Abstract
Objectives
The ability to identify the early neurobiological markers to predict the clinical response to a course of chronic psychotropic drug treatment motivated the early development of neurochemical brain imaging methods. The present study tested the hypothesis that lower baseline glucose metabolism and greater acute cerebral metabolic responses to a single, intravenous dose of the selective serotonin reuptake inhibitor (SSRI) citalopram would be associated with greater antidepressant response to twelve weeks of citalopram treatment in geriatric depression.
Methods
Sixteen geriatric depressed patients underwent two scans to measure cerebral glucose metabolism after administration of either a saline placebo or citalopram infusion (40mg, IV). Then, the patients were treated with the oral medication for twelve weeks.
Results
Greater improvement of depressive symptoms was associated with lower baseline metabolism in anterior cingulate, superior, middle and inferior frontal gyri (bilaterally), inferior parietal lobule (bilaterally), precuneus (right), insula (left), parahippocampal gyrus (right), caudate (bilaterally) and putamen (left) regions. Greater improvement of depressive symptoms was associated with greater reductions in metabolism after acute citalopram administration in similar brain regions, including additional posterior cortical regions.
Conclusions
Lower baseline cerebral metabolism and greater decreases with acute citalopram administration are associated with better response to chronic citalopram treatment. These data are consistent with previous studies of total sleep deprivation and suggest that dynamic, early adaptive changes or normalization of cerebral metabolism may represent early neurobiological markers of chronic SSRI treatment response in geriatric depression.
OBJECTIVES
The early development of in vivo neurochemical brain imaging methods was strongly motivated by an interest in developing early neurobiological markers of psychotropic drug response in psychotic and affective disorders. The majority of studies measured the effects of a single drug dose or acute interventions on global measures of brain function (cerebral glucose metabolism and blood flow) or, more directly, on endogenous neurotransmitter activity (1, 2). Studies to measure serotonin transporter occupancy by antidepressants or D2 occupancy by antipsychotics have not shown associations with affective or psychotic symptom improvement, respectively (e.g. \3, 4). These occupancy measures reflect whether the medications act on the primary target sites of action. The secondary effects of medication, measured by the dynamic response of the brain (global brain function or endogenous neurotransmitter activity), may be better associated with clinical outcome than measures of transporter or receptor occupancy. The underlying assumption is that the initial, dynamic brain response to acute treatment is indicative of the capacity of the brain to respond to a course of chronic treatment and thus, greater clinical improvement.
Research over the past decade has focused on developing early neurobiological markers of antidepressant (e.g. total sleep deprivation or selective serotonin reuptake inhibitor, SSRI) treatment response in geriatric depression (as reviewed in 5). Early indicators of treatment response are particularly important in geriatric patients as more than half of geriatric depressed patients are either mixed/partial responders or non-responders to an adequate course of treatment (6). The ability to identify which patients would respond best to an SSRI versus those who would require another class of antidepressant medication or more intensive treatment would have a significant impact on the clinical management of geriatric depression. This is especially important for some classes of antidepressants that are associated with greater side effects (e.g. selective noradrenergic reuptake inhibitors; 7). Thus far, neuropsychological, structural neuroimaging and genetic methods have been applied to distinguish treatment responders from non-responders. The neurobiological measures associated with poorer response to antidepressant (SSRI) treatment include: poorer baseline executive function, magnetic resonance (MR) measures of decreased anterior cingulate volumes (Brodmann area [BA] 24, 32) and greater cerebrovascular burden (hyperintensities in the white matter and deep grey matter structures), decreased functional connectivity of white matter pathways in cortical and limbic regions and the s allele of the serotonin transporter promoter (5HTTLPR). These data suggest that dysfunction in cortical and limbic structures, as well as decreased serotonin function may be associated with poorer antidepressant response (8–12).
With respect to the predictive value of dynamic measures of brain function in geriatric depression, the cerebral metabolic response to total sleep deprivation (TSD), as well as one night of recovery sleep was associated with the clinical antidepressant response to twelve weeks of treatment with the SSRI paroxetine (1). Greater reductions in cerebral glucose metabolism in the rostral anterior cingulate (BA 24, 32), superior and middle frontal gyri and precuneus after TSD and recovery sleep was associated with greater decreases in depressive symptoms after chronic paroxetine treatment. These data suggest that acute metabolic responses may represent an early neurobiological marker of chronic treatment response in geriatric depression. It is important to note that TSD is not a neurochemically selective intervention but increases monoamine concentrations, as well as concentrations of acetylcholine and trophic factors (as reviewed in 1). The present study was undertaken to evaluate whether a neurochemically selective acute intervention, the most selective of the SSRIs, citalopram, would produce similar changes in neural circuitry associated with antidepressant treatment response. The cerebral glucose metabolic response to acute citalopram administration (40mg, IV) was measured in geriatric depressed patients who then, underwent a twelve week treatment trial with the oral medication.
Based on prior observations of increased pre-treatment cerebral glucose metabolism in geriatric depression (13) and reductions in metabolism with treatment (1, 14, 15), it was hypothesized that lower pre-treatment metabolism in the rostral anterior cingulate gyrus (BA 24), superior and middle frontal cortex and precuneus would be associated with greater improvement of depressive symptoms. Greater reductions in cerebral metabolism after acute citalopram in these brain regions were hypothesized to be associated with a greater antidepressant effect after twelve weeks of citalopram treatment.
METHODS
Subjects
Sixteen depressed patients were enrolled in the study (seven male and nine female), who met DSM-IV criteria for major depression (non-bipolar and non-psychotic; mean age, 65.3 ± 9.1 years; mean education level, 14.3 ± 2.9 years; mini mental status exam score [MMSE, Folstein et al., 1975], 28.7 ± 1.1). The mean age at onset of depression was 60.0 ± 4.4 and the mean duration of the current episode was 7.1 months ± 2.8. There were no significant differences in these demographic variables as a function of gender (age: males 66.3 ± 8.5; female 64.4 ± 9.9, age at onset: males 66.3 ± 8.5; female 61.4 ± 9.6 duration: males 6.9 ±2.7; female 7.2± 3.0, education level: males 14.0 ± 1.5; female 13.7 ± 3.4, MMSE: males 29.1 ± 0.7; female 28.3 ± 1.2; p > 0.1). Subjects were recruited through the Geriatric Psychiatry Outpatient Clinic of the Zucker Hillside Hospital and by advertisements in the community. The protocol and consent forms were approved by the Institutional Review Board and the Radiation Safety Committee of the North Shore-Long Island Jewish Health System. The clinical and neuroimaging data from this patient sample have been reported previously (1, 14, 15).
Participants underwent psychiatric screening using the Structured Clinical Interview for DSM-IV Axis 1 Disorders – Patient Edition (SCID-I/P, 16). Depression severity was measured using the Hamilton Depression Rating Scale - 24 item (HDRS, 17), Beck Depression Inventory (BDI, 18), and Geriatric Depression Scale (GDS, 19). Participants underwent laboratory testing (CBC, blood chemistry, glucose levels, thyroid function, toxicology) and MR imaging to rule out structural abnormalities (e.g. stroke, brain tumor; GE 1.5T Magnetom Vision, General Electric Medical Systems, Milwaukee, Wisconsin). Exclusionary criteria were: 1) past history or current other DSM-IV, axis 1 disorders, 2) unstable medical conditions or insulin dependent diabetes, 3) use of medications with central nervous system effects within the past two weeks (including beta blockers and over the counter medications). 25% of the patients were taking anti-hypertensive medications (either an angiotensin converting enzyme inhibitors or a calcium channel blocker, since beta blockers were an exclusion). The mean blood pressure for the patients was: systolic 130.0 ± 15.3 and diastolic 75.3 ± 11.3. The majority of patients enrolled in this study were never treated previously with an antidepressant or other psychotropic medication (12 patients or 75%). Three of four previously-medicated subjects had been treated with sertraline, but were medication free for six months or more prior to study enrollment. The fourth subject was treated with nortriptyline and was not responding to treatment. She was tapered off the medication two weeks prior to the first PET scan and did not have detectable plasma nortriptyline levels at the time of scanning.
Citalopram Administration and PET Scan Procedures
The study procedures, including the positron emission tomography (PET) scans, have been described previously (14, 15, 20). Prior to starting citalopram treatment, patients underwent PET scans on two consecutive days, after placebo infusion (250ml saline) on Day 1 and citalopram infusion (40mg, IV) on Day 2. The study was single-blind in that the investigators, not the patients, were aware of the identity of the infusions. The placebo was always administered on Day 1 and citalopram on Day 2. It has been estimated that the residual effects of intravenous citalopram may last as long as three weeks (as reviewed in 20). Such a long interval between scans might introduce greater variability into the measurements, as well as the clinical state of the patients, and treatment would be delayed for several weeks. Thus, the decision was made to administer the placebo consistently on day 1.
The PET scans were acquired on a GE Advance Tomograph (General Electric Medical Systems, Milwaukee, Wisconsin) in the Center for Neurosciences, Feinstein Institute for Medical Research. On each of the days of the PET scans, subjects arrived at the laboratory and catheters were placed in each arm for placebo/citalopram and radiotracer infusion and for blood sampling (opposite arm). The radiotracer was injected approximately 30 minutes after the end of infusion, at which time the greatest effect of citalopram on cerebral metabolism has been observed (20). 5 ± 10% mCi of [18F]-2-deoxy-2-fluoro-D-glucose (FDG) was injected as an intravenous bolus. Then, a 25 minute radiotracer uptake period occurred in a quiet, dimly lit room with eyes and ears unoccluded. Then, the subjects were positioned in the PET scanner. A 10 minute transmission scan was performed followed by a five minute, two-dimensional emission scan for attenuation correction. A 10 minute, three-dimensional emission scan was performed approximately 40 minutes after the injection of the radiotracer. At the completion of the scan, subjects were removed from the scanner and debriefed as to their perceptions of the study. 20% of the subjects experienced transient headache and nausea that coincided with the peak plasma concentration of citalopram, from the end of infusion to 30 minutes post infusion.
Two days after the acute, intravenous administration of a single dose of citalopram, the patients began treatment with the oral medication at a dose of 10mg per day for 3 days. The dose was increased to 20mg on the 4th day. If significant clinical improvement was not observed at the 20mg dose after 4 weeks of treatment [measured as a rating of ≥3 on the Clinical Global Impression Scale (CGI, 21), the dose was increased to 30mg, and then, if needed, to 40mg. One patient was titrated to a dose of 50mg. Patients were monitored on a weekly basis in the Geriatric Outpatient Clinic of the Zucker Hillside Hospital, at which time clinical ratings were administered and side effects were assessed. All patients who began treatment completed the twelve week course of treatment and were included in this report.
PET Data and Image Analyses
The quantification of glucose metabolism was performed on a voxel wise basis using validated methods as described previously (1, 13–15, 20). Image pre-processing and voxel-wise statistical analyses were performed with SPM5 (Institute of Neurology, London, United Kingdom; 22). Images were smoothed using an isotropic Gaussian kernel (full-width half-maximum [FWHM] 8mm for all directions). Global normalization of the images to a mean of 50 was performed after confirming that the global means for the two conditions (placebo/citalopram) did not differ significantly (p > .05). Two primary analyses were performed: 1) the paired t-test option with change in HDRS as a covariate was used to correlate baseline cerebral metabolism with change in HDRS score from baseline to 12 weeks, and 2) the flexible factorial option was used to compare change in cerebral metabolism from pretreatment to the acute citalopram challenge and reduction in HDRS score from baseline to 12 weeks. Thus, treatment response (change in HDRS score) was treated as a continuous variable. Results are reported for a t threshold > 3.51 (z > 2.98, p < .001, uncorrected for multiple independent comparisons) and a cluster size of greater than 50 voxels. To address the possibility that the magnitude of reduction in HDRS score following treatment may be related to greater baseline depression severity, a correlation was computed between baseline HDRS scores and changes in HDRS scores. The relationship between the baseline scores and the magnitude of change was not significant (r = 0.016, p = 0.953).
RESULTS
Clinical Data
The bi-weekly clinical ratings and citalopram doses are shown in Table 1. The patients demonstrated significantly decreased observer and self report depression ratings after 12 weeks of citalopram treatment. Significant reductions in HDRS scores (Baseline: 27.8 ± 3.7, Treatment Week 12: 6.6 ± 5.6; Range: −27 to −12 [F=302.4, df=1, p < 0.001]), BDI scores (Baseline: 11.4 ± 3.1, Treatment Week 12: 3.3 ±3.1; Range: −13 to 5 [F=53.9, df=1, p < 0.001]) and GDS scores were observed (Baseline: 20.44 ± 5.6 Treatment Week 12: 8.0 ± 8.1; Range: −21 to 5 [F= 52.1, df=1, p < 0.001]).
Table 1.
Clinical data (mean ± standard deviation)
| HDRS Score | HARS Score | BDI Score | GDS Score | Citalopram Dose | |
|---|---|---|---|---|---|
| Week 0 | 25.6 ± 4.1 | 13.1 ± 5.4 | 11.4 ± 3.2 | 20.6 ± 5.2 | 10.0 ± 0.0 |
| Week 2 | 17.3 ± 7.2 | 9.1 ± 4.7 | 7.5 ± 4.6 | 15.8 ± 7.9 | 20.0 ± 0.0 |
| Week 4 | 12.6 ± 8.6 | 7.3 ± 5.6 | 5.9 ± 3.9 | 13.9 ± 8.7 | 26.3 ± 9.6 |
| Week 6 | 10.0 ± 7.7 | 4.9 ± 3.5 | 4.4 ± 3.6 | 11.5 ± 8.1 | 29.4 ± 7.7 |
| Week 8 | 8.6 ± 6.4 | 5.3 ± 3.7 | 4.4 ± 4.3 | 10.6 ± 8.4 | 31.9 ± 7.5 |
| Week 10 | 7.3 ± 6.2 | 4.4 ± 3.4 | 4.0 ± 3.8 | 9.1 ± 8.1 | 35.0 ± 7.3 |
| Week 12 | 6.8 ± 5.6 | 4.6 ± 3.1 | 3.3 ± 3.1 | 8.0 ± 8.2 | 35.0 ± 8.9 |
Note: HDRS: Hamilton Depression Rating Scale; HARS: Hamilton Anxiety Rating Scale; BDI: Beck Depression Inventory; GDS: Geriatric Depression Scale.
While treatment response status was treated as a continuous variable in the statistical analysis, to compare with the literature, the patients were characterized as responders or non-responders. The definition of response was a HDRS change of 50% and a score of less than 10 at week 12 of citalopram treatment. On this basis, 25% of patients met criteria for non-response. There were no significant differences in the demographic variables as a function of treatment response (age: responders 65.6 ± 7.8; non-responders 64.3 ± 13.6, age at onset: responders 64.7 ± 7.8; non-responders 60.3 ± 13.3, duration: responders 6.5 ± 2.1; non-responders 8.8 ± 4.3, education level: responders 13.9 ± 2.9; non-responders 13.5 ± 1.9, MMSE: responders 28.6 ± 1.2; non-responders 29.0 ± 0.8, and week 12 citalopram dose: responders 32.5 ± 8.7; non-responders 42.5 ± 5.0; p > 0.05). In fact, at the end of the study, the non-responders were taking a higher dose of citalopram than responders.
Correlations between baseline metabolism and change in HDRS score from pretreatment to week 12 of citalopram treatment
The correlations between baseline metabolism and change in HDRS score from pre-treatment to week 12 of citalopram treatment are shown in Table 2 and Figure 1. Positive correlations (higher baseline metabolism associated with greater reductions in HDRS score with treatment) were observed in the left medial frontal gyrus, right superior temporal gyrus, left fusiform gyrus, and cerebellum (culmen, bilaterally).
Table 2a.
Positive correlations (higher baseline glucose metabolism is associated with greater reductions in HDRS scores during treatment)
| LEFT HEMISPHERE | Brain Region | RIGHT HEMISPHERE | ||||||
|---|---|---|---|---|---|---|---|---|
| Talairach Coordinates | Talairach Coordinates | |||||||
| X (mm) | Y (mm) | Z (mm) | Z-Score | X (mm) | Y (mm) | Z (mm) | Z-Score | |
| −8 | −21 | 61 | 3.48 | Medial Frontal Gyrus | ||||
| Superior Temporal Gyrus(BA 22) | 62 | −18 | 1 | 3.05 | ||||
| −44 | −14 | −23 | 3.93 | Fusiform Gyrus | ||||
| −8 | −46 | −16 | 3.58 | Cerebellum (Culmen) | 20 | −53 | −19 | 3.72 |
Figure 1.
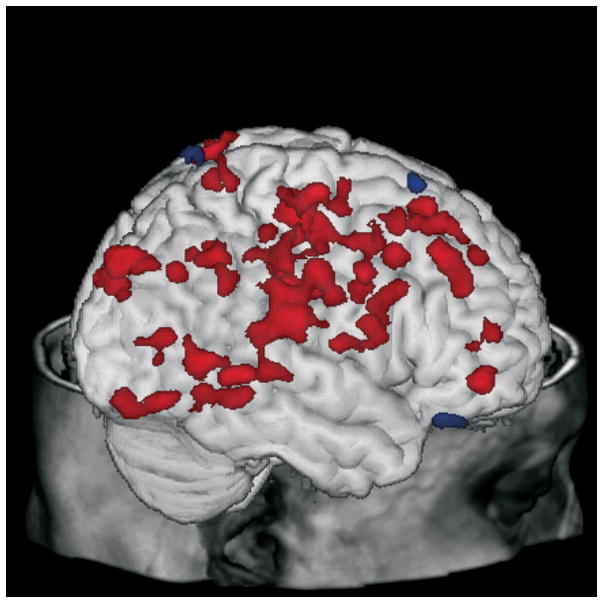
Correlations between lower (green) or higher (red) baseline metabolism and improvement of depressive symptoms displayed on a three-dimensional MR rendering of a representative subject.
Negative correlations (lower baseline metabolism associated with greater reductions in HDRS score with treatment) were observed in the anterior cingulate gyrus (BA 24/32, bilaterally), superior frontal gyrus (bilaterally), middle frontal gyrus (bilaterally), inferior frontal gyrus (bilaterally), precentral gyrus (bilaterally), left superior parietal lobule, inferior parietal lobule (bilaterally), right postcentral gyrus, right precuneus, left cuneus, right parahippocampal gyrus, left insula, caudate (bilaterally), and left putamen.
Correlations between change in metabolism from placebo to acute citalopram administration and change in HDRS score from pretreatment to week 12 of citalopram treatment
The correlations between change in metabolism from placebo to acute citalopram administration and change in HDRS score from pretreatment to week 12 of citalopram treatment are shown in Table 3 and Figure 2. Positive correlations (greater reductions in metabolism from baseline to acute challenge are associated with greater reductions in HDRS score with treatment) were observed in the anterior cingulate gyrus (BA 23/31; bilaterally), right anterior cingulate (BA32), superior and medial frontal gyri (bilaterally), right inferior frontal gyrus, left precentral gyrus, right superior parietal lobule, inferior parietal lobule (bilaterally), postcentral gyrus (bilaterally), left precuneus, right supramarginal gyrus, middle occipital gyrus (bilaterally), cuneus (bilaterally), left lingual gyrus, right fusiform gyrus, left superior temporal gyrus, left middle temporal gyrus, right parahippocampal gyrus, left insula, and culmen (bilaterally). Negative correlations (increases in metabolism from baseline to acute challenge are associated with greater reductions in HDRS score with treatment) were observed in the left middle frontal gyrus.
Table 3a.
Positive correlations (greater reductions in cerebral glucose metabolism following the acute citalopram challenge are associated with greater reductions in HDRS score with citalopram treatment)
| LEFT HEMISPHERE | Brain Region | RIGHT HEMISPHERE | ||||||
|---|---|---|---|---|---|---|---|---|
| Talairach Coordinates | Talairach Coordinates | |||||||
| X (mm) | Y (mm) | Z (mm) | Z-Score | X (mm) | Y (mm) | Z (mm) | Z-Score | |
| −2 | −26 | 32 | 3.49 | Cingulate Gyrus (BA 23/31) | 8 | −24 | 43 | 3.73 |
| Anterior Cingulate (BA32) | 11 | 45 | 1 | 4.2 | ||||
| −15 | 21 | 50 | 4.14 | Superior Frontal Gyrus (BA 6) | 19 | 28 | 54 | 3.85 |
| −2 | 11 | 46 | 3.36 | Medial Frontal Gyrus (BA 6) | 4 | −17 | 58 | 4.35 |
| Inferior Frontal Gyrus | 45 | 3 | 25 | 3.23 | ||||
| −52 | −7 | 45 | 3.18 | Precentral Gyrus (BA 4) | ||||
| Superior Parietal Lobule | 28 | −59 | 53 | 3.37 | ||||
| −38 | −55 | 45 | 3.15 | Inferior Parietal Lobule (BA 40) | 60 | −35 | 39 | 3.21 |
| −42 | −23 | 40 | 3.69 | Postcentral Gyrus (BA 2) | 48 | −27 | 32 | 3.81 |
| −5 | −49 | 57 | 3.29 | Precuneus (BA 7) | ||||
| Supramarginal Gyrus | 59 | −49 | 38 | 3.29 | ||||
| −26 | −80 | 17 | 3.11 | Middle Occipital Gyrus | 44 | −74 | 3 | 3.18 |
| −6 | −84 | 17 | 3.35 | Cuneus (BA 18) | 12 | −81 | 31 | 5.4 |
| −22 | −64 | −5 | 3.72 | Lingual Gyrus | ||||
| Fusiform Gyrus | 29 | −63 | −10 | 3.11 | ||||
| −45 | −59 | 29 | 3.48 | Superior Temporal Gyrus (BA 39) | ||||
| −49 | −62 | 11 | 3.39 | Middle Temporal Gyrus | ||||
| Parahippocampal Gyrus | 22 | −36 | −9 | 3.48 | ||||
| −33 | −23 | 15 | 3.96 | Insula (BA 13) | ||||
| −12 | −52 | −9 | 3.91 | Culmen | 21 | −51 | −9 | 3.3 |
Figure 2.
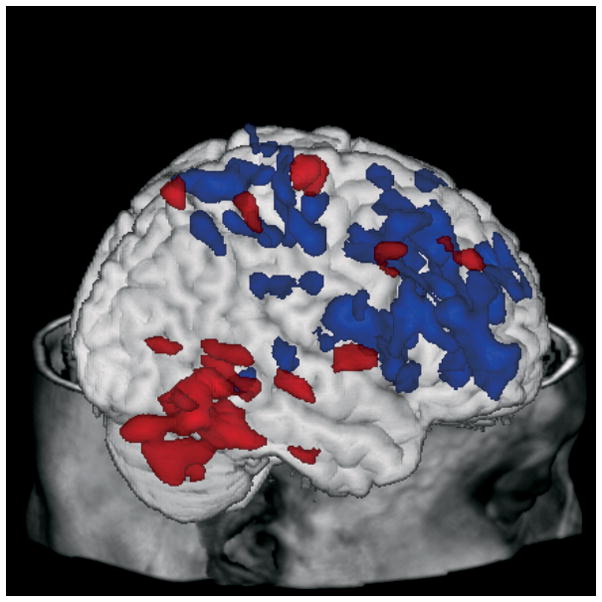
Correlations between increases (red) or decreases (green) in patients following the acute citalopram challenge are associated with the improvement of depressive symptoms displayed on a three-dimensional MR rendering of a representative subject.
While gender differences in the baseline or change in HDRS score with citalopram treatment were not observed, the correlations between the change in metabolism and change in HDRS scores were significantly greater in males than females. Significantly greater positive correlations in males were observed in the right (BA 24) and left (BA 32) anterior cingulate gyrus (right 8 37 −2; 3.03; left −7 36 18; 3.82: Talairach Coordinates; z- score), right medial frontal gyrus (27 26 −27; 3.15), bilateral pre-central gyrus (right 58 1 29 3.01; left −46, 0 49; 3.19), right superior temporal gyrus (BA 22; 66 −28 13; 4.05), right post-central gyrus (22 −29 67; 3.49)and left precuneus (BA 7: −15 −58 55; 3.32). Significantly greater negative correlations in males than females were observed in the left occipital cortex (BA 17; −7 −83 12; 3.44).
CONCLUSIONS
In the present study, greater improvement of depressive symptoms (reductions in HDRS score) with chronic citalopram treatment was associated with lower pre-treatment cerebral glucose metabolism and greater reductions in cerebral glucose metabolism after acute, intravenous citalopram administration. At baseline, higher glucose metabolism in the rostral anterior cingulate (BA 24, 32), frontal and parietal cortices, striatum (caudate, putamen) and limbic/paralimbic regions (insula, parahippocampal gyrus) was associated with less improvement of depressive symptoms with citalopram treatment. This finding is consistent with observations of cortical hypermetabolism in geriatric depressed patients relative to controls and of correlations between higher metabolism and greater severity of depression and anxiety symptoms in the patients prior to treatment (13). Several regions including the left medial frontal gyrus, right superior temporal gyrus, left fusiform gyrus and bilateral cerebellum showed the opposite association (greater improvement associated with higher metabolism), which may represent a compensatory response in these mainly sensory and motor regions for change in other brain regions.
The reductions in cerebral metabolism after acute citalopram administration that were correlated with improvement of depressive symptoms occurred in many of the same regions in which the baseline associations were observed. The notable differences between the baseline and acute citalopram analyses were that the striatal regions showed correlations with baseline metabolism only, whereas more extensive changes in posterior cortical regions were observed in the acute citalopram condition only (e.g. right supramarginal and fusiform gyri, left lingual gyrus, left superior and middle temporal gyri). The correlations between less depressive symptom improvement and higher striatal metabolism in the baseline condition and not the acute citalopram condition suggest that the underlying mechanism may be another neurotransmitter aside from serotonin. Other neurochemical mechanisms including increased glutamate concentrations or structural pathology in fronto-striatal circuitry that might result in an increase by disinhibition of striatal metabolism may be involved (11). The left middle frontal gyrus was the only region that showed the opposite association (increased metabolism was associated with greater improvement of depressive symptoms. This was the only region that showed decreased metabolism in the geriatric depressed patients compared to controls which may explain this opposite finding (13). With respect to gender differences, the male depressed patients showed significant greater correlations between metabolic responses and clinical improvement than females even though the magnitude of clinical improvement did not differ significantly between groups. The greater association between clinical and metabolic responses in males is consistent with reports of greater serotonin metabolism in males than females (23) and may be associated with the greater vulnerability of females than males to depression (24).
In comparing the correlations with treatment response for the acute citalopram study to the earlier study of correlations between the metabolic effects of TSD and paroxetine response (1), similar anterior cortical regions show correlations in both studies. The acute citalopram data show a more extensive brain network of correlations including more posterior cortical regions. This observation may be explained by a greater neurochemical effect of acute citalopram compared to TSD or possibly differences in patient characteristics, although the samples are similar in such variables as magnitude and rate of treatment response. Neurochemical brain imaging studies of the acute effects of intravenous citalopram have shown significant serotonin transporter occupancy in striatum, thalamus, brainstem, amygdala and hippocampus, as well as increases in striatal dopamine concentrations (25,26). Thus, both primary and secondary neurochemical effects of citalopram have been observed in the same time frame as the cerebral metabolic effects observed in the present study. Given the regional distribution of the correlations, the alterations in cerebral metabolism may reflect the effects of serotonin transporter occupancy on cortico-cortico circuits that are likely to be mediated through a glutaminergic mechanism (27, 28). In addition, many of the regions that are “hypermetabolic” at baseline and are affected by citalopram treatment are regions that comprise the “default network” and that demonstrate beta-amyloid deposition in demented and non-demented elderly (29). As increased glutamate activity is observed in amyloid transgenic mouse models and serotonin inhibits cortical glutamate (30,31), a secondary consequence of beta-amyloid deposition and decreased serotonin functional integrity could be glutamate hyperactivity which would increase glucose metabolism (28). Citalopram treatment may decrease metabolism by decreasing glutamate concentrations (31). Thus, correlations in the resting state and after acute citalopram administration provide different functional neuroanatomic and mechanistic information associated with depressive symptom improvement.
The majority of studies that have evaluated baseline or acute cerebral metabolism or neuroreceptor measures relative to clinical treatment outcome have been performed in mid-life depressed patients. There is some evidence, consistent with findings of the present study, that baseline hypermetabolism of between the pregenual and subgenual cingulate cortices (BA 24/32) predicts worse treatment response to venlafaxine or cognitive behavioral therapy (CBT; 32). Lower midbrain metabolism was also associated with better antidepressant treatment response (33). Other studies of SSRI treatment and TSD in mid-life depressed patients have reported opposite findings in rostral anterior cingulate cortex and frontal cortex (34, 35). As described, the findings in older depressed patients may be a secondary consequence of neuropathological processes, in contrast to findings in some studies of mid-life depressed patients (34,35). Magnetic resonance spectroscopy (MRS) studies suggest that bioenergetic abnormalities related to mitochondrial dysfunction may be associated with treatment response. These studies have shown that 1) lower basal ganglia beta nucleoside triphosphate and purine intensities (in females) was associated with better treatment response to fluoxetine (36) and 2) higher baseline phosphocreatine was associated with better treatment response to triiodothyronine (T3) augmentation (37). Serotonin imaging studies have shown that increased 5-HT1A binding in cortical and limbic regions and the raphe nuclei and lower serotonin transporter (5HTT) binding in the anterior cingulate, amygdala and midbrain were associated with poorer SSRI treatment response (38,39). As increased 5-HT1A binding may represent an upregulation due to decreased serotonin concentrations and the lower transporter binding may suggest a loss of serotonin projections, these observation suggest serotonin hypofunction in non-responders. These observations are consistent with the findings of the present study that suggest a blunted metabolic response to acute citalopram is associated with a poorer antidepressant response.
Several issues should be considered in the interpretation of the data from the present study. As described in the methods section, the acute citalopram administration was performed in a fixed order. The chronic citalopram treatment phase was open label and a placebo treated group was not included. It is important to note that the rate of treatment non-response to citalopram in this study (25%) was similar to that of placebo controlled trials (6). Another important consideration is that the HDRS measures the core symptoms of depressed mood, in addition to the vegetative signs of depression, so the correlations represent metabolic alterations associated with the net effect on different aspects of depressive symptomatology.
In summary, lower pretreatment cerebral glucose metabolism in the rostral anterior cingulate, prefrontal cortex, striatum and limbic/paralimbic regions was associated with greater improvement of depressive symptoms in geriatric depressed patients. Furthermore, greater decreases in cerebral glucose metabolism following administration of a serotonergic challenge agent were associated with a greater antidepressant effect of citalopram. The regions affected uniquely in the acute citalopram condition compared to baseline metabolism were temporal, parietal and occipital cortical regions. The anterior cortical findings are consistent with the prior study that showed an association between the cerebral metabolic effects of TSD and the antidepressant response (1). The regional pattern of the acute citalopram effects in cortical and limbic regions includes the targets of serotonergic projections which suggest that the changes in metabolism reflect the functional integrity of the serotonin system or serotonin modulation of glutamate concentrations (40). These regional cerebral metabolic findings are consistent with the results of other studies in geriatric depression that have shown correlations between poorer treatment response and white matter connectivity in similar cortical and limbic pathways (11). The results of the present study suggest that the dynamic response of the brain to an acute increase in serotonin, the cerebral metabolic response to acute citalopram, is indicative of the capacity of the brain to respond to a course of chronic citalopram treatment. Future studies should evaluate the extent to which the acute metabolic and neurochemical changes associated with other pharmacologic classes of antidepressants (e.g. selective noradrenergic reuptake inhibitors), somatic treatments (electroconvulsive therapy, transcranial magnetic stimulation) and psychotherapy are associated with improvement in depressive symptoms to determine whether the early metabolic neurochemical changes represent biomarkers of treatment response. While the integration of neuroimaging studies into clinical trials is logistically challenging, such studies are critical to developing neuroimaging biomarkers of treatment response in psychiatric disorders.
Table 2b.
Negative correlations (Lower baseline glucose metabolism is associated with greater reductions in HDRS scores during treatment)
| LEFT HEMISPHERE | Brain Region | RIGHT HEMISPHERE | ||||||
|---|---|---|---|---|---|---|---|---|
| Talairach Coordinates | Talairach Coordinates | |||||||
| X (mm) | Y (mm) | Z (mm) | Z-Score | X (mm) | Y (mm) | Z (mm) | Z-Score | |
| Cingulate Gyrus (BA 32) | 8 | 12 | 34 | 3.26 | ||||
| −4 | −3 | 42 | 3.2 | Cingulate Gyrus (BA 24) | 15 | −33 | 43 | 3.25 |
| −7 | 29 | 25 | 3.87 | Anterior Cingulate (BA 32) | 9 | 36 | 10 | 3 |
| −13 | 48 | 39 | 3.89 | Superior Frontal Gyrus (BA 8) | 15 | 53 | 33 | 3.3 |
| −29 | 36 | −7 | 3.29 | Middle Frontal Gyrus (BA 47) | 40 | 48 | 16 | 4.41 |
| −45 | 20 | 11 | 4.08 | Inferior Frontal Gyrus (BA 45) | 50 | 19 | 11 | 3.38 |
| −42 | −17 | 39 | 3.54 | Precentral Gyrus (BA 4) | 39 | −21 | 51 | 3.57 |
| −31 | −63 | 46 | 3.77 | Superior Parietal Lobule | ||||
| −35 | −50 | 45 | 3.58 | Inferior Parietal Lobule (BA 40) | 39 | −48 | 55 | 3.37 |
| Postcentral Gyrus (BA 3) | 27 | −30 | 63 | 3.97 | ||||
| Precuneus (BA 7) | 7 | −60 | 60 | 3.22 | ||||
| −20 | −88 | 37 | 3.18 | Cuneus (BA 19) | ||||
| Parahippocampal Gyrus | 26 | −39 | −4 | 3.3 | ||||
| −33 | −15 | 16 | 3.04 | Insula | ||||
| −10 | 17 | −2 | 3.9 | Caudate | 11 | 15 | 0 | 3.39 |
| −23 | 0 | 8 | 4.3 | Putamen | ||||
Table 3b.
Negative correlations (Increases in cerebral glucose metabolism following the acute citalopram challenge are associated with greater reductions in HDRS score with treatment)
| LEFT HEMISPHERE | Brain Region | RIGHT HEMISPHERE | ||||||
|---|---|---|---|---|---|---|---|---|
| Talairach Coordinates | Talairach Coordinates | |||||||
| X (mm) | Y (mm) | Z (mm) | Z-Score | X (mm) | Y (mm) | Z (mm) | Z-Score | |
| −29 | 17 | 53 | 3.45 | Middle Frontal Gyrus | ||||
Acknowledgments
The present study was supported in part by National Institute of Health: MH 01621 (GSS), MH 49936 (GSS), MH 57078 (GSS), MH 64823 (GSS), M01 RR 018535 (Chiorazzi). Citalopram medication was provided by Forest Laboratories. David Bjelke, CNMT and Claude Margouleff, B.S. are gratefully acknowledged for their contribution to the conduct of the PET studies. Bruce G. Pollock, M. D., Ph.D., Margaret Kirshner, B.S., Denise Soriso, B.S. and Kimberly A. Huber, M.P.H., Geriatric Psychopharmacology Laboratory, Department of Psychiatry, University of Pittsburgh School of Medicine are acknowledged for providing the intravenous formulation of citalopram.
References
- 1.Smith GS, Reynolds CF, Houck PR, et al. Glucose metabolic response to total sleep deprivation, recovery sleep, and acute antidepressant treatment as functional neuroanatomic correlates of treatment outcome in geriatric depression. Am J Geriatr Psychiat. 2002;10:561–567. [PubMed] [Google Scholar]
- 2.Lahti AC, Weiler MA, Holcomb HH, et al. Modulation of Limbic Circuitry Predicts Treatment Response to Antipsychotic Medication: A Functional Imaging Study in Schizophrenia. Neuropsychopharm. 2009 doi: 10.1038/npp.2009.94. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolkin A, Barouche F, Wolf AP, et al. Dopamine blockade and clinical response: evidence for two biological subgroups of schizophrenia. Am J Psychiat. 1989;146:905–908. doi: 10.1176/ajp.146.7.905. [DOI] [PubMed] [Google Scholar]
- 4.Meyer JH, Wilson AA, Ginovart N, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiat. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- 5.Smith GS, Gunning-Dixon FM, Lotrich FE, et al. Translational research in late-life mood disorders: Implications for future intervention and prevention research. Neuropsychopharm. 2007;32:1857–1875. doi: 10.1038/sj.npp.1301333. [DOI] [PubMed] [Google Scholar]
- 6.Dew MA, Reynolds CF, Houck PR, et al. Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry. 1997;54:1016–1024. doi: 10.1001/archpsyc.1997.01830230050007. [DOI] [PubMed] [Google Scholar]
- 7.Wu E, Greenberg P, Yang E, et al. Comparison of treatment persistence, hospital utilization and costs among major depressive disorder geriatric patients treated with escitalopram versus other SSRI/SNRI antidepressants. Curr Med Res Opin. 2008;24:2805–2813. doi: 10.1185/03007990802336780. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Kiosses DN, Heo M, et al. Executive dysfunction and the course of geriatric depression. Biol Psychiat. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Gunning FM, Cheng J, Murphy CF, et al. Anterior cingulate cortical volumes and treatment remission of geriatric depression. Int J Geriatr Psychiat. 2009;24:829–836. doi: 10.1002/gps.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen PS, McQuoid DR, Payne ME, et al. White matter and subcortical gray matter lesion volume changes and late-life depression outcome: A 4-year magnetic resonance imaging study. Int Psychogeriatr. 2006;18:445–456. doi: 10.1017/S1041610205002796. [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiat. 2008;165:238–244. doi: 10.1176/appi.ajp.2007.07050744. [DOI] [PubMed] [Google Scholar]
- 12.Murphy GM, Hollander SB, Rodrigues HE, et al. Effects of the serotonin transporter gene promoter polymorphism on mirtazapine and paroxetine efficacy and adverse events in geriatric major depression. Arch Gen Psychiat. 2004;61:1163–1169. doi: 10.1001/archpsyc.61.11.1163. [DOI] [PubMed] [Google Scholar]
- 13.Smith GS, Kramer E, Ma Y, et al. The functional neuroanatomy of geriatric depression. Int J Geriatr Psychiat. 2009;24:798–808. doi: 10.1002/gps.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith GS, Kramer E, Hermann C, et al. Serotonin Modulation of Cerebral Glucose Metabolism in Geriatric Depression. Am J Geriatr Psychiat. 2002;45:105–112. [Google Scholar]
- 15.Smith GS, Kramer E, Hermann C, et al. Serotonin modulation of cerebral glucose metabolism in depressed older adults. Biol Psychiatr. 2009;66:259–266. doi: 10.1016/j.biopsych.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis 1 Disorders-Patient Edition (SCIP-I/P) New York: New York Psychiatric Institute; 1995. [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck AT, Steer RA, Ball R, et al. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 19.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 20.Smith GS, Ma Y, Dhawan V, et al. Serotonin modulation of cerebral glucose metabolism measured with positron emission tomography (PET) in human subjects. Synapse. 2002;45:105–112. doi: 10.1002/syn.10088. [DOI] [PubMed] [Google Scholar]
- 21.Guy W. ECDEU: An assessment manual for psychopharmacology. US Department of Health Education and Welfare Publication (ADM) 1976;76:336. [Google Scholar]
- 22.Friston KJ, Ashburner JT, Kiebel SJ, et al., editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London: Academic Press; 2007. [Google Scholar]
- 23.Sakai Y, Nishikawa M, Leyton M, et al. Cortical trapping of alpha-[(11)C]methyl-l-tryptophan, an index of serotonin synthesis, is lower in females than males. Neuroimage. 2006;33:815–24. doi: 10.1016/j.neuroimage.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Piccinelli M, Wilkinson G. Gender differences in depression. Critical review. Br J Psychiatry. 2000;177:486–92. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- 25.Hinz R, Selvaraj S, Murthy NV, et al. Effects of citalopram infusion on the serotonin transporter binding of [11C]DASB in healthy controls. J Cereb Blood Flow Metab. 2008;28:1478–1490. doi: 10.1038/jcbfm.2008.41. [DOI] [PubMed] [Google Scholar]
- 26.Smith GS, Ma Y, Dhawan V, et al. Selective Serotonin Reuptake Inhibitor (SSRI) modulation of striatal dopamine measured with [11C]-raclopride and positron emission tomography (PET) Synapse. 2009;63:1–6. doi: 10.1002/syn.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maura G, Marcoli M, Tortarolo M, et al. Glutamate release in human cerebral cortex and its modulation by 5-hydroxtryptamine acting at h 5-HT1D receptors. British J Pharmacol. 2009;123:45–50. doi: 10.1038/sj.bjp.0701581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 29.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters O, Schipke CG, Philipps A, et al. Astrocyte Function is Modified by Alzheimer’s Disease-like Pathology in Aged Mice. J Alzheimers Dis. 2009;18:177–89. doi: 10.3233/JAD-2009-1140. [DOI] [PubMed] [Google Scholar]
- 31.Golembiowska K, Dziubina A. Effect of acute and chronic administration of citalopram on glutamate and aspartate release in the rat prefrontal cortex. Polish J Pharmacol. 2000;52:441–8. [PubMed] [Google Scholar]
- 32.Konarski JZ, Kennedy SH, Segal ZV, et al. Predictors of nonresponse to cognitive behavioural therapy or venlafaxine using glucose metabolism in major depressive disorder. J Psychiatry Neurosci. 2009;34:175–180. [PMC free article] [PubMed] [Google Scholar]
- 33.Milak MS, Parsey RV, Lee L, et al. Pretreatment regional brain glucose uptake in the midbrain on PET may predict remission from a major depressive episode after three months of treatment. Psychiatry Res. 2009;173:63–70. doi: 10.1016/j.pscychresns.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 35.Saxena S, Brody AL, Ho ML, et al. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatr. 2003;160:522–532. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- 36.Renshaw PF, Parow AM, Hirashima F, et al. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry. 2001;158:2048–55. doi: 10.1176/appi.ajp.158.12.2048. [DOI] [PubMed] [Google Scholar]
- 37.Iosifescu DV, Bolo NR, Nierenberg AA, et al. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry. 2008;63:1127–34. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 38.Parsey RV, Olvet DM, Oquendo MA, et al. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: Preliminary data from a naturalistic study. Neuropsychopharm. 2006;31:1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- 39.Miller JM, Oquendo MA, Ogden RT, et al. Serotonin transporter binding as a possible predictor of one-year remission in major depressive disorder. J Psychiatr Res. 2008;42:1137–1144. doi: 10.1016/j.jpsychires.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varnäs K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]


