Abstract
Context
Depression is a consistent predictor of recurrent events and mortality in ACS patients, but it has 2 core diagnostic criteria with distinct biological correlates—depressed mood and anhedonia.
Objective
To determine if depressed mood and/or anhedonia (loss of pleasure or interest) predict 1-year medical outcomes for patients with Acute Coronary Syndrome (ACS).
Design
Observational cohort study of post-ACS patients hospitalized between May 2003 and June 2005. Within one week of admission, patients underwent a structured psychiatric interview to assess clinically impairing depressed mood, anhedonia, and major depressive episode (MDE); also assessed were the Global Registry of Acute Coronary Events risk score, Charlson comorbidity index, left ventricular ejection fraction, antidepressant use, and depressive symptom severity.
Setting
Coronary care and cardiac care step-down units of 3 university hospitals in New York and Connecticut.
Participants
Consecutive sample of 453 ACS patients (aged 25–93 years; 42% women).
Main Outcomes Measures
All-cause mortality (ACM) and documented major adverse cardiac events (MACE; myocardial infarction, hospitalization for unstable angina, or urgent revascularization) were actively surveyed for 1 year after admission.
Results
There were 67 events (16 deaths and 51 MACE; 14.8%). 108 (24%) and 77 (17%) patients with anhedonia and depressed mood, respectively. After controlling for sex, age, and medical covariates, anhedonia (adjusted hazard ratio, 1.58; 95% confidence interval, 1.16–2.14; P<.01) and MDE (adjusted hazard ratio, 1.48; 95% confidence interval, 1.07–2.04; P=.02) were significant predictors of combined MACE/ACM, but depressed mood was not. Anhedonia continued to significantly predict outcomes controlling for MDE diagnosis and depressive symptom severity, each of which were no longer significant.
Conclusions
Anhedonia identifies risk for MACE/ACM beyond that of established medical prognostic indicators. Biological correlates of anhedonia may add to the understanding of the link between depression and heart disease.
Keywords: Acute coronary syndromes, mortality, depressio, anhedonia
INTRODUCTION
Systematic reviews have concluded that depression, identified either by self-reported depressive symptom severity or by psychiatric interview, confers an independent mortality and morbidity risk in acute coronary syndrome (ACS) patients.1–3 Moreover, in patients with established coronary heart disease (CHD), the presence of a depression diagnosis is more important than CHD severity in predicting quality of life and overall perceived health.4, 5 This risk has been observed in recent studies and meta-analyses, despite continued improvement in cardiology interventions, medications, and care.3, 6–14 Recent calls have been made for the recognition of depression as an independent risk marker for ACS patients,15 and evidence-based patient grand rounds16 are now being provided to assess and manage this predictor of poor cardiac prognosis. Despite growing acceptance of depression as a cardiac risk factor,17 initial attempts to prevent mortality or recurrence by treating depression in ACS patients have not been successful.18, 19 In addition, some doubt remains about the power of depression as an independent risk marker beyond the power of established clinical disease markers, since many prognostic studies have not adequately controlled for clinical and cardiovascular covariates such as left ventricular ejection fraction.13
To better understand how depression predicts cardiac risk, some have suggested searching for subtypes of depression that are particularly cardiotoxic.1, 14, 20 Major depression is a complex phenotype encompassing a wide range of symptoms, not all of which are present in all patients. To receive a diagnosis of major depression, 1 of 2 core functionally impairing criteria must be present—depressed mood (sadness and the report of feeling depressed) or anhedonia (markedly diminished interest or pleasure in all, or almost all, activities). These symptoms must be present most of the day, nearly every day. Depressed mood and anhedonia have been posited to have distinct biological correlates; while depressed mood is typically associated with central serotonergic dysfunction, anhedonia has recently been linked to cathecholaminergic dysfunction.21 Thus, it is possible that depressed mood and/or anhedonia may differentially predict clinical outcomes in post-ACS patients.
The aim of the present study was to determine the independent prognostic significance the 2 core criteria of depression, as judged by a trained psychiatric interviewer, for major adverse cardiac events (MACE; myocardial infarction [MI], hospitalization for unstable angina, or urgent revascularization) or all-cause mortality (ACM) in ACS patients. We chose as covariates established clinical prognostic markers of recurrent clinical events and mortality in ACS patients:13, 22, 23 the Global Registry of Acute Coronary Events (GRACE) risk score, a composite prediction score recently shown to predict the 12-month risk of MACE or ACM in post-ACS patients discharged from the hospital24, 25; the Charlson comorbidity index23; left ventricular ejection fraction (LVEF); age; sex; and antidepressant use. In addition, we assessed whether depressed mood and anhedonia carried independent prognostic risk beyond that associated with previously established markers of depression—clinical diagnosis of major depressive episode (MDE) and self-reported depressive symptom severity.
METHODS
Study Sample
The study included post-ACS patients who were admitted to the coronary care and cardiac care step-down units of 3 university hospitals (Mount Sinai Hospital, New York, NY, and Yale–New Haven Hospital and Hospital of St. Raphael, New Haven, CT) between May 2003 and June 2005. The institutional review boards of each hospital approved the study.
Inclusion Criteria
Patients who were aged 18 years or older and spoke English or Spanish were asked to provide informed consent within 1 week of admission to hospital for the index ACS event. Patients were eligible if they met the criteria for ACS (either acute MI with or without ST-segment elevation or unstable angina) verified by the study cardiologists using standard ACS criteria26 and had valid scores (0–4, indicating minimal depressive symptoms, or ≥10, indicating at least mild depressive symptoms) on the Beck Depression Inventory (BDI)27 assessed within 1 week after the index ACS event.28 Patients with BDI scores between 5 and 9 were excluded to more clearly delineate depressed and nondepressed group at baseline. Patients were also ineligible for study participation if they had a terminal illness (life expectancy <1 year), were active substance abusers, or had cognitive impairment; if the screening could not be completed within 1 week of the initial hospitalization date; or if they were unavailable for follow-up visits.
Depression Measures
In addition to the self-report measure of depressive symptom severity (BDI), all patients underwent the semistructured diagnostic interview developed for the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) trial29 to determine the presence of MDE according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).30 When trained interviewers use this interview and quality assurance is conducted, the concordance with other structured psychiatric interviews is excellent.29 In our study, one clinical psychologist and one psychiatrist independently reviewed the trained interviewers’ audiotapes and written notes for each interview and verified all diagnoses. The presence of depressed mood and anhedonia was determined for patients according to the standard criteria used in psychiatric interviews—that the symptom be clinically impairing and present for at least 2 weeks, most of the day, nearly every day.
Medical Covariates
GRACE Risk Score, Charlson Comorbidity Index, and Antidepressant Use
We employed the GRACE risk score to measure established mortality risk factors in post-ACS patients.25 Prognostic markers in the final GRACE model are advanced age, history of MI and heart failure, elevated pulse rate and systolic blood pressure at presentation to hospital, elevated initial serum creatinine level, elevated initial cardiac enzyme level, ST-segment depression evident on electrocardiogram at presentation, and percutaneous coronary intervention (PCI) performed in hospital. The GRACE risk score ranges from 1 to 263 points, with higher scores indicating higher mortality risk. A GRACE risk score of 80 predicts a 1% mortality rate at 6 months; 100, a 2% mortality rate; and higher than 210, a mortality rate greater than 50%. 25 Data for the GRACE risk score, the Charlson comorbidity index,23, 31 and antidepressant use at discharge from hospital were collected from the medical records or patient history.
Left Ventricular Ejection Fraction Values
Values for LVEF were measured quantitatively by left ventriculogram during cardiac catheterization (43% of patients), by echocardiogram (50% of patients), or by nuclear study (7% of patients). If multiple variables were available, the value from the ventriculogram was used. Values for LVEF were then categorized into 2 groups: normal to mild dysfunction (LVEF ≥40%) and moderate to severe dysfunction (LVEF <40%).
Ascertainment of MACE and ACM
The primary endpoint of the study was either the first occurrence of a MACE (hospitalization for nonfatal MI, urgent cardiac revascularization, or unstable angina) or ACM by 12 months. Study participants were proactively contacted to complete follow-up assessments at 1, 3, 6, and 18 months either by telephone or in person. For any patient-reported hospitalization, supporting documentation of the event was requested from the hospital’s records. An endpoint committee consisting of 2 board-certified cardiologists independently reviewed and classified each hospitalization; in case of disagreement, a third board-certified cardiologist adjudicated the final endpoint. All cardiologists were unaware of the participants’ depression status. For participants who could not be contacted or were reported deceased by a relative, the Social Security Death Index was searched to verify vital status, and death certificates were obtained.
Statistical Analysis
The t test for continuous variables and χ2 analysis for categorical variables tested baseline variables for differences between those with and without depressed mood or anhedonia. The familywide α error underwent Bonferroni correction within variable grouping (eg, demographic variables, Charlson comorbidity index, and GRACE risk score components). When baseline medical covariate data were missing, a regression-based approach was used to impute the best linear-predicted score based on the nonmissing items.
Cox proportional hazards models were used to estimate the hazard ratios for MACE/ACM, stratified by hospital. The depression predictors (depressed mood, anhedonia, and MDE) were first tested separately, adjusting for specified covariates: GRACE risk score, Charlson comorbidity index, LVEF value (<40% vs ≥40%), antidepressant use, age, and sex. We followed suggestions for apriori selection for covariate inclusion.32, 33 First, we listed the covariates from published findings that might confound the Depression—MACE/ACM association: age, sex, left ventricular ejection fraction, medical comorbidities (Charlson), clinical prognostic index (GRACE), and anti-depressant use. We then calculated how many covariates we could include in the model based on a ratio of at least 10 events per covariate; as we had 67 events, we could include up to 6 covariates. Depressed mood and anhedonia were then tested to determine if they predicted outcomes over and above the power of MDE and the selected covariates. This was followed by tests of these 2 predictors over and above BDI scores to rule out the possibility that they are simply markers of depressive symptom severity.
The proportional hazards assumption was met for all presented models. Follow-up was censored at 12 months after the baseline interview. All analyses were performed using SPSS (version 16; SPSS Inc, Chicago, IL).
RESULTS
During medical eligibility screening (no terminal illness, no alcoholism/drug addiction, no dementia, available for follow-up), we identified 677 patients for the study. Of these, 211 (31%) had BDI scores of 5 to 9, 4 were suicidal and were referred for treatment, and 5 withdrew before the baseline examination was completed. Of the 457 enrolled patients, 4 were excluded from the current analysis because the psychiatric interview was not completed within the designated time. Patients who were unavailable or refused to be screened differed significantly from patients who participated in the study; they were older (mean [SD] age, 65.2 [11.9] vs 61.5 [12.2] years; P<.001), and more were Hispanic by self-report (24.3% vs 12.5%; P=.01). No other differences were noted.
The remaining 453 patients had an average age of 61 years (range, 25–93 years), and 42% were women, 80% white, and 10% Hispanic. Twenty-one percent of the patients were diagnosed with myocardial infarction with ST-segment elevation; 46%, unstable angina; and 33%, myocardial infarction with non–ST-segment elevation. For the depression variables, 48 patients (11%) met the criteria for MDE, 108 (24%) were rated as having depressed mood, and 77 (17%) were rated as having anhedonia. Furthermore, 39 patients (36%) with depressed mood and 35 patients (45%) with anhedonia met the criteria for MDE. Anhedonia and depressed mood were highly intercorrelated (r = .74, P < .001).
Demographic and medical variables at baseline according to depressed mood and anhedonia are shown in Table 1. Those with depressed mood were significantly more likely to be female, to have higher Charlson comorbidity index scores, to be younger, to have an MDE, to have higher rates of depressive symptoms, and to have an antidepressant prescribed. Patients with anhedonia were significantly more likely to be female and to have a higher Charlson comorbidity index score. They did not differ from patients without anhedonia on GRACE risk score, but they did receive significantly fewer percutaneous interventions while in the hospital, and they were significantly younger. Finally, they were significantly more likely to have an MDE, to have more severe depressive symptoms, and to have an antidepressant prescribed.
Table 1.
Demographic and Clinical Characteristics of 453 Patients with Severe Anhedonia or Depressed Mooda
| Depressed Mood | Anhedonia | |||||
|---|---|---|---|---|---|---|
| Characteristic | Absent (n=345) |
Present (n=108) |
P | Absent (n=376) |
Present (n=77) |
P |
| Demographic Characteristicsc | ||||||
| Women, No. (%) | 132 (38.3) | 56 (51.9) | .01 | 146 (38.9) | 42 (555) | .01 |
| Age, y | 62 (12.3) | 58 (12.6) | .001 | 62 (12.3) | 55 (11.6) | <.001 |
| Medical Covariates | ||||||
| LVEF <40% | 51 (14.8) | 18 (16.7) | .63 | 56 (14.9) | 13 (17) | .66 |
| Charlson comorbidity index23; | 1.21 (1.4) | 1.85 (1.9) | .002b | 1.28 (1.5) | 1.79 (1.8) | .009 |
| GRACE24, 25 risk scorec | 92.2 (30.6) | 88.0 (33.0) | .23 | 92.5 (31.2) | 84.64 (30.8) | .04 |
| Prior MI | 0.35 (0.7) | 0.50 (0.8) | .06 | 0.37 (0.7) | 0.47 (0.8) | .15 |
| Prior congestive heart failure | 33 (9.6) | 10 (9.9) | .92 | 35 (9.4) | 8 (11.0) | .68 |
| Heart rate at admission | 76.1 (18.6) | 80.7 (17.6) | .03 | 76.3 (18.5) | 81.42 (18.0) | .03 |
| Systolic blood | 143.9 (30.0) | 137.2 (24.2) | .046 | 143.6 (29.5) | 136.1 (24.7) | .046 |
| pressure at admission | ||||||
| ST-segment deviation | 57 (17.1) | 15 (15.0) | .62 | 61 (16.8) | 11 (15.9) | .87 |
| Serum creatinine level at admission | 1.18 (0.6) | 1.17 (0.7) | .94 | 1.18 (0.6) | 1.16 (0.7) | .84 |
| Elevated serum cardiac | 106 (33.8) | 29 (30.5) | .56 | 111 (32.6) | 24 (34.8) | .73 |
| enzyme levels at admission | ||||||
| PCI during hospitalization | 250 (72.9) | 60 (58.8) | .007 | 269 (72.5) | 41 (55.4) | .003b |
| Antidepressant prescribed at | 58 (16.8) | 43 (39.8) | <.001 | 64 (17.0) | 64 (17.0) | <.001 |
| discharge, No. (%) | ||||||
| SSRI | 38 (11.0) | 31 (28.7) | <.001 | 42 (11.1) | 27 (35) | <.001 |
| Non-SSRI | 20 (5.8) | 12 (11.1) | .06 | 22 (5.9) | 10 (13) | .03 |
| Depression Variables | ||||||
| MDE, No. (%) | 9 (2.6) | 39 (36.1) | <.001 | 13 (3.5) | 35 (45) | <.001 |
| BDI28 score ≥10, No. (%) | 110 (31.9) | 102 (94.4) | <.001 | 138 (36.7) | 74 (96) | <.001 |
Abbreviations: BDI, Beck Depression Inventory; GRACE, Global Registry of Acute Coronary Events; LVEF, left ventricular ejection fraction; MDE, major depressive episode; MI, myocardial infarction; PCI, percutaneous coronary intervention; SSRI, selective serotonin reuptake inhibitor.
Values are mean (SD) unless otherwise indicated. Denominators for variables vary slightly due to missing responses for some variables.
Significant difference between groups after Bonferroni correction.
Bonferroni correction for demographic variables, α≤ .003; Bonferroni correction for GRACE risk components , α≤.004
During a mean follow up of 10.4 months (range, <1–12 months), there were 67 confirmed events (16 deaths and 51 ACS recurrences; 14.8%), which is comparable to the finding of another study (15.1% over 1 year).24 Table 2 displays age-adjusted associations between the covariates and MACE and ACM. All medical covariates were significant predictors of MACE and ACM within 12 months. Major adverse cardiac events or ACM occurred in 23 patients (29.9%) with anhedonia compared with 44 patients (11.7%) without anhedonia; in 27 patients (25.0%) with depressed mood compared with 40 patients (11.6%) without depressed mood; and in 17 patients (35.4%) with MDE compared with 50 patients (12.3%) without MDE.
Table 2.
Age- Adjusted Association of Each Covariate with 12Month MACE or ACM in 453 Patients
| Predictor | Category of Exposure |
Age-Adjusted Hazard Ratio 95% Confidence Interval) |
P |
|---|---|---|---|
| Demographic Characteristic | |||
| Sex | Men (vs women) | 1.02 (0.80–1.31) | .87 |
| Medical Covariates | |||
| Left ventricular ejection fraction |
< 40 (vs ≥ 40) | 1.39 (1.06–1.84) | .02 |
| Charlson comorbidity index23 |
Per SD (1 SD = 1.57) | 1.38 (1.16–1.64) | <.001 |
| GRACE24, 25 risk score | Per SD (1 SD = 31.21) | 1.45 (1.01–2.10) | .03 |
| Antidepressant use | Yes | 1.34 (1.03–1.74) | .02 |
Abbreviations: ACM, all-cause mortality; GRACE, Global Registry of Acute Coronary Events; MACE, major adverse cardiac event.
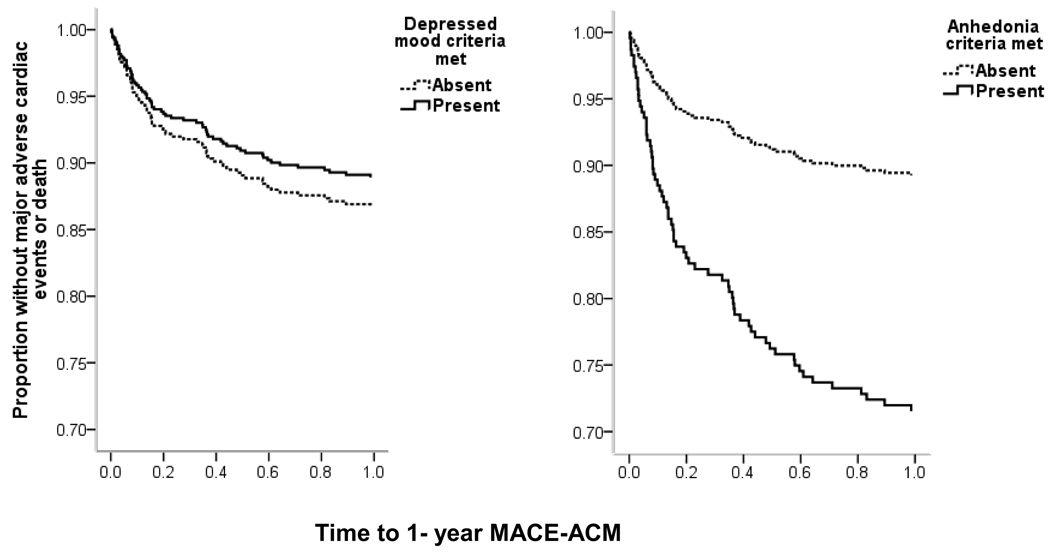
Major depressive episode and depressive symptom severity assessed by BDI were significant predictors of age-adjusted MACE and ACM within 12 months, but only MDE remained a significant predictor after adjusting for age, sex, and medical covariates (Table 3). Furthermore, both depressed mood and anhedonia significantly predicted MACE and ACM in the age-adjusted model (Table 3) but only anhedonia remained a significant predictor of MACE and ACM after adjusting for age, sex, and medical covariates. When both anhedonia and depressed mood were in the multivariable adjusted model, only anhedonia was a significant predictor of MACE/ACM (adjusted hazard ratio [HR], 1.69; 95% confidence interval [CI], 1.07–2.68; P=.03; see Figure 1).
Table 3.
Adjusted Hazard Ratios for Anhedonia and Depressed Mood
| Predictor | Age-Adjusted Hazard Ratio (95% Confidence Interval) |
P | Multivariable Adjusted Hazard Ratio (95% Confidence Interval)a |
P |
|---|---|---|---|---|
| Depressive symptom severity (BDI28 score <5 vs ≥10) |
1.33 (1.03–1.72) | .03 | 1.23 (0.94–1.62) | .14 |
| Major Depressive Episode |
1.71 (1.27–2.29) | <.001 | 1.48 (1.07–2.04) | .02 |
| Depressed mood | 1.41 (1.08–1.85) | .01 | 1.28 (0.96–1.71) | .09 |
| Anhedonia | 1.69 (1.28–2.24) | <.001 | 1.58 (1.16–2.14) | <.01 |
| Depressed mood | 0.99 (0.65–1.51) | .95 | 0.92 (0.59–1.43) | .70 |
| Anhedonia | 1.71 (1.10–2.65) | .02 | 1.69 (1.07–2.68) | .03 |
Figure 1.
Kaplan-Meier curves for patients with and without clinically impairing anhedonia (left; markedly diminished interest or pleasure in all or almost all activities) or depressed mood (right; sadness and the report of feeling depressed) most of the day, nearly every day. Adjusted for site, age, sex, Charlson comorbidity index score23, GRACE [Global Registry of Acute Coronary Events] risk score,25 left ventricular ejection fraction, and antidepressant use at discharge and the other depression core criteria. Abbreviations: MACE, major adverse cardiac events; ACM, all-cause mortality. Time is percentage of one year.
Anhedonia remained a significant predictor after adjusting for all covariates and MDE (HR, 1.45; 95% CI, 1.03–2.05; P=.04), and it also remained significant after adjusting for covariates and severity of depressive symptoms (HR, 1.52; 95% CI, 1.09–2.11; P=.01). With either MDE or depressive symptom severity in the model, depressed mood was not a significant predictor of outcome. Furthermore, with anhedonia in the model, neither MDE (HR, 1.25; 95% CI, 0.87–1.79; P=.23) nor depressive symptom severity (HR, 1.09; 95% CI, 0.81–1.47; P=.57) was a significant predictor of outcome. A final model with anhedonia and all covariates as well as PCI, the only GRACE risk score component that significantly differed between those with and without anhedonia, and anhedonia remained a significant predictor of outcome (P= .002).
DISCUSSION
The finding in the present study replicates and augments the finding of previous research in patients with established heart disease that depression is a significant risk factor for adverse clinical outcomes,2, 3 even when adjusting for demographic characteristics and known medical prognostic disease markers and comorbid conditions.13 We further demonstrated that of the 2 core criteria of depression—depressed mood and anhedonia—only anhedonia predicted the risk of MACE and ACM beyond the power of all covariates and other depression indices.
Improving Risk Stratification
Major depressive episode is a heterogeneous clinical entity with diverse somatic and cognitive symptoms that include fatigue, feelings of guilt, sleep problems, and weight gain or loss. Not all these symptoms may mark those at increased risk of MACE or ACM. As others have pointed out, it is important to move beyond the broad phenotype of depression and delineate its “cardiotoxic” components.34 In a previous study with post-MI patients, de Jonge and colleagues35 identified 3 symptom dimensions of the BDI—somatic or affective, cognitive or affective, and appetitive—and found that only somatic or affective symptoms predicted future death or cardiac events after controlling for somatic health indicators. However, the somatic or affective dimension still contained symptoms that are clinically (and likely, etiologically) distinct, such as insomnia, indecisiveness, and both depressed mood and anhedonia. Recent analyses from the Women’s Ischemia Syndrome Evaluation (WISE) study used a similar approach to evaluate the cardiac prognostic impact of symptom dimensions from the Beck depression inventory (BDI) in women with suspected myocardial ischemia.36 This study revealed that somatic symptoms of depression predicted cardiovascular events and mortality, and cognitive/affective did not. Interestingly, the two BDI items that assess anhedonia, had low factor loadings (< .50) on the somatic factor as well as the cognitive/affective factor, indicating that anhedonia is neither a purely ‘cognitive’, nor a purely ‘somatic’ depression symptom in their analyses. We chose a different approach by analyzing the two core symptoms of depression that are also known as ‘endophenotypes’ of depression21 that are clinically distinct and may have distinct biological bases.
Anhedonia is described as the loss of the ability to experience pleasure in all or almost all activities37 and is viewed as different from depressed mood, which is marked by sadness, tearfulness, and sometimes visible distress. This clinical distinction within the complex phenotype of major depression has been noted for centuries.38, 39 A diagnosis of MDE requires the presence of either anhedonia or depressed mood, and excellent screening tools for MDE exist that assess just these 2 criteria.40, 41 Anhedonia is not specific to depression; it occurs in other psychiatric disorders as well.42 Moreover, as with all symptoms of psychiatric disease, not all patients with anhedonia fulfill the diagnostic criteria for MDE. In fact, almost half the patients with severe anhedonia in our sample did not have MDE, yet anhedonia proved to be the most persistent predictor of MACE and ACM, better even than the clinical diagnosis of MDE. When we controlled for depression symptom severity, anhedonia still predicted MACE or ACM, ruling out the possibility that patients with anhedonia are at increased risk merely because they have more severe depressive symptoms overall. These findings suggest the possibility that examining the particular criteria of anhedonia can provide new insights concerning mechanisms that increase risk of adverse cardiac events in post-ACS patients.
Understanding Mechanisms
Numerous biological and behavioral mechanisms have been proposed for the link between depression and adverse cardiovascular events in post-ACS patients 43, 44. For example, we found increased platelet reactivity in depressed patients 45, and others found hypercoaguability, inflammatory activation, and endothelial or autonomic dysfunction among depressed patients with cardiac disease.46–50 More recently, we demonstrated that depressed post-ACS patients comply less well with recommended medications and other treatments.51, 52 The mechanisms for the relationship between anhedonia (as opposed to the more complex phenotype of MDE) and poor outcome after ACS have not yet been studied.
Patients with anhedonia tend to have perturbations of sleep, satisfaction, appetite, weight, and libido. Animal models of experimentally induced anhedonia suggest that cytokine profiles are disrupted.53 A recent comparison of MDE patients and interferon-alpha induced depressed patients determined that anhedonia was more prevalent in the latter, as were other neurovegetative symptoms.54 Perturbations in the dopaminergic system, inflammatory processes, circadian rhythms, and melatonin production have also been observed in anhedonic patients.38, 55, 56 Hasler and colleagues suggested that anhedonia, but not depressed mood, is associated with catecholaminergic dysfunction,21 a mechanism that has been proposed to explain increased morbidity and mortality due to cardiovascular disease.46, 57 High catecholamine levels are toxic to cardiac myocytes,58 can trigger tachyarrhythmia,53, 59, 60 and promote platelet aggregation.61, 62 Among patients with congestive heart failure, the extent of plasma catecholamine elevation correlates directly with cardiac mortality.63 Further study of these mechanisms is needed in anhedonic cardiac patients.
Directions for Future Treatments
If previous depression trials had already demonstrated that depression treatment offsets the increased risk of adverse cardiac events found for depressed patients, it would be moot to explore the type of depression symptom that increases MACE/ACM risk. Two previous trials, SADHART, which used sertraline,64 and ENRICHD, which predominantly used cognitive behavioral therapy,65 demonstrated a statistically significant (but not large) reduction of depressive symptoms in post-MI patients. Although the ENRICHD study was sufficiently powered to determine whether depressive symptom reduction improved post-MI survival, it was unable to do so. A recent trial of antidepressant medication use in depressed post-MI patients also did not show a reduction in mortality risk.66 Depressed patients with anhedonia have long been thought to respond well to antidepressant treatment,67 but evidence for this clinical intuition is limited to a few studies in psychiatric patient samples.68 Mechanisms of action in antidepressant treatments are not fully understood. A recent study of experimentally induced myocardial infarction in rats found that sertraline blocked the anhedonia and despair equivalent in this animal model, and modulated the inflammatory response to the infarction; the effect was also noted with desipramine.69 If we hypothesize that a cytokine release precipitates anhedonia in some ACS patients, then rapid treatment may be indicated, as it is possible that the timing of the depression treatment is critical for preventing the anhedonia comorbidity we discovered in humans. Treatments that focus on reducing anhedonia through modulating upregulated proinflammatory cytokines may be of particular interest.56, 70, 71 More recent efforts have focused on the functional interaction between the serotonergic and dopaminergic systems72, 73and these may also offer new treatments to test in anhedonic ACS patients. Whether reducing anhedonia in post-ACS patients will reduce their risk of MACE or ACM is not yet known.
Study Limitations
There are a number of limitations in the present study. Although anhedonia has previously been found to predict in-hospital mortality in medical patients74 and major clinical events after stent placement,75 our finding that anhedonia is uniquely associated with increased risk of MACE and ACM in post-ACS patients must be replicated. Furthermore, the current study was not sufficiently powered to rule out the possibility that depressed mood is not a moderate prognostic marker, and its usefulness in this regard should be retested with a larger sample size. Events or deaths within this study occurred primarily within the first 3 months after discharge, so the association of depressed mood and anhedonia with longer-term outcomes was also not determined.
It is possible that the post-ACS patients who reported loss of interest in activities are those with the most severe forms of heart disease, and it is the severity of their disease that is causing a poor prognosis, rather than a particular symptom of depression—anhedonia. As shown in Table 1, patients with anhedonia were significantly younger than those without anhedonia, and there were no significant differences between the 2 groups in GRACE risk score variables other than the frequency of PCI. When frequency of PCI was added to the model, anhedonia remained a significant predictor of clinical outcomes. Anhedonic patients also had higher Charlson comorbidity index scores, but this difference was controlled for in analyses. It remains possible, however, that anhedonia is a marker for some medical confounder not assessed in the current study that predicts clinical outcome.
CONCLUSIONS
The current finding replicates the finding of prior research that MDE is a significant and independent risk factor for adverse clinical outcomes in patients with ACS. It also demonstrates that, of the 2 criteria of MDE, depressed mood and anhedonia, only anhedonia was a significant predictor, after adjusting for severity of depression and clinical diagnosis of MDE. Focusing on anhedonia in post-ACS patients may better identify those at risk, may provide insight into the mechanisms underlying the association between depression and poor outcomes in patients with heart disease, and ultimately may inform the design of novel treatments to improve post-ACS prognosis.
Acknowledgements
Data collection and manuscript preparation were financially supported by grants HC-25197, HL-076857, HL-084034, HL-04458, HL088117 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Dr. Schwartz conducted the analyses and contributed to the interpretation of the data. Dr. Schwartz is independent of any commercial funding agency, and had full access to all the data in the study and he takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr. Lespérance receives unrestricted grant support from Isodis Natura. He is a consultant to Servier Canada.
Abbreviations list
- ACS
Acute Coronary Syndrome
- GRACE
Global Registry of Acute Coronary Events
- MACE
Major Adverse Cardiac Events
- MDE
Major Depressive Episode
REFERENCES
- 1.Davidson KW, Rieckmann N, Rapp M. Definitions and distinctions among depressive syndromes and symptoms: Implications for a better understanding of the depression--cardiovascular disease association. Psychosom Med. 2005;67 Suppl 1:S6–S9. doi: 10.1097/01.psy.0000162257.19266.fc. [DOI] [PubMed] [Google Scholar]
- 2.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a Risk Factor for Mortality in Patients With Coronary Heart Disease: A Meta-analysis. Psychosom Med. 2004;66(6):802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 3.van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66(6):814–822. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 4.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290(2):215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parashar S, Rumsfeld JS, Spertus JA, et al. Time course of depression and outcome of myocardial infarction. Arch Intern Med. 2006;166(18):2035–2043. doi: 10.1001/archinte.166.18.2035. [DOI] [PubMed] [Google Scholar]
- 6.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction.[see comment] Am J Cardiol. 2001;88(4):337–341. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 7.Bush DE, Ziegelstein RC, Patel UV, et al. Post-myocardial infarction depression. Agency for Healthcare Research and Quality Evidence Report: Technology Assessment. 2005;123:1–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery.[see comment] Lancet. 2003;362(9384):604–609. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 9.Carney RM, Blumenthal JA, Catellier D, et al. Depression as a risk factor for mortality after acute myocardial infarction. Am J Cardiol. 2003;92(11):1277–1281. doi: 10.1016/j.amjcard.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 10.de Jonge P, van den Brink RH, Spijkerman TA, Ormel J. Only incident depressive episodes after myocardial infarction are associated with new cardiovascular events. J Am Coll Cardiol. 2006;48(11):2204–2208. doi: 10.1016/j.jacc.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 11.Frasure-Smith N, Lesperance F. Recent evidence linking coronary heart disease and depression. Can J Psychiatry. 2006;51(12):730–737. doi: 10.1177/070674370605101202. [DOI] [PubMed] [Google Scholar]
- 12.Grace SL, Abbey SE, Kapral MK, Fang J, Nolan RP, Stewart DE. Effect of depression on five-year mortality after an acute coronary syndrome. Am J Cardiol. 2005;96(9):1179–1185. doi: 10.1016/j.amjcard.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 14.Rutledge T, Reis SE, Olson MB, et al. Depression symptom severity and reported treatment history in the prediction of cardiac risk in women with suspected myocardial ischemia: The NHLBI-sponsored WISE study. Arch Gen Psychiatry. 2006;63(8):874–880. doi: 10.1001/archpsyc.63.8.874. [DOI] [PubMed] [Google Scholar]
- 15.Rumsfeld JS, Ho PM. Depression and cardiovascular disease: a call for recognition. Circulation. 2005;111(3):250–253. doi: 10.1161/01.CIR.0000154573.62822.89. [DOI] [PubMed] [Google Scholar]
- 16.Whooley MA. Depression and cardiovascular disease: healing the broken-hearted. JAMA. 2006;295(24):2874–2881. doi: 10.1001/jama.295.24.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118(17):1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 18.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 19.Honig AMDP, Kuyper AMGMD, Schene AHMDP, et al. Treatment of Post-Myocardial Infarction Depressive Disorder: A Randomized, Placebo-Controlled Trial With Mirtazapine. Psychosom Med. 2007;69(7):606–613. doi: 10.1097/PSY.0b013e31814b260d. [DOI] [PubMed] [Google Scholar]
- 20.de Jonge P, Ormel J. Heterogeneity of patients with coronary artery disease and distress and the need to identify relevant subtypes. Arch Gen Psychiatry. 2008;65(7):851–852. doi: 10.1001/archpsyc.65.7.851. [DOI] [PubMed] [Google Scholar]
- 21.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 22.Eagle KA, Kline-Rogers E, Goodman SG, et al. Adherence to evidence-based therapies after discharge for acute coronary syndromes: an ongoing prospective, observational study. Am J Med. 2004;117(2):73–81. doi: 10.1016/j.amjmed.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.de Araujo Goncalves P, Ferreira J, Aguiar C, Seabra-Gomes R. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS.[see comment] Eur Heart J. 2005;26(9):865–872. doi: 10.1093/eurheartj/ehi187. [DOI] [PubMed] [Google Scholar]
- 25.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 26.Cannon CP, Battler A, Brindis RG, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38(7):2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 28.Beck AT, Ward CH, Mendelson M. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 29.Freedland KE, Skala JA, Carney RM, et al. The Depression Interview and Structured Hamilton (DISH): rationale, development, characteristics, and clinical validity. Psychosom Med. 2002;64(6):897–905. doi: 10.1097/01.psy.0000028826.64279.29. [DOI] [PubMed] [Google Scholar]
- 30.Association AP. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition (Text Revision) Arlington: American Psychiatric Publishing Inc; 2000. [Google Scholar]
- 31.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105(9):1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 32.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66(3):411–421. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 33.Steyerberg EW, Eijkemans MJC, Harrell FE, Jr, Habbema JDF. Prognostic Modeling with Logistic Regression Analysis: In Search of a Sensible Strategy in Small Data Sets. Med Decis Making. 2001;21(1):45–56. doi: 10.1177/0272989X0102100106. [DOI] [PubMed] [Google Scholar]
- 34.Denollet J, Pedersen SS. Anger, Depression, and Anxiety in Cardiac Patients: The Complexity of Individual Differences in Psychological Risk. J Am Coll Cardiol. 2009;53(11):947–949. doi: 10.1016/j.jacc.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 35.de Jonge P, Ormel J, van den Brink RH, et al. Symptom dimensions of depression following myocardial infarction and their relationship with somatic health status and cardiovascular prognosis. Am J Psychiatry. 2006;163(1):138–144. doi: 10.1176/appi.ajp.163.1.138. [DOI] [PubMed] [Google Scholar]
- 36.Linke SE, Rutledge T, Johnson BD, et al. Depressive symptom dimensions and cardiovascular prognosis among women with suspected myocardial ischemia: A report from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation. Arch Gen Psychiatry. 2009;66(5):499–507. doi: 10.1001/archgenpsychiatry.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry. 1983;40(1):79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- 38.Leventhal AM, Rehm LP. The empirical status of melancholia: implications for psychology. Clin Psychol Rev. 2005;25(1):25–44. doi: 10.1016/j.cpr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Shorter E. The doctrine of the two depressions in historical perspective. Acta Psychiatr Scand Suppl. 2007;433:5–13. doi: 10.1111/j.1600-0447.2007.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 41.Thombs BD, de Jonge P, Coyne JC, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300(18):2161–2171. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- 42.Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, Bradshaw JL, White OB. Negative symptoms: A review of schizophrenia, melancholic depression and Parkinson's disease. Brain Res Bull. 2006;70(4–6):312–321. doi: 10.1016/j.brainresbull.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: A review of potential mechanisms. J Psychosom Res. 2002;53(4):897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 44.Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 45.Shimbo D, Child J, Davidson K, et al. Exaggerated serotonin-mediated platelet reactivity as a possible link in depression and acute coronary syndromes. Am J Cardiol. 2002;89(3):331–333. doi: 10.1016/s0002-9149(01)02236-6. [DOI] [PubMed] [Google Scholar]
- 46.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67 Suppl 1:S29–S33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 47.Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 2005;67 Suppl 1:S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- 48.Bruce EC, Musselman DL. Depression, alterations in platelet function, and ischemic heart disease. Psychosom Med. 2005;67 Suppl 1:S34–S36. doi: 10.1097/01.psy.0000164227.63647.d9. [DOI] [PubMed] [Google Scholar]
- 49.Kop WJ, Gottdiener JS. The role of immune system parameters in the relationship between depression and coronary artery disease. Psychosom Med. 2005;67 Suppl 1:S37–S41. doi: 10.1097/01.psy.0000162256.18710.4a. [DOI] [PubMed] [Google Scholar]
- 50.Licinio J, Wong ML. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry. 1999;4(4):317–327. doi: 10.1038/sj.mp.4000586. [DOI] [PubMed] [Google Scholar]
- 51.Rieckmann N, Gerin W, Kronish IM, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study.[see comment] J Am Coll Cardiol. 2006;48(11):2218–2222. doi: 10.1016/j.jacc.2006.07.063. [DOI] [PubMed] [Google Scholar]
- 52.Kronish IM, Rieckmann N, Halm EA, et al. Persistent Depression Affects Adherence to Secondary Prevention Behaviors After Acute Coronary Syndromes. J Gen Intern Med. 2006 doi: 10.1111/j.1525-1497.2006.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98(13):1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 54.Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009 doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues in Clinical Neuroscience. 2008;10(3):291–299. doi: 10.31887/DCNS.2008.10.3/pgorwood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55(7):580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 58.Singh K, Communal C, Sawyer DB, Colucci WS. Adrenergic regulation of myocardial apoptosis. Cardiovasc Res. 2000;45(3):713–719. doi: 10.1016/s0008-6363(99)00370-3. [DOI] [PubMed] [Google Scholar]
- 59.Mann JJ, McBride PA, Anderson GM, Mieczkowski TA. Platelet and whole blood serotonin content in depressed inpatients: correlations with acute and life-time psychopathology. Biol Psychiatry. 1992;32(3):243–257. doi: 10.1016/0006-3223(92)90106-a. [DOI] [PubMed] [Google Scholar]
- 60.Lampert R, Jain D, Burg MM, Batsford WP, McPherson CA. Destabilizing effects of mental stress on ventricular arrhythmias in patients with implantable cardioverter-defibrillators. Circulation. 2000;101(2):158–164. doi: 10.1161/01.cir.101.2.158. [DOI] [PubMed] [Google Scholar]
- 61.Podrid PJ, Fuchs T, Candinas R. Role of the sympathetic nervous system in the genesis of ventricular arrhythmia. Circulation. 1990;82 2 Suppl:I103–I113. [PubMed] [Google Scholar]
- 62.Meredith IT, Broughton A, Jennings GL, Esler MD. Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. N Engl J Med. 1991;325(9):618–624. doi: 10.1056/NEJM199108293250905. [DOI] [PubMed] [Google Scholar]
- 63.Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311(13):819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 64.Glassman AH, O'Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288(6):701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 65.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA: Journal of the American Medical Association. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 66.van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460–466. doi: 10.1192/bjp.bp.106.028647. [DOI] [PubMed] [Google Scholar]
- 67.Klein DF. Endogenomorphic depression. A conceptual and terminological revision. Arch Gen Psychiatry. 1974;31(4):447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- 68.Rush AJ, Weissenburger JE. Melancholic symptom features and DSM-IV. Am J Psychiatry. 1994;151(4):489–498. doi: 10.1176/ajp.151.4.489. [DOI] [PubMed] [Google Scholar]
- 69.Wann BP, Bah TM, Kaloustian S, et al. Behavioural signs of depression and apoptosis in the limbic system following myocardial infarction: effects of sertraline. J Psychopharmacol. 2008 doi: 10.1177/0269881108089820. 0269881108089820. [DOI] [PubMed] [Google Scholar]
- 70.Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pace TWW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21(1):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Esposito E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr Drug Targets. 2006;7(2):177–185. doi: 10.2174/138945006775515455. [DOI] [PubMed] [Google Scholar]
- 73.Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113(2):296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Furlanetto LM, Cavanaugh SV, Bueno JR, Creech SD, Powell LH. Association between depressive symptoms and mortality in medical inpatients. Psychosomatics. 2000;41(5):426–432. doi: 10.1176/appi.psy.41.5.426. [DOI] [PubMed] [Google Scholar]
- 75.Denollet J, Pedersen SS, Daemen J, de Jaegere P, Serruys PW, van Domburg RT. Reduced positive affect (anhedonia) predicts major clinical events following implantation of coronary-artery stents. J Intern Med. 2008;263(2):203–211. doi: 10.1111/j.1365-2796.2007.01870.x. [DOI] [PubMed] [Google Scholar]