Abstract
Objectives
Both the Young Laplace law and finite element (FE) based methods have been used to calculate left ventricular (LV) wall stress. We tested the hypothesis that the Young Laplace law is able to reproduce results obtained with FE method.
Methods
Magnetic resonance (MRI) images with non-invasive tags were used to calculate 3D myocardial strain in five sheep 16 weeks after anteroapical myocardial infarction and in one of those sheep 6 weeks after a Dor procedure. Animal specific FE models were created from the remaining five animals using MRI images obtained at early diastolic filling. FE based stress in the fiber, cross fiber and circumferential directions was calculated and compared to stress calculated with (Young Laplace law) and without (Modified Laplace) the assumption that wall thickness is very much less than the radius of curvature.
Results
First,circumferential stress calculated with the Modified Laplace law is closer to results obtained with the FE method than stress calculated with the Young Laplace law. However, there are pronounced regional differences with the largest difference between Modified Laplace and FE occurring especially in the inner and outer layers of the infarct borderzone. Also, stress calculated with Modified Laplace is very different than stress in the fiber and cross fiber direction calculated with FE. As a consequence, the Modified Laplace law is inaccurate when used to calculate the effect of the Dor procedure on regional ventricular stress.
Conclusion
The FE method is necessary to determine stress in the LV with post infarct and surgical ventricular remodeling.
Keywords: Myocardial Infarction, Strain, Stress, Finite Element, Laplace
II. INTRODUCTION
Regional coronary blood flow, [1] myocardial oxygen consumption, hypertrophy, [2] and remodeling are all determined by ventricular wall stress. In addition, a reduction in wall stress has been used to justify a number of new cardiac operations including the Batista operation [3], the Acorn cardiac support device [4] and the Myocor Myosplint. [5] However, Huisman previously showed that it is impossible to directly measure regional in vivo myocardial stress because of tethering from surrounding myocardium. [6, 7] As a consequence, ventricular wall stress is usually calculated with force balance methods such as the Young Laplace law. [8, 9] Unfortunately, localized shape change in and around the myocardial infarction (MI) and regional changes in systolic and diastolic material properties [10] make the left ventricle (LV) a mechanically complex structure. The accuracy of force balance based calculation of LV stress may be, therefore, severely limited.
An alternative approach to quantifying ventricular wall stress and stiffness is mathematical modeling based on the conservation laws of continuum mechanics, the most versatile of which is the finite element (FE) method. [11] The FE method used in this study for continuum analysis of the heart includes several features that are uncommon in conventional FE methods. First, the constitutive relationship is non-linear and anisotropic [12, 13] with direction based directly on measured 3-D myofiber angle distributions. [14, 15] Next, the models undergo large or finite deformation with difference in dimensions between the end-diastole (ED) and end-systole (ES) that is greater than 10%. More recent models now also include the transmural heterogeneity of cellular excitation-contraction coupling mechanisms. [16]
We calculated stress with (Young Laplace law) and without (Modified Laplace) the assumption that wall thickness is very much less than the radius of curvature with large deformation finite element methods using data collected from sheep after antero-apical myocardial infarction (MI) and after Dor procedure. We tested the hypothesis that stress in the circumferential direction calculated with Laplace law is equal to values obtained with the FE method. Since stress in the cross fiber direction is likely to cause volume overload hypertrophy and stress in the fiber direction is likely to cause pressure overload hypertrophy, we also compared stress calculated using force balance with the magnitude of stress in the fiber and cross fiber directions calculated with the FE method.
II. MATERIAL AND METHODS
Animals used in this study were treated in compliance with the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, National Research Council, and published by the National Academy Press, revised 1996. Magnetic resonance (MRI) images with non-invasive tags used to calculate 3D myocardial strain in five sheep 16 weeks after anteroapical myocardial infarction and in one of those sheep 6 weeks after Dor procedure.[17] These experimental results were previously reported. [17, 18]
A. Force balance calculations
i. The Young Laplace law
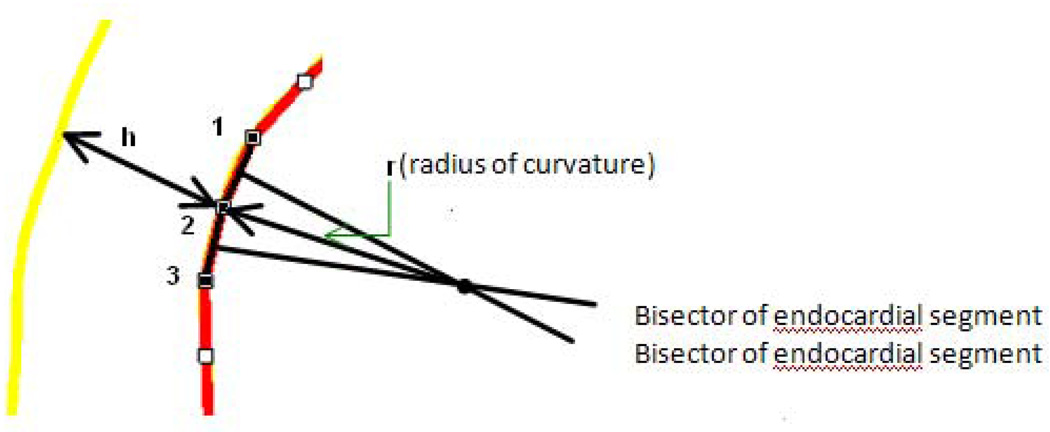
The endocardial contour was divided into 32 points. As seen in Figure 2, 3 adjacent points on the endocardial contour were identified and used to determine the radius of curvature. [21] Two line segments were created by connecting point 1 and point 2, point 2 and point 3, where two bisectors to these two line segments were then constructed. The radius of curvature for point 2 was calculated from the distance from point 2 to the bisector’s intersection. Note that short axis contours near the apex (infarct area) were not used because the image plane in that area cuts the LV wall obliquely.
Figure 2.
Method of measuring endocardial radius of curvature. r is the radius of curvature and h is the wall thickness.
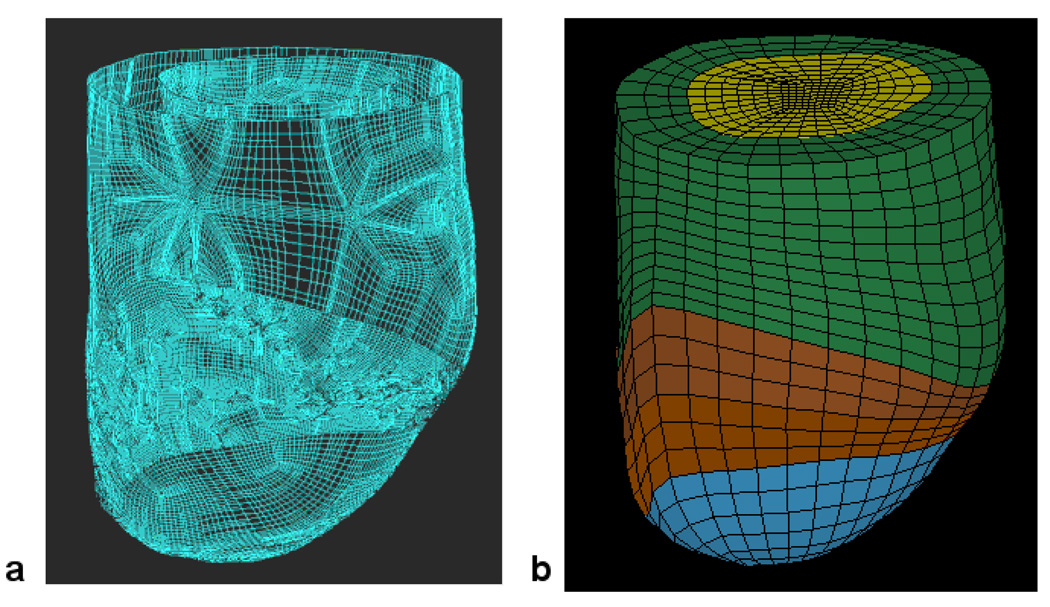
Figure 3.
Finite element model of the LV with anteroapical MI. Animal specific contours were generated from MRI (A). Solid mesh (B) was broken into four regions (green = remote, brown and red = borderzone).
The derivation of the Young-Laplace law is as follows:
| (1) |
where P is intracavitary pressure, r is the endocardial radius of curvature and h is the wall thickness. Equation 1 simplifies to:
| (2) |
if h≪r Equation 2 is reduced to the Young Laplace law:
| (3) |
ii. Modified Laplace law
Throughout the paper we refer to Equation 3 as ‘Young Laplace’ and Equation 2 as ‘Modified Laplace’.
B. FE Model
Analysis of borderzone function using FE models has been previously reported. [22]
i. Finite element method
Animal specific FE models were created from MRI images at the early diastole, which was considered as the initial unloaded reference state.[23]. Representative surface and finite element meshes are seen in Figure 3. Myofiber angles of −37°, 23° and 83° were assigned at the epicardium, midwall and endocardium, respectively, in the remote and borderzone regions [24]. At the aneurysm region, fiber angles were set to 0° [25]. Circumferential displacement of basal epicardial nodes was constrained.
ii. Loading
The inner endocardium wall was loaded to the measured in-vivo ED and ES LV pressures.
iii. Constitutive relationship
Passive [13] and active myocardial [26] constituitive relationships have been previously described. The active myocardial material property law was implemented using a user-defined material subroutine in LS-DYNA (Livermore Software Technology Corporation, Livermore, CA). Diastolic [27] and systolic [26] material parameters have been previously reported.
iv. Material property optimization
The method of myocardial material property optimization was previously described by Sun et al. [29] Specifically, the commercial FE optimization software, LS-OPT [30], was used to find the optimal value of Tmax for each region.
Simulations were performed on a small Linux cluster (7 nodes; each node with two AMD Opteron 240 processors and 1 gB memory).
C. Statistical analysis
All values are expressed as mean ± standard deviation (SD) and compared by repeated measures analysis using a mixed model to test for both fixed and random effects (Systat Software, Inc., Chicago, IL). The statistical model was as follows:
| (5) |
where Method, Region and Layer are dummy variables and where Method is either Laplace or FE, Region is either BZ or remote and Layer is either Endo, MID or Epi. The statistical significance of individual group comparisons was tested using the Student t test with the Bonferoni correction for multiple comparisons. Significance was set at p less than 0.05.
III. RESULTS
Computational time was recorded while performing the FE method on a single FE model. Time for FE simulation was 385 seconds.
A. Average circumferential stress
Table 1 shows average LV wall stress in the circumferential direction calculated using the Young Laplace law, the Modified Laplace law and the finite element method. The average wall thickness to radius of curvature ratio was 0.22 ± 0.022 at end diastole and 0.24 ± 0.030 at end systole. As a consequence, circumferential stress calculated with the Young Laplace law and the Modified Laplace law is significantly different.
Table 1.
Average stress in the circumferential direction at end diastole and end systole. Units are kPa. Values are mean ± standard deviation.
| End-diastole | End-systole | |
|---|---|---|
| Young Laplace | 2.69 ± 0.95a | 22.38 ± 7.97a |
| Modified Laplace | 2.35 ± 0.85a,b | 19.43 ± 7.93a |
| Finite element | 1.87 ± 1.25b | 15.00 ± 10.27 |
is p < 0.05 between Young Laplace and Modified Laplace
is p < 0.05 between Modified Laplace and Finite element.
Furthermore, circumferential stress calculated with the Modified Laplace is closer to results obtained with the FE method than stress calculated with the Young Laplace law. Specifically, average Modified Laplace stress was 26% higher than FE based calculation of circumferential stress at end-diastole (p=0.006) but was not different at end-systole.
B. Regional variation
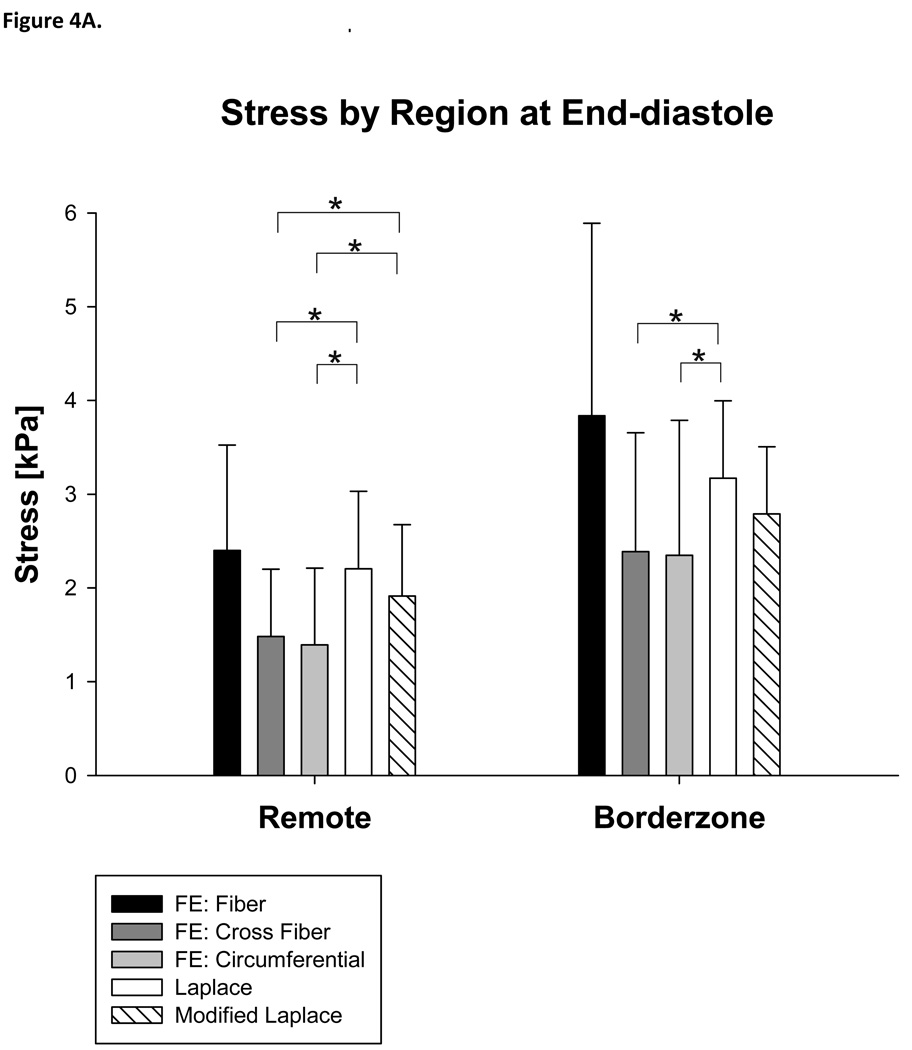
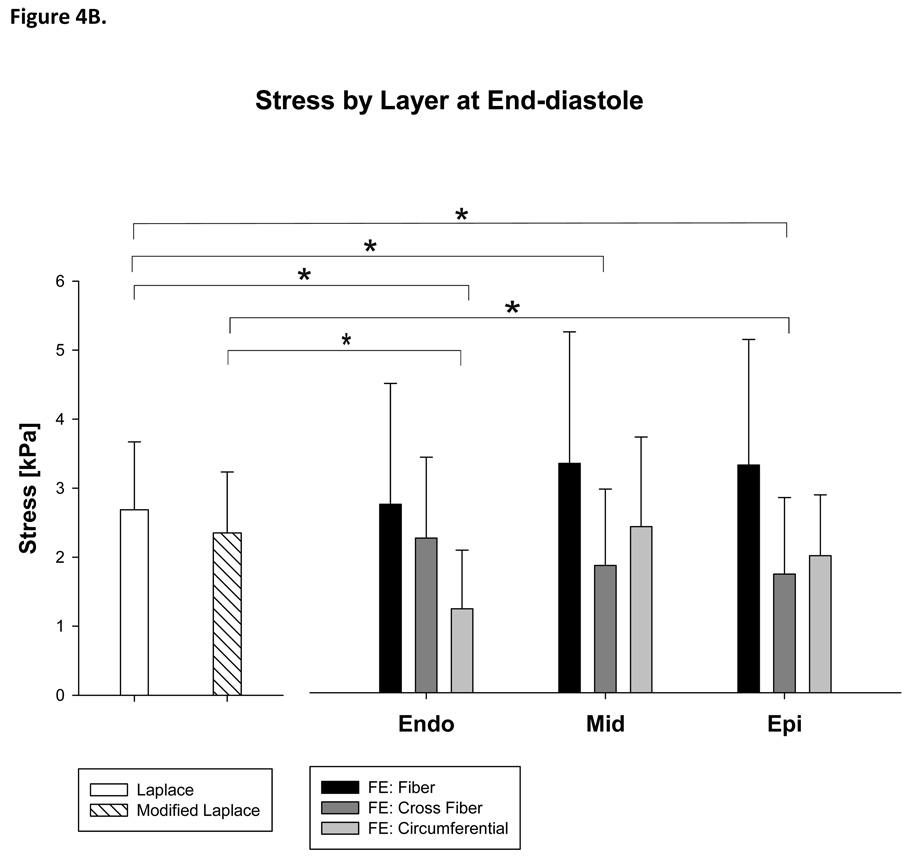
There were significant regional differences. Stress calculated with the Modified Laplace was higher than circumferential stress calculated with the FE method in the remote (37%, p=0.048) but not borderzone regions (Figure 4A) and higher than circumferential stress calculated with the FE method in the endocardial layer (93%, p<0.001) at end-diastole (Figure 4B).
Figure 4.
Stress calculated with Laplace and finite element methods at end-diastole by region (A) and layer of the LV wall (B). Note that both the Young Laplace’s law and Modified Laplace law overestimate stress in the circumferential and cross fiber directions in the remote myocardium at end-diastole.
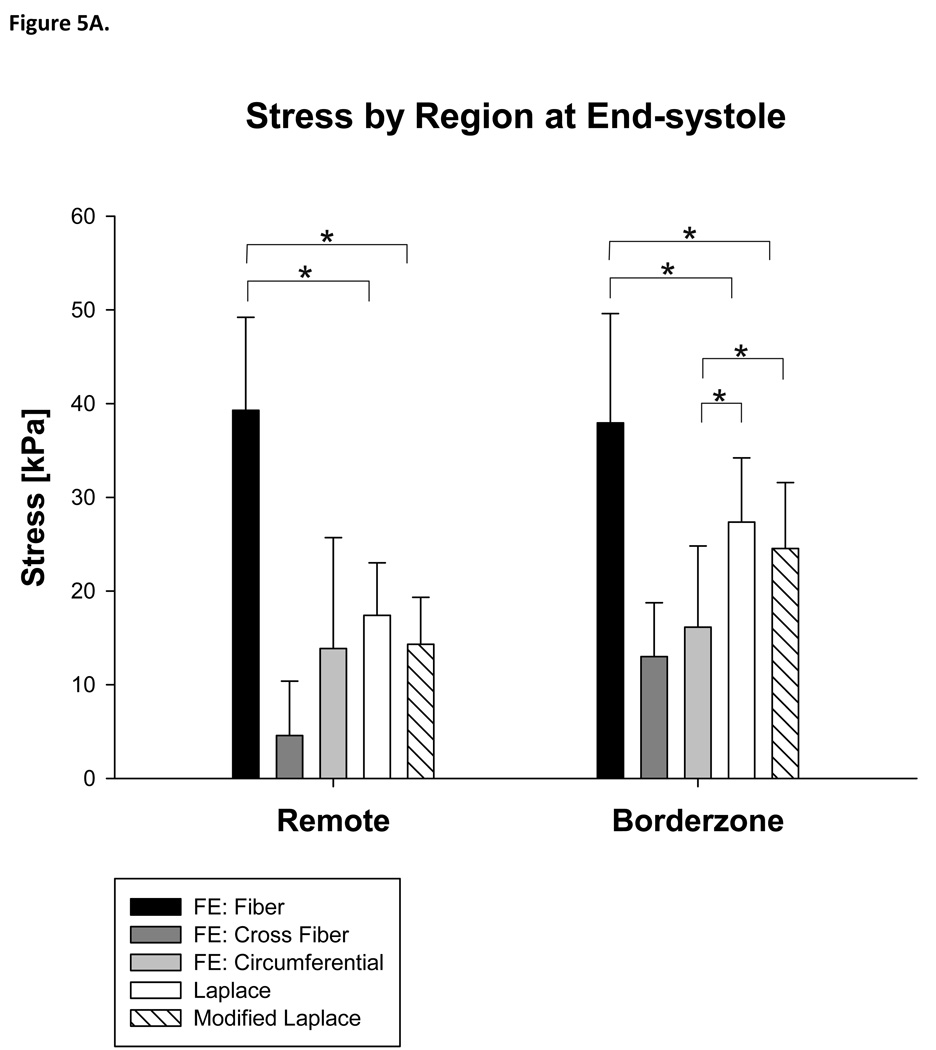
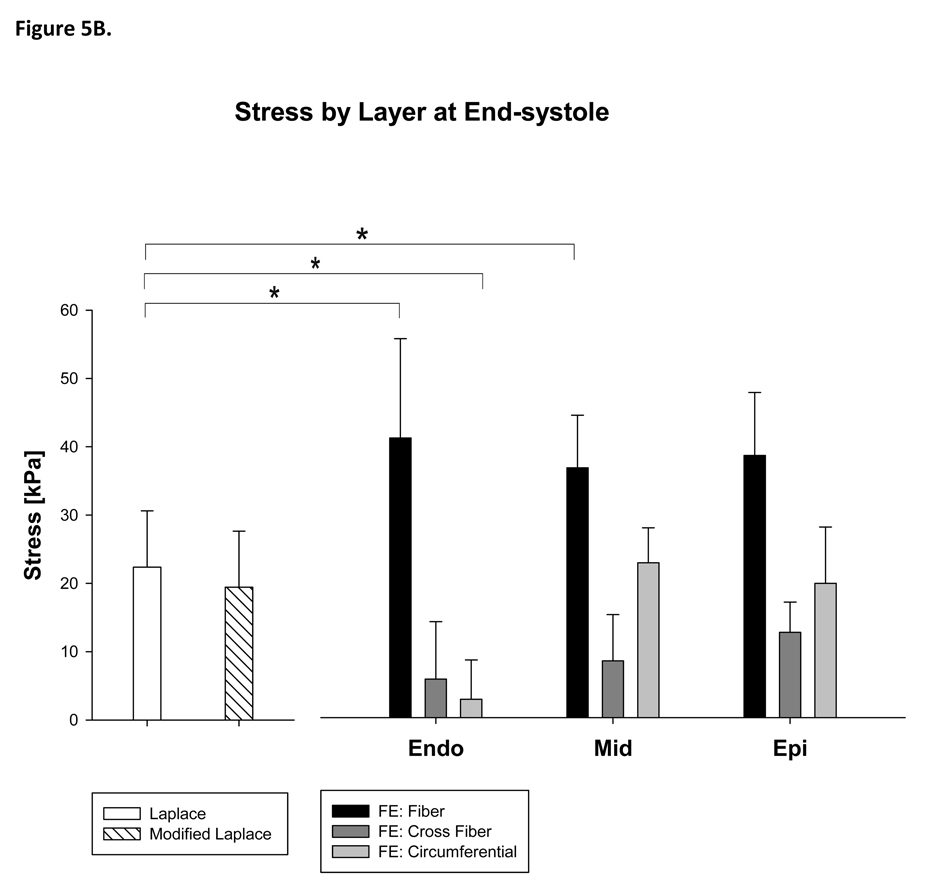
Furthermore, stress calculated with the Modified Laplace law was higher than circumferential stress calculated with the FE method in the borderzone (Modified Laplace: 52%, p=0.048) but not in the remote myocardium (Figure 5A) and higher than circumferential stress calculated with the FE method in the endocardial layer (93%, p<0.001) at end-systole (Figure 5B).
Figure 5.
Stress calculated with Laplace and finite element methods at end-systole by region (A) and layer of the LV wall (B). Note that the Young Laplace law underestimates stress in the fiber direction at end-systole. The Young Laplace law is not different than circumferential stress in the remote myocardium but fails to account for transmural variation in stress. Statistically significant comparisons with cross fiber stress are not marked. See the text for those details.
C. Comparison with fiber and cross fiber stress calculated with the FE method
The magnitude of stress calculated with the Modified Laplace law was very different than stress in the fiber and cross fiber direction. Average fiber stress with the FE method was 3.119 ± 1.784 kPa at end-diastole and 38.618 ± 10.653 kPa at end-systole. Average cross fiber stress with the FE method was 1.935 ± 1.112 kPa at end-diastole and 8.800 ± 7.102 kPa at end-systole. Average Modified Laplace stress was 22% higher than FE based calculation of cross fiber stress end-diastole (p<0.001) and 121% higher at end-systole (p<0.001).
Once again, there were significant regional differences. Stress calculated with the Modified Laplace law was higher than cross fiber stress calculated with the FE method in the remote region (22%, p=0.012) at end-diastole (Figure 4A). Stress calculated with both the Modified Laplace law was substantially lower than fiber stress calculated with the FE method in both remote (Modified Laplace: 64%, p<0.001) and borderzone regions (Modified Laplace: 35%, p=0.012) at end systole (Figure 5A). Finally, Stress calculated with the Modified Laplace law was lower than fiber stress at all layers (p<0.001) at end-systole (Figure 5B).
D. Change in stress after Dor procedure
Stress at end systole calculated with the Young Laplace law, Modified Laplace law and the finite element in the infarct borderzone before and after Dor procedure is seen in Table 2. The reduction in average borderzone stress calculated with the Young Laplace and Modified Laplace law was −23.5 and −26.2% respectively. However, the change in regional stress calculated with the finite element method was quite different with a substantial increase in stress in the inner layer (Δ in circumferential stress +196.7%; Δ in fiber stress +15%) and a more pronounced decrease in the outer layer (Δ in circumferential stress −70.4%; Δ in fiber stress −49.7%).
Table 2.
Stress at end systole calculated with the Young Laplace law, Modified Laplace law and the finite element in the infarct borderzone before and after Dor procedure. Data is from a single sheep. FE is finite element. Note that the change in regional circumferential and fiber stress calculated with the finite element method was quite different especially in the inner and outer layers of the borderzone.
| Inner | Midwall | Outer | Average | ||
|---|---|---|---|---|---|
| Before Dor (16 weeks after MI) |
FE (Fiber) | 28.39 | 26.60 | 26.76 | 27.25 |
| FE (Cross Fiber) | 6.43 | 9.16 | 11.82 | 9.14 | |
| FE (Circumferential) | 3.63 | 17.72 | 14.40 | 11.91 | |
| Young LaPlace | 19.71 | ||||
| Modified LaPlace | 17.57 | ||||
| After Dor | FE (Fiber) | 32.65 | 20.22 | 13.45 | 22.10 |
| FE (Cross Fiber) | 8.33 | 7.30 | 6.39 | 7.34 | |
| FE (Circumferential) | 10.77 | 12.66 | 8.45 | 10.63 | |
| Young LaPlace | 15.08 | ||||
| Modified LaPlace | 12.96 | ||||
IV. Comments
The principal findings of our study are 1) circumferential stress calculated with the Modified Laplace is closer to results obtained with the FE method than stress calculated with the Young Laplace law, 2) there are pronounced regional differences between stress calculated with the Modified Laplace law and the finite element method and 3) the magnitude of stress calculated with the Modified Laplace law is very different than stress in the fiber and cross fiber directions calculated with the finite element method.
A. The Finite Element Method as the ‘Gold Standard’
The decision to use the large deformation finite element method as the reference or gold standard is reasonable given that the finite element models were optimized using measured myocardial strain in addition to end-diastolic and end-systolic volumes. A comparison of Laplace’s law with the finite element method in a physical model constructed from materials with known material properties, such as silicone [31] or silicone with embedded elastic fibers, and that has simplified geometry would be interesting and we propose to carry out this study in the future. However, even when completed, physical models of that sort will lack the ability to simulate active contraction.
B. Limitations of the Young Laplace law
There are a number of inaccuracies and limitations associated with the application of Laplace type calculations of left ventricular wall stress [32] An inaccuracy specific to the Young Laplace law is the restriction that the wall thickness (h) be very much less than the radius of curvature (r). However, the h/r ratio in this study was 0.22 ± 0.022 at end diastole and 0.24 ± 0.030 at end systole. This suggests that Equation 2 (Modified Laplace) which does not have the h ≪ r restriction is more reasonable. In fact, we found in all cases that stress calculated with Equation 2 was closer to finite element based calculation of circumferential stress than stress calculated with the Young Laplace law.
C. Stress variation across the LV wall
When a thick-walled pressure vessel is inflated, most of the deformation in the circumferential and longitudinal directions occurs at the inner surface and decreases monotonically towards the outer surface. Thus, we would expect circumferential and longitudinal stress to vary transmurally in a similar manner although the actual transmural variation in these stress components will depend on the relationship between stress and strain (constitutive relation) of the myocardium. For instance, Guccione et al previously measured fiber stress in the LV of a normal dog using a finite deformation finite element method similar to that used in the current study. [33], Guccione found the transmural gradient in fiber stress was 3.3 kPa at the base and 4.6 kPa at the apex at end diastole. [33] The transmural gradients were small near the LV base during systole were as high as 43 kPa between the mid ventricle and apex. [33] In the present study, transmural gradients in fiber stress were smaller but still significant with a gradient of 1.55 kPa in the remote myocardium at end diastole and 7.23 kPa at end systole. The Young Laplace law is based on a global force balance, which ignores myocardial material properties. Thus, the Young Laplace law can be used to estimate only average stress across the full wall thickness in the circumferential and longitudinal directions.
D. The importance of fiber and cross fiber stress
Another limitation of the Young Laplace and Modified Laplace laws is that they cannot be used to calculate stress in the local muscle fiber or cross fiber direction since stress in the fiber and cross fiber directions are probably the causes of hypertrophy. There is increasing evidence that stress in the cross fiber direction causes eccentric or volume overload type hypertrophy. [34, 35] Although evidence is less clear, it is probable that end systolic fiber stress causes concentric or pressure overload type hypertrophy.
E. Implications for the Dor procedure
Regional differences between stress calculated the Young Laplace law and finite element methods occurs especially in the inner and outer layers of the infarct BZ. When coupled with inability of Laplace type methods to measure fiber and cross fiber stress, this leads the Young Laplace and Modified Laplace laws to miscalculate the change in stress in the infarct BZ after Dor procedure. Since the rationale for the Dor procedure in specific and surgical ventricular remodeling in general is reduction in wall stress [36], accurate knowledge of stress in the fiber and cross fiber directions is of obvious importance. Furthermore, in our opinion, improvement in BZ contractility is the primary therapeutic target of the Dor procedure. [22]
F. Utility of the finite deformation type finite element model of the left ventricle
The FE method can be used to simulate the effect of surgical ventriculoplasty [37] and passive constraint on the LV. For instance, Dang and colleagues previously used a finite element model of the finite deformation type to calculate the effect of surgical remodeling on stroke volume and mean fiber stress. [37] Force balance methods such as the Young Laplace law can only calculate stress from an existing LV image obtained during animal experiments or from patients. Thus, it cannot be used as a predictive theoretical tool. In our opinion, this is the greatest limitation of the Young Laplace law.
V. CONCLUSIONS
Circumferential stress calculated with the Modified Laplace law was superior to the Young Laplace law although stress calculated with the Modified Laplace law remained statistically different (higher) than finite element based calculation of circumferential stress at end-diastole.
On the other hand, the magnitude of stress calculated with the Modified Laplace law is very different than stress in the fiber and cross fiber directions calculated with the finite element method. Also, there are pronounced regional differences with the largest differences between Modified Laplace and finite element methods occurring in the inner and outer layers of the infarct BZ. As a consequence, both the Modified Laplace law is inaccurate when used to calculate the effect of the Dor procedure on regional ventricular stress. The finite element method is necessary to determine stress in the LV with post infarct and surgical ventricular remodeling.
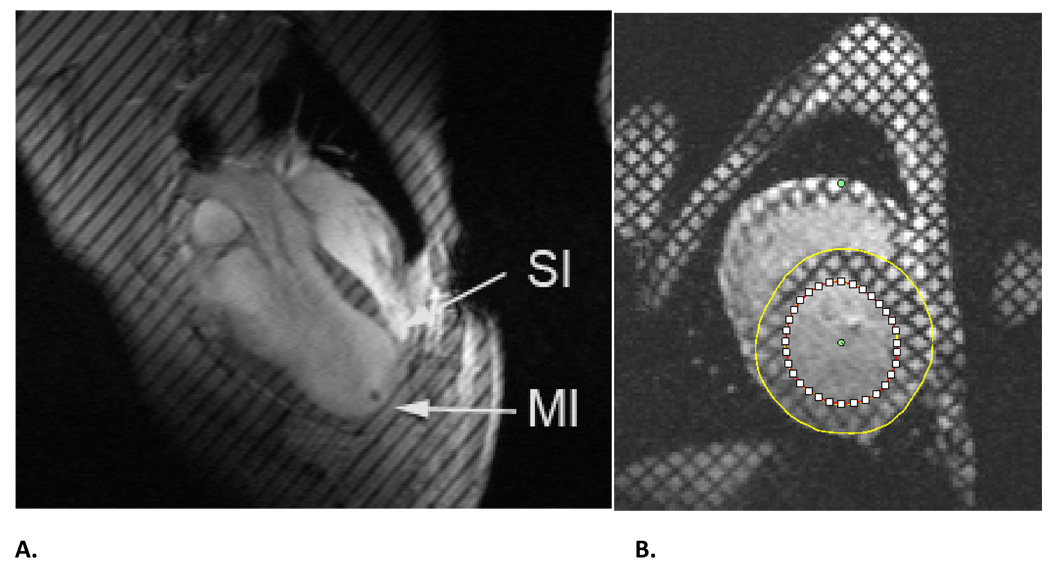
Figure 1.
A. Long axis MR images from sheep with anteroapical MI. B. Short axis MR image. Both images were obtained 16 weeks after MI. Both images have non invasive tissue tags and both images were obtained at end-systole. MI=dykinetic infarct; SI=septal infarct. Note that Panel A has been previously reported. [17]
ACKNOWLEDGMENTS
This study was supported by NIH grant R01-HL-77921 (Dr. Guccione), and R01-HL-63348 (Dr. Ratcliffe). This support is gratefully acknowledged.
References
- 1.Jan KM. Distribution of myocardial stress and its influence on coronary blood flow. Journal Of Biomechanics. 1985;18(11):815–820. doi: 10.1016/0021-9290(85)90478-6. [DOI] [PubMed] [Google Scholar]
- 2.Grossman W. Cardiac hypertrophy: useful adaptation or pathologic process? Am J Med. 1980;69(4):576–584. doi: 10.1016/0002-9343(80)90471-4. [DOI] [PubMed] [Google Scholar]
- 3.Batista RJ, Verde J, Nery P, et al. Partial left ventriculectomy to treat end-stage heart disease. Ann Thorac Surg. 1997;64(3):634–638. doi: 10.1016/s0003-4975(97)00779-0. [DOI] [PubMed] [Google Scholar]
- 4.Mann DL, Acker MA, Jessup M, et al. Rationale, design, and methods for a pivotal randomized clinical trial for the assessment of a cardiac support device in patients with New York health association class III–IV heart failure. J Card Fail. 2004;10(3):185–192. doi: 10.1016/j.cardfail.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy PM, Takagaki M, Ochiai Y, et al. Device-based change in left ventricular shape: a new concept for the treatment of dilated cardiomyopathy. J Thorac Cardiovasc Surg. 2001;122(3):482–490. doi: 10.1067/mtc.2001.115240. [DOI] [PubMed] [Google Scholar]
- 6.Huisman RM, Elzinga G, Westerhof N, Sipkema P. Measurement of left ventricular wall stress. Cardiovascular Research. 1980;14(3):142–153. doi: 10.1093/cvr/14.3.142. [DOI] [PubMed] [Google Scholar]
- 7.Yin FCP. Ventricular wall stress. Circ Res. 1986;49:829–842. doi: 10.1161/01.res.49.4.829. [DOI] [PubMed] [Google Scholar]
- 8.Fung YC. Biomechanics: Mechanical properties of living tissues. New York: Springer-Verlag; 1981. [Google Scholar]
- 9.Woods RH. A Few Applications of a Physical Theorem to Membranes in the Human Body in a State of Tension. J Anat Physiol. 1892;26(Pt 3):362–370. [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta KB, Ratcliffe MB, Fallert MA, Edmunds LHJ, Bogen DK. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation. 1994;89:2315–2326. doi: 10.1161/01.cir.89.5.2315. [DOI] [PubMed] [Google Scholar]
- 11.McCulloch A, Waldman L, Rogers J, Guccione J. Large-scale finite element analysis of the beating heart. Crit Rev Biomed Eng. 1992;20(5–6):427–449. [PubMed] [Google Scholar]
- 12.Guccione JM, McCulloch AD. Mechanics of active contraction in cardiac muscle: Part I--Constitutive relations for fiber stress that describe deactivation. J Biomech Eng. 1993;115(1):72–81. doi: 10.1115/1.2895473. [DOI] [PubMed] [Google Scholar]
- 13.Guccione JM, McCulloch AD, Waldman LK. Passive material properties of intact ventricular myocardium determined from a cylindrical model. J Biomech Eng. 1991;113(1):42–55. doi: 10.1115/1.2894084. [DOI] [PubMed] [Google Scholar]
- 14.Streeter DD, Spotnitz HM, Patel DP, Ross JRJ, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circulation Research. 1969;24:339–347. doi: 10.1161/01.res.24.3.339. [DOI] [PubMed] [Google Scholar]
- 15.Walker JC, Guccione JM, Jiang Y, et al. Helical myofiber orientation after myocardial infarction and left ventricular surgical restoration in sheep. J Thorac Cardiovasc Surg. 2005;129(2):382–390. doi: 10.1016/j.jtcvs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Saucerman JJ, Brunton LL, Michailova AP, McCulloch AD. Modeling beta-adrenergic control of cardiac myocyte contractility in silico. J Biol Chem. 2003;278(48):47997–48003. doi: 10.1074/jbc.M308362200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P, Guccione JM, Nicholas SI, et al. Endoventricular patch plasty for dyskinetic anteroapical left ventricular aneurysm increases systolic circumferential shortening in sheep. J Thorac Cardiovasc Surg. 2007;134(4):1017–1024. doi: 10.1016/j.jtcvs.2007.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P, Guccione JM, Nicholas SI, et al. Left ventricular volume and function after endoventricular patch plasty for dyskinetic anteroapical left ventricular aneurysm in sheep. J Thorac Cardiovasc Surg. 2005;130(4):1032–1038. doi: 10.1016/j.jtcvs.2005.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guttman MA, Zerhouni EA, McVeigh ER. Analysis and visualization of cardiac function from MR images. IEEE Comp Graph Appl. 1997;17:30–38. doi: 10.1109/38.576854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozturk C, McVeigh ER. Four-dimensional B-spline based motion analysis of tagged MR images: introduction and in vivo validation. Phys Med Biol. 2000;45(6):1683–1702. doi: 10.1088/0031-9155/45/6/319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson BM, Gorman JH, 3rd, Salgo IS, et al. Border zone geometry increases wall stress after myocardial infarction: contrast echocardiographic assessment. Am J Physiol Heart Circ Physiol. 2003;284(2):H475–H479. doi: 10.1152/ajpheart.00360.2002. [DOI] [PubMed] [Google Scholar]
- 22.Sun K, Zhang Z, Suzuki T, et al. Dor procedure for dyskinetic anteroapical myocardial infarction fails to improve contractility in the border zone. J Thorac Cardiovasc Surg. 2010 doi: 10.1016/j.jtcvs.2009.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moustakidis P, Maniar HS, Cupps BP, et al. Altered left ventricular geometry changes the border zone temporal distribution of stress in an experimental model of left ventricular aneurysm: a finite element model study. Circulation. 2002;106(12 Suppl 1):I168–I175. [PubMed] [Google Scholar]
- 24.Omens JH, May KD, McCulloch AD. Transmural distribution of three-dimensional strain in the isolated arrested canine left ventricle. Am J Physiol. 1991;261(3 Pt 2):H918–H928. doi: 10.1152/ajpheart.1991.261.3.H918. [DOI] [PubMed] [Google Scholar]
- 25.Moonly S. Department of Bioengineering. San Francisco, CA: University of California, San Francisco with University of California, Berkeley; 2003. Experimental and computational analysis of left ventricular aneurysm mechanics. [Google Scholar]
- 26.Guccione JM, Waldman LK, McCulloch AD. Mechanics of active contraction in cardiac muscle: Part II--Cylindrical models of the systolic left ventricle. J Biomech Eng. 1993;115(1):82–90. doi: 10.1115/1.2895474. [DOI] [PubMed] [Google Scholar]
- 27.Walker JC, Ratcliffe MB, Zhang P, et al. MRI-based finite-element analysis of left ventricular aneurysm. Am J Physiol Heart Circ Physiol. 2005;289(2):H692–H700. doi: 10.1152/ajpheart.01226.2004. [DOI] [PubMed] [Google Scholar]
- 28.Usyk TP, Mazhari R, McCulloch AD. Effect of laminar orthotropic myofiber architecture on regional stress and strain in the canine left ventricle. Journal of Elasticity. 2000;61(1–3):143–164. [Google Scholar]
- 29.Sun K, Stander N, Jhun C-S, et al. A computationally efficient formal optimization of regional myocardial contractility in a sheep with left ventricular aneurysm. J Biomech Eng. 2009;131 doi: 10.1115/1.3148464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stander N, Roux W, Eggleston T, Craig K. LS-OPT user's manual version 3.2. 2007 [Google Scholar]
- 31.Dokos S, LeGrice IJ, Smaill BH, Kar J, Young AA. A triaxial-measurement shear-test device for soft biological tissues. J Biomech Eng. 2000;122(5):471–478. doi: 10.1115/1.1289624. [DOI] [PubMed] [Google Scholar]
- 32.Moriarty TF. The law of Laplace. Its limitations as a relation for diastolic pressure, volume, or wall stress of the left ventricle. Circ Res. 1980;46(3):321–331. doi: 10.1161/01.res.46.3.321. [DOI] [PubMed] [Google Scholar]
- 33.Guccione JM, Costa KD, McCulloch AD. Finite element stress analysis of left ventricular mechanics in the beating dog heart. J Biomech. 1995;28(10):1167–1177. doi: 10.1016/0021-9290(94)00174-3. [DOI] [PubMed] [Google Scholar]
- 34.Gopalan SM, Flaim C, Bhatia SN, et al. Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol Bioeng. 2003;81(5):578–587. doi: 10.1002/bit.10506. [DOI] [PubMed] [Google Scholar]
- 35.Senyo SE, Koshman YE, Russell B. Stimulus interval, rate and direction differentially regulate phosphorylation for mechanotransduction in neonatal cardiac myocytes. FEBS Lett. 2007;581(22):4241–4247. doi: 10.1016/j.febslet.2007.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkhoff D, Wechsler AS. Surgical ventricular remodeling: a balancing act on systolic and diastolic properties. J Thorac Cardiovasc Surg. 2006;132(3):459–463. doi: 10.1016/j.jtcvs.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 37.Dang AB, Guccione JM, Zhang P, et al. Effect of ventricular size and patch stiffness in surgical anterior ventricular restoration: a finite element model study. Ann Thorac Surg. 2005;79(1):185–193. doi: 10.1016/j.athoracsur.2004.06.007. [DOI] [PubMed] [Google Scholar]