Abstract
Metastatic melanoma is an aggressive cancer with very low response rate against conventional chemotherapeutic agents such as dacarbazine (DTIC). Inhibitor of Rb-Raf-1 interaction (RRD-251) was tested against the melanoma cell lines SK-MEL-28, SK-MEL-5 and SK-MEL-2. RRD-251 was found to be a potent inhibitor of melanoma cell proliferation, irrespective of V600E B-Raf mutation status of the cell lines. In a SK-MEL-28 xenograft experiment, RRD-251 exerted a significant suppression of tumor growth compared to vehicle (p=0.003). Similar to in vitro effects, tumors from RRD-251 treated animals showed decreased Rb-Raf-1 interaction in vivo. Growth suppressive effects of RRD-251 were associated with induction of apoptosis as well as a G1 arrest, with an accompanying decrease in S-phase cells. RRD-251 inhibited Rb phosphorylation, and downregulated E2F1 protein levels in these cells. Real-time PCR analysis showed that RRD-251 caused downregulation of cell cycle regulatory genes thymidylate synthase (TS) and cdc6 as well as anti-apoptotic gene Mcl-1. Combinatorial treatment of RRD-251 and DTIC resulted in a significantly higher apoptosis in DTIC resistant cell lines SK-MEL-28 and SK-MEL-5, as revealed by increased Caspase-3 activity and PARP cleavage. Since aberrant Rb/E2F pathway is associated with melanoma progression and resistance to apoptosis, these results suggest that the Rb-Raf-1 inhibitor could be an effective agent for melanoma treatment, either alone or in combination with DTIC.
Keywords: Rb-E2F pathway, Melanoma, Apoptosis, Cell cycle, Dacarbazine
INTRODUCTION
The incidence of melanoma is continuously rising and has increased more than six fold over the last 50 years (1, 2). Melanoma is highly resistant to the conventional chemotherapeutic agent dacarbazine (DTIC) and has a response rate of only 15-20% with the median response duration of only four months (3-5). In addition, the new treatments have also failed to significantly improve the survival time (6-8). Thus, it is essential to identify new therapeutic targets for better treatment of the disease.
Several studies have suggested that in human melanoma cells all three Rb family pocket proteins (pRb, p107 and p130) are hyper-phosphorylated and several E2F family members (E2F1, E2F2, E2F3 and E2F4) are present in unbound, transcriptionally active forms (9-13) The retinoblastoma tumor suppressor protein, Rb, plays a vital role in regulating mammalian cell proliferation primarily by its interaction with the E2F family of transcription factors (14, 15). Rb binds to E2Fs 1, 2, and 3 and suppresses their transcriptional activity, inhibiting their ability to drive the expression of proliferative promoters (16, 17). In response to mitogenic signals, Rb is hyper-phosphorylated through a cascade of phosphorylation events, which leads to its inactivation and dissociation from E2Fs. Free E2Fs can actively participate in the transcription of target genes such as cdc6 and thymidylate synthase (TS), facilitating S-phase entry and cell cycle progression (17).
Several kinases can phosphorylate Rb. For example, cyclin-dependent kinases such as CDK4 and CDK2 phosphorylate Rb and this is essential for the G1 to S phase transition (18). Recently, we had shown that the signaling kinase Raf-1 can physically bind and phosphorylate Rb very early in the cell cycle, facilitating its further hyper-phosphorylation by CDKs and eventual inactivation (19, 20). We have previously reported that the disruption of Rb-Raf-1 interaction by an eight-amino acid peptide (corresponding to Raf-1 residues 10-18) prevented Rb phosphorylation even in the later stages of G1. This suggests that preventing the binding of Raf-1 to Rb keeps Rb in a functional, hypophosphorylated form with its tumor suppressor role intact (20). Our attempts to identify a small molecule disruptor of Rb-Raf-1 interaction resulted in the characterization of RRD-251, which had potent anti-proliferative, anti-angiogenic and anti-tumor activity against non-small cell lung carcinoma cells in vitro and in vivo (21). Here we have explored the efficacy of RRD-251 in targeting melanoma cells SK-MEL-28, SK-MEL-5 and SK-MEL-2.
We find that RRD-251 can inhibit the growth of melanoma cells by induction of apoptosis as well as cell cycle arrest. We further show that pre-treatment of cells with DTIC enhanced the pro-apoptotic effects of RRD-251 on these cells. Overall, these data suggest that RRD-251 possesses potent anticancer activity against melanoma in vitro and in vivo. Furthermore, it appears that the combination of DTIC and RRD-251 would be a viable strategy to combat the growth and progression of melanoma.
MATERIALS and METHODS
Cell lines and reagents
The metastatic melanoma cell lines, SK-MEL-28, SK-MEL-5 and SK-MEL-2 were purchased from ATCC (Manassas, VA) and cultured in MEM containing 10% fetal bovine serum (FBS; Mediatech) and maintained in 5% CO2 at 37 °C. While these cell lines were originally purchased from ATCC, we did not revalidate them. DTIC was obtained from Sigma and 100 mM stock solution was prepared by dissolving the drug in DMSO. RRD-251 was synthesized by our in-house chemistry laboratory. It is a benzyl-isothiourea compound with chloride as the counter ion (19). The chemical structures of RRD-251 and DTIC are shown in Supplementary Figure 1. Unless not indicated, cells were treated with 50 μmol/L concentration of DTIC and RRD-251. Primary antibodies against PARP and Caspase-3 were obtained from Cell Signaling Technology, Rb and Raf-1 were obtained from BD Transduction Laboratories and Mcl-1, Bcl-2 and Bax were obtained from Santa Cruz Biotechnology.
Proliferation and soft agar colony formation assay
Actively growing human melanoma cells were plated in 96-well plates at a density of 7500 cells/well in triplicates. After 24 hr, cells were treated with 20 and 50 μmol/L concentrations of RRD-251 or the solvent (DMSO). Cell proliferation assay was carried out with MTT (Thiazolyl Blue Tetrazolium Bromide) after 24 hr of drug treatment. Briefly, cells were incubated with the 1 mg/mL MTT solution at 37 °C for 1 hr. The reaction was terminated with DMSO that solubilizes the formazan product formed. Absorbance at 540 nm was recorded using plate reader. A soft agar colony-formation assay was used as described by Kinkade et. al. 2008 (21). Over the bottom agarose (0.5%), 5,000 logarithmically growing melanoma cells were mixed with 0.3% agarose along with the indicated doses of RRD-251. Drugs were added twice weekly in complete media to the respective wells. After incubating for 14 days, colonies were stained with MTT (1 mg/mL) for 1 hr at 37°C. The experiment was repeated twice in duplicate.
In vivo xenograft experiments in nude mice
SK-MEL-28 cells were harvested in PBS and mixed with equal volume of Matrigel (BD Biosciences, Bedford, MA) and implanted subcutaneously into the right and left flanks (10 × 106 cells per flank) of 8-wk-old female athymic nude mice (Charles River) as described previously (21). When xenograft tumor growth was established, treatment by intra-peritoneal injection of 0.1 ml of the drug or the vehicle was initiated. Tumor volumes were determined by measuring the length (L) and the width (W) and calculating the volume (V = L × W2 / 2). Statistical significance between control and treated animals were evaluated using Student’s t test.
Cell cycle analysis by flow cytometry
Cells were treated with indicated dose of RRD-251 for given time. Following treatment, cells were scrapped and collected. Cell pellet was washed in PBS and resuspended in 0.1 ml of citrate/DMSO buffer (250 mmol/L sucrose, 40 mmol/L Na3C6H5O7.2H2O, 5% DMSO, pH 7.6). The pellets were then frozen at −80°C. Samples were processed as suggested in Vindelov method and cell cycle analysis was performed by flow cytometry (22).
Lysate preparation, immunoprecipitation and Western blotting
Cell lysates were prepared by lysis-buffer containing NP40, as described earlier (20) (21). Tumor lysates were prepared with T-Per tissue lysis buffer (Pierce) and a Fisher PowerGen 125 dounce homogenizer (21). Physical interaction between proteins in the tumors was analyzed by immunoprecipitation–western blot experiments using 200 μg of lysate and 1 μg of the indicated antibody (20).
Real-time PCR
Melanoma cells were treated with RRD-251 for indicated time. DMSO treated cells were used as vehicle-control. Total RNA was isolated by an RNeasy miniprep kit from QIAGEN following the manufacturer’s protocol. One microgram of RNA was used for first-strand cDNA synthesis using the iScript cDNA synthesis kit (Bio-Rad). A fraction (1/20) of the final cDNA reaction volume was used in each PCR. Primer sequences are as follows: 5’-CTG CCA GCT GTA CCA GAG AT-3’ (TS forward primer), 5’-ATG TGC ATC TCC CAA AGT GT-3’ (TS reverse primer), 5’-CCC CAT GAT TGT GTT GGT AT-3’ (Cdc6 forward primer), 5’-TTC AAC AGC TGT GGC TTA CA-3’ (Cdc6 reverse primer), 5’-ATG CTT CGG AAA CTG GAC AT-3’ (Mcl-1 forwad primer), 5’-TCC TGA TGC CAC CTT CTA GG-3’ (Mcl-1 reverse primer), 5’-CTC AAC ACG GGA AAC CTC AC-3’ (18S forward primer), and 5’-AAA TCG CTC CAC CAA CTA AGA A-3’ (18S reverse primer). Real-time PCR was performed on a Bio-Rad iCycler and analyzed by ddCt method.
Caspase 3/7 activity assay and TUNEL assay
Cells were grown in eight well chamber slides and treated with RRD-251 for 24 hr. TUNEL staining was performed using DeadEnd™ TUNEL Assay kit (Promega) as per the manufacture’s instructions. Actively growing melanoma cells were plated in clear bottom 96-well plates at a density of 7500 cells/well in triplicates. After 24 hr, cells were treated with 50 μmol/L concentrations of DTIC or the solvent (DMSO). 48 hr post-DTIC treatment, RRD-251 was added in the respective wells and further incubated for 4 hr. Caspase activity were measured by quantitative luminescence assays using caspase-3/7–activatable DEVD-aminoluciferin (Caspase-Glo 3/7 kit, Promega). Assays were done according to the manufacturer’s protocol and plates were read in a luminometer (Perkin Elmer).
Comet assay
The Comet assay was performed in its alkaline version following published protocols (23, 24). Cells were treated with 50 μmol/L DTIC for 48 hr. Afffter treatment, cells were mixed with 0.8% (w/v) low melting point agarose and the mixture spread onto two slides pre-coated with 1.5% (w/v) normal melting point agarose. The slides were covered with coverslips and were refrigerated for 10 min to solidify the agarose. Next, the coverslips were removed and the slides were immersed in lysis solution (2.5 Mol/L NaCl, 100 mmol/L EDTA, 10 mmol/L Tris-HCl (pH 10) containing 1% (v/v) Triton X-100) for 60 min at 4 °C. Subsequently, the slides were placed in an alkaline buffer (1 mmol/L EDTA, 300 mmol/L NaOH, pH > 13) for 20 min at 4 °C for the DNA to unwind. For electrophoresis slides were equalibarated in 1× TBE for 10 minutes following electrophoresis in the same buffer at 0.52 V/cm for 5 min. Slides were washed in water, dried at room temperature and fixed in 100% ethanol for 5 min. The slides were stained with 80 μL of 2 μg/ml ethidium bromide and rinsed with water. Randomly selected cells from each sample and %DNA damage was analyzed using LAI Automated Comet Assay Analysis System (Loats Associates Inc., Westminster, MD).
RESULTS
In-vitro and in-vivo anti-melanoma effects of RRD-251
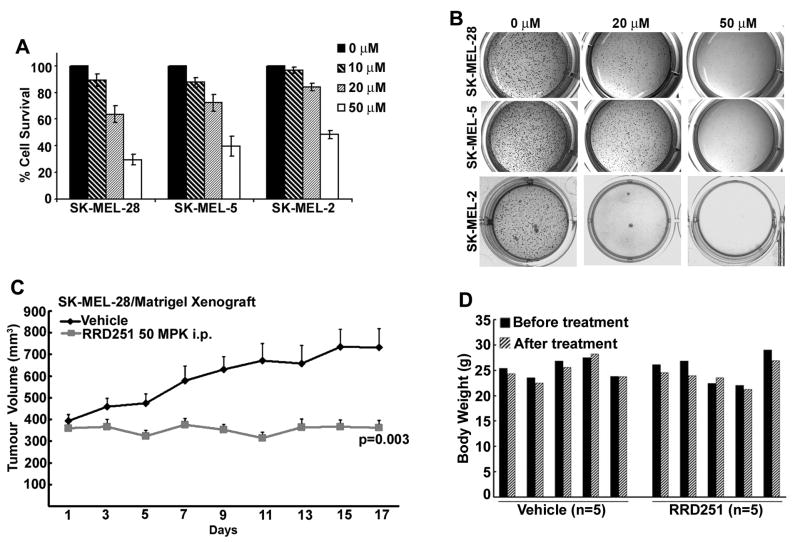
Melanoma cells harbor persistently hyperphosphorylated Rb proteins and this appears to be associated with melanoma progression and resistance to apoptosis. We, therefore, investigated the effects of Raf/Rb disruptor RRD-251 on melanoma tumors. As a first step, the effect of RRD-251 on cell survival and anchorage-dependent cell growth was tested by a MTT assay. SK-MEL-28, SK-MEL-5 and SK-MEL-2 cells were grown in the absence or presence of increasing concentrations (10, 20 and 50 μmol/L) of RRD-251 for 24 hr. RRD-251 treatment resulted in a dose dependent decrease in cell survival in all the three cell lines (Fig. 1A). The IC50 value for SK-MEL-28, SK-MEL-5 (both carrying a V600E mutant B-Raf) and SK-MEL-2 (carrying wild-type B-Raf) were found to be 28.7μmol/L, 37.3μmol/L and 48 μmol/L respectively. Next, the effect of RRD-251 on the anchorage-independent growth of these cell lines in soft agar was examined. As shown in Fig. 1B, RRD-251 could significantly suppress anchorage-independent colony formation of SK-MEL-28 and SK-MEL-5 and SK-MEL-2 cells in a dose dependent manner. These data show that RRD-251 treatment produces strong inhibition of viability and proliferation of melanoma cells, irrespective of their B-Raf status.
Figure 1. RRD-251 inhibits melanoma growth in-vitro and in-vivo.
(A) MTT assay showing the viability of SK-MEL-28, SK-MEL-5 and SK-MEL-2 cells treated with either DMSO or 10, 20, 50 μmol/L of RRD-251 for 24 hr. Absorbance given by vehicle treated cells was taken as 100% cell survival. Data represent the average ± S.D. from three independent experiments. (B) RRD-251 inhibits the anchorage independent growth of SK-MEL-28, SK-MEL-5 and SK-MEL-2 cells in soft agar. Photographs shown are representative of three independent experiments. (C) RRD-251 inhibits the growth of SK-ME-28 xenograft in nude mice. SK-MEL-28 cells mixed with Matrigel were xenotransplanted bilaterally into the flanks of athymic nude mice and allowed to grow for 14 days until xenograft were established. Data represents the average tumor volume ± S.E. (D) Daily administration of RRD-251 for 15 days did not show any treatment associated body weight loss. Data represent the body weight of 5 different mice, before and after the treatment.
Given the ability of RRD-251 to exert anti-proliferative effects in cultured cells, we next examined whether RRD-251 could inhibit tumor growth in vivo in nude mouse xenograft models. SK-MEL-28 cells mixed with matrigel were implanted by subcutaneous injection in nude mice, and the tumors were allowed to reach an average size of 376 mm3 in size before intraperitoneal (i.p.) administration of RRD-251 or vehicle. As shown in Fig. 1C, tumors from vehicle treated mice grew to an average size of 733±86 mm3. In contrast, tumors did not grow significantly (average tumor size at the end of treatment was 362±34 mm3) in mice treated with RRD-251. This anti-tumor effect of RRD-251 was statistically significant after 9 days of treatment and remained significant throughout the duration of the experimental period. The body weight of vehicle or RRD-251 treated mice were not affected (Fig. 1D). This set of experiments suggests that RRD-251 has anti-cancer activities in vitro and in vivo on melanomas.
RRD-251 inhibits Rb-Raf-1 interaction and Rb-phosphorylation
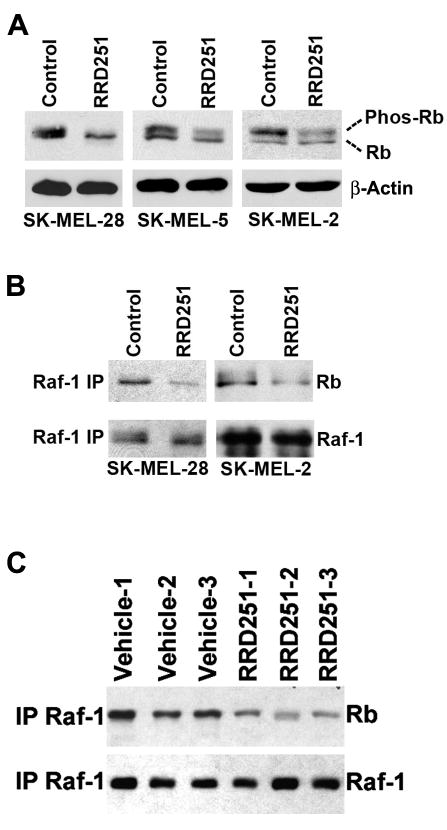
Previous studies from our laboratory had shown that RRD-251 inhibits Rb-Raf-1 interaction and Rb phosphorylation in non-small cell lung cancer cells (20, 21). Here, we extended our study to check the effects of RRD-251 on Rb phosphorylation in melanoma cells having different B-Raf (V600E) mutation status. SK-MEL-28 and SK-MEL-5 cells with mutant B-Raf as well as SK-MEL-2 cells with wild type B-Raf were treated with 50 μmol/L RRD-251 for 2 hr. Phosphorylation of Rb was ascertained by western blotting. As shown in Fig. 2A, treatment with RRD-251 resulted in reduced Rb phosphorylation in all the three cell lines; there was a slight reduction in the levels of Rb protein as well. To examine whether RRD-251 inhibits Rb phosphorylation due to the disruption of the physical association of Raf-1 with Rb (21), we immunoprecipitated Raf-1 from lysates prepared from RRD-251-treated or untreated cells and Rb was detected by western blotting. Fig. 2B shows that the binding of Raf-1 to Rb was inhibited by RRD-251 in both B-Raf wild type (SK-MEL-2) and mutant (SK-MEL-28) cells. Overall, these results suggest that RRD-251 disrupts Rb-Raf-1 association and inhibits Rb phosphorylation, irrespective of B-Raf mutation status in melanoma cells. Next, to assess whether RRD-251 affected its target in vivo, tumors lysates were prepared from vehicle-treated and RRD-251-treated mice and Rb-Raf-1 interaction was assessed by immunoprecipitation-western blots. As shown in Fig. 2C, tumors from vehicle treated mice showed robust Rb-Raf-1 interaction whereas RRD-251 treatment reduced the Rb-Raf-1 interaction in SK-MEL-28 xenografts. This shows that the drug is affecting the intended target in vivo.
Figure 2. RRD-251 inhibits Rb-Raf-1 interaction in-vitro and in-vivo.
(A) RRD-251 inhibits Rb phosphorylation in melanoma cells. Logarithmically growing SK-MEL-28, SK-MEL-5 and SK-MEL-2 cells were treated with 50 μmol/L RRD-251 for 2 hr. Western blot analysis showed the depletion of phosphorylated-Rb (migrates slower in SDS-page) in RRD-251 treated cells. β-Actin was used as loading control. (B) Co-immunoprecipitation-Western blot analysis revealed decreased Rb-Raf-1 interaction in RRD-251 treated cells. (C) After 14 days of treatment at the dose of 50 mpk, tumors from vehicle and RRD-251 treatment group were processed for immunoprecipitation-Western blot experiment. Tumors from RRD-251 treated animals showed decreased Rb-Raf-1 interaction as compared to vehicle treatment.
Induction of apoptosis and cell cycle arrest in melanoma cells by RRD-251
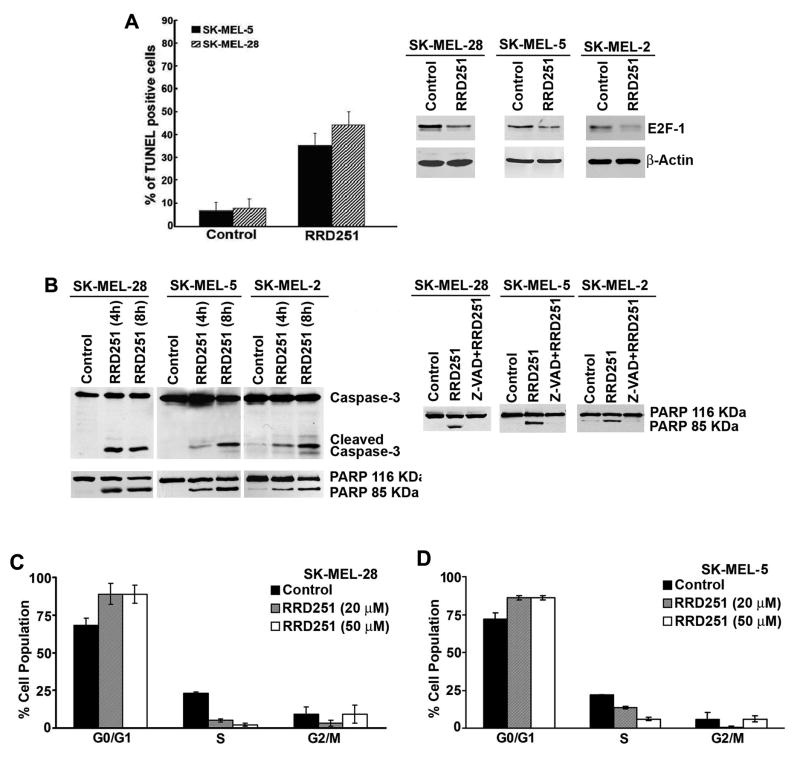
To elucidate the molecular mechanisms behind the anticancer property of RRD-251, we next determined its ability to induce cell cycle arrest and/or apoptosis in melanoma cells. First, SK-MEL-28 and SK-MEL-5 cells were treated with 50 μmol/L of RRD-251 for 18 hr and TUNEL staining was performed. RRD-251 treatment induced apoptosis in both cell lines as demonstrated by a 30-40% increase in TUNEL-positive cells as compared to DMSO treated control cells (Fig. 3A, left panel). Since there was induction of apoptosis by RRD-251, we evaluated whether the level of E2F1 was affected by RRD-251 treatment. It was found that RRD-251 inhibited Rb hyper-phosphorylation and decreased the levels of E2F1 after 4 hr treatment in SK-MEL-28, SK-MEL-5 and SK-MEL-2 cells (Fig. 3A, right panel). The induction of apoptosis was further verified by examining the induction of caspase-3 and PARP cleavage. Towards this purpose, SK-MEL-28, SK-MEL-5 and SK-MEL-2 cells were treated with 50 μmol/L of RRD-251; whole cell lysates were prepared at different time intervals and western blotting was performed to detect the presence of cleaved caspase-3 and PARP. The cleaved band of caspase-3 and PARP was detected as early as 4 hr after RRD-251 treatment in all the three cell lines (Fig. 3B, left panel). Induction of PARP cleavage by RRD-251 was observed in eight additional melanoma cell lines, including two carrying a wild-type B-Raf gene (data not shown). The involvement of caspases in RRD-251-induced apoptosis was next tested, using the pan-caspase inhibitor Z-VAD. Cells were pretreated with 20 μmol/L of Z-VAD for 30 minutes, following which the cells were treated with RRD-251 for 8 hr. The induction of apoptosis was monitored by the detection of PARP cleavage. As can be seen in Fig. 3B, right panel, pan-caspase inhibitor Z-VAD completely suppressed RRD-251-induced apoptosis as indicated by the absence of PARP cleavage in all three cell lines.
Figure 3. RRD-251 induces apoptosis and cell cycle arrest.
(A, left panel) TUNEL staining of SK-MEL-28 and SK-MEL-5 cells treated with RRD-251 (50 μmol/L) for 24 hr. Data represents the average number of TUNEL positive cells from five fields. (A, right panel) Western blot analysis of cells treated with RRD-251 at 50 μmol/L for 4 hr showed decrease in E2F1 protein levels. (B, left panel) RRD-251 induced apoptosis is an early event as evident by the detection of cleaved Caspase-3 and PARP by western blot analysis. (B, right panel) Apoptosis induced by RRD-251 could be completely blocked by pan-caspase inhibitor Z-VAD-fmk as indicated by inhibition of PARP cleavage. (C and D) Flow cytometry analysis in response to RRD-251 treatment. SK-MEL-28 and SK-MEL-5 cells were treated with 20 and 50 μmol/L RRD-251 and subjected to flow cytometry analysis. The percentage of cells in the different phases of the cell cycle is shown.
Since decrease in E2F1 levels (Fig. 3A) should result in cell cycle perturbations, we next assessed how RRD-251 treatment alters cell cycle progression in these melanoma cells. Cells were treated with 20μmol/L and 50 μmol/L of RRD-251 for 18 hr and cell cycle distribution was evaluated by flow cytometry. As shown in Fig. 3C and 3D, RRD-251 treatment resulted in a dose dependent inhibition of cell cycle progression in SK-MEL-28 and SK-MEL-5 cells, respectively. Treatment with 50 μmol/L of RRD-251 increased the G0/G1 population from an average of 68% in untreated cells to 89% in SK-MEL-28 cells and from 72% to 86% in SK-MEL-5 cells after 18 hr. A concomitant decrease in S-phase population was observed, from an average of 23% to 2% and 22% to 6% in SK-MEL-28 and SK-MEL-5 cells respectively. Interestingly, data demonstrated that RRD-251 induces apoptosis at the same dose where we also observe reduction of E2F1 levels and cell cycle rearrangement in melanoma cells.
RRD-251 alters the expression of cell cycle and apoptosis regulatory protein
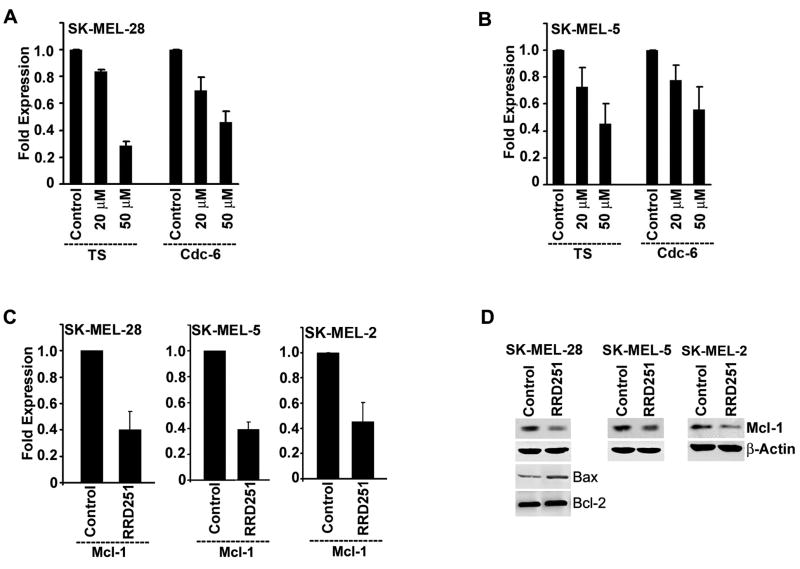
Further studies were done to evaluate the expression of E2F1 transcriptional targets involved in cell cycle progression in response to RRD-251 treatment. E2F1 regulates G1-S phase transition through transcriptional regulation of a variety of genes like cdc6 and TS involved in DNA synthesis and replication. To examine how the expression of these genes is affected by RRD-251, we checked the mRNA levels of CDC6 and TS in response to RRD-251 treatment by quantitative real-time PCR. Results revealed a reduction in the transcript levels of these genes when SK-MEL-28 and SK-MEL-5 cells were treated with 20 and 50 μmol/L of RRD-251 (Fig. 4A and B).
Figure 4. RRD-251 alters the expression of cell cycle and apoptosis regulators.
(A and B) Real-time PCR analysis for TS and cdc6 from the mRNA prepared after 18 hr of RRD-251 treatment in SK-MEL-28 (A) and SK-MEL-5 (B). Expression of these genes was decreased in a dose dependent manner. (C) RRD-251 treatment was done for 4 hr and the mRNA expression of Mcl-1 was analyzed by real-time PCR method. (D) Western blot analysis for Mcl-1 and Bax after 4 hr treatment of RRD-251.
Earlier studies have reported that the overexpression of anti-apoptotic proteins like Bcl-2 and Mcl-1 in melanoma cells is associated with their progression (25-29). Since expression of many of the Bcl-2 family members are regulated through the Rb-E2F pathway, we next examined the expression of Bcl-2 family members, Bax and Mcl-1 in response to RRD-251 treatment. RRD-251 significantly downregulated the mRNA levels of the pro-survival protein Mcl-1 in SK-MEL-28, SK-MEL-5 and SK-MEL-2 cells after 4 hr of treatment (Fig. 4C). Decrease in mRNA expression resulted in decreased level of Mcl-1 protein also in all the three cell lines (Fig. 4D). In addition to changes in Mcl-1 expression, SK-MEL-28 cells also showed increased expression of the pro-apoptotic protein Bax, whereas Bcl-2 expression was not affected. SK-MEL-5 and SK-MEL-2 cells did not show any change in the expression of Bax or Bcl-2 in RRD-251 treated cells (data not shown). Collectively, these results suggest that RRD-251 treatment modulates the expression of vital genes involved in the regulation of the cell cycle and apoptosis, facilitating its anti-cancer effects.
DTIC enhances the efficacy of RRD-251
As mentioned earlier, DTIC is the most commonly used chemotherapeutic agent for the treatment of advanced melanoma but the response rate of melanoma to this conventional agent is low (3). Many studies have tried to explore different DTIC based combinations to improve its clinical efficacy, but have failed to obtain better clinical response and overall survival (4, 5). Here we evaluated whether a combination of DTIC and RRD-251 had an enhanced effect on metastatic melanoma cells lines.
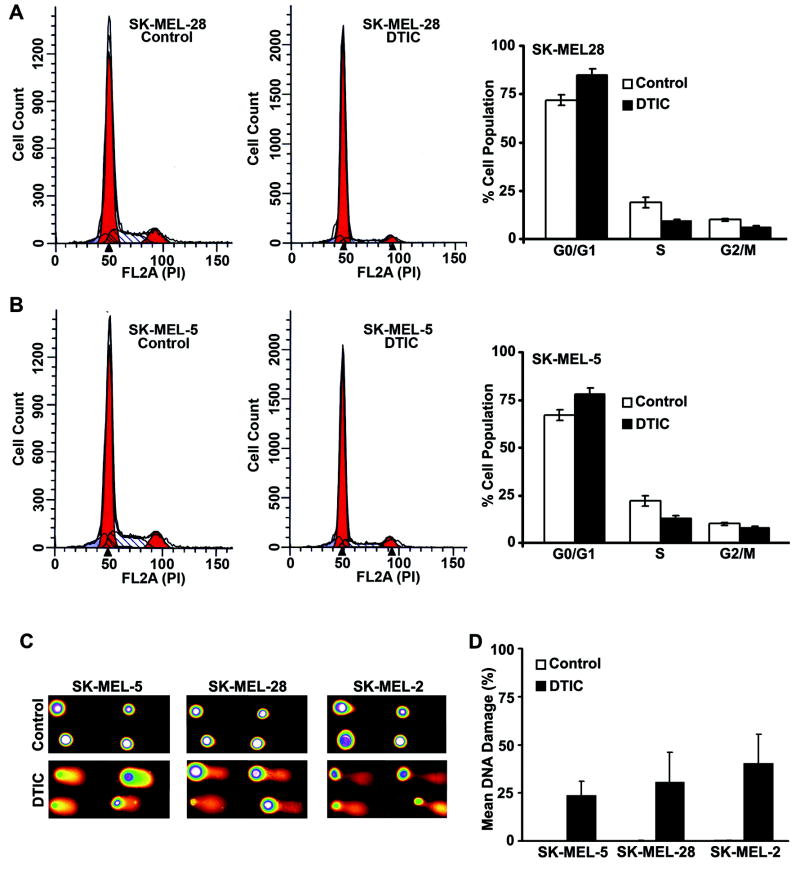
Being an alkalyting agent, DTIC induces DNA-damage to exert its physiological affects as an anti-cancer drug (30). We examined the effect of DTIC on its ability to induce apoptosis or cell cycle arrest in SK-MEL-28, SK-MEL-5 and SK-MEL-2 cells. Cells treated with DTIC at 50 μmol/L concentration for 48 hr failed to induce apoptosis in SK-MEL-5 and SK-MEL-28 cell lines, whereas, SK-MEL-2 cells showed sensitivity against DTIC treatment (data not shown and see following sections). We next examined how DTIC affects the cell cycle distribution of melanoma cells under similar conditions of treatment, using flow cytometry. Representative cell cycle profile of SK-MEL-28 and SK-MEL-5 cells are given in Fig. 5A and B respectively. Treatment with DTIC for 48 hr resulted in an increase in G0/G1 phase cells with a partial decrease in S and G2/M phase cells in both the cell lines. This data suggests that DTIC, despite being a pro-drug, has a certain amount of cell cycle regulatory effects on melanoma cells in culture. Since, SK-MEL-2 cells were sensitive to DTIC, we did not perform cell cycle analysis of this cell line.
Figure 5. DTIC partially inhibits the cell cycle progression.
(A and B) SK-MEL-28 (A) and SK-MEL-5 (B) cell were treated with 50 μmol/L dose of DTIC for 48 h and subjected for Flow cytometry analysis. The representative cell cycle profile is shown. Histograms on the right represent the percentage of cells in the different phases of the cell cycle form three independent experiments. (C) Damaged DNA was visualyzed by the Comet assay in SK-Mel-5, SK-Mel28 and SK-MEL-2 cells following exposure to 50 μmol/L DTIC for 48 h. Representative micrograph of fluorescent DNA stain of control cells, showing undamaged DNA remaining. whereas DTIC-treated cells showing denatured DNA fragments migrating out from cell. (D) %DNA damage was quantified and mean is represented with standered deviation as error bars.
Further, the effect of DTIC on DNA-damage was checked by comet assay. Cells were treated with DTIC for 48 hr at 50 μM concentration. The representative pictures of each cell line are shown in Fig. 5C. All the three cells lines showed mild to moderate DNA-damage with the tail length of approximately one or two times to the diameter of the head. The scored DNA-damage accounted the mean of approximately 25% in SK-MEL-5 and SK-MEL-28 and approximately 40% in SK-MEL-2 cells. Untreated cells had no DNA damage in any cell type (Fig. 5D).
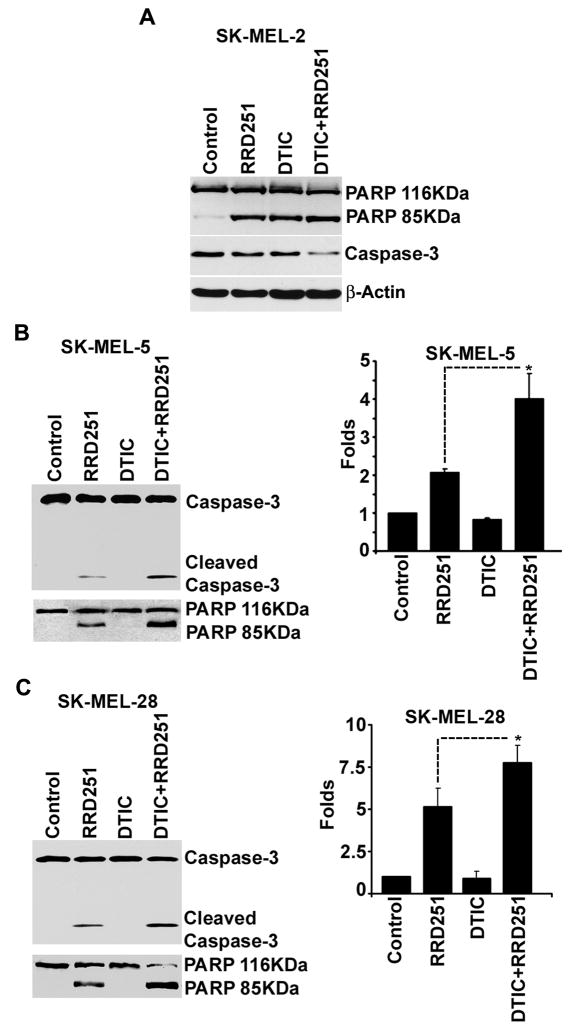
Since DTIC failed to induce apoptosis as a single agent in SK-MEL-28 and SK-MEL-5 cell lines, we next evaluated its ability to interact with RRD-251 induced apoptosis in all the three cell lines. SK-MEL-2, SK-MEL-5 and SK-MEL-28 cells were pretreated with 50 μmol/L DTIC for 48 hr before treatment with RRD-251 for a period of 4 hr. The induction of apoptosis was assessed by detecting caspase-3 as well as PARP-cleavage by Western blotting. As mentioned above, treatment with DTIC alone caused apoptosis in SK-MEL-2 cells as detected by decrease in caspase-3 and PAPR cleavage (Fig. 6A; DTIC lane). Under similar conditions DTIC did not induce any apoptotic effect in SK-MEL-5 and SK-MEL-28 cell lines (Fig. 6B and C; DTIC lane). At the same time, pretreatment of DTIC resulted in increased apoptosis (DTIC+RRD-251 lane) as compared to RRD-251 alone as indicated by the detection of enhanced cleavage of PARP and caspase-3 in all the three cell lines. It is also important to note that treatment of DTIC and RRD-251 together for 4 hr did not result in increased apoptosis as compared to RRD-251 treatment alone (data not shown). Overall, data suggests that DTIC pretreatment can enhance the apoptotic potential of RRD-251 in melanoma cells.
Figure 6. DTIC enhances the efficacy of RRD-251.
(A) SK-MEL-2 cell were pretreated with 50 μmol/L of DTIC for 48 before the exposure to RRD-251 for further 4 hr. Apoptosis was detected by the appearance of decreased caspase-3 and cleavage of PARP by western blot analysis. DTICas well as RRD-251 alone resulted in apoptosis. (B and C) SK-MEL-28 (B; left panel) and SK-MEL-5 (C; left panel) cell were pretreated with 50 μmol/L of DTIC for 48 before the exposure to RRD-251 for further 4 hr. Apoptosis was detected by the appearance of cleaved caspase-3 and PARP by western blot analysis. DTIC alone did not result in apoptosis (DTIC-lane). Pretreatment of DTIC before RRD-251 addition resulted in increased apoptosis as compared to RRD-251 treatment alone (DTIC+RRD-251-lane Vs RRD-251-lane) in both the cells. (B and C; right panels) The caspase-3 activity was quantified using Caspase Glo 3/7 kit in SK-MEL-28 and SK-MEL-5 cells. The pretreatment of DTIC before RRD-251 exposure resulted in significantly higher (p value <0.05) caspase activity than these treatments alone in both the cell lines.
DTIC-mediated enhanced efficacy of RRD-251 was further assessed by quantifying the levels of active caspase-3/7 using a luminescent-based assay. As shown in right panels of Fig. 6B and C, both the cell lines did not show any increased caspase activity after treatment with DTIC for 48 hr, treatment with RRD-251 alone resulted in an average increase of caspase activity by 5±1 fold in SK-MEL-28 and 2±0.1 fold in SK-MEL-5 cells as compared to untreated cells. When the cells were pre-treated with DTIC for 48 hr prior to RRD-251 treatment for 4 hr, both the cell lines showed a statistically significant increase in caspase activity (p<0.05) as compared to RRD-251 treatment alone. Overall, these results strongly suggested that DTIC pretreatment enhances the apoptotic effect of RRD-251 in melanoma cells.
DISCUSSION
Incidence of melanoma has been increasing at an alarming rate with very limited treatment choices and low overall disease free survival. The fact that hyper-phosphorylation of Rb and its subsequent aberrant E2F activation are associated with melanoma progression and poor prognosis prompted us to evaluate the anti-tumor efficacy of the Rb/Raf-1 interaction disruptor RRD-251 in melanoma. Our studies demonstrate that RRD-251 disturbed cell cycle progression and apoptosis signaling pathways, inhibited anchorage-dependent and –independent cell growth and suppress melanoma tumor growth in vivo. Many reports have shown that the tumor suppressor function of Rb is deregulated at multiple levels in melanoma (10, 12, 13). Rb and its homologues, p107 and p130 are highly expressed in melanoma cells as compared to normal melanocytes and are inactivated by phosphorylation through the persistent cyclin dependent kinases (CDKs) activity (11, 13). Since Rb is rarely disabled by mutation in melanoma cells, novel approaches can be devised to reactivate its tumor suppressive functions. Recently, flavopiridol, a potent inhibitor of CDKs 1, 2, 4 and 7 activity was used in one of such approaches. Flavopiridol is shown to inhibit the progression of cell cycle and induction of apoptosis in melanoma cells (12), but failed to improve the status of melanoma patients at clinical level (31). Whereas, CDKs phosphorylate Rb in mid to late G1 phase (32-34), our lab has demonstrated that Raf-1 kinase binds and phosphorylates Rb early in the G1 phase (19). Disruption of this Rb-Raf-1 interaction by an eight–amino acid peptide (corresponding to Raf-1 residues 10–18) prevented Rb phosphorylation even in the late-G1 phase, suggesting that the binding of Raf-1 is necessary for the eventual phosphorylation and complete inhibition of Rb by CDKs (20). In addition, our results also demonstrated that inhibition of Rb-Raf-1 interaction by RRD-251 could also suppress the proliferation of cells harboring mutations in the Rb regulatory pathway genes like Ras, PTEN, p16INK4, as well as receptor tyrosine kinases (21). However, whether RRD-251 can suppress proliferation of tumor cells harboring mutations relevant to melanoma is not known. For example, oncogenic V600E mutation in B-Raf is prevalent in ~63% of melanoma tumor (35). Interestingly, here we show that RRD-251 was able to inhibit Rb phosphorylation even in melanoma cells that harbor the V600E mutation. We had observed earlier that while B-Raf could associate with Rb in cells, this interaction did not appear to have any significant regulatory effect on Rb function and RRD-251 could not disrupt the Rb-B-Raf interaction (20, 21). Melanoma cell lines with and without V600E mutation were sensitive to RRD-251 anti-proliferative and pro-apoptotic effects. Furthermore, treatment of female nu/nu mice bearing SK-MEL-28 (V600E positive) xenograft tumors with 50 mg of RRD-251/kg of body weight resulted in a highly significant (p=0.003) decrease in tumor growth. Taken together, these results provide evidences for the development of RRD-251 as a potential agent against metastatic melanoma.
Inhibition of apoptosis is the major molecular event associated with melanoma resistance against chemotherapy (25). Our results show that RRD-251 induces apoptosis in melanoma cells, as evident by TUNEL staining, activation of caspase3 and cleavage of PARP. Recent studies have suggested that expression of anti-apoptotic Mcl-1-protein expression is critically associated with inhibition of apoptosis in melanoma (25) (36). Here we show that melanoma cells treated with RRD-251 resulted in decreased expression of Mcl-1 at both transcriptional and translational levels. Additionally, SK-MEL-28 cells also demonstrated an increased expression of pro-apoptotic protein Bax in RRD-251 treated melanoma cells. Moreover, in addition to the induction of apoptosis we also found that RRD-251 blocks the S-phase entry of the cells. Data suggested that in addition to decrease in Rb phosphorylation, RRD-251 treatment downregulated the expression of E2F1 and its downstream cell cycle regulatory genes TS and cdc6 in melanoma cells. This occurs possibly through the degradation of the E2F1 protein rather than a transcriptional effect, given the fast kinetics of the reduction in E2F1 protein. It has been shown that E2F family members like E2F1 and E2F6, are deregulated in melanoma and provide growth advantage to the cells (37). Therefore, as demonstrated here, the ability of RRD-251 to downregulate E2F1 and its downstream target genes CDC6 and TS, highlights the importance of the potential use of RRD-251 in controlling the cell cycle progression in melanoma cells.
DTIC either as a single agent, or as in combination with two or three-drugs has failed to show any advantage in terms of response or survival of melanoma patients (38, 39). Results illustrated here suggest that DTIC has a mild to moderate DNA damage effect as well as partial effect on cell cycle distribution in SK-MEL-28 and SK-MEL-5 cells, however, these cells were completely resistance for the induction of apoptosis for up to 48 hr at 50 μmol/L concentration. This is in accordance with the earlier studies from other groups who have also demonstrated the resistance of SK-MEL-28 cells against DTIC treatment (40, 41). Interestingly, results from our study showed that cells pretreated with DTIC resulted in a statistically significant enhancement of the ability of RRD-251 to induce caspase activity and apoptosis. At the same time, since DTIC alone had no pro-apoptotic activity, it was synergistically enhanced by RRD-251 treatment in SK-MEL-5 and SK-MEL-28 cells. Notably, these results suggest that after DTIC exposure, DTIC-sensitive SK-MEL-2 as well as DTIC-resistant melanoma cells like SK-MEL-28 and SK-MEL-5 cells show better sensitivity towards RRD-251 induced apoptosis. Thus, this combination may be promising for the treatment of cancer patients with DTIC-resistant melanoma.
In conclusion, this work shows that RRD-251 has potent anti-tumoral activity against metastatic melanoma in vitro and in vivo. Results indicate that the decrease in Rb phosphorylation, downregulation of E2F1 and its downstream cell cycle regulators such as TS and cdc6 as well as decrease in anti-apoptotic protein Mcl-1 and upregulation of pro-apoptotic protein Bax may be involved in RRD-251 induced dual effects with cell cycle arrest as well as induction of apoptosis. Furthermore, we demonstrate that the induction of apoptosis in melanoma cell lines was significantly enhanced by the combined treatment of DTIC and RRD-251 as compared to either treatment used alone. Overall, these results provide a rationale for the clinical evaluation of RRD-251 in combination with DTIC in advanced melanoma patients. Further elucidation of the apoptotic pathways and in vivo studies will aid in the effective implementation of this combination-based therapy against metastatic melanoma.
Supplementary Material
Acknowledgments
This study was supported by the Donald Adams Comprehensive Melanoma Center as well as the grant CA118210 from the NCI. We thank Michael Damit for technical assistance. Support from the Core Facilities at Moffitt Cancer Center is acknowledged.
Grant Support: CA1182110 (SC, SS, NL)
Abbreviations List
- DTIC
Dacarbazine
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Oliveria S, Dusza S, Berwick M. Issues in the epidemiology of melanoma. Expert Rev Anticancer Ther. 2001;1:453–9. doi: 10.1586/14737140.1.3.453. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Sun W, Schuchter LM. Metastatic melanoma. Curr Treat Options Oncol. 2001;2:193–202. doi: 10.1007/s11864-001-0033-5. [DOI] [PubMed] [Google Scholar]
- 4.Atallah E, Flaherty L. Treatment of metastatic malignant melanoma. Curr Treat Options Oncol. 2005;6:185–93. doi: 10.1007/s11864-005-0002-5. [DOI] [PubMed] [Google Scholar]
- 5.Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer. 2004;40:1825–36. doi: 10.1016/j.ejca.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Stein JA, Brownell I. Treatment approaches for advanced cutaneous melanoma. J Drugs Dermatol. 2008;7:175–9. [PubMed] [Google Scholar]
- 7.Eberle J, Kurbanov BM, Hossini AM, Trefzer U, Fecker LF. Overcoming apoptosis deficiency of melanoma-hope for new therapeutic approaches. Drug Resist Updat. 2007;10:218–34. doi: 10.1016/j.drup.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Lorigan P, Eisen T, Hauschild A. Systemic therapy for metastatic malignant melanoma--from deeply disappointing to bright future? Exp Dermatol. 2008;17:383–94. doi: 10.1111/j.1600-0625.2007.00673.x. [DOI] [PubMed] [Google Scholar]
- 9.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 10.Nelson MA, Reynolds SH, Rao UN, et al. Increased gene copy number of the transcription factor E2F1 in malignant melanoma. Cancer Biol Ther. 2006;5:407–12. doi: 10.4161/cbt.5.4.2512. [DOI] [PubMed] [Google Scholar]
- 11.Bartkova J, Lukas J, Guldberg P, et al. The p16-cyclin D/Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. Cancer Res. 1996;56:5475–83. [PubMed] [Google Scholar]
- 12.Halaban R, Cheng E, Smicun Y, Germino J. Deregulated E2F transcriptional activity in autonomously growing melanoma cells. J Exp Med. 2000;191:1005–16. doi: 10.1084/jem.191.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halaban R. Rb/E2F: a two-edged sword in the melanocytic system. Cancer Metastasis Rev. 2005;24:339–56. doi: 10.1007/s10555-005-1582-z. [DOI] [PubMed] [Google Scholar]
- 14.Hiebert SW, Chellappan SP, Horowitz JM, Nevins JR. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992;6:177–85. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- 15.Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–61. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 16.Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000;2:E65–7. doi: 10.1038/35008695. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen ES, Buckmaster C, Chen TT, Feramisco JR, Wang JY. Inhibition of DNA synthesis by RB: effects on G1/S transition and S-phase progression. Genes Dev. 1998;12:2278–92. doi: 10.1101/gad.12.15.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leone G, DeGregori J, Jakoi L, Cook JG, Nevins JR. Collaborative role of E2F transcriptional activity and G1 cyclindependent kinase activity in the induction of S phase. Proc Natl Acad Sci U S A. 1999;96:6626–31. doi: 10.1073/pnas.96.12.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Ghosh RN, Chellappan SP. Raf-1 physically interacts with Rb and regulates its function: a link between mitogenic signaling and cell cycle regulation. Mol Cell Biol. 1998;18:7487–98. doi: 10.1128/mcb.18.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dasgupta P, Sun J, Wang S, et al. Disruption of the Rb--Raf-1 interaction inhibits tumor growth and angiogenesis. Mol Cell Biol. 2004;24:9527–41. doi: 10.1128/MCB.24.21.9527-9541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinkade R, Dasgupta P, Carie A, et al. A small molecule disruptor of Rb/Raf-1 interaction inhibits cell proliferation, angiogenesis, and growth of human tumor xenografts in nude mice. Cancer Res. 2008;68:3810–8. doi: 10.1158/0008-5472.CAN-07-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vindelov LL, Christensen IJ, Nissen NI. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983;3:323–7. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- 23.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 24.Tice RR, Agurell E, Anderson D, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–21. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 25.Tang L, Tron VA, Reed JC, et al. Expression of apoptosis regulators in cutaneous malignant melanoma. Clin Cancer Res. 1998;4:1865–71. [PubMed] [Google Scholar]
- 26.Leiter U, Schmid RM, Kaskel P, Peter RU, Krahn G. Antiapoptotic bcl-2 and bcl-xL in advanced malignant melanoma. Arch Dermatol Res. 2000;292:225–32. doi: 10.1007/s004030050479. [DOI] [PubMed] [Google Scholar]
- 27.Wong RP, Khosravi S, Martinka M, Li G. Myeloid leukemia-1 expression in benign and malignant melanocytic lesions. Oncol Rep. 2008;19:933–7. [PubMed] [Google Scholar]
- 28.Zhuang L, Lee CS, Scolyer RA, et al. Mcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanoma. Mod Pathol. 2007;20:416–26. doi: 10.1038/modpathol.3800750. [DOI] [PubMed] [Google Scholar]
- 29.Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. Mcl-1 is required for melanoma cell resistance to anoikis. Mol Cancer Res. 2009;7:549–56. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SM, Thatcher N, Dougal M, Margison GP. Dosage and cycle effects of dacarbazine (DTIC) and fotemustine on O6-alkylguanine-DNA alkyltransferase in human peripheral blood mononuclear cells. Br J Cancer. 1993;67:216–21. doi: 10.1038/bjc.1993.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burdette-Radoux S, Tozer RG, Lohmann RC, et al. Phase II trial of flavopiridol, a cyclin dependent kinase inhibitor, in untreated metastatic malignant melanoma. Invest New Drugs. 2004;22:315–22. doi: 10.1023/B:DRUG.0000026258.02846.1c. [DOI] [PubMed] [Google Scholar]
- 32.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–69. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 33.Knudsen ES, Wang JY. Differential regulation of retinoblastoma protein function by specific Cdk phosphorylation sites. J Biol Chem. 1996;271:8313–20. doi: 10.1074/jbc.271.14.8313. [DOI] [PubMed] [Google Scholar]
- 34.Knudsen ES, Wang JY. Dual mechanisms for the inhibition of E2F binding to RB by cyclin-dependent kinase-mediated RB phosphorylation. Mol Cell Biol. 1997;17:5771–83. doi: 10.1128/mcb.17.10.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 36.Keuling AM, Felton KE, Parker AA, Akbari M, Andrew SE, Tron VA. RNA silencing of Mcl-1 enhances ABT-737-mediated apoptosis in melanoma: role for a caspase-8-dependent pathway. PLoS One. 2009;4:e6651. doi: 10.1371/journal.pone.0006651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halaban R, Miglarese MR, Smicun Y, Puig S. Melanomas, from the cell cycle point of view (Review) Int J Mol Med. 1998;1:419–25. doi: 10.3892/ijmm.1.2.419. [DOI] [PubMed] [Google Scholar]
- 38.Huncharek M, Caubet JF, McGarry R. Single-agent DTIC versus combination chemotherapy with or without immunotherapy in metastatic melanoma: a meta-analysis of 3273 patients from 20 randomized trials. Melanoma Res. 2001;11:75–81. doi: 10.1097/00008390-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Mouawad R, Sebert M, Michels J, Bloch J, Spano JP, Khayat D. Treatment for metastatic malignant melanoma: Old drugs and new strategies. Crit Rev Oncol Hematol. 2009 doi: 10.1016/j.critrevonc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Lillehammer T, Engesaeter BO, Prasmickaite L, Maelandsmo GM, Fodstad O, Engebraaten O. Combined treatment with Ad-hTRAIL and DTIC or SAHA is associated with increased mitochondrial-mediated apoptosis in human melanoma cell lines. J Gene Med. 2007;9:440–51. doi: 10.1002/jgm.1036. [DOI] [PubMed] [Google Scholar]
- 41.Munoz-Alonso MJ, Gonzalez-Santiago L, Zarich N, et al. Plitidepsin has a dual effect inhibiting cell cycle and inducing apoptosis via Rac1/c-Jun NH2-terminal kinase activation in human melanoma cells. J Pharmacol Exp Ther. 2008;324:1093–101. doi: 10.1124/jpet.107.132662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.