Abstract
Our group previous demonstrated a strong association between elevated plasma soluble CD13 enzyme activity and newly diagnosed extensive chronic graft-versus-host disease (cGVHD) in children. Since cytotoxic anti-CD13 antibodies have been documented after blood and marrow transplant in association with cytomegalovirus infection and cGVHD, we hypothesized soluble CD13 contributes to cGVHD pathogenesis by induction of CD13 reactive antibodies and that anti-CD13 antibodies could be additional biomarkers for newly diagnosed pediatric extensive cGVHD. Using prospectively collected plasma samples from pediatric allogeneic blood and marrow transplant subjects with cGVHD and controls without cGVHD enrolled in a large multi-institution Children's Oncology Group cGVHD therapeutic trial we evaluated whether soluble CD13 correlates with induction of anti-CD13 antibodies. We found that CD13 reactive antibodies are present in a proportion of patients after allogeneic BMT, but did not appear to correlate with the presence of soluble CD13. Anti-CD13 antibodies also did not meet our criteria as a diagnostic biomarker for cGVHD. These data are not able to confirm that induction of CD13 reactive antibodies is a mechanism for cGVHD in children nor are part of the pathogenesis of cGVHD associated with elevated soluble CD13. The exact role of CD13 in cGVHD remains to be determined.
Keywords: anti-CD13 antibodies, CD13, aminopeptidase N, biomarkers, chronic graft-versus-host disease, blood and marrow transplantation
Introduction
Chronic graft-versus-host disease (cGVHD) is a multisystem alloimmune and autoimmune disorder complicating 40-70% of patients following allogeneic blood and marrow transplant (allo-BMT) [1-6]. The Children's Oncology Group (COG) trial ASCT0031 (“Phase III Trial of Hydroxychloroquine plus Standard Therapy for Chronic Graft-Versus-Host Disease”) a newly discovered plasma biomarker, elevated soluble CD13 (aminopeptidase N) in subjects developing cGVHD early after allo-BMT [7, 8]. CD13 is an integral membrane protein with enzymatic activity, ubiquitously expressed on a number of tissue types affected by cGVHD including smooth muscle, endothelial, epithelial, and fibroblast cells [9, 10]. The mechanism by which soluble CD13 enzyme activity becomes elevated in cGVHD is unclear. One mechanistic possibility is that soluble CD13 becomes immunogenic once in the plasma, producing cytotoxic anti-CD13 antibodies that incite further tissue damage. Cytotoxic anti-CD13 antibodies have previously been described in a small series of patients following allo-BMT in association with cGVHD and cytomegalovirus (CMV) infection [11, 12]. Similar cGVHD mechanisms have also been reported for antibodies reactive with H-Y minor antigens [13] and platelet-derived growth factor receptor [14]. We sought to determine whether (1) soluble CD13 was immunogenic after allo-BMT, leading to the production of anti-CD13 antibodies, and (2) whether anti-CD13 antibodies met our previously defined criteria [7] as a biomarker of pediatric extensive cGVHD.
Materials and Methods
Subjects
Peripheral blood samples were collected and evaluated prospectively from subjects enrolled on the COG trial ASCT0031, a phase III randomized, placebo-controlled, double blind trial evaluating two treatment regimens for pediatric subjects with newly diagnosed extensive cGVHD as previously described [7]. Subjects enrolled and treated on ASCT0031 formed the experimental group (n=52). To control for immune reconstitution following allo-BMT, a time matched comparison to the control group (n=28) was performed for the subjects with cGVHD, with days after allo-BMT at the time of cGVHD diagnosis used to divide the experimental group into early onset cGVHD (3-8 months after transplantation) or late onset cGVHD (≥9 months after transplantation) and compared to control BMT patients without cGVHD from 6 (early) and 12 months (late) after BMT. Healthy volunteer blood donor controls (n=6) not undergoing transplantation were evaluated for comparison.
Samples Evaluated
Peripheral blood was collected from subjects with cGVHD at study entry (time of cGVHD diagnosis) and in control subjects collected at 6 and 12 months (after transplantation). The sample was separated into cells and plasma after centrifugation and stored at −80°C. Frozen plasma was defrosted in a waterbath at 37°C and ultracentrifuged (15,000 rpm for 5 minutes) to rid of precipitate when ready for use. Following our previous analysis of biomarkers [7], a minority of subjects did not have adequate volume of plasma remaining for anti-CD13 antibody analysis and were not included with this analysis. Adequate plasma was available for anti-CD13 antibody analysis (45 cGVHD samples and 26 controls by ELISA; 42 cGVHD samples and 23 controls by Cellomics).
Enzymatic Assay of Soluble CD13 (Aminopeptidase N)
Plasma samples were tested for soluble CD13 (Aminopeptidase N) activity as we had previously described [7]. Results of soluble CD13 activity previously reported by our group [7] were used for correlative studies against anti-CD13 antibodies.
ELISA for Anti-CD13
100 μg of purified porcine aminopeptidase N / CD13 (Sigma-Aldrich, 83% amino acid homology with human CD13) was diluted in 10 ml of 0.1 M carbonate-bicarbonate buffer (pH 9.5). One hundred μl was added to each well of a polystyrene flat-bottom ELISA plate, incubated overnight at 4°C, washed three times with Tris buffered saline containing 0.05% Tween 20, pH 7.4. The nonspecific binding of sera proteins was prevented by adding 200 μl of 1.5% bovine serum albumin in PBS followed by 2 hr incubation at room temperature. Plates were washed as above and 100 μl of 1:100 diluted serum samples were added in duplicate and incubated for 2 hours at room temperature. Plates were washed and 100 μl of horseradish peroxidase conjugated goat anti-human polyspecific immunoglobulin (diluted based on manufacturer's recommendation; Sigma-Aldrich) was added to each well. After 1 hr incubation at room temperature, plates were washed with TBS/T five times and 100 μl of the specific substrate, TMB (Sigma-Aldrich) was added. The enzyme reaction was stopped by adding 50 μl of 1M H2SO4 and the optical density (OD) was read at 450 nm with a microtiter reader. To detect nonspecific binding, several control wells containing all reagents except human serum or CD13, in addition to wells containing mouse sera, were used.
Cellomics for Anti-CD13
Samples (30 μL) were diluted 1:5 in PBS (120 μL) and added to 96-well plates containing non-transfected murine hemangioendothelioma endothelial cells (EOMA cell line) and incubated for 2 hours at room temperature to reduce non-specific binding. Plates were centrifuged (800 rpm for 5 minutes) and 100 μL of supernatant harvested. The supernatant was added to 96-well plates containing plasmid transfected EOMA cells expressing human CD13 antigen and incubated for 2 hours at room temperature. Wells were then washed three times with PBS and murine anti-human immunoglobulin (with specificity for human IgG) conjugated to FITC (BDBiosciences, Mississauga, ON, Canada) was added, incubated for 30 minutes, and washed with PBS. Monoclonal antibody WM15 was used as a positive control (1:50 dilution using 2 μL WM15 and 98 μL PBS) and IgG1 mouse isotype monoclonal antibody (Ancell, Bayport, MN, USA) was used as a negative control followed by FITC conjugated anti-mouse IgG1. HOECHST 33342 dye (Sigma-Aldrich) at 1 μg/ml was used to detect EOMA cell DNA. The FITC intensity of each well was read by ArrayScan® Reader 4.5 (Cellomics, Pittsburgh, PA, USA) and the average FITC intensity / cell used as a marker of anti-CD13 antibodies.
Statistical Analysis
As defined previously, anti-CD13 antibodies were considered biologically and clinically important if two criteria were met: (1) 100% higher or 50% lower compared to control (+/-5%) and (2) statistical significance with a p value <0.05 [7]. Descriptive statistics were generated on all data using Prism version 5 for PC (GraphPad Software, San Diego, CA, USA) or SPSS (version 15.0). Significance of observed changes was determined using Student's t-test and 95% confidence intervals of the difference expressed. All p values <0.05 were considered statistically significant.
Results
Subject Characteristics
Subjects were compared for differences in age, sex, donor source, donor type, and presence of acute GVHD and were previously reported [7]. No significant differences were detected except cGVHD was more likely in unrelated donor transplantation (29/51 (57%) vs 8/28 (29%); p= 0.02).
Anti-CD13 Antibodies
Mean anti-CD13 antibodies by ELISA assay were not different comparing early cGVHD (those patients with onset before 9 months) to early controls (no cGVHD at 6 at 9 months and samples collected at 6 months post BMT) (0.40 OD ± 0.28 vs. 0.39 OD ± 0.24 (SD); p=0.2) or late cGVHD (onset ≥9 months after BMT) to late controls (samples collected at 12 months in patients who did not develop cGVHD by 18 months) (0.53 OD ± 0.33 vs. 0.57 OD ± 0.21 (SD); p=0.4) (Figure 1A). Since the ELISA assay used porcine CD13 we hypothesized human anti-CD13 antibodies may not bind to the porcine antigen. Anti-CD13 detection was therefore repeated by Cellomics using plasmid transfected EOMA cells (provided by Dr. Shapiro) expressing human CD13 on the cell surface. Similarly, no difference in mean antibody levels were detected comparing early cGVHD to early controls (1063 average FITC intensity/cell ± 639 vs. 1008 average FITC intensity/cell ± 528 (SD); p=0.19) or late cGVHD to late controls (1367 average FITC intensity/cell ± 620 vs. 1178 average FITC intensity/cell ± 378 (SD); p=0.34) (Figure 1B). As a continuous variable, anti-CD13 antibodies were not different between cGVHD or control groups (no cGVHD) and did not meet our criteria as a biomarker of cGVHD.
Figure 1.
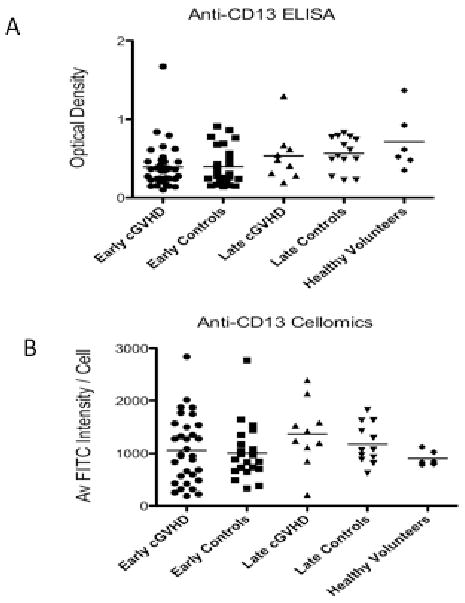
Comparison of anti-CD13 antibodies by (A) ELISA and (B) Cellomics between subject groups (p=NS for all). The ELISA assay used porcine CD13 as the antigen and optical density to measure relative anti-CD13 antibody. The Cellomics assay used human CD13 as the antigen and average FITC intensity / cell to measure relative anti-CD13 antibody.
Some of the samples from both cGVHD and controls in the Cellomics assay had high anti-CD13 levels compared to healthy volunteers (Figure 1B). Using a positive anti-CD13 cut-off as the mean FITC intensity/cell plus 2 standard deviations for the healthy volunteers, we found no significant difference of positive anti-CD13 antibodies between early cGVHD (14/32; 44%) and early controls (5/22; 23%) (difference 21%; 95% CI: -3.5% to +45.6%). Similarly, there was no significant difference in late cGVHD (7/10; 70%) compared to late controls (5/12; 42%) (difference 28%; 95% CI: -11.5% to +68.1%). No association could be found between anti-CD13 antibodies (positive or negative) and past history of acute GVHD, nor any particular site of cGVHD involvement (skin, oral, ocular, gastrointestinal, hepatic, pulmonary, or musculoskeletal) (data not shown).
Correlation of Anti-CD13 Antibodies with sCD13 Activity
To evaluate whether soluble CD13 correlates with induction of anti-CD13 antibody a correlation analysis was performed using Cellomics data and soluble CD13 enzyme activity. There was no correlation with soluble CD13 enzyme activity and anti-CD13 antibody (Figure 2. r values: 0.03, -0.06, -0.34, 0.15 for early cGVHD, early controls, late cGVHD, late controls, respectively.).
Figure 2.
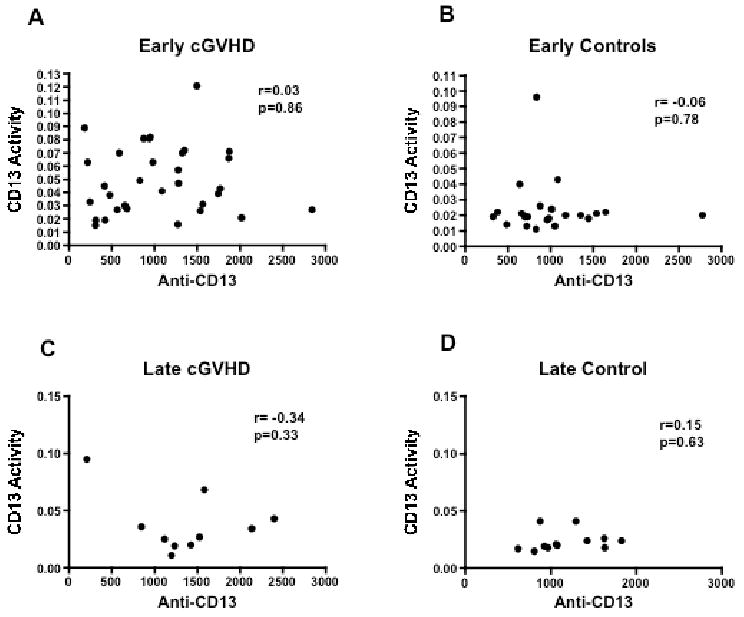
Comparison of anti-CD13 antibody levels by Cellomics with sCD13 activity for subjects with (A) early-onset cGVHD (B) allo-BMT but no cGVHD (blood taken at 6 months after BMT; early controls) (C) late-onset cGVHD (D) allo-BMT but no cGVHD (blood taken 12 months after BMT; late controls).
Discussion
Having recently shown that soluble CD13 is strongly correlated with newly diagnosed extensive cGVHD in children [7], combined with the previously identified association of anti-CD13 antibodies with CMV infection [11] and cGVHD [12] following blood and marrow transplantation, we attempted to determine whether soluble CD13 induced CD13 reactive antibodies as a mechanism underlying cGVHD. Our analysis demonstrates soluble CD13 does not contribute to cGVHD pathogenesis through the induction of CD13 reactive antibodies nor are anti-CD13 antibodies are biomarkers for extensive cGVHD.
Our prevalence of anti-CD13 antibodies (23-70%) was consistent with previous reports of anti-CD13 antibodies (45%) after BMT [11]. In this previous study, anti-CD13 antibodies were found only with CMV viremia or disease. Data on the CMV donor / recipient serostatus and CMV infection patterns after BMT in our study was not collected, making it impossible to evaluate the relationship of anti-CD13 to CMV infection [15]. Anti-CD13 neutralizes CMV infection by interacting with virion-associated CD13 [16] and these antibodies appears with CMV detection [11]. Therefore, the presence of anti-CD13 antibodies may be related to CMV reactivation after allo-BMT as opposed to cGVHD.
The mechanism of elevated soluble CD13 in cGVHD remains unclear. Membrane bound CD13 is extensively expressed on multiple tissue types including those affected by cGVHD [9, 10]. Soluble CD13 may be a non-specific byproduct of intense tissue inflammation. An alternative is that Aminopeptidase N is co-expressed with major histocompatibility class II (MHC II) molecules on a number of professional antigen presenting cells [17] and the NH2-terminal sequence pattern of MHC class II bound peptides may affect in vivo antigen processing [18].
In conclusion we demonstrate that soluble CD13 in cGVHD does not induce anti-CD13 antibodies, and although antibodies are present in some subjects after allo-BMT. The mechanism of CD13 in cGVHD pathogenesis remains unclear but may involve mediation of inflammatory responses by T cells.
Acknowledgments
The authors would like to thank Ruth Milner for her expert statistical help and Dr. Kaiji Hu for assistance with Cellomics.
This work was supported by grant RO1-CA84137 from the National Cancer Institute of the National Institutes of Health and by the Canadian Institutes of Health Research/Wyeth Clinical Research Chair in Transplantation.
Financial Support: Supported by: Chair's U10 CA98543, R01-CA84137 and CIHR/Wyeth Clinical Research Chair in Transplantation.
References
- 1.Remberger M, Beelen DW, Fauser A, Basara N, Basu O, Ringden O. Increased risk of extensive chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation using unrelated donors. Blood. 2005;105:548–551. doi: 10.1182/blood-2004-03-1000. [DOI] [PubMed] [Google Scholar]
- 2.Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 3.Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versushost disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 4.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 5.Fraser CJ, Bhatia S, Ness K, Carter A, Francisco L, Arora M, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108:2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Przepiorka D, Anderlini P, Saliba R, Cleary K, Mehra R, Khouri I, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 2001;98:1695–1700. doi: 10.1182/blood.v98.6.1695. [DOI] [PubMed] [Google Scholar]
- 7.Fujii H, Cuvelier G, She K, Aslanian S, Shimizu H, Kariminia A, et al. Biomarkers in newly diagnosed pediatric extensive chronic graft-versus-host disease: A report from the Children's Oncology Group. Blood. 2008;111:3276–85. doi: 10.1182/blood-2007-08-106286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.She K, Gilman AL, Aslanian S, Shimizu H, Krailo M, Chen Z, et al. Altered toll-like receptor 9 responses in circulating B cells at the onset of extensive chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:386–97. doi: 10.1016/j.bbmt.2006.12.441. [DOI] [PubMed] [Google Scholar]
- 9.Luan Y, Xu W. The structure and main functions of aminopeptidase N. Curr Med Chem. 2007;14:639–47. doi: 10.2174/092986707780059571. [DOI] [PubMed] [Google Scholar]
- 10.Dixon J, Kaklamanis L, Turley H, Hickson ID, Leek RD, Harris AL, et al. Expression of aminopeptidase-n (CD13) in normal tissues and malignant neoplasms of epithelial and lymphoid origin. J Clin Pathol. 1994;47:43–47. doi: 10.1136/jcp.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soderberg C, Sumitran-Karuppan S, Ljungman P, Moller E. CD13-specific autoimmunity in cytomegalovirus-infected immunocompromised patients. Transplantation. 1996;61:594–600. doi: 10.1097/00007890-199602270-00014. [DOI] [PubMed] [Google Scholar]
- 12.Soderberg C, Larsson S, Rozell B, Sumitran-Karuppan S, Ljungman P, Moller E. Cytomegalovirus-induced CD13 specific autoimmunity – a possible cause of chronic graft-versus-host disease. Transplantation. 1996;61:600–9. doi: 10.1097/00007890-199602270-00015. [DOI] [PubMed] [Google Scholar]
- 13.Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svegliati S, Olivieri A, Campelli N, Luchetti M, Poloni A, Trappolini S, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110:237–241. doi: 10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- 15.Soderberg C, Giugni TD, Zaia JA, Larsson S, Wahlberg JM, Moller E. CD13 (human aminopeptidase N) mediates human cytomegalovirus infection. J Virol. 1993;67:6576–85. doi: 10.1128/jvi.67.11.6576-6585.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giugni TD, Soderberg C, Ham DJ, Bautista RM, Hedlund KO, Moller E, et al. Neutralization of human cytomegalovirus by human CD13-specific antibodies. J Infect Dis. 1996;173:1062–1071. doi: 10.1093/infdis/173.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen AS, Noren O, Sjostrom H, Werdelin O. A mouse aminopeptidase N is a marker for antigen presenting cells and appears to be co-expressed with major histocompatibility complex class II molecules. Eur J Immunol. 1993;23:2358–64. doi: 10.1002/eji.1830230946. [DOI] [PubMed] [Google Scholar]
- 18.Falk K, Rotzschke O, Stavanovic S, Jung G, Rammensee HG. Pool sequencing of natural HLA-DR, DQ and DP ligands reveal detailed peptide motifs, constraints of processing, and general rules. Immunogenetics. 1994;39:230–42. doi: 10.1007/BF00188785. [DOI] [PubMed] [Google Scholar]


