriguez et al 05), use “end-feet” specializations to prevent the direct contact of GnRH terminals with the portal vasculature (Kozlowski and Coates 85;King and Letourneau 94). This blockade is lifted at the time of the preovulatory surge of gonadotropins; the end-feet retract allowing the GnRH terminals to reach the endothelial wall, presumably resulting in greater GnRH release into the portal blood (King and Rubin 96) [reviewed in (Prevot 02)]. Several studies have shed light into the cellular mechanisms underlying this plasticity. In one of these studies, TGFα-mediated activation of erbB1 receptors in tanycytes of the ME was shown to promote tanycytic outgrowth, and a PGE2-dependent production of TGFβ1 (Fig. 1), which then elicitedretraction of the tanycytic processes (Prevot et al 03a). By first promoting the outgrowth of tanycytic processes, and then the retraction of the processes via TGFβ1 release, TGFα mimics the morphological plasticity displayed by tanycytes during the preovulatory surge of GnRH.
Another study showed that purified endothelial cells of the ME induce acute actin cytoskeleton remodeling in ependymoglial cells, and demonstrated that this plasticity is mediated by nitric oxide (NO), a diffusible factor released from endothelial cells (De Seranno et al 04). Both soluble guanylyl cyclase and cyclooxygenase products were shown to mediate this endothelial-dependent control of ependymoglial cytoarchitecture. Very recently, these concepts have been expanded by the demonstration that estradiol at preovulatory levels acts on both endothelial cells and tanycytes to induce two key signaling events that ultimately result in retraction of tanycytic processes from their points of contact with endothelial cells. One of these events is the production of NO by endothelial cells; the other is the production of PGE2 by tanycytes (de Seranno S. et al 10). Synthesis of PGE2 in the ME was shown to be essential for tanycytic plasticity to be manifested and for estrous cyclicity to occur. These results established the concept that endothelial cells play a critical role in regulating glial plasticity in the ME, and strongly supported the notion that PGE2 produced in this region is required for the cyclic, periodic release of GnRH controlling reproductive cyclicity (Ojeda et al 75;Ojeda and Campbell 82).
Molecules mediating glial-GnRH neuron adhesiveness
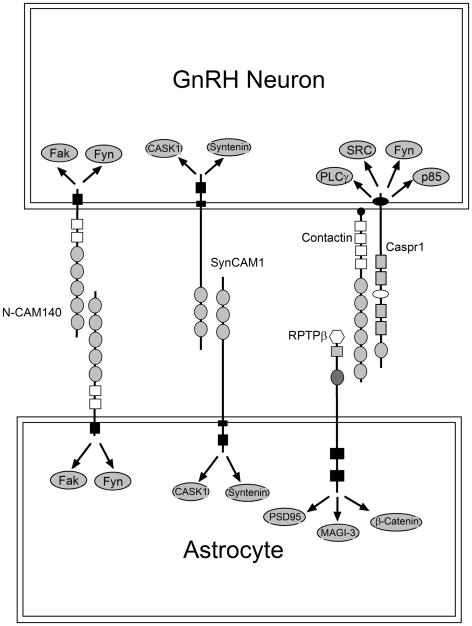
Although GnRH neurons and astrocytes maintain an intimate contact throughout development and adult life, the cell-surface molecules that may contribute to this adhesiveness remain largely unknown. Earlier immunohistochemical studies suggested that the sialylated form of the neural cell adhesion molecule NCAM (PSA-NCAM) is involved in this process (Perera et al 93;Parkash and Kaur 05). PSA-NCAM is abundant in brain regions endowed with a high degree of postnatal plasticity (Gascon et al 07), such as the medial basal hypothalamus-ME region (Perera et al 93). PSA-NCAM is also present in GnRH nerve terminals and glial cells of the ME (Parkash and Kaur 05), suggesting an involvement in the control of GnRH nerve terminal-glial adhesiveness (Fig. 2). This interaction may be, to a significant extent, mediated by NCAM-140, an isoform expressed in both neurons and glial cells (Povlsen et al 08).
Figure 2.
Molecules postulated to mediate glia-GnRH neuron adhesive communication in the hypothalamus. GnRH neurons adhere to astrocytes via at least three mechanisms: 1) Homophilic interactions involving NCAM140, 2) Homophilic interactions mediated by SynCAM1, and 3) heterophilic interactions mediated by the binding of contactin present in GnRH neurons to the receptor RPTPβ expressed in glial cells. Each of these systems has signaling capabilities suggesting that, in addition to providing an adhesive interaction, they can regulate astrocyte and GnRH neuron intracellular processes. This regulation is likely to occur via a variety of intracellular signaling molecules, some of which are shown in the figure.
Recent studies used mechanistic approaches to identify molecules mediating glia-GnRH neuron adhesiveness. One set of such molecules utilizes the glycosylphosphatidyl inositol (GPI)-anchored protein contactin (a cell-surface neuronal protein required for axonal-glial adhesiveness), and Receptor-like Protein Tyrosine Phosphatase β (RPTP β, a transmembrane phosphatase with structural similarities to cell adhesion molecules) as adhesive partners (Parent et al 07). Single cell RT-PCR of EGFP-tagged GnRH neurons revealed that 80% of GnRH neurons express the contactin gene. Hypothalamic astrocytes express the RPTPβ gene instead. The most abundant RPTPβ mRNA species found in these cells encodes an RPTPβ isoform (short RPTPβ) that uses its carbonic anhydrase (CAH) extracellular subdomain to interact with neuronal contactin. Immunoreactive contactin was found to be abundant in GnRH nerve terminals projecting toboth the organum vasculosum of the lamina terminalis (OVLT) and the ME, suggesting that GnRH axons are an important site of contactin-dependent cell adhesiveness. This notion is supported by the finding that immortalized GnRH-producing cells (GT1-7) adhere to the CAH domain of RPTPβ and extend neurites on this substrate. Disruption of contactin GPI anchoring or immunoneutralization of contactin inhibited GT1-7 cell adhesiveness and neurite growth on the CAH substrate, indicating that the adhesive effect of RPTPβ on GnRH neurons is mediated by contactin (Parent et al 07) (Fig. 2). Because the abundance of short RPTPβ mRNA increases in the female mouse hypothalamus before puberty, an enhanced interaction between GnRH axons and astrocytes mediated by heterophilic RPTPβ-contactin complexes may represent a major mechanism of GnRH neuron-glia communication during female sexual development.
Another cell membrane-spanning protein involved in GnRH-glia adhesive communication was identified using quantitative proteomics in a mouse model of delayed puberty (Prevot et al 03b). These mutant mice exhibit an astrocytic-specific defect in erbB4 signaling caused by the transgenic expression of a dominant-negative erbB4 receptor targeted to astrocytes by the GFAP promoter (Prevot et al 03b). The content of SynCAM, an adhesion molecule previously shown to be important for synaptic assembly (Biederer et al 02), was prominently reduced in the mutant hypothalamus in comparison with wild-type mice. SynCAM1 was shown to be abundant in hypothalamic astrocytes and to be strikingly diminished in astrocytes with impaired erbB4 signaling (Sandau, US, AE Mungenast, A Lomniczi, A-S Parent, SP Sardi, AI Fogel, T Biederer, G Corfas and SR Ojeda, in preparation). Ligand-dependent activation of astroglial erbB4 receptors results in rapid association of erbB4 with SynCAM1 and activation of SynCAM1 gene transcription. SynCAM1 is also expressed in GnRH neurons, and promotes astrocyte-GnRH neuron adhesiveness via hemophilic, extracellular domain-mediated, interactions (Fig. 2). To determine if astrocytic SynCAM1-dependent intracellular signaling is required for normal female reproductive function, transgenic mice were generated to express in an astrocyte-specific manner a dominant-negative form of SynCAM1 (DN-SynCAM1) lacking the intracellular domain. The mutant protein was correctly targeted to the cell membrane and was functionally viable as shown by its ability to block intracellular CASK redistribution, a major SynCAM1-mediated event. DN-SynCAM1 female mice had a delayed onset of puberty, disrupted estrous cyclicity and reduced fecundity(Sandau et al., in preparation). These results suggest that one of the mechanisms underlying erbB4 receptors facilitation of glial-neuronal interactions in the neuroendocrine brain involves SynCAM1-dependent signaling, and that this interaction is required for normal female reproductive function.
Additional adhesive molecules may also be involved. For instance, the presence of three multigene families of adhesion/signaling molecules with complementary functions has been reported in both the prepubertal female monkey hypothalamus and the GnRH-secreting cell line GT1-7 (Mungenast and Ojeda 05). One of these families is composed of a large number of synaptic specifiers termed neurexins; another is formed by protocadherins, a group of membrane-anchored proteins that function as synaptic adhesion molecules. The third family consists of members of the contactin-associated protein (Caspr) gene family (Mungenast and Ojeda 05). Despite the unmistakable presence of these molecules in the neuroendocrine brain, the contribution they have to the adhesiveness/plasticity of GnRH neurons and glial cells remain to be established. It should be noted, however, that contactin has been shown to interact in cis with Caspr1, whose cytoplasmic domain contains a proline-rich sequence with a canonical SH3 domain that associates with at least four SH domain-containing proteins, including Src, Fyn, p85 and PLCγ (Peles et al 95;Zisch et al 95).
Altogether, these results indicate that GnRH neurons adhere to astrocytes using both heterophilic (contactin/RPTPβ) and homophilic (SynCAM/SynCAM) interactions (Fig. 2). Because both systems have signaling capabilities (Peles et al 97;Biederer et al 02), it would appear that in addition to providing an adhesive interaction, these molecules can activate intracellular signaling cascades in both astrocyte and GnRH neurons [reviewed in (Lomniczi and Ojeda 09)] (Fig. 2).
Neuron-to-Glia Communication in the Hypothalamus
Neurons regulate glial activity using a variety of bioactive molecules, including neurotransmitters, ATP, and neuropeptides (Newman 03;Hertz and Zielke 04;Fields and Burnstock 06). Hypothalamic astrocytes respond to glutamate via both metabotropic receptors (mGluRs) of the mGluR5 subtype and ionotropic α-a-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid(AMPA) receptors. These receptors form a complex with erbB1 and erbB4 receptors (Dziedzic et al 03); simultaneous stimulation of metabotropic and AMPA receptors causes mobilization of erbB receptors to the cell surface, association of thesereceptors withtheir respective ligands TGFαand NRGs, and erbB receptor phosphorylation (Dziedzic et al 03) (Fig. 2).
For TGFα and NRG to interact with their respective receptors, their precursors need to be first cleaved by a metalloproteinase activity (Dziedzic et al 03). The metalloproteinase involved in processing the TGFα precursor has been shown to be tumor necrosis factor-α converting enzyme (TACE) (Peschon et al 98). In hypothalamic astrocytes, coactivation of astrocytic AMPA and metabotropic receptors causes extracellular Ca2+ influx, a Ca2+/protein kinase C-dependent increase in TACE-like activity, and enhanced release of TGFα (Lomniczi et al 06). TACE is abundant in astrocytes of the ME and becomes more active in this region at the time of the first preovulatory surge of gonadotropins. Inhibition of TACE activity in the ME decreases GnRH secretion and delays puberty indicating that an increased TACE activity in this region is necessary for the pubertal activation of GnRH secretion to take place (Lomniczi et al 06).
Conclusions
Both astrocytes and tanycytes participate in the control of GnRH secretion (Fig. 1). They physically engage GnRH neurons by adhering to the GnRH neuronal cell membrane, a process that is highly dynamic and subjected to sex steroid regulation. This cell-cell interaction appears to require cell membrane molecules endowed with both adhesive and signaling capabilities (Fig. 2). In addition, astrocytes and tanycytes release of a variety of substances that, directly or indirectly, stimulate GnRH secretion. Among these substances, growth factors of the EGF family play a central role. Glial cells integrate stimulatory inputs to the GnRH neuronal network by regulating glutamate availability for synaptic transmission and transducing glutamatergic signals into growth factor-mediated glia-to GnRH neuron signaling pathways.
Acknowledgments
This work was supported by grants from the National Institutes of Health HD25123, U54 HD18185 through cooperative agreement as part of the Specialized Cooperative Center’s Program in Reproduction and Infertility Research, National Institute of Child Health and Human Development /NIH, and RR000163 for the operation of the Oregon National Primate Research Center.
References
- 1.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 2.Barbour B, Szatkowski M, Ingledew N, Attwell D. Arachidonic acid induces a prolonged inhibition of glutamate uptake into glial cells. Nature. 1989;342:918–920. doi: 10.1038/342918a0. [DOI] [PubMed] [Google Scholar]
- 3.Beerli RR, Graus-Porta D, Woods-Cook K, Chen X, Yarden Y, Hynes NE. Neu differentiation factor activation of ErbB-3 and ErbB-4 is cell specific and displays a differential requirement for ErbB-2. Mol Cell Biol. 1995;15:6496–6505. doi: 10.1128/mcb.15.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 5.Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 6.Blutstein T, Devidze N, Choleris E, Jasnow AM, Pfaff DW, Mong JA. Oestradiol up-regulates glutamine synthetase mRNA and protein expression in the hypothalamus and hippocampus: implications for a role of hormonally responsive glia in amino acid neurotransmission. J Neuroendocrinol. 2006;18:692–702. doi: 10.1111/j.1365-2826.2006.01466.x. [DOI] [PubMed] [Google Scholar]
- 7.Brann DW. Glutamate: A major excitatory transmitter in neuroendocrine regulation. Neuroendocrinology. 1995;61:213–225. doi: 10.1159/000126843. [DOI] [PubMed] [Google Scholar]
- 8.Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 9.Burden S, Yarden Y. Neuregulins and their receptors: A versatile signaling module in organogenesis and oncogenesis. Neuron. 1997;18:847–855. doi: 10.1016/s0896-6273(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- 11.Carraway KL, Weber JL, Unger MJ, Ledesma J, Yu N, Gassmann M, Lai C. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature. 1997;387:512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- 12.Cashion AB, Smith MJ, Wise PM. The morphometry of astrocytes in the rostral preoptic area exhibits a diurnal rhythm on proestrus: relationship to the luteinizing hormone surge and effects of age. Endocrinology. 2003;144:274–280. doi: 10.1210/en.2002-220711. [DOI] [PubMed] [Google Scholar]
- 13.Chang H, Riese DJ, Gilbert W, Stern DF, McMahan UJ. Ligands for ErbB-family receptors encoded by a neuregulin-like gene. Nature. 1997;387:509–516. doi: 10.1038/387509a0. [DOI] [PubMed] [Google Scholar]
- 14.Cotrina ML, Lin JHC, Lopez-Garcia JC, Naus CCG, Nedergaard M. ATP-mediated glia signaling. J Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cottrell EC, Campbell RE, Han SK, Herbison AE. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:3652–3661. doi: 10.1210/en.2006-0296. [DOI] [PubMed] [Google Scholar]
- 16.de Seranno S, d’Anglemont de Tassigny X, Estrella C, Loyens A, Kasparov S, Leroy D, Ojeda SR, Beauvillain JC, Prevot V. Role of estradiol in the dynamic control of tanycyte plasticity mediated by vascular endothelial cells in the median eminence. Endocrinology. 2010;151:1760–1772. doi: 10.1210/en.2009-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Seranno S, Estrella C, Loyens A, Cornea A, Ojeda SR, Beauvillain SC, Prevot V. Vascular endothelial cells promote acute ependymoglial cell plasticty. J Neurosci. 2004;24:10353–10363. doi: 10.1523/JNEUROSCI.3228-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dungan HM, Clifton DK, Steiner RA. Minireview: Kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- 19.Dziedzic B, Prevot V, Lomniczi A, Jung H, Cornea A, Ojeda SR. Neuron-to-glia signaling mediated by excitatory amino acid receptors regulates erbB receptor function in astroglial cells of the neuroendocrine brain. J Neurosci. 2003;23:915–926. doi: 10.1523/JNEUROSCI.23-03-00915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erecinska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 21.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 22.Fellin T, Sul JY, D’Ascenzo M, Takano H, Pascual O, Haydon PG. Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP. Purinergic signalling in Neuron-Glia Interactions (Norvartis Foundation Symposium 276); Chichester: Wiley; 2006. pp. 208–221. [DOI] [PubMed] [Google Scholar]
- 23.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fields RD, Stevens B. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 2000;23:625–633. doi: 10.1016/s0166-2236(00)01674-x. [DOI] [PubMed] [Google Scholar]
- 25.Galbiati M, Zanisi M, Messi E, Cavarretta I, Martini L, Melcangi RC. Transforming growth factor-β and astrocytic conditioned medium influence luteinizing hormone-releasing hormone gene expression in the hypothalamic cell line GT1. Endocrinology. 1996;137:5605–5609. doi: 10.1210/endo.137.12.8940390. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Segura LM, McCarthy MM. Minireview: Role of glia in neuroendocrine function. Endocrinology. 2004;145:1082–1086. doi: 10.1210/en.2003-1383. [DOI] [PubMed] [Google Scholar]
- 27.Gascon E, Vutskits L, Kiss JZ. Polysialic acid-neural cell adhesion molecule in brain plasticity: From synapses to integration of new neurons. Brain Res Rev. 2007;56:101–118. doi: 10.1016/j.brainresrev.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006;26:8881–8891. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatton GI, Perlmutter LS, Salm AK, Tweedle CD. Dynamic neuronal-glial interactions in hypothalamus and pituitary: Implications for control of hormone synthesis and release. Peptides. 1984;5:121–138. doi: 10.1016/0196-9781(84)90271-7. [DOI] [PubMed] [Google Scholar]
- 30.Haydon PG. Glia: Listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 31.Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Hiney JK, Ojeda SR, Dees WL. Insulin-like growth factor-I: A possible metabolic signal involved in the regulation of female puberty. Neuroendocrinology. 1991;54:420–423. doi: 10.1159/000125924. [DOI] [PubMed] [Google Scholar]
- 33.Jung H, Carmel P, Schwartz MS, Witkin JW, Bentele KHP, Westphal M, Piatt JH, Costa ME, Cornea A, Ma YJ, Ojeda SR. Some hypothalamic hamartomas contain transforming growth factor alpha, a puberty-inducing growth factor, but not luteinizing hormone-releasing hormone neurons. J Clin Endocrinol Metab. 1999;84:4695–4701. doi: 10.1210/jcem.84.12.6185. [DOI] [PubMed] [Google Scholar]
- 34.Junier MP, Hill DF, Costa ME, Felder S, Ojeda SR. Hypothalamic lesions that induce female precocious puberty activate glial expression of the epidermal growth factor receptor gene: Differential regulation of alternatively spliced transcripts. J Neurosci. 1993;13:703–713. doi: 10.1523/JNEUROSCI.13-02-00703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Junier MP, Ma YJ, Costa ME, Hoffman G, Hill DF, Ojeda SR. Transforming growth factor alpha contributes to the mechanism by which hypothalamic injury induces precocious puberty. Proc Natl Acad Sci USA. 1991;88:9743–9747. doi: 10.1073/pnas.88.21.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karunagaran D, Tsahar E, Beerli RR, Chen X, Graus-Porta D, Ratzkin BJ, Seger R, Hynes NE, Yarden Y. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: Implications for breast cancer. EMBO J. 1996;15:254–264. [PMC free article] [PubMed] [Google Scholar]
- 37.King JC, Letourneau RL. Luteinizing hormone-releasing hormone terminals in the median eminence of rats undergo dramatic changes after gonadectomy, as revealed by electron microscopic image analysis. Endocrinology. 1994;134:1340–1351. doi: 10.1210/endo.134.3.8119174. [DOI] [PubMed] [Google Scholar]
- 38.King JC, Rubin BS. Recruitment of LHRH neurons and increased access of LHRH terminals to portal capillaries: Integral mechanisms for LH surge induction. Ann d’ Endocrinol (Paris) 1996;57(Suppl 4):72. [Google Scholar]
- 39.Kordon C, Drouva SV, Martinez de la Escalera G, Weiner RI. Role of classic and peptide neuromediators in the neuroendocrine regulation of luteinizing hormone and prolactin. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. Vol. 1. Raven Press; New York: 1994. pp. 1621–1681. [Google Scholar]
- 40.Kozlowski GP, Coates PW. Ependymoneuronal specializations between LHRH fibers and cells of the cerebroventricular system. Cell Tissue Res. 1985;242:301–311. doi: 10.1007/BF00214542. [DOI] [PubMed] [Google Scholar]
- 41.Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J Neurosci. 2006;26:8537–8548. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lomniczi A, Cornea A, Costa ME, Ojeda SR. Hypothalamic tumor necrosis factor-α converting enzyme (TACE) mediates excitatory amino acid-dependent neuron-to-glia signaling in the neuroendocrine brain. J Neurosci. 2006;26:51–62. doi: 10.1523/JNEUROSCI.2939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lomniczi A, Ojeda SR. A role for glial cells of the neuroendocrine brain in the central control of female sexual development. In: Parpura V, Haydon P, editors. Astrocytes in (Patho)Physiology of the Nervous System. Springer; NY: 2009. pp. 487–511. [Google Scholar]
- 44.Ma YJ, Berg-von der Emde K, Rage F, Wetsel WC, Ojeda SR. Hypothalamic astrocytes respond to transforming growth factor alpha with secretion of neuroactive substances that stimulate the release of luteinizing hormone-releasing hormone. Endocrinology. 1997;138:19–25. doi: 10.1210/endo.138.1.4863. [DOI] [PubMed] [Google Scholar]
- 45.Ma YJ, Dissen GA, Merlino G, Coquelin A, Ojeda SR. Overexpression of a human transforming growth factor alpha (TGFα) transgene reveals a dual antagonistic role of TGFα in female sexual development. Endocrinology. 1994a;135:1392–1400. doi: 10.1210/endo.135.4.7925101. [DOI] [PubMed] [Google Scholar]
- 46.Ma YJ, Hill DF, Creswick KE, Costa ME, Ojeda SR. Neuregulins signaling via a glial erbB2/erbB4 receptor complex contribute to the neuroendocrine control of mammalian sexual development. J Neurosci. 1999;19:9913–9927. doi: 10.1523/JNEUROSCI.19-22-09913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma YJ, Hill DF, Junier MP, Costa ME, Felder SE, Ojeda SR. Expression of epidermal growth factor receptor changes in the hypothalamus during the onset of female puberty. Mol Cell Neurosci. 1994b;5:246–262. doi: 10.1006/mcne.1994.1029. [DOI] [PubMed] [Google Scholar]
- 48.Ma YJ, Junier MP, Costa ME, Ojeda SR. Transforming growth factor alpha (TGFα) gene expression in the hypothalamus is developmentally regulated and linked to sexual maturation. Neuron. 1992;9:657–670. doi: 10.1016/0896-6273(92)90029-d. [DOI] [PubMed] [Google Scholar]
- 49.Mahesh VB, Dhandapani KM, Brann DW. Role of astrocytes in reproduction and neuroprotection. Mol Cell Endocrinol. 2006;246:1–9. doi: 10.1016/j.mce.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Melcangi RC, Galbiati M, Messi E, Piva F, Martini L, Motta M. Type 1 astrocytes influence luteinizing hormone-releasing hormone release from the hypothalamic cell line GT1–1: Is transforming growth factor-β the principle involved? Endocrinology. 1995;136:679–686. doi: 10.1210/endo.136.2.7835301. [DOI] [PubMed] [Google Scholar]
- 51.Mong JA, Blutstein T. Estradiol modulation of astrocytic form and function: implications for hormonal control of synaptic communication. Neuroscience. 2006;138:967–975. doi: 10.1016/j.neuroscience.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 52.Mong JA, McCarthy MM. Steroid-induced developmental plasticity in hypothalamic astrocytes: implications for synaptic patterning. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 53.Montero JC, Yuste L, Diaz-Rodriguez E, Esparis-Ogando A, Pandiella A. Differential shedding of transmembrane neuregulin isoforms by the tumor necrosis factor-alpha-converting enzyme. Mol Cell Neurosci. 2000;16:631–648. doi: 10.1006/mcne.2000.0896. [DOI] [PubMed] [Google Scholar]
- 54.Mungenast A, Ojeda SR. Expression of three gene families encoding cell-cell communication molecules in the prepubertal nonhuman primate hypothalamus. J Neuroendocrinol. 2005;17:208–219. doi: 10.1111/j.1365-2826.2005.01293.x. [DOI] [PubMed] [Google Scholar]
- 55.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Newman EA. New roles for astrocytes: Regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- 57.Ojeda SR, Campbell WB. An increase in hypothalamic capacity to synthesize prostaglandin E2 precedes the first preovulatory surge of gonadotropins. Endocrinology. 1982;111:1031–1037. doi: 10.1210/endo-111-4-1031. [DOI] [PubMed] [Google Scholar]
- 58.Ojeda SR, Harms PG, McCann SM. Effect of inhibitors of prostaglandin synthesis on gonadotropin release in the rat. Endocrinology. 1975;97:843–854. doi: 10.1210/endo-97-4-843. [DOI] [PubMed] [Google Scholar]
- 59.Ojeda SR, Prevot V, Heger S, Lomniczi A, Dziedzic B, Mungenast A. Glia-to neuron signaling and the neuroendocrine control of female puberty. Ann Med. 2003;35:244–255. doi: 10.1080/07853890310005164. [DOI] [PubMed] [Google Scholar]
- 60.Ojeda SR, Skinner MK. Puberty in the rat. In: Neill JD, editor. The Physiology of Reproduction. 3. Academic Press/Elsevier; San Diego: 2006. pp. 2061–2126. [Google Scholar]
- 61.Ojeda SR, Terasawa E. Neuroendocrine regulation of puberty. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain and Behavior. Vol. 4. Elsevier; New York: 2002. pp. 589–659. [Google Scholar]
- 62.Ojeda SR, Urbanski HF, Costa ME, Hill DF, Moholt-Siebert M. Involvement of transforming growth factor α in the release of luteinizing hormone-releasing hormone from the developing female hypothalamus. Proc Natl Acad Sci USA. 1990;87:9698–9702. doi: 10.1073/pnas.87.24.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olson BR, Scott DC, Wetsel WC, Elliot SJ, Tomic M, Stojilkovic S, Nieman LK, Wray S. Effects of insulin-like growth factors I and II and insulin on the immortalized hypothalamic GT1–7 cell line. Neuroendocrinology. 1995;62:155–165. doi: 10.1159/000127000. [DOI] [PubMed] [Google Scholar]
- 64.Parent AS, Mungenast AE, Lomniczi A, Sandau US, Peles E, Bosch MA, Ronnekleiv OK, Ojeda SR. A contactin-receptor-like protein tyrosine phosphatase beta complex mediates adhesive communication between astroglial cells and gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2007;19:847–859. doi: 10.1111/j.1365-2826.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 65.Parkash J, Kaur G. Neuronal-glial plasticity in gonadotropin-releasing hormone release in adult female rats: role of the polysialylated form of the neural cell adhesion molecule. J Endocrinol. 2005;186:397–409. doi: 10.1677/joe.1.06156. [DOI] [PubMed] [Google Scholar]
- 66.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 67.Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, Clary DO, Schilling J, Barnea G, Plowman GD, Grumet M, Schlessinger J. The carbonic anhydrase domain of receptor tyrosine phosphatase β is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- 68.Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, Plowman GD, Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perera AD, Lagenaur CF, Plant TM. Postnatal expression of polysialic acid-neural cell adhesion molecule in the hypothalamus of the male rhesus monkey (Macaca mulatta) Endocrinology. 1993;133:2729–2735. doi: 10.1210/endo.133.6.7694845. [DOI] [PubMed] [Google Scholar]
- 70.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Koslosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 71.Plant TM. Neurophysiology of puberty. J Adolesc Health. 2002;31:185–191. doi: 10.1016/s1054-139x(02)00484-6. [DOI] [PubMed] [Google Scholar]
- 72.Plant TM, Witchel SF. Puberty in nonhuman primates and humans. In: Neill JD, editor. The Physiology of Reproduction. 3. Academic Press/Elsevier; San Diego: 2006. pp. 2177–2230. [Google Scholar]
- 73.Povlsen GK, Berezin V, Bock E. Neural cell adhesion molecule-180-mediated homophilic binding induces epidermal growth factor receptor (EGFR) down-regulation and uncouples the inhibitory function of EGFR in neurite outgrowth. J Neurochem. 2008;104:624–639. doi: 10.1111/j.1471-4159.2007.05033.x. [DOI] [PubMed] [Google Scholar]
- 74.Prevot V. Glial-neuronal-endothelial interactions are involved in the control of GnRH secretion. J Neuroendocrinol. 2002;14:247–255. doi: 10.1046/j.0007-1331.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- 75.Prevot V, Cornea A, Mungenast A, Smiley G, Ojeda SR. Activation of erbB-1 signaling in tanycytes of the median eminence stimulates transforming growth factor β 1 release via prostaglandin E2 production and induces cell plasticity. J Neuroscience. 2003a;23:10622–10632. doi: 10.1523/JNEUROSCI.23-33-10622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prevot V, Croix D, Bouret S, Dutoit S, Tramu G, Stefano GB, Beauvillain JC. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: Implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience. 1999;94:809–819. doi: 10.1016/s0306-4522(99)00383-8. [DOI] [PubMed] [Google Scholar]
- 77.Prevot V, Lomniczi A, Corfas G, Ojeda SR. ErbB-1 and erbB-4 receptors act in concert to facilitate both female sexual development and mature reproductive function. Endocrinology. 2005;146:1465–1472. doi: 10.1210/en.2004-1146. [DOI] [PubMed] [Google Scholar]
- 78.Prevot V, Rio C, Cho GJ, Lomniczi A, Heger S, Neville CM, Rosenthal NA, Ojeda SR, Corfas G. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci. 2003b;23:230–239. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rage F, Hill DF, Sena-Esteves M, Breakefield XO, Coffey RJ, Costa ME, McCann SM, Ojeda SR. Targeting transforming growth factor α expression to discrete loci of the neuroendocrine brain induces female sexual precocity. Proc Natl Acad Sci USA. 1997;94:2735–2740. doi: 10.1073/pnas.94.6.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riese DJ, Bermingham Y, van Raaij TM, Buckley S, Plowman GD, Stern DF. Betacellulin activates the epidermal growth factor receptor and erbB-4, and induces cellular response patterns distinct from those stimulated by epidermal growth factor or neuregulin-beta. Oncogene. 1996a;12:345–353. [PubMed] [Google Scholar]
- 81.Riese DJ, Kim ED, Elenius K, Buckley S, Klagsbrun M, Plowman GD, Stern DF. The epidermal growth factor receptor couples transforming growth factor-α heparin-binding epidermal growth factor-like factor, and amphiregulin to neu, ErbB-3 and ErbB-4. J Biol Chem. 1996b;271:20047–20052. doi: 10.1074/jbc.271.33.20047. [DOI] [PubMed] [Google Scholar]
- 82.Rimer M. Neuregulins: primary or secondary signals for the control of synapse-specific gene expression. J Neurocytol. 2003;32:665–675. doi: 10.1023/B:NEUR.0000020615.79831.51. [DOI] [PubMed] [Google Scholar]
- 83.Rodriguez EM, Blazquez JL, Pastor FE, Pelaez B, Pena P, Peruzzo B, Amat P. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 84.Roth C, Leonhardt S, Theiling K, Lakomek M, Jarry H, Wuttke W. Ontogeny of the GnRH-, glutaminase- and glutamate decarboxylase-gene expression in the hypothalamus of female rats. Brain Res Dev Brain Res. 1998;110:105–114. doi: 10.1016/s0165-3806(98)00102-3. [DOI] [PubMed] [Google Scholar]
- 85.Roth CL, McCormack AL, Lomniczi A, Mungenast AE, Ojeda SR. Quantitative proteomics identifies a major change in glial glutamate metabolism at the time of female puberty. Mol Cell Endocrinol. 2006;254–255:51–59. doi: 10.1016/j.mce.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 86.Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peshon J, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shelly M, Pinkas-Kramarski R, Guarino BC, Waterman H, Wang LM, Lyass L, Alimandi M, Kuo A, Bacus SS, Pierce HJ, Andrews GC, Yarden Y. Epiregulin is a potent pan-ErbB ligand that preferentially activates heterodimeric receptor complexes. J Biol Chem. 1998;273:10496–10505. doi: 10.1074/jbc.273.17.10496. [DOI] [PubMed] [Google Scholar]
- 88.Silverman A-J, Livne I, Witkin JW. The gonadotropin-releasing hormone (GnRH), neuronal systems: Immunocytochemistry and in situ hybridization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. Vol. 1. Raven Press; New York: 1994. pp. 1683–1709. [Google Scholar]
- 89.Stella N, Tence M, Glowinski J, Premont J. Glutamate-evoked release of arachidonic acid from mouse brain astrocytes. J Neurosci. 1994;14:568–575. doi: 10.1523/JNEUROSCI.14-02-00568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Terasawa E. Role of ATP in synchronization of Ca2+ oscillations in LHRH neurons in vitro. 5th Int’l Congress of Neuroendocrinology (S20); August 30-September 6, 2002; Bristol, UK. 2002. Ref Type: Abstract. [Google Scholar]
- 91.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 92.Terasawa E, Keen KL, Grendell RL, Golos TG. Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol. 2005;19:2736–2747. doi: 10.1210/me.2005-0034. [DOI] [PubMed] [Google Scholar]
- 93.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2008;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsai PS, Moenter SM, Postigo HR, El Majdoubi M, Pak TR, Gill JC, Paruthiyil S, Werner S, Weiner RI. Targeted expression of a dominant-negative fibroblast growth factor (FGF) receptor in gonadotropin-releasing hormone (GnRH) neurons reduces FGF responsiveness and the size of GnRH neuronal population. Mol Endocrinol. 2005;19:225–236. doi: 10.1210/me.2004-0330. [DOI] [PubMed] [Google Scholar]
- 95.Tsai PS, Werner S, Weiner RI. Basic fibroblast growth factor is a neurotropic factor in GT1 gonadotropin-releasing hormone neuronal cell lines. Endocrinology. 1995;136:3831–3838. doi: 10.1210/endo.136.9.7649090. [DOI] [PubMed] [Google Scholar]
- 96.Voigt P, Ma YJ, Gonzalez D, Fahrenbach WH, Wetsel WC, Berg-von der Emde K, Hill DF, Taylor KG, Costa ME, Seidah NG, Ojeda SR. Neural and glial-mediated effects of growth factors acting via tyrosine kinase receptors on LHRH neurons. Endocrinology. 1996;137:2593–2605. doi: 10.1210/endo.137.6.8641214. [DOI] [PubMed] [Google Scholar]
- 97.Wetsel WC, Hill DF, Ojeda SR. Basic fibroblast growth factor regulates the conversion of pro-luteinizing hormone-releasing hormone (LHRH) to LHRH in immortalized hypothalamic neurons. Endocrinology. 1996;137:2606–2616. doi: 10.1210/endo.137.6.8641215. [DOI] [PubMed] [Google Scholar]
- 98.Witkin JW, Ferin M, Popilskis SJ, Silverman AJ. Effects of gonadal steroids on the ultrastructure of GnRH neurons in the rhesus monkey: Synaptic input and glial apposition. Endocrinology. 1991;129:1083–1092. doi: 10.1210/endo-129-2-1083. [DOI] [PubMed] [Google Scholar]
- 99.Witkin JW, O’Sullivan H, Ferin M. Glial ensheathment of GnRH neurons in pubertal female rhesus macaques. J Neuroendocrinol. 1995;7:665–671. doi: 10.1111/j.1365-2826.1995.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 100.Zhang D, Sliwkowski MX, Mark M, Frantz G, Akita R, Sun Y, Hillan K, Crowley C, Brush J, Godowski PJ. Neuregulin-3 (NGR3): A novel neural tissue-enriched protein that binds and activates ErbB4. Proc Natl Acad Sci USA. 1997;94:9562–9567. doi: 10.1073/pnas.94.18.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zisch AH, D’Alessandri L, Amrein K, Ranscht B, Winterhalter KH, Vaughan L. The glypiated neuronal cell adhesion molecule contactin/F11 complexes with src-family protein tyrosine kinase Fyn. Mol Cell Neurosci. 1995;6:263–279. doi: 10.1006/mcne.1995.1021. [DOI] [PubMed] [Google Scholar]
- 102.Zonta M, Sebelin A, Gobbo S, Fellin T, Pozzan T, Carmignoto G. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J Physiol. 2003;553:407–414. doi: 10.1113/jphysiol.2003.046706. [DOI] [PMC free article] [PubMed] [Google Scholar]