Abstract
In the current study we examined a specific aspect of executive abilities, strategic processing, in 32 children with early-treated phenylketonuria (PKU) and 41 typically-developing control children. To do so, clustering and switching were assessed during semantic (animal, food/drink) and phonemic (S, F) fluency tasks. Specifically, number of words generated, number of subcategory clusters, number of words in subcategory clusters, and number of switches between subcategories were analyzed to provide a refined analysis of strategic processing. Compared with controls, children with PKU generated significantly fewer words and made significantly fewer switches between subcategories in the food/drink trial and the phonemic fluency condition. Number of switches was associated with number of words generated in these tasks. In addition, a significant interaction between age and group in number of switches for the food/drink trial reflected a greater increase in number of switches for the control than PKU group as a function of increasing age. These results suggest impairment in frontally-mediated aspects of strategic processing in children with early-treated PKU and indicate that strategic processing should be evaluated carefully as these children age.
Keywords: executive, strategic, phenylketonuria, children, neuropsychology, cognitive
Introduction
Executive abilities encompass higher order cognitive processes including shifting in response to changing task demands, updating information in working memory, and inhibiting inappropriate responses (Miyake et al., 2000). Strategy use during any given task requires at least one of these processes (Miyake, et al., 2000). For example, during the Wisconsin Card Sorting Test (Heaton, 1981), a widely used measure of executive abilities, cards must be sorted on the basis of the color, form, and number of stimuli presented. To strategically and efficiently complete this task, one must shift to a new sorting principle when required, update the correct sorting principle in working memory, and inhibit the previously correct sorting principle. Thus, shifting, working memory, and inhibition are all necessary for optimal performance.
Verbal fluency tasks, during which words must be generated in response to specified categories, are also widely used to assess executive abilities. In phonemic and semantic fluency conditions, words are generated in response to letters (e.g., F, A, S) and semantic categories (e.g., animals), respectively. In most studies, the number of words correctly reported is the primary variable of interest, with a greater number of words presumed to reflect better executive abilities.
It is possible, however, to conduct refined analyses to evaluate strategic processing more directly. In their seminal study, Troyer, Moscovitch, and Winocur (1997) used an approach wherein the degree of clustering and switching was examined during verbal fluency performance in healthy adults. Clustering refers to the consecutive report of two or more words within the same semantic or phonemic subcategories, whereas switching refers to shifts between subcategories. For example, report of lion, giraffe, gazelle / snake / mouse, rat / lizard / duck, chicken, goose reflects consecutive clustering within three animal subcategories (African animal, rodent, bird) and four switches (/) between animal subcategories (African animal / reptile / rodent / reptile / bird). In another example, report of lion, giraffe / snake / mouse / lizard / duck / rat / chicken / gazelle / goose reflects consecutive clustering within one animal subcategory (African animal) and seven switches between animal subcategories (African animal / reptile / rodent / reptile / bird / rodent / bird / African animal / bird). Although in each instance 10 words are generated, there is a substantial difference in organizational quality, with the better strategic approach reflected by the first example.
Findings from the Troyer et al. (1997) study demonstrated that clustering and switching are at least partially dissociable processes that differentially contribute to semantic and phonemic fluency. Specifically, clustering and switching made equal contributions to semantic fluency. In contrast, switching made a greater contribution to phonemic fluency than did clustering.
In terms of possible neuroanatomical underpinnings, a large body of research indicates that the prefrontal cortex subserves executive abilities, which include strategic processing (for an overview, see Fuster, 2008). Specific to strategic processing, neuroimaging studies reveal that this brain region is activated during strategic tasks such as the Wisconsin Card Sorting Test (Berman, Ostrem, Randolph, & Gold, 1995) and word list learning (Miotto et al., 2006; Strangman et al., 2009). Patient studies are also informative, as frontal lobe lesions are related to poorer strategic processing during word list learning (Stuss et al., 1994).
With regard to verbal fluency, findings from a number of studies point to dissociations in the possible neuroanatomical underpinnings of phonemic and semantic fluency. For example, using a behavioral interference task in healthy adults, Martin et al. (1994) showed that phonemic fluency was reduced more than semantic fluency when participants concurrently performed a frontally-mediated motor sequencing task; in contrast, semantic fluency was reduced more than phonemic fluency when participants concurrently performed a temporally-mediated object-decision task. Neuroimaging and patient studies have also shown that frontal brain regions play a more prominent role in phonemic fluency (for an overview, see Alvarez & Emory, 2006; Henry & Crawford, 2004; Moscovitch, 1994), whereas temporal brain regions play a more prominent role in semantic fluency (Henry & Crawford, 2004; Monsch et al., 1994; Mummery, Patterson, Hodges, & Wise, 1996; Rosser & Hodges, 1994).
Strategic components of verbal fluency (i.e., clustering and switching) have also been examined from a neuroanatomical perspective. During semantic and phonemic fluency tasks, Troyer et al. (1997) administered a concurrent finger-tapping task presumed to be frontally-mediated. Consistent with the findings of Martin et al. (1994), the concurrent task reduced phonemic but not semantic fluency. In addition, the concurrent task decreased switching during phonemic fluency but did not affect clustering. Troyer et al. (1997) interpreted these findings as evidence that switching is a frontally-mediated process. It should be noted, however, that the concurrent task did not universally disrupt switching, as switching during semantic fluency remained unaffected. These findings point to a difficulty in clearly delineating the neuroanatomical underpinnings of clustering and switching at this point in time. For the most part, clustering has been studied within the context of semantic fluency, whereas switching has been studied within the context of phonemic fluency.
In addition to studies of healthy adults, a number of studies have been conducted in which strategic processing during verbal fluency has been investigated in adults with brain damage. Patients with Parkinson disease (Troyer, Moscovitch, Winocur, Leach, & Freedman, 1998), Huntington disease (Ho et al., 2002), and frontal lobe lesions (Troyer, Moscovitch, Winocur, Alexander, & Stuss, 1998) have shown intact clustering during semantic fluency but impaired switching during phonemic fluency. Patients with dementia of the Alzheimer type (Troyer, Moscovitch, Winocur, Leach, et al., 1998) and temporal lobe lesions (Troyer, Moscovitch, Winocur, Alexander, et al., 1998), however, have shown impaired clustering during semantic fluency but intact switching during phonemic fluency. These findings have been taken as indications that clustering during verbal fluency is more temporally mediated, whereas switching is more frontally mediated.
Neuroimaging studies have also been informative, particularly with regard to clarifying the underpinnings of switching. In one such study, Hirshorn and Thompson-Schill (2006) identified activation in the left inferior frontal gyrus during switching on a semantic fluency task (unfortunately, phonemic fluency was not assessed). The role of prefrontal brain regions during switching has also been demonstrated in functional neuroimaging and lesion studies using neuropsychological tasks other than verbal fluency (Kumada & Humphreys, 2006; Robinson, Heaton, Lehman, & Stilson, 1980; for an overview, see Shallice, Stuss, Picton, Alexander, & Gillingham, 2008).
In addition to these studies of adults, researchers have recently explored the development of clustering and switching during verbal fluency in children. Regarding switching, a relatively clear pattern has emerged in which switching during phonemic fluency increases as children age (Kavé, Kigel, & Kochva, 2008; Sauzéon, Lestage, Raboutet, N'Kaoua, & Claverie, 2004). During semantic fluency, however, findings are inconsistent, with both age-related decrease (Sauzéon, et al., 2004) and increase (Hurks et al., 2010; Kavé, et al., 2008) in switching reported. For clustering, findings are also inconsistent, with reports of age-related increase, decrease, and no change during semantic and phonemic fluency (Hurks, et al., 2010; Kavé, et al., 2008; Koren, Kofman, & Berger, 2005; Sauzéon, et al., 2004).
Taken together, studies regarding the development of strategic processing during verbal fluency point to a clear need for further research. In addition, strategic processing during verbal fluency has rarely been examined in clinical pediatric populations (for a study of children with Down syndrome, see Nash & Snowling, 2008). To extend our knowledge in this area, we explored clustering and switching during verbal fluency in children with phenylketonuria (PKU).
PKU is a hereditary disorder that affects approximately 1 in 15,000 children in the United States (National Institutes of Health Consensus Developmental Panel, 2001). The disorder is characterized by a deficiency in phenylalanine hydroxylase, resulting in excess phenylalanine and deficient dopamine (for overview, see Scriver, 2007), which particularly affects frontal brain function (Diamond, Prevor, Callender, & Druin, 1997). White matter abnormalities have also been identified in individuals with early-treated PKU (P. J. Anderson et al., 2007; P. J. Anderson et al., 2004; Ding et al., 2008; Scarabino et al., 2009; Vermathen et al., 2007; White et al., 2010), which may affect connectivity within frontal brain regions and between frontal and other brain regions. From a cognitive perspective, the neuropathology associated with early-treated PKU is thought to result in particular impairments in executive abilities (for overview, see Christ, Huijbregts, de Sonneville, & White, 2010).
With regard to verbal fluency, clustering and switching have not been previously investigated in individuals with PKU. The total number of words generated, however, has been examined in individuals with early-treated PKU, with mixed results. Impaired phonemic fluency has been reported in some (V. Anderson, Anderson, Northam, Jacobs, & Mikiewicz, 2002; Brumm et al., 2004; Channon, German, Cassina, & Lee, 2004; White, Nortz, Mandernach, Huntington, & Steiner, 2001) but not all (P. J. Anderson, et al., 2007; Luciana, Hanson, & Whitley, 2004; Moyle, Fox, Bynevelt, Arthur, & Burnett, 2007; Smith, Klim, & Hanley, 2000; VanZutphen et al., 2007) studies. Similarly, some studies have identified impaired semantic fluency (Brumm, et al., 2004; Welsh, Pennington, Ozonoff, & Rouse, 1990), whereas others have not (Moyle, et al., 2007; VanZutphen, et al., 2007; White, et al., 2001). Further, a meta-analysis by DeRoche and Welsh (2008) demonstrated a large effect size (1.15) indicating impaired set shifting in individuals with PKU across a range of cognitive tasks, such as the Contingency Naming Test, Intradimensional/Extradimensional Set-Shifting Task, and Wisconsin Card Sorting Test.
In the current investigation, we predicted that children with early-treated PKU would exhibit deficits in verbal fluency and strategic processing. More specifically, given research on the neural bases of verbal fluency and PKU, we hypothesized that children with early-treated PKU would demonstrate specific impairments in phonemic fluency and switching.
Methods
Participants
The study sample included 32 children with early- and continuously-treated PKU (18 girls, 14 boys) and 41 typically developing control children (23 girls, 18 boys). Children in the PKU group ranged from 7 to 18 years of age (M = 12.2, SD = 3.9) and were recruited through the Division of Medical Genetics/Department of Pediatrics at St. Louis Children’s Hospital in Missouri and through the Metabolic Clinic of the Child Development and Rehabilitation Center at Doernbecher Children’s Hospital in Portland, Oregon. Children in the control group also ranged from 7 to 18 years of age (M = 13.1, SD = 3.2) and were recruited from the St. Louis and Portland communities. Years of education ranged from 1 to 12 (M = 6.3, SD = 3.6) for the PKU group and from 1 to 14 (M = 7.2, SD = 3.3) for the control group. General intellectual ability was estimated using the Wechsler Abbreviated Scale of Intelligence (Psychological Corporation, 1999). Full Scale IQ ranged from 86 to 122 (M = 105.8, SD = 9.6) for the PKU group and from 84 to 124 (M = 108.5, SD = 10.0) for the control group. There were no significant between-group differences in gender (Χ2(1, N = 73) < 0.001, p = .99, ns), age (t(71) = 1.08, p = .28, ns), race (Χ2(4, N = 73) = 6.47, p = .16, ns), education (t(71) = 1.10, p = .27, ns), or Full Scale IQ (t(71) = 1.15, p = .25, ns). The parents of all 67 participants under the age of 17.5 were asked to report their years of education and household income; 65 responses (97%) were received for parent education and 55 responses (82%) were received for household income. No significant between-group differences existed for average level of parent education (PKU: M = 14.4, SD = 2.3; Control: M = 15.5, SD = 2.5), t(63) = 1.82, p = .07, ns, or total household income (PKU: M = 76.5, SD = 58.3; Control: M = 87.5, SD = 56.3), t(53) = 0.70, p = .49, ns. Based on findings from a health and demographic information questionnaire, no child in the study had a history of mental retardation, learning disorder (e.g., ADHD, reading disorder), or major medical disorder (e.g., traumatic brain injury, diabetes) unrelated to PKU. No child with PKU was being treated with or had ever been treated with sapropterin dihydrochloride (Kuvan) at the time of testing, or had a phenylalanine level ≥ 2 standard deviations from the group mean. Children in the PKU group were diagnosed near the time of birth and were treated early and continuously for the disorder through dietary restrictions to manage phenylalanine intake. Phenylalanine levels recorded closest to the time of participation in the study (typically the same day) ranged from 2 to 20 mg/dl (M = 8.4 mg/dl, SD = 4.7 mg/dl).
Procedure
Children were administered the Verbal Fluency subtest of the NEPSY (Korkman, Kirk, & Kemp, 1998), which comprises two semantic fluency (animal and food/drink) and two phonemic fluency (words beginning with S and F) trials. For each trial, children were asked to orally generate as many words as possible for a period of one minute. The total number of words correctly generated was recorded for each trial.
Additional variables were also calculated to assess strategic processing. For each semantic fluency trial, number of semantic clusters, number of words in semantic clusters, and number of semantic switches were calculated. For each phonemic fluency trial, number of phonemic clusters, number of words in phonemic clusters, and number of phonemic switches were calculated. The definitions of clusters and switches, as well as the scoring criteria, were based on the work of Troyer et al. (1997). As previously described, semantic clusters comprised two or more consecutively reported words sharing a semantic subcategory, whereas semantic switches comprised shifts to a new subcategory. Single words were not considered clusters, as suggested by Abwender, Swan, Bowerman, & Connolly (2001) and Koren, Kofman, & Berger (2005). Similarly, phonemic clusters comprised two or more consecutively reported words sharing a phonemic subcategory, whereas phonemic switches comprised shifts to a new subcategory. Phonemic subcategories included words that rhyme, differ by a single vowel sound, have the same beginning sound consisting of at least two letters, or are homophones. For example, in the S trial, report of sit, set / shelf, sharp, ship / slate, skate was recorded as three phonemic clusters, seven words in phonemic clusters, and two phonemic switches.
Results
Statistical analyses included repeated measures analyses of variance (ANOVA), t tests, and hierarchical regression. Significant results reflected p < .05. Means and standard deviations for each fluency variable generated are reported in Table 1.
Table 1.
Means and standard deviations for control and PKU groups across animal, food/drink, and phonemic fluency conditions.
| Condition | Control |
PKU |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Animal | ||||
| Total Words | 15.9 | 6.0 | 17.1 | 5.5 |
| Clusters | 4.3 | 1.8 | 4.3 | 1.8 |
| Words in Clusters | 11.6 | 5.5 | 12.9 | 5.8 |
| Switches | 7.4 | 2.5 | 7.3 | 2.8 |
| Food/Drink | ||||
| Total Words* | 19.8 | 6.9 | 16.6 | 5.6 |
| Clusters | 4.6 | 1.9 | 3.9 | 1.9 |
| Words in Clusters | 13.4 | 6.1 | 11.0 | 5.9 |
| Switches** | 10.4 | 3.3 | 8.4 | 2.5 |
| Phonemic | ||||
| Total Words** | 10.3 | 3.5 | 8.0 | 3.4 |
| Clusters | 2.2 | 1.4 | 1.9 | 1.2 |
| Words in Clusters | 4.6 | 2.7 | 3.9 | 2.5 |
| Switches** | 7.0 | 2.6 | 5.2 | 2.5 |
Note: *and ** indicate significant between-group differences at p < .05 and p < .01, respectively.
Total Number of Words Generated
As is typical in verbal fluency studies, we evaluated the number of words correctly generated in 60 seconds. Repeated measures ANOVA was first used to determine whether it was possible to collapse the two semantic fluency trials into a single semantic fluency condition and the two phonemic fluency trials into a single phonemic fluency condition. For semantic fluency, a significant group by condition interaction, F(1,71) = 14.53, p < .001, ηp2 = .17, indicated that group had a differential effect on number of words generated in the animal and food/drink trials. Therefore, in further analyses these trials were examined separately. For phonemic fluency, there was no significant interaction between group and condition, F(1,71) = 0.30, p = .58, ns. Therefore, S and F trials were averaged to form new phonemic fluency condition variables for further analyses.
Results of t tests indicated that the PKU and control groups generated a comparable number of words in the animal fluency trial, t(71) = −0.86, p = .40, ns. In contrast, the PKU group generated fewer words than the control group in the food/drink trial, t(71) = 2.17, p = .03, d = 0.52, and phonemic fluency condition, t(71) = 2.77, p < .01, d = 0.66.
Strategic Processing
To further explore findings of poorer word generation by the PKU group in the food/drink trial and phonemic fluency condition, clustering and switching variables were examined using t tests. For food/drink, number of semantic switches was significantly less for the PKU than control group, t(71) =2.76, p < .01, d = 0.66. There were, however, no significant between-group differences in number of semantic clusters, t(71) = 1.45, p = .15, ns, or number of words in semantic clusters, t(71) = 1.70, p = .09, ns. Similarly, for phonemic fluency, number of phonemic switches was significantly less for the PKU than control group, t(71) = 3.03, p < .01, d = 0.72, but there were no significant between-group differences in number of phonemic clusters, t(71) = 0.90, p = .37, ns, or number of words in phonemic clusters, t(71) = 1.08, p = .29, ns. Although there was no significant difference between the PKU and control groups in number of words generated in the animal trial, strategic processing variables were examined in this trial for the sake of thoroughness; as shown in Table 1, no significant between-group differences were observed for any variables (p > .05 in all instances), verifying the comparability of animal fluency performance in the PKU and control groups.
We next conducted hierarchical regression analyses to determine whether group accounted for a significant proportion of the variance in number of words generated after accounting for the variance attributable to number of switches in the food/drink trial and phonemic fluency condition. In these analyses, number of words generated served as the dependent variable. For independent variables, number of switches was entered in a first step, followed by group in a second step. We found that, for the food/drink trial, number of semantic switches explained a significant portion of the variance in number of words generated, R2 = .45, F(1,71) = 57.72, p < .001. Similarly, for the phonemic fluency condition, number of phonemic switches explained a significant portion of the variance in number of words generated, R2 = .84, F(1,71) = 366.40, p < .001. In neither instance did group account for additional variance in number of words generated beyond that attributable to number of switches. Overall, these results suggest that poorer word generation for the PKU than control group was driven by poorer switching in both the food/drink trial and the phonemic fluency condition.
It seemed likely that our pattern of findings reflected the generation of more single, non-clustered words by the control than PKU group, which resulted in between-group differences in number of words generated and a larger number of switches but no differences in number of words in clusters or number of clusters. T tests revealed that, for phonemic fluency, the PKU group (M = 4.11, SD = 2.14) produced significantly fewer single, non-clustered words, t(71) = 2.87, p < .01, d = 0.68, than the control group (M = 5.68, SD = 2.46). For food/drink, there was no between-group difference in number of single, non-clustered words, t(71) = 1.27, p = .21, ns; examination of absolute values, however, suggested a trend in which the control group (M = 6.41, SD = 3.03) produced more single, non-clustered words than the PKU group (M = 5.60, SD = 2.31).
Age Effects
Using hierarchical regression, we next examined whether there was a differential effect of age on food/drink and phonemic fluency performance for the PKU and control groups. In separate analyses, the dependent variables were number of words generated, number of clusters, number of words in clusters, and number of switches for the food/drink trial and phonemic condition. For independent variables, in each analysis age was entered in the first step, group (PKU, control) in the second step, and the interaction between age and group in the final step. (In earlier modeling the effects of age2 and the interactions between age2 and group were entered; because there were no significant age2 findings, these variables were eliminated from the final models.)
Not surprisingly, results showed that age accounted for a significant proportion of the variance in all variables for both the food/drink trial and phonemic fluency condition. Specifically, number of words generated, number of clusters, number of words in clusters, and number of switches increased with age (R2s ranged from .10 to .40, p < .01 in all instances). In addition, consistent with the findings reported earlier, group accounted for a significant proportion of the variance in number of switches for both the food/drink trial, ΔR2 = .06, ΔF(1,70) = 6.51, p = .01, and the phonemic fluency condition, ΔR2 = .07, ΔF(1,70) = 8.57, p < .01, with a greater number of switches for the control than PKU group. The interaction between age and group was not significant for the number of switches in the phonemic fluency condition. The interaction between age and group, however, accounted for a significant proportion of the variance in number of switches for food/drink, ΔR2 = .06, ΔF(1,69) = 7.42, p < .01. This interaction reflected a greater increase in number of switches for the control than PKU group as a function of increasing age.
Clinical Relevance
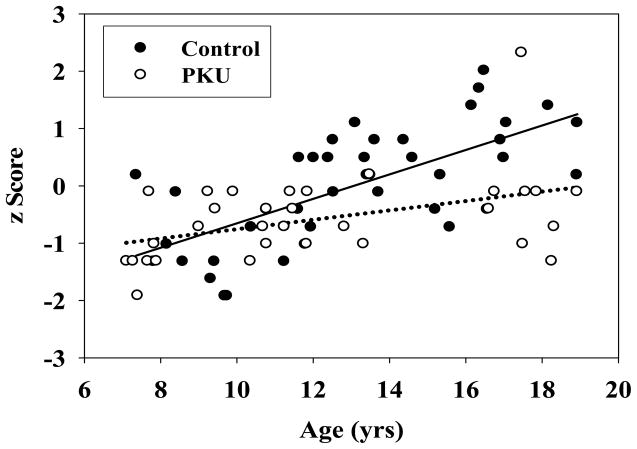
In addition to statistical analyses, the clinical relevance of our findings was assessed. For the four key variables on which significant between-group differences were found, z-scores were calculated for each participant based on control group means and standard deviations. Mean z scores for the PKU group were −.47 for number of words reported in the food/drink trial, −.58 for number of semantic switches in the food/drink trial, −.65 for number of words reported in the phonemic fluency condition, and −.71 for number of phonemic switches in the phonemic fluency condition. Thus, on average, performance of the PKU group was 0.6 standard deviations below that of the control group. Due to the interaction between age and group in the earlier regression analysis examining number of switches for food/drink, we plotted z scores for this variable as a function of age. As illustrated in Figure 1, the oldest participants with PKU scored approximately 1.5 standard deviations below the oldest controls.
Figure 1.
Number of switches as a function of age for control and PKU groups in the food/drink task.
Phenylalanine Levels and Cognitive Performance
Correlations between blood phenylalanine levels and verbal fluency performance were examined for children with PKU. Phenylalanine level closest to the time of testing (typically same day), average phenylalanine level over the three months prior to testing, and highest phenylalanine level on record during the lifetime were examined. Verbal fluency variables included number of words generated, number of switches, number of clusters, and number of words in clusters for the animal, food/drink, and phonemic fluency tasks. No significant correlations were found between any of the phenylalanine and verbal fluency variables.
Discussion
In the current study, we administered semantic (animal, food/drink) and phonemic (S, F) fluency tasks to test the hypotheses that children with early-treated PKU have specific impairments in phonemic fluency and switching. Our results provided strong support for both hypotheses. At the broadest level of analysis, children with PKU generated fewer words than typically-developing control children in both the food/drink trial and phonemic fluency condition, although in the animal trial the number of words generated was comparable for the two groups. The finding of impaired phonemic fluency was predicted on the basis of previous research indicating that this type of fluency is largely mediated by frontal brain regions (for overview, see Alvarez & Emory, 2006) which are thought to be compromised in children with PKU. The differential findings for animal and food/drink fluency are more intriguing, because semantic fluency is thought to be mediated largely by temporal brain regions (Henry & Crawford, 2004; Martin, et al., 1994; Monsch, et al., 1994; Moscovitch, 1994; Mummery, et al., 1996; Rosser & Hodges, 1994). Further research will be necessary to reach definitive conclusions, but it is possible that the differential findings across the two semantic fluency trials are related to the fact that only one category was presented in animal fluency, whereas two subcategories were presented in food/drink fluency (foods and drinks). This hypothesis is consistent with the following discussion of our switching findings, as it is possible that control children switched more efficiently between food and drink subcategories (thereby generating more words) than children with PKU.
Because fewer words were generated by the PKU than control group in the food/drink trial and phonemic condition, we next examined specific strategic processing variables for food/drink and phonemic fluency. For number of clusters and number of words in clusters, performance was comparable for the PKU and control groups. In contrast, children with PKU made fewer switches between subcategories than controls in both the food/drink trial and phonemic fluency condition. Findings from hierarchical regression analyses further suggested that between-group differences in number of words generated for food/drink and phonemic fluency were driven by between-group differences in number of switches. Although neuroimaging was not conducted in our study, these findings are consistent with the notion that switching is subserved by frontal brain regions (Hirshorn & Thompson-Schill, 2006; Troyer, et al., 1997; Troyer, Moscovitch, Winocur, Alexander, et al., 1998) which are compromised in children with PKU. These results are also consistent with those of a meta-analysis indicating that shifting is impaired in individuals with PKU across a range of cognitive tasks (DeRoche & Welsh, 2008).
Further analyses also demonstrated that control children generated more single, non-clustered words than children with PKU. This is consistent with a suggestion by Kave et al. (2008) that the adults in their study may have generated more single words that were not in clusters than adolescents, resulting in more overall words produced but no differences in clustering variables. The authors proposed that switching is therefore a better measure of efficient, strategic processing than clustering (also see Koren, et al., 2005).
Given previous findings of differential effects of age on executive performance in children with PKU versus controls (White, et al., 2001; White, Nortz, Mandernach, Huntington, & Steiner, 2002), we next examined each strategic processing variable as a function of age for the food/drink trial and phonemic fluency condition. For the clustering variables, the relationship between age and performance was comparable for the two groups. Similarly, although children with PKU made fewer switches than controls in the phonemic fluency condition, there was no differential effect of age. Of greater interest, in the food/drink trial, the control group produced more switches between subcategories as age increased than did the children with PKU. Although longitudinal research is needed for verification, it is possible that impaired switching during food/drink fluency emerges as children with PKU age. The differential effect of age on food/drink but not phonemic fluency also requires further study, but it is possible that the inherent switching component of the food/drink trial makes it particularly susceptible to disruptions in frontal brain development which are reflected in age effects on performance.
Taken together, our results indicate that children with early-treated PKU exhibit impaired switching during verbal fluency performance, which is thought to be a frontally-mediated aspect of strategic processing. Our findings suggest that strategic processing should be evaluated carefully as children with PKU age. There are, however, limitations to our study. For example, converging evidence from multiple strategic processing tasks using verbal and nonverbal materials would foster greater confidence in the generalizability of our findings. In addition, our speculation regarding the neural underpinnings of impaired strategic processing in children with PKU requires verification through neuroimaging research. Future research should also be conducted to examine real-world implications such as the developmental relationship between strategic processing and academic achievement in children with PKU.
Acknowledgments
This research was supported by the National Institute of Child Health and Human Development grant R01HD044901. Desiree A. White serves as a consultant for Merck Serono S.A. Desiree A. White and Dorothy K. Grange serve as consultants for and receive research funding from BioMarin Pharmaceutical Inc. Robert D. Steiner served as a consultant for BioMarin Pharmaceutical Inc. in the past. The authors wish to thank Suzin Blankenship, Laurie Sprietsma, and Tina Marrone for their contributions to this study
References
- Abwender DA, Swan JG, Bowerman JT, Connolly SW. Qualitative analysis of verbal fluency output: Review and comparison of several scoring methods. Assessment. 2001;8(3):323–336. doi: 10.1177/107319110100800308. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review. 2006;16(1):17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Wood SJ, Francis DE, Coleman L, Anderson V, Boneh A. Are neuropsychological impairments in children with early-treated phenylketonuria (PKU) related to white matter abnormalities or elevated phenylalanine levels? Developmental Neuropsychology. 2007;32(2):645–668. doi: 10.1080/87565640701375963. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Wood SJ, Francis DE, Coleman L, Warwick L, Casanelia S, et al. Neuropsychological functioning in children with early-treated phenylketonuria: Impact of white matter abnormalities. Developmental Medicine & Child Neurology. 2004;46:230–238. doi: 10.1017/s0012162204000386. [DOI] [PubMed] [Google Scholar]
- Anderson V, Anderson PJ, Northam E, Jacobs R, Mikiewicz O. Relationships between cognitive and behavioral measures of executive function in children with brain disease. Child Neuropsychology. 2002;8(4):231–240. doi: 10.1076/chin.8.4.231.13509. [DOI] [PubMed] [Google Scholar]
- Berman KF, Ostrem JL, Randolph C, Gold J. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: A positron emission tomography study. Neuropsychologia. 1995;33(8):1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- Brumm V, Azen C, Moats R, Stern A, Broomand C, Nelson M, et al. Neuropsychological outcome of subjects participating in the PKU Adult Collaborative Study: A preliminary review. Journal of Inherited Metabolic Disease. 2004;27:549–566. doi: 10.1023/b:boli.0000042985.02049.ff. [DOI] [PubMed] [Google Scholar]
- Channon S, German E, Cassina C, Lee P. Executive functioning, memory, and learning in phenylketonuria. Neuropsychology. 2004;18(4):613–620. doi: 10.1037/0894-4105.18.4.613. [DOI] [PubMed] [Google Scholar]
- Christ SE, Huijbregts S, de Sonneville L, White DA. Executive function in early-treated phenylketonuria: Profile and underlying mechanisms. Molecular Genetics and Metabolism. 2010;99(Supplement 1):22–32. doi: 10.1016/j.ymgme.2009.10.007. [DOI] [PubMed] [Google Scholar]
- DeRoche K, Welsh M. Twenty-five years of research on neurocognitive outcomes in early-treated phenylketonuria: Intelligence and executive function. Developmental Neuropsychology. 2008;33(4):474–504. doi: 10.1080/87565640802101482. [DOI] [PubMed] [Google Scholar]
- Diamond A, Prevor MB, Callender G, Druin DP. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monographs of the Society for Research in Child Development. 1997;62(1–205) [PubMed] [Google Scholar]
- Ding XQ, Fiehler J, Kohlschutter B, Wittkugel O, Grzyska U, Zeumer H, et al. MRI abnormalities in normal-appearing brain tissue of treated adult PKU patients. Journal of Magnetic Resonance Imaging. 2008;27(5):998–1004. doi: 10.1002/jmri.21289. [DOI] [PubMed] [Google Scholar]
- Fuster J. Human neuropsychology: Executive functions. In: Fuster J, editor. The prefrontal cortex. London: Elsevier, Ltd; 2008. pp. 178–192. [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Manual. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004;18(2):284–295. doi: 10.1037/0894-4105.18.2.284. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: Neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44(12):2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Ho AK, Sahakian BJ, Robbins TW, Barker RA, Rosser AE, Hodges JR. Verbal fluency in Huntington's disease: A longitudinal analysis of phonemic and semantic clustering and switching. Neuropsychologia. 2002;40(8):1277–1284. doi: 10.1016/s0028-3932(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Hurks P, Schrans D, Meijs C, Wassenberg R, Feron F, Jolles J. Developmental changes in semantic verbal fluency: Analyses of word productivity as a function of time, clustering, and switching. Child Neuropsychology. 2010;16(4):366–387. doi: 10.1080/09297041003671184. [DOI] [PubMed] [Google Scholar]
- Kavé G, Kigel S, Kochva R. Switching and clustering in verbal fluency tasks throughout childhood. Journal of Clinical and Experimental Neuropsychology. 2008;30(3):349–359. doi: 10.1080/13803390701416197. [DOI] [PubMed] [Google Scholar]
- Koren R, Kofman O, Berger A. Analysis of word clustering in verbal fluency of school-aged children. Archives of Clinical Neuropsychology. 2005;20(8):1087–1104. doi: 10.1016/j.acn.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY: A developmental neuropsychological assessment. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- Kumada T, Humphreys G. Dimensional weighting and task switching following frontal lobe damage: Fractionating the task switching deficit. Cognitive Neuropsychology. 2006;23(3):424–447. doi: 10.1080/02643290542000058. [DOI] [PubMed] [Google Scholar]
- Luciana M, Hanson KL, Whitley CB. A preliminary report on dopamine system reactivity in PKU: Acute effects of haloperidol on neuropsychological, physiological, and neuroendocrine functions. Psychopharmacology. 2004;175:18–25. doi: 10.1007/s00213-004-1775-0. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Lalonde Fo, Mack C. Word retrieval to letter and semantic cues: A double dissociation in normal subjects using interference tasks. Neuropsychologia. 1994;32(12):1487–1494. doi: 10.1016/0028-3932(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Miotto EC, Savage GR, Evans JJ, Wilson BA, Martins MGM, laki S, et al. Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Human Brain Mapping. 2006;27:288–295. doi: 10.1002/hbm.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman N, Emerson M, Witzki A, Howerter A, Wager T. The unity and diversity of executive functions and their contributions to complex "frontal lobe" tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Paulsen JS, Salmon DP, Brugger P, et al. A comparison of category and letter fluency in Alzheimer's disease and Huntington's disease. Neuropsychology. 1994;8:25–30. [Google Scholar]
- Moscovitch M. Cognitive resources and dual-task interference effects at retrieval in normal people: The role of the frontal lobes and medial temporal cortex. Neuropsychology. 1994;8(4):524–534. [Google Scholar]
- Moyle JJ, Fox AM, Bynevelt M, Arthur M, Burnett JR. A neuropsychological profile of off-diet adults with phenylketonuria. Journal of Clinical and Experimental Neuropsychology. 2007;29(4):436–441. doi: 10.1080/13803390600745829. [DOI] [PubMed] [Google Scholar]
- Mummery C, Patterson K, Hodges JR, Wise R. Generating 'tiger' as an animal name or a word beginning with T: Differences in brain activation. Proceedings of the Royal Society of London--Series B: Biological Sciences. 1996;263(1373):989–995. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Nash H, Snowling M. Semantic and phonological fluency in children with Down syndrome: Atypical organization of language or less efficient retrieval strategies? Cognitive Neuropsychology. 2008;25(5):690–703. doi: 10.1080/02643290802274064. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Developmental Panel. Phenylketonuria: Screening and management, October 16–18, 2000. Pediatrics. 2001;108:972–982. doi: 10.1542/peds.108.4.972. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Robinson A, Heaton RK, Lehman R, Stilson D. The utility of the Wisconsin Card Sorting Test in detecting and localizing frontal lobe lesions. Journal of Consulting and Clinical Psychology. 1980;48(5):605–614. doi: 10.1037//0022-006x.48.5.605. [DOI] [PubMed] [Google Scholar]
- Rosser A, Hodges JR. Initial letter and semantic category fluency in Alzheimer's disease, Huntington's disease, and progressive supranuclear palsy. Journal of Neurology, Neurosurgery & Psychiatry. 1994;57(11):1389–1394. doi: 10.1136/jnnp.57.11.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauzéon H, Lestage P, Raboutet C, N'Kaoua B, Claverie B. Verbal fluency output in children aged 7–16 as a function of the production criterion: Qualitative analysis of clustering, switching processes, and semantic network exploitation. Brain and Language. 2004;89(1):192–202. doi: 10.1016/S0093-934X(03)00367-5. [DOI] [PubMed] [Google Scholar]
- Scarabino T, Popolizio T, Tosetti M, Montanaro D, Giannatempo GM, Terlizzi R, et al. Phenylketonuria: white-matter changes assessed by 3.0-T magnetic resonance (MR) imaging, MR spectroscopy and MR diffusion. La Radiologia Medica. 2009;114(3):461–474. doi: 10.1007/s11547-009-0365-y. [DOI] [PubMed] [Google Scholar]
- Scriver CR. The PAH gene, phenylketonuria, and a paradigm shift. Human Mutation. 2007;28:831–845. doi: 10.1002/humu.20526. [DOI] [PubMed] [Google Scholar]
- Shallice T, Stuss D, Picton T, Alexander M, Gillingham S. Mapping task switching in frontal cortex through neuropsychological group studies. Frontiers in Neuroscience. 2008;2(1):79. doi: 10.3389/neuro.01.013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Klim P, Hanley WB. Executive function in school-aged children with phenylketonuria. Journal of Developmental and Physical Disabilities. 2000;12(4):317–332. [Google Scholar]
- Strangman G, Goldstein R, O'Neil-Pirozzi T, Kelkar K, Supelana C, Burke D, et al. Neurophysiological alterations during strategy-based verbal learning in traumatic brain injury. Neurorehabilitation and Neural Repair. 2009;23(3):226–236. doi: 10.1177/1545968308324225. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Palumbo C, Buckle L, Sayer L, Pogue J. Organizational strategies of patients with unilateral or bilateral frontal lobe injury in word list learning tasks. Neuropsychology. 1994;8:355–373. [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: Evidence from younger and older healthy adults. Neuropsychology. 1997;11(1):138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G, Alexander MP, Stuss D. Clustering and switching on verbal fluency: The effects of focal frontal- and temporal-lobe lesions. Neuropsychologia. 1998;36(6):499–504. doi: 10.1016/s0028-3932(97)00152-8. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G, Leach L, Freedman M. Clustering and switching on verbal fluency tests in Alzheimer's and Parkinson's disease. Journal of the International Neuropsychological Society. 1998;4(2):137–143. doi: 10.1017/s1355617798001374. [DOI] [PubMed] [Google Scholar]
- VanZutphen K, Packman W, Sporri L, Needham M, Morgan C, Weisiger K, et al. Executive functioning in children and adolescents with phenylketonuria. Clinical Genetics. 2007;72(1):13–18. doi: 10.1111/j.1399-0004.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- Vermathen P, Robert-Tissot L, Pietz J, Lutz T, Boesch C, Kreis R. Characterization of white matter alterations in phenylketonuria by magnetic resonance relaxometry and diffusion tensor imaging. Magnetic Resonance in Medicine. 2007;58(6):1145–1156. doi: 10.1002/mrm.21422. [DOI] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF, Ozonoff S, Rouse B. Neuropsychology of early-treated phenylketonuria: Specific executive function deficits. Child Development. 1990;61(6):1697–1713. [PubMed] [Google Scholar]
- White DA, Connor LT, Nardos B, Shimony JS, Archer R, Snyder AZ, et al. Age-related decline in the microstructural integrity of white matter in chkldren with early- and continously-treated PKU: a DTI study of the corpus callosum. Molecular Genetics and Metabolism. 2010;99(Supplement 1):S41–S46. doi: 10.1016/j.ymgme.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Nortz MJ, Mandernach T, Huntington K, Steiner RD. Deficits in memory strategy use related to prefrontal dysfunction during early development: Evidence from children with phenylketonuria. Neuropsychology. 2001;15(2):221–229. doi: 10.1037//0894-4105.15.2.221. [DOI] [PubMed] [Google Scholar]
- White DA, Nortz MJ, Mandernach T, Huntington K, Steiner RD. Age-related working memory impairments in children with prefrontal dysfunction associated with phenylketonuria. Journal of the International Neuropsychological Society. 2002;8(1):1–11. [PubMed] [Google Scholar]