Abstract
We propose that a C-type lectin receptor, SIGNR-1, plays a role in conditioning gastrointestinal lamina propria (LP) DC subset for the induction of oral tolerance in a model of food-induced anaphylaxis. Oral delivery of bovine serum albumin (BSA) bearing 51 mols of mannosides (Man51-BSA) significantly reduced the levels of BSA-induced anaphylactic response. Man51-BSA was found to, selectively, target the LPDC subset expressing a member of the CLRs, SIGNR1, and induce the expression of IL-10, but not IL-6 and IL-12p70. This was noted also in Man51-BSA-treated IL-10-GFPknockin (tiger) mice. The Man51-BSA–SIGNR1 axis in LPDCs, both in vitro and in vivo, promoted the generation of CD4+ Tr1-like cells expressing IL-10 and IFN-γ, in a SIGNR-1- and IL-10-dependent manner, but not of CD4+CD25+Foxp3+ Tregs. The in vivo-generated Tr1-like cells were capable of transferring tolerance. These results suggest the potential utility of sugar-modified antigen in oral tolerance through targeting of SIGNR1 and LPDCs.
Keywords: Food allergy, Dendritic cells, Oral tolerance, C-type lectin receptor, Lamina propria
Introduction
Analyses of DCs within tissues of the gastrointestinal tract have identified different subtypes with diverse functions, depending, in part, on the nature of the subset, maturation state and the initial stimulus from the microenvironment1. Indeed, disturbances at different steps in homeostasis result in a defective tolerance state and gastrointestinal hypersensitivity, such as seen in food allergy and anaphylaxis2–4, but the underlying mechanism remains obscure. At present, strict avoidance of allergen sources is the only option for alleviating potentially fatal food anaphylaxis, highlighting the need for understanding of the mechanism of gastrointestinal homeostasis and developing strategies to effectively induce oral tolerance.
Mucosal DCs are known to be critical in the induction of immunity and tolerance, through, in part, their capacity in generating regulatory cytokines, and depending on the cytokine milieu functionally diversified T cells can be generated5. DCs with regulatory functions have been demonstrated in Peyer's patches (PPs), lamina propria (LP) and mesenteric lymph nodes (MLN). While both PP and MLN have been shown to be crucial in the T-cell priming events by DCs, DCs from LP and MLN appear to be critical in the induction of oral tolerance6–10. DCs may achieve oral tolerance through the induction of T cells with regulatory activities, including naturally occurring, CD4+CD25+Foxp3+ Treg cells, Th3 cells or T-regulatory-1 (Tr1) cells11–13. This has been highlighted by the recent observation that DCs in both MLN and LP can convert CD4+ T cells into Foxp3+ Treg in vitro14,15, or induce IL-10-producing CD4+ Tr1-like cells16. While mucosal DCs are known to be critical in the induction of immunity and tolerance, the “decision-making mechanism” to generate this dichotomized response remains to be fully defined.
In this regard, DCs are known to utilize their innate pattern-recognition TLRs and CLRs to generate innate immunity and influence adaptive response17,18. CLRs recognize complex glycan structures on various pathogens through their C-type carbohydrate-recognition domains (CRDs) and have evolved to facilitate the endocytosis of pathogens19,20, but the exact expression profiles for various CLRs in DC subsets remain to be defined. Among the murine homologues of human DC-SIGN, it appears that SIGNR1, with binding specificity similar to human DC-SIGN and its homologue, L-SIGN, is primarily expressed in the peritoneal macrophages and lymphoid endothelium21–23, although its expression level and cell-type specificity remain unclear. Also, depending on their tissue localization and differentiation state, distinct DC subsets with different sets of CLRs may recognize distinct classes of infectious agents to induce tolerance or activate immunity, wherein complex glycan structures on pathogens may play a key role18. The efficiency and specificity of the ligand-CLR interaction may be major contributing factors determining the immune outcomes, and can potentially be harnessed into a targeting strategy for tolerance induction. The goal of this study was to test a hypothesis that targeting the intestinal DCs with neoglyco-antigen (sugar-modified antigen), mimicking highly mannosylated structures on pathogens, leads to oral tolerance via CLRs in a prophylactic model of food allergy. We showed here that oral delivery of chemically mannose-modified BSA (Man51-BSA) selectively targeted LPDCs through SIGNR1 and induced the expression of IL-10, leading to the induction of tolerance.
Results
Sugar-modified BSA protected mice from BSA-induced anaphylaxis
To investigate whether BSA, when coupled with mannoside, mediates differential immune response in vivo, a mouse model of food allergy24 was tested, wherein mannosylated BSA with 51 mols of mannosides (Man51-BSA; Supplemental Fig. 1; refs. 25 and 26) was utilized as a model. The results showed that while C3H/hej mice sensitized orally once weekly for seven weeks with BSA (200μg/mouse) and cholera toxin (CTX; 10 μg/mouse), a commonly used adjuvant, showed signs [mean symptom severity score 4 out of 5 (fatality); N=8–14 mice] of hypersensitivity following challenge, mice sensitized with Man51-BSA (200μg/mouse plus 10 μg/mouse of CTX) did not develop any signs of hypersensitivity following challenge with BSA (Fig. 1a) or with Man51-BSA (data not shown). Importantly, BSA-sensitized mice pretreated with Man51-BSA (200μg/mouse without CTX), but not BSA, showed significantly reduced severity of anaphylaxis following challenge with BSA (p < 0.05; Fig. 1b).
Figure 1.
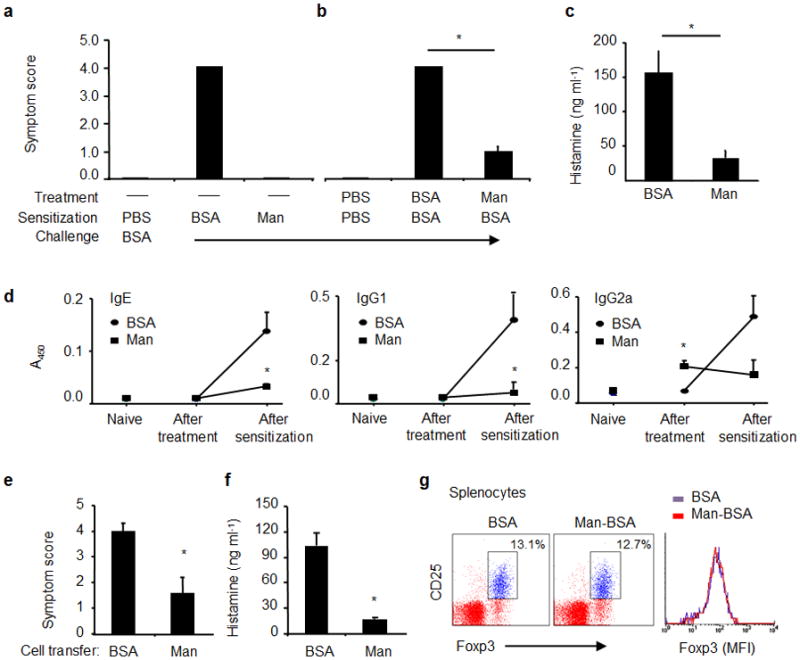
Antigen-induced anaphylaxis in C3H/Hej mice. (a) Mice were sensitized with PBS, BSA (200 μg/mouse) or Man51-BSA (Man, 200 μg/mouse) with CTX (10 μg/mouse) and challenged with BSA (1 mg/mouse). The severity of anaphylactic responses was scored 30 min after antigen challenge by the scoring system24- from 0, no sign of shock to 5, death. (b) Man51-BSA suppressed BSA-induced anaphylaxis. Mice were pretreated daily on three consecutive days with BSA or Man51-BSA (Man) at 200 μg/mouse, followed by oral sensitization and challenge with BSA. *p < 0.05. (c) The levels of plasma histamine in the same groups of mice as in (b). (d) The relative levels of specific IgE, IgG1 and IgG2a Abs in naïve mice and those after the treatment (before BSA sensitization) with BSA or Man51-BSA, or those after BSA sensitization. *p < 0.05 vs BSA. 8–14 mice per group. Adoptive transfer of splenocytes suppressed BSA-induced anaphylaxis. (e) Symptom scores and (f) plasma histamine levels in sensitized and challenged mice receiving spleen cell transfer from BSA- or Man51-BSA (Man)-treated mice. *p < 0.05. 6–8 mice per group. (g) Representative flow analysis of splenic Foxp3+ Tregs in BSA- or Man51-BSA (Man-BSA)-treated mice. The % of CD25+Foxp3+ cells gated on CD4+ cells and the relative intensity (MFI) of Foxp3 are shown. 3–5 mice per group. Results are representative of 2 independent experiments.
Moreover, the reduced anaphylactic response in the Man51-BSA-treated mice was associated with significantly decreased levels of plasma histamine (p < 0.05; Fig. 1c) and of vascular permeability upon challenge (Supplemental Fig. 2). When BSA-specific Ab levels were measured, while mice receiving BSA sensitization and challenge revealed significant levels of IgE, IgG1 and IgG2a (p < 0.05 in all cases), mice pretreated with Man51-BSA did not show any appreciable increase in the levels of IgE and IgG1 antibodies. Interestingly, Man51-BSA-treated mice showed modest enhancement in the level of IgG2a prior to sensitization with BSA (Fig. 1d), but no further increase was noted after BSA sensitization.
Further, mice receiving adoptive transfers of splenocytes from Man51-BSA-treated mice showed significantly reduced levels of anaphylactic response (p < 0.05; Fig. 1e) and plasma histamine (p < 0.05; Fig. 1f) following sensitization and challenge, when compared to those receiving cells from BSA-treated mice. This effect was not due to the development of naturally occurring, CD4+CD25+Foxp3+ regulatory Tregs in Man51-BSA-treated mice, as no significant increase in the levels of Tregs and Foxp3 expression before and after sensitization with BSA was noted in PPs, MLN (Supplemental Fig. 3) and spleens (Fig. 1g, as a representation) 5 days after the last oral administration of BSA or Man51-BSA, at the time when splenocytes were shown to be able to transfer tolerance.
Man51-BSA targeted LP DCs
To determine if Man51-BSA was able to target mucosal DCs in vivo, naïve mice were orally administered with fluorophore (FITC)-labeled Man51-BSA or BSA, and 6 hrs later, flow cytometry analyses of the cells isolated from LP, PP, MLN and spleen were performed. The results showed that significant staining of DCs co-expressing CD11c and CD11b was found in LP, with around 50% of those cells staining positive for FITC-Man51-BSA (Fig. 2a). In contrast, very little, if any, positively stained cells were found in other cell subsets, including CD11c−CD11b+ macrophages (Fig. 2a for representation) in the LP and in cells from MLN, PPs and spleens (data not shown). Immunocytochemical analyses of small intestine also demonstrated that the majority of the cells taking up FITC-Man51-BSA were CD11c+ DCs (Fig. 2b), as judged by significant dual-color staining of CD11c+ cells in the LPs of mice receiving Man51-BSA. In contrast, no apparent co-stained cells were noted in the LPs of BSA-treated mice. In fact, at this time point the majority of the labeled BSA was localized in the epithelium with diffuse staining patterns (Fig. 2b). Also, phenotypic analysis of this CD11c+CD11b+ LPDC subset revealed its expression of I-A, CD80, CD40, a chemokine receptor, CCR7, as well as the α integrin CD103, while this DC subset was negative for CD8α, B220, CD45RB and F4/80 expression (Supplemental Fig. 4).
Figure 2.
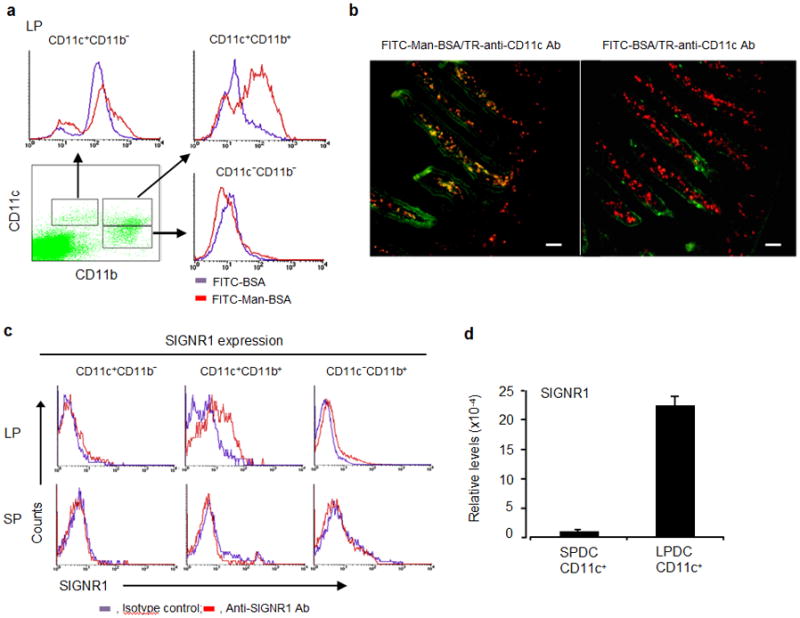
Man51-BSA targeted LPDC subset in lamina propria. (a) A representative flow analysis of three major LP DC or macrophage subsets in mice receiving oral administration of FITC-BSA or FITC-Man51-BSA (Man-BSA). (b) Immunocytochemical analysis of small intestine samples from the same groups of mice as above. Two-color merge pictures are shown. Scale bar, 50 μm. (c) Flow analysis of SIGNR1 expression in three cell subsets from lamina propria (LP) and spleen (SP). (d) The relative levels of SIGNR1 gene expression in LP CD11c+ or splenic CD11+ DCs, measured by the use of quantitative RT-PCR.
Expression of SIGNR1 in mucosal DCs
To determine which CLR is responsible for taking up Man51-BSA, flow analyses of intestinal DCs were first performed for profiling of the expression patterns of selected CLRs, including SIGNR1 and mannose receptor (CD206), both of which are known to bind mannose-bearing antigens. Analyses of LPDCs demonstrated selective expression of SIGNR1 in the CD11c+CD11b+ DC subset (Fig. 2c), while DCs from SPs showed no apparent expression of SIGNR1, confirmed by quantitative PCR analyses (Fig. 2d). From these analyses, selective expression of CD205 was noted in the CD11c+CD11b− DC subset, while Dectin-1, yeast zymosan receptor, appeared to be primarily expressed in the CD11c+CD11b+ subset, and variable levels of expression for CD206 and Dectin-2 were noted (Supplemental Fig. 5).
To examine whether, indeed, Man51-BSA is a ligand for SIGNR1, the binding activities of Man51-BSA and other sugar-modified BSA derivatives, including galactosylated BSA (Gal34-BSA), to SIGNR1 were analyzed. The results showed that a significant binding of Man51-BSA to mouse SIGNR1 was observed, whereas the binding of BSA and Gal34-BSA was negligible (Fig. 3a, representative). To confirm its binding, a cell-based, competition analysis of SIGNR1-transfectant cells was performed, using FITC-labeled dextran, a known ligand for SIGNR123, and varying concentrations of neoglyco-antigens as competitors. The results showed that Man51-BSA inhibited, in a dose-dependent manner, the labeled dextran’s binding to SIGNR1 (Fig. 3b), but no significant competition was found for BSA (not shown), Gal34-BSA and a mannosylated BSA derivative, Man8-BSA, with much lower density of mannosides.
Figure 3.
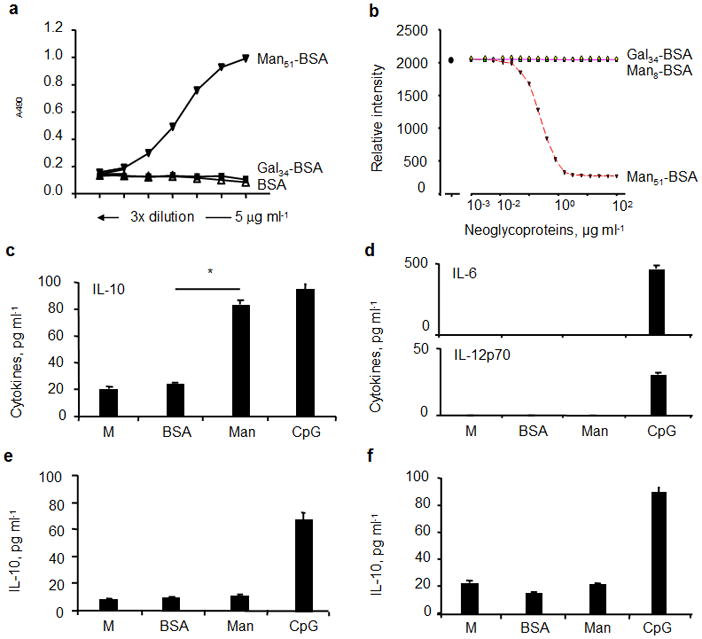
Binding analysis of neoglyco-antigens. (a) Solid-phase binding analyses. The relative binding activity was expressed as absorbance at 490 nm after subtracting the background. (b) Representative competition analysis. The mean fluorescence intensity (MFI) of SIGNR1-transfectants stained with FITC-labeled dextran (10 μg/ml; Dextran-FITC) in the presence or absence of varying concentrations of neoglyco-antigens as competitors as indicated, using flow cytometry. Man51-BSA selectively induced IL-10 in LPDCs. The levels of (c) IL-10, (d) IL-6 and IL-12p70 in CD11c+ DCs from LPs. (e) The levels of IL-10 in LP CD11c−CD11b+ cells and (f) in CD11c+ DCs of spleens (SP). Cells were stimulated with medium alone (M), BSA (20 μg/ml), Man51-BSA (Man, 20 μg/ml), or 1 μM of CpG for 24 hrs.*p < 0.05.
Man51-BSA induced, selectively, IL-10 expression in LPDCs
To examine the functional effect of the (Man51-BSA)–SIGNR1 axis, the levels of cytokines in LP CD11c+ DCs stimulated with BSA or Man51-BSA were examined. The results showed first that as expected, a TLR-9 agonist, CpG, induced the expression of IL-10, IL-12 and IL-6 in all subsets. In LP CD11c+ DCs, Man51-BSA significantly induced the expression of IL-10 (p < 0.05; Fig. 3c), but not IL-6, IL-12p70 (Fig. 3d), IL-27 and TGF-β (not shown); in contrast, CD11c−CD11b+ cells from LP (Fig. 3e) and CD11c+ SPDCs (Fig. 3f) showed no detectable levels of IL-10 expression following the stimulation with Man51-BSA.
Significantly, the addition of SIGNR1-specific blocking antibody or mannan (a weak SIGNR1 antagonist) significantly reduced the levels of IL-10 in Man51-BSA-treated LP DCs (p < 0.05; Fig. 4a). Moreover, the presence of CD206-specific blocking antibody did not inhibit Man51-BSA-induced IL-10 expression (Fig. 4b), nor did the addition of an inhibitor for MyD88, a TLR-signaling molecule, as compared to that seen in medium control or in cultures with an irrelevant peptide (Fig. 4c). In contrast, the induction of IL-10 in LPDCs by a TLR9 agonist, CpG, was significantly inhibited in the presence of MyD88 peptide inhibitor. Further, Man51-BSA-induced IL-10 expression in LPDCs was associated with activation of a member of the MAPK family, JNK, but not p38 and ERK (Supplemental Fig. 6), consistent with those noted in a mouse RAW cell line over-expressing SIGNR1-FLAG fusion genes and activated with FLAG-specific antibody27. Moreover, analysis of intestinal DCs from IL-10-GFPtiger mice28 corroborated the finding of LPDCs as the primary Man51-BSA-targeted cells, in which increased percentage of GFP+CD11c+ DCs, from 1.7% to 7.3%, was found in LP of mice receiving Man51-BSA (Fig. 4d), whereas SP contained far fewer GFP+ cells and no difference was noted in cells from MLN and PP at this time point (data not shown).
Figure 4.
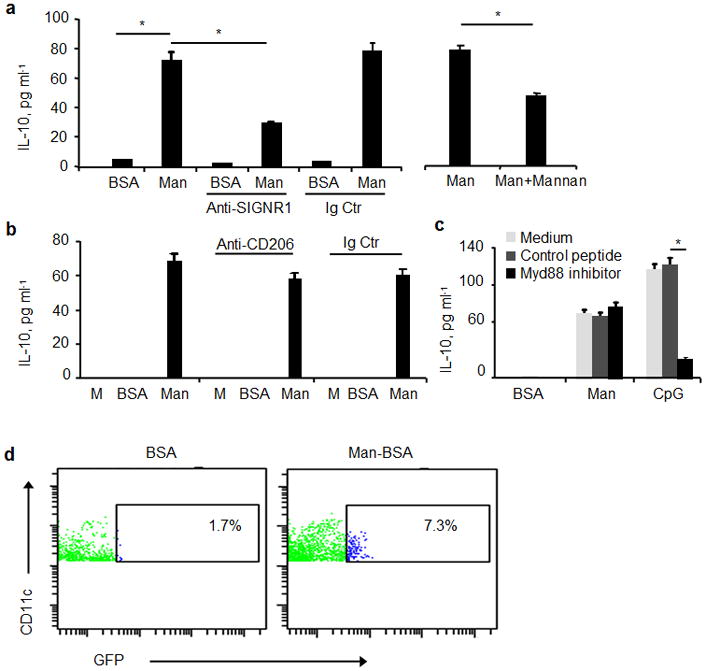
Inhibition analyses of Man51-BSA-induced IL-10 in LPDCs. The levels of IL-10 in CD11c+ LPDCs (2×105/condition) stimulated with medium alone (M), BSA (20 μg/ml) or Man51-BSA (Man, 20 μg/ml) with or without the addition of (a) a blocking SIGNR1-specific antibody (50μg/ml), isotype control (Ig Ctr), or in separate assays, mannan (20 μg/ml), or (b) antibody specific to mannose receptor (CD206; 50 μg/ml), isotype control (Ig Ctr), or (c) pretreated with a MyD88 peptide inhibitor or a control peptide (200 μM). Also, DCs were stimulated with 1 μM CpG in separate cultures for comparison. The levels of IL-10 in LPDCs after 24 hrs simulation were measured with ELISA. *p < 0.05. (d) Flow analysis of CD11c+GFP+ (IL-10+) cells in lamina propria (LP) of IL-10-GFPtiger mice.
Man51-BSA-treated DCs promote the generation of Tr1-like T cells
Next, the in vitro effects of Man51-BSA-treated LPDCs on the T-cell differentiation pathway were examined. Significantly elevated levels of IL-10 and IFN-γ were found in T cells cultured with Man51-BSA-pulsed LPDCs (p < 0.05; Fig. 5), as compared to those seen in BSA-pulsed cells. This effect was abated in the presence of SIGNR1 blocking antibody (Fig. 5a) or IL-10 neutralizing antibody (Fig. 5b), but not TGF-β neutralizing antibody (data not shown). In parallel, the frequency of Foxp3+ T cells in the co-cultures remained similar throughout the assay period (data not shown). When CpG, a TLR9 agonist, was used as an additional stimulus, no significant difference in the levels of cytokines was found in T cells when co-cultured with either BSA- or Man51-BSA-pulsed DCs (data not shown).
Figure 5.
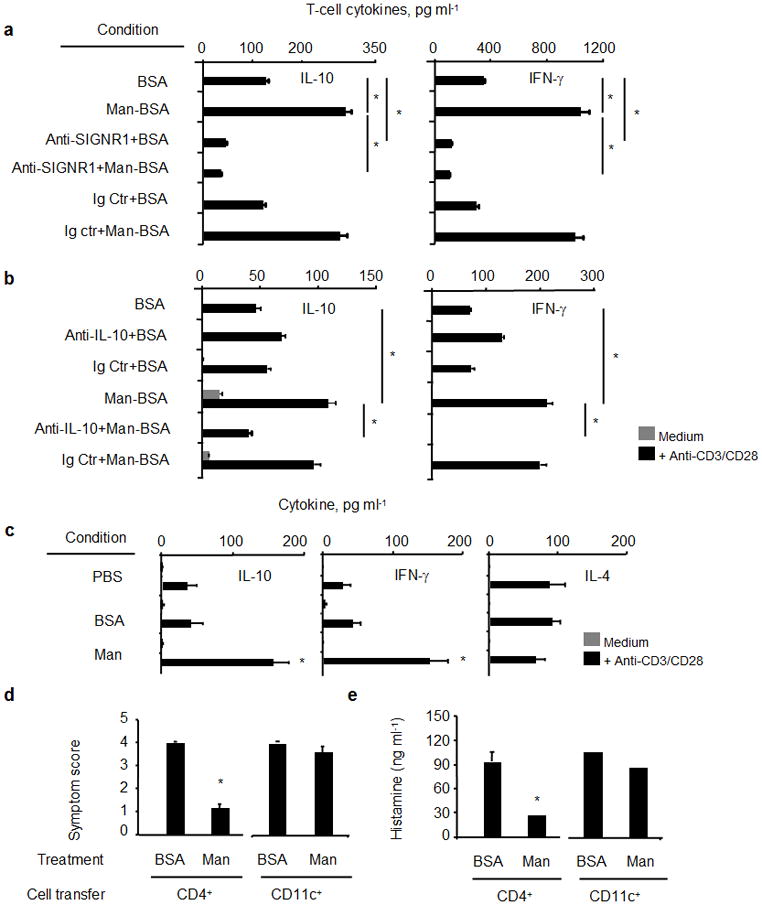
Increased IL-10- and IFN-γ expression in T cells in vitro when co-cultured with Man51-BSA (Man) pulsed LPDCs (CD11c+), in the presence or absence of (a) SIGNR1-specific or isotype control (Ig Ctr) antibodies, (b) neutralizing IL-10-specific (5 μg/ml) or isotype control (Ig Ctr) antibodies. T-cell cytokines, IL-10 and IFN-γ, were measured by ELISA. (c) Increased IL-10-and IFN-γ-expressing T cells from mice receiving oral administration of PBS, BSA or Man51-BSA (Man, 200 μg/mouse). *p < 0.05 vs PBS or BSA. Adoptive transfer of splenic CD4+ T cells suppressed BSA-induced anaphylaxis. (d) Symptom scores and (e) plasma histamine levels in sensitized and challenged mice receiving transfers of CD4+ T cells or CD11c+ DCs from BSA- or Man51-BSA (Man)-treated mice. *p < 0.05. 6–8 mice per group.
Furthermore, a similar pattern of T-cell differentiation into IL-10/IFN-γ expressing cells in vivo was also noted in those CD4+ T cells from the Man51-BSA-, but not BSA-, treated mice after stimulation (Fig. 5c). Significantly, mice receiving adoptive transfers of CD4+ T cells, but not CD11c+ SPDCs, from Man51-BSA-, but not BSA-, treated mice showed reduced levels of anaphylactic response (p < 0.05; Fig. 5d) and plasma histamine (p < 0.05; Fig. 5e).
SIGNR1 and IL-10 are necessary for Man51-BSA-mediated tolerance
To determine whether SIGNR1 was, indeed, required for Man51-BSA-induced tolerance, the generation of splenic Tr1-like T cells in vivo was analyzed in SIGNR1-deficient and wild-type mice (B6 background). The results showed that Man51-BSA failed to induce Tr1-like T cells expressing IL-10 (Fig. 6a) and IFN-γ (Fig. 6b) in Man51-BSA-treated SIGNR1-deficient mice as compared to those seen in Man51-BSA-treated wild-type mice. Moreover, while B6 mice are known to be a poor IgE/Th2 responder strain and the severity of anaphylaxis tends to be milder than that seen in the C3H/hej strain, it was found that when compared to those seen in wild-type mice, significant reversal of reduced symptom severity (p < 0.05; Fig. 6c) and plasma histamine levels (p < 0.05; Fig. 6d) was noted in Man51-BSA-treated SIGNR1-deficient mice after sensitization and challenge with antigen.
Figure 6.
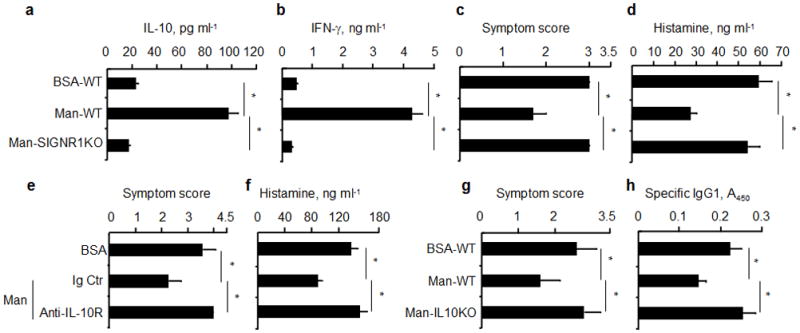
Reversal of Man51-BSA-mediated tolerance in SIGNR1- and IL-10-deficient mice. Analysis of (a) IL-10 and (b) IFN-γ in splenic CD4+ T cells from wild-type (WT) or SIGNR1-deficient (SIGNR1KO) mice receiving oral administration of BSA or Man51-BSA (Man) and stimulated. *p < 0.05. (c) Symptom scores and (d) plasma histamine levels in sensitized and challenged wild-type and SIGNR1-deficient mice. *p<0.05. 6–8 mice per group. (e) Symptom scores and (f) plasma histamine levels in BSA- or Man51-BSA (Man)-treated C3H/hej mice receiving IL-10R-specific or isotype control (Ig Ctr) antibodies as indicated. *p < 0.05. 4–6 mice per group. Antigen-induced (g) symptom scores and (h) specific IgG1 response in mice (wild-type) receiving adoptive transfer of splenic CD4+ T cells from BSA- or Man51-BSA (Man)-treated IL-10-deficient (IL10KO) or wild-type (WT) mice. *p < 0.05. 4–6 mice per group.
Furthermore, Man51-BSA-mediated suppression of antigen-induced anaphylactic severity (Fig. 6e) and plasma histamine levels (Fig. 6f) was no longer apparent when C3H/hej mice received IL-10R-specific, but not isotype control, antibody one day prior to and two weeks after the initial antigen sensitization. The failure of tolerance induction by Man51-BSA was also evident in mice receiving transfer of splenic CD4+ T cells from Man51-BSA-treated, IL-10-deficient, but not wild-type, mice, as no significant reduction in symptom scores (Fig. 6g) and specific IgG1 levels (Fig. 6h) was noted. Also, Man51-BSA-mediated tolerance to antigen-induced IgG1 response was lost in IL-10-deficient mice, as compared with that of wild-type mice (data not shown). These results suggest, therefore, the importance of IL-10 from Man51-BSA-modified LPDCs for the generation of Tr1-like T cells and in conferring the suppressive activity of Tr1-like T cells on antigen-induced anaphylactic response.
Discussion
In a model of food allergy, evidence is provided suggesting that a sugar-modified antigen, Man51-BSA, is capable of inducing oral tolerance by a sequential process originating from targeting of LPDCs by Man51-BSA and leading to the development of CD4+, Tr1-like T cells expressing IL-10 and IFN-γ in the spleen, wherein SIGNR1 and IL-10 appeared to be critical. Our results showed that while the expression patterns of CCR7 and CD103 in the CD11c+CD11b+ LPDC subset appeared to be indistinguishable from those of the CD11c+CD11b− DC subset, the pattern of CLR expression showed inter-subset variation. Notably, SIGNR1 expression seemed to mark the CD11c+CD11b+CD8− subset and served as the primary CLR in mediating Man51-BSA’s effect. Functionally, the (Man51-BSA)–SIGNR1 axis was shown to be able to induce tolerogenic DCs through the induction of IL-10, and Man51-BSA-mediated tolerance required SIGNR1 and IL-10.
The finding that SPDCs from Man51-BSA-treated mice were not able to transfer tolerance is consistent with their inability to respond to Man51-BSA in vitro (Fig. 3f). This is due, perhaps, to their low or lack of SIGNR1 expression (Fig. 2), and also to the possibility that they are spleen resident, not migratory, DCs. These results suggest that the mucosal immune response, particularly in villous lamina propria which accounts for the majority of protein uptake from the intestine9, can be pre-programmed for the induction of tolerance through the likely important mediation of the CLR–ligand interaction. It is, at present, unclear as to where Tr1-like T cells were primed by Man51-BSA-stimulated DCs. It is noted that the Man51-BSA-targeted CD11c+CD11b+ LPDCs subset expressed CCR7, suggesting their possible migration into the MLN with subsequent priming of Tr1-like T cells. This possibility is supported by the finding that CCR7-dependent migration of DCs from the LP to MLN is required for the induction of oral tolerance29. A detailed kinetic analysis is required to track the DC migratory patterns and the development of Tr1-like T cells in situ.
A recent report by Awasthi A et al30 suggests that spleen-derived DCs with plasmacytoid phenotype (CD45RB+B220+) can be modified by TGF-β-induced Treg cells, with subsequent generation of IL-27 which, in turn, promotes the development of IL-10-expressing Tr1 cells. In contrast, Man51-BSA-targeted CD11c+CD11b+ LPDCs were negative for the expression of CD45RB and B220, were unable to secrete IL-27 and TGF-β following activation with Man51-BSA and showed no impact on the generation of Tregs. These phenotypic and functional features, therefore, distinguish LPDCs from those spleen-derived, CD45RB+B220+ plasmacytoid-like DCs, and those mucosal DCs capable of inducing Tregs through TGF-β14. It is noted also that while in the absence of TLR signaling, Man51-BSA was shown to be able to elicit IL-10 production in LPDCs with subsequent generation of Tr1-like T cells, its selective effect on IL-10 expression in T cells was lost when a TLR9 agonist, CpG, was added to the culture. Therefore, it is likely that the nature of the ligand–receptor axis in DC priming may determine the functional outcomes. The selective expression of SIGNR1 and its candidacy as a target for induction of IL-10 in LPDCs may thus represent a novel feature of tolerogenic DCs at the site of intestinal mucosa in maintaining the homeostatic state.
There has been an intense interest in determining the mechanisms by which DCs are programmed to generate immunity or induce tolerance, wherein the DC-specific CLRs, with their diverse roles in Ag uptake and DC-T cell interactions, have been considered as attractive targets. For example, co-injection of yeast zymosan plus ovalbumin (OVA) in mice induces T-cell tolerance through TLR2 and dectin-1, a CLR31. Also, targeting of DEC-205 by specific antibody has been shown to generate T-cell tolerance32. Similar tolerogenic activity has also been found for human DC-SIGN33. Further, DCs can be rendered tolerogenic when exposed to galectin-1, a glycan-binding protein, promoting IL-27-dependent and IL-10-mediated T cell tolerance34. These studies, combined with the finding that DC-specific immune receptor (DCIR), a member of the CLR family, is shown to be a negative regulator of DC expansion35, support the importance of CLR–ligand axis in immune modulation.
Moreover, studies have suggested the potential application of pro- and pre-biotics (for review, see ref. 36) in alleviating allergic responses, while in most studies, the detailed mechanisms are unclear and the potential development of anaphylaxis upon delivery remains a major concern. The engineering with relatively simple synthetic chemistry of sugar-modified antigens for targeting tolerogenic DCs would potentially circumvent these limitations and offer safer and more efficacious designs. It is of interest to note that feeding of prebiotic formula with oligosaccharides to infants with a parental history of atopy was associated with a lower incidence of allergic diseases37. The potential modulatory effect of oligosaccharides and, perhaps, microbials38 may be mediated through the ligand–CLR axis, providing a ‘conditioning’ effect on mucosal DCs. How mucosal DCs integrate the CLR signaling in controlling innate immunity and adaptive responses may thus represent an important mechanism in controlling mucosal immunity, and may help in the design of effective strategy for alleviating the increasingly common and potentially fatal problem of food allergies.
Methods
Mice, sensitization and challenge
Four- to six-week-old female mice [C3H/Hej, C57BL/6, IL-10-deficient (B6.129P2-IL-10tm1Cgn/J), IL-10GFPknockin (tiger) from the Jackson Laboratory and SIGNR1-deficient mice] were maintained under specific pathogen-free conditions, and all experiments were approved by the Animal Care and Use Committee, Johns Hopkins University School of Medicine. Mice received 7 weekly sensitizations with PBS, BSA (200 μg/mouse) plus cholera toxin (CTX, B subunit; 10 μg/mouse) as adjuvant, and one week after final sensitization, were challenged intraperitoneally (i.p.) with 1 mg of BSA. In some cases, C3H/hej mice were injected i.p. with antibody for IL-10 receptor (IL-10R; 0.25 mg/mouse; clone 1B1.3a) one day prior to and two weeks after the initial sensitization.
Coupling of BSA with mannosides or galactosides was performed as previously described25,26. Mice were orally administered with 200 μg/mouse of BSA or Man51-BSA for 3 successive days prior to first sensitization. Systemic anaphylaxis-like symptoms were evaluated blindly within 30 min after challenge using a scoring system24. Plasma histamine levels were determined using enzyme immunoassays, and serum Ab levels were analyzed by ELISA24 before and after sensitization.
Binding analysis and analyses of immunological responses (see also Supplemental Methods for details)
The binding activity of various neoglyco-antigens to SIGNR1 was determined by solid-phase binding assays as described39. A cell-based competition analysis of SIGNR1-transfectant BaF3 cells was performed using varying concentrations of neoglyco-antigens as competitors in the presence of FITC-labeled dextran (2000 kd, 10μg/ml), a known ligand for SIGNR123.
Single cell suspensions of intestinal LPs and PPs were enriched for CD11c+ cells using anti-CD11c MACS beads and CD11c−CD11b+ cells were positively selected from the flow through cells. For flow cytometric analysis of Man51-BSA’s cell target, cells were isolated from mice 6 hrs after receiving oral administration of 200 μg of Man51-BSA or BSA labeled with fluorophore (FITC), stained with biotinylated FITC-specific antibody and FITC-conjugated streptavidin. For analysis of SIGNR1 expression, the cells were stained with Alexa-fluor®488-conjugated-antibody to SIGNR1 or isotype control, gated on the expression of CD11c (N418) and CD11b (M1/70). The relative levels of Signr1 transcripts were evaluated by quantitative RT-PCR.
For analysis of Man51-BSA target cells, frozen sections of small intestines of mice receiving FITC-conjugated BSA or Man51-BSA were incubated with CD11c-specific antibody (Hamster IgG, clone N418), Texas red conjugated anti-hamster IgG, rabbit antibody to FITC, isotype control antibody, FITC-conjugated antibody to Rabbit IgG. Total LP cells were isolated from IL-10-GFP tiger mice on day 4 after oral administration of BSA or Man51-BSA, and the percentage of GFP+ cells determined by gating on the CD11c+ cells. For analysis of Man51-BSA-induced cytokines, CD11c+ and CD11c−CD11b+ cells (2×105/condition) were stimulated with medium alone, BSA (20 μg/ml), Man51-BSA (20 μg/ml), or 1 μM of CpG (ODN1668), in the presence or absence of antibody to SIGNR1 (50 μg/ml; ER-TR9), isotype control antibody, mannan (20 μg/ml), antibody to mannose receptor (50 μg/ml), 200 μM of a MyD88 peptide inhibitor or a control peptide, for 24 hrs, and cytokine levels analyzed with ELISA.
For adoptive cell transfer experiments, total spleen cells, CD4+ or CD11c+ cells positively selected from pooled spleen cells of the same group were isolated from mice five days after receiving three daily oral administrations of 200 μg of BSA or Man51-BSA, and were transferred into naive mice via the tail veins 1 day before the first sensitization followed by 7 times weekly sensitization and the challenge as described above. In all cases, the purity of isolated cells was >95%, as assessed by flow cytometry. The numbers of transferred cells were 107 for whole spleen cells, 2×106 for isolated CD4+ cells or 106 for isolated CD11c+ cells.
For analysis of T-cell cytokines, splenic CD4+CD62L+ T cells (1×106/condition) from naïve mice were co-cultured with CD11c+ LPDCs (1×105/condition) pulsed with BSA (20 μg/ml) or Man51-BSA (20 μg/ml), in the presence or absence of antibody to SIGNR1 (50 μg/ml; ER-TR9), neutralizing antibodies to IL-10 (5 μg/ml; JES5-16E3) for 96 hrs, followed by stimulation with or without a combination of antibody to CD3 and CD28 (1 μg/ml each; 145-2C11 and 37.51, respectively) for 24 hrs, and the levels of cytokines measured by ELISA. In some cases, splenic CD4+ T cells were isolated (1×106 cells/condition) from mice on day 8 after receiving oral administration of PBS, BSA or Man51-BSA (200 μg/mouse) and stimulated with antibodies for CD3 and CD28 Abs as above for 24 hrs.
Statistical analysis
Data are expressed as the mean ± SEM for each subject group. Differences in each of the quantitative parameters were analyzed by t-test, and Wilcoxon signed-rank test was used to analyze differences in the anaphylactic symptom scores. Differences among groups of mice were determined using analysis of variance (StatView). If differences among groups were significant (p<0.05), Fisher's protected least significant difference test was used to distinguish between pairs of groups.
Supplementary Material
Acknowledgments
We thank A. Myers and H. Rohde for technical assistance, and K. Takahara (Kyoto University, Japan) for providing SIGNR1-transfectant cells. We are also grateful of the Consortium for Functional Glycomics for providing SIGNR1-deficient mice. This work was supported, in part, by NIH grants (AI052468 and AI073610).
Abbreviations
- DCs
dendritic cells
- CLR
C-type lectin receptor
- SIGNR1 (Cd209b)
DC-specific intracellular adhesion molecule-3-grabbing nonintegrin-related 1
- Man
mannose
- Lex
Lewis-x trisaccharides
- LP
lamina propria
- PP
Peyer’s patch
- MLN
mesenteric lymph node
- SP
spleen
Footnotes
AUTHOR CONTRIBUTIONS
Y.Z. conducted experiments involving characterizing mucosal DC subsets, their regulatory role, peformed data analysis and wrote manuscripts; H.K. performed in vivo experiments for antigen-induced anaphylactic responses, data analysis and wrote manuscript; S.-C.H. conducted experiments involving binding analyses of neoglyco-antigens; R.T.L. carried out the synthesis of neoglyco-antigens and wrote manuscript; X.Y. conducted in vitro experiments characterizing T-cell responses; B.P. performed flow cytometric experiments and edited manuscript; J.F. conducted in vivo cell transfer experiments; K.Y. contributed to the design and preparation of neoglyco-antigens; Y.C.L. designed, prepared neoglyco-antigens and supervised the work involving their synthesis; S.-K.H. planned, designed, supervised and coordinated the overall research efforts
References
- 1.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–46. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115:3–12. doi: 10.1016/j.jaci.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Poulsen LK. In search of a new paradigm: mechanisms of sensitization and elicitation of food allergy. Allergy. 2005;60:549–58. doi: 10.1111/j.1398-9995.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 4.Finkelman FD. Anaphylaxis: Lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–15. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–38. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 6.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–41. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 7.Chirdo FG, Millington OR, Beacock-Sharp H, Mowat AM. Immunomodulatory dendritic cells in intestinal lamina propria. Eur J Immunol. 2005;35:1831–40. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 8.Spahn TW, et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur J Immunol. 2002;32:1109–13. doi: 10.1002/1521-4141(200204)32:4<1109::AID-IMMU1109>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 9.Worbs T, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rescigno M, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–7. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 12.Taams LS, Palmer DB, Akbar AN, Robinson DS, Brown Z, Hawrylowicz CM. Regulatory T cells in human disease and their potential for therapeutic manipulation. Immunology. 2006;118:1–9. doi: 10.1111/j.1365-2567.2006.02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–42. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilsborough J, George TC, Norment A, Viney JL. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology. 2003;108:481–92. doi: 10.1046/j.1365-2567.2003.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taked K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 18.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 19.Cambi A, Figdor CG. Levels of complexity in pathogen recognition by C-type lectins. Curr Opin Immunol. 2005;17:345–51. doi: 10.1016/j.coi.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C- type lectin receptors. Curr Opin Immunol. 2005;17:18–24. doi: 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koppel EA, van Gisbergen KP, Geijtenbeek TB, van Kooyk Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell Microbiol. 2005;7:157–65. doi: 10.1111/j.1462-5822.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 22.Takahara K, et al. Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int Immunol. 2004;16:819–29. doi: 10.1093/intimm/dxh084. [DOI] [PubMed] [Google Scholar]
- 23.Galustian C, et al. High and low affinity carbohydrate ligands revealed for murine SIGN-R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN-R3 and langerin. Int Immunol. 2004;16:853–66. doi: 10.1093/intimm/dxh089. [DOI] [PubMed] [Google Scholar]
- 24.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan--DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5:387–91. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 25.Lee RT, Lee YC. Neoglycoproteins. In: Montreuil J, Vliegenthart JFG, Schachter H, editors. Glycoproteins II. Elsevier; Amsterdam, The Netherlands: 1997. pp. 601–20. [Google Scholar]
- 26.Stowell CP, Lee YC. Neoglycoproteins: The preparation and application of synthetic glycoproteins. Adv in Carbohydr Chem and Biochem. 1980;37:225–81. doi: 10.1016/s0065-2318(08)60022-0. [DOI] [PubMed] [Google Scholar]
- 27.Numazaki M, Kato C, Kawauchi Y, Kajiwara T, Ishii M, Kojima N. Cross-linking of SIGNR1 activates JNK and induces TNF-alpha production in RAW264.7 cells that express SIGNR1. Biochem Biophys Res Commun. 2009;386:202–6. doi: 10.1016/j.bbrc.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Kamanaka M, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–52. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Jang MH, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol. 2006;176:803–10. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 30.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10- producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 31.Dillon S, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–28. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ilarregui JM, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–91. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 35.Fujikado N, et al. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med. 2008;14:176–80. doi: 10.1038/nm1697. [DOI] [PubMed] [Google Scholar]
- 36.Mine Y, Yang M. Recent advances in the understanding of egg allergens: basic, industrial, and clinical perspectives. J Agric Food Chem. 2008;56:4874–900. doi: 10.1021/jf8001153. [DOI] [PubMed] [Google Scholar]
- 37.Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138:1091–5. doi: 10.1093/jn/138.6.1091. [DOI] [PubMed] [Google Scholar]
- 38.Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002;169:3284–92. doi: 10.4049/jimmunol.169.6.3284. [DOI] [PubMed] [Google Scholar]
- 39.Hsu SC, et al. Functional interaction of common allergens and a C-type lectin receptor, DC-specific ICAM3-grabbing non-integrin (DC-sign), on human dendritic cells. J Biol Chem. 2010;285:7903–10. doi: 10.1074/jbc.M109.058370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


