Abstract
Objective
The primary objective of this study was to examine the potential interaction between sphingosine-1-phosphate (S1P), a pleiotropic lipid mediator, and CTGF/CCN2 a secreted multimodular protein, in the process of endothelial cell migration. The second objective was to determine whether C- and N-terminal domains of CTGF/CCN2 have specific function in cell migration.
Materials and Methods
Migration of human dermal microvascular endothelial cells (HDMECs) was examined in monolayer wound healing “scratch” assay, while capillary-like tube formation was examined in 3 dimensional collagen co-culture assays.
Results
We observed that S1P stimulates HDMECs migration concomitant with upregulation of CTGF/CCN2 expression. Furthermore, the blockade of endogenous CTGF/CCN2 via siRNA abrogated S1P induced HDMECs migration and capillary-like tube formation. Full length CTGF induced cell migration and capillary-like tube formation with potency similar to that of S1P, while C-terminal domain of CTGF was slightly less effective. However; N-terminal domain had only a residual activity in inducing capillary-like tube formation.
Conclusions
This study revealed that CTGF/CCN2 is required for the S1P induced endothelial cell migration, which suggests that CTGF/CCN2 may be an important mediator of S1P induced physiological and pathological angiogenesis. Moreover, this study shows that the pro-migratory activity of CTGF/CCN2 is located in the C-terminal domain.
Keywords: HDMECs, S1P, CTGF/CCN2, N-terminus of CTGF, C-terminus of CTGF
INTRODUCTION
Angiogenesis is a process of new vessel formation from preexisting capillaries or venules. It is a multi-stage process where the principle cell type, endothelial cells, are required to undergo proliferation, migration, differentiation, and coalescence assembly into cord-like structures with a central lumen (8). The molecular and cellular mechanisms that regulate these processes are not fully understood. Sphingosine-1-phosphate (S1P) is a pleiotropic bioactive lipid released by activated platelets, known to exhibit many biological activities in different cell types (42). S1P through interactions with G-protein-coupled receptors (GPCR) termed S1P1-6 receptors (formerly known as Edg receptors) regulates functions of endothelial and smooth muscle cells. The S1P1 knockout mice reveal lack of peri-endothelial support cells, which results in weakened vasculature with disrupted and leaky vessels leading to massive hemorrhage, edema followed by embryonic death (1, 2, 35). Furthermore, S1P has been shown to play an important role in tumorigenesis (9). Visentin and collaborators demonstrated that inhibition of S1P using anti-S1P mAb reduced tumor cell proliferation and invasion, and diminished tumor-associated angiogenesis, thus validating S1P as a target for therapy (51). In addition, in vitro studies support the role of S1P as a potent inducer of proliferation, migration and survival of endothelial cells (29, 39). S1P has also been shown to stimulate endothelial tube/lumen formation in 3D collagen and fibrin matrices through the integrin-dependent pathway (5), and in matrigel through the Ras/Raf1-dependent ERK signaling (37).
It has been reported that S1P induces expression of other proangiogenic factors such as IL-8, Ang-2 and Connective Tissue Growth Factor (CTGF/CCN2) (27, 28, 46). CTGF/CCN2, a member of the CCN family of multifunctional factors, has been shown to play a role in developmental regulation of chondrogenesis, osteogenesis, and angiogenesis, and in pathologcal processes, including, fibrosis, and tumorigenesis (7, 10, 44, 47). This diverse spectrum of biological properties of CTGF has been attributed to its characteristic structure encompassing four functional modules. The N domain contains two modules, the first one homologous to the IGF binding protein and the second containing von Willebrand-like motif. The C domain that is separated from the N domain by a hinge region, is comprised of a module containing thrombospondin type 1 repeats and a carboxy terminal (CT) module (14). Hinge region can be cleaved by a number of proteases, while elastase and plasmin have also been shown to cleave the individual modules. Full lengths CTGF, as well as the clipped modules, are present in cultured cells and in human extracellular fluids including plasma, skin interstitial fluid, peritoneal cavity fluid, and vitreous fluid (15, 23, 31, 53). Although a number of studies support a proangiogenic function of CTGF/CCN2, the specific role played by this factor during physiological and pathological angiogenesis, has not been defined. It has been reported that application of rCTGF in collagen pellets induces neovascularization in the backs of mice in vivo (48). Studies using cultured human umbilical vein endothelial cells (HUVEC) have demonstrated that CTGF enhances production of the ECM-degrading Matrix Metalloproteinases (MMPs), which facilitates cell migration toward angiogenic stimulus (7, 32). It has also been reported that CTGF promotes cell adhesion and increases proliferation in choroidal endothelial cells (20). Furthermore, it has been shown that Fisp12 (a mouse ortholog of CTGF) stimulates adhesion and migration of human dermal microvascular endothelial cells (HDMVECs) through αvβ3 integrin dependent pathway (3).
Given that both S1P and CTGF/CCN2 have been shown to regulate process of angiogenesis, this study was undertaken to elucidate the involvement of CTGF/CCN2 in mediating angiogenic effect of S1P. In addition, since preteolytically cleaved CTGF/CCN2 fragments have been reported to possess biological activity, we compared the angiogenic function of full length molecule and its N and C domains in two representative in vitro angiogenic assays, cell migration and capillary-like structure formation. We show, for the first time, that in HDMVECs CTGF/CCN2, and in particular its C domain, is required for the promigatory function of S1P.
MATERIALS AND METHODS
Cell culture
Human dermal microvascular endothelial cells (HDMECs) were isolated from foreskins as previously described (45). The cells were cultured on collagen type I - coated flasks in the presence of endothelial cell growth medium EBM2-MV with supplements (Cambrex) and incubated at 37°C with humidified 95% air/ 5% CO2. Human dermal fibroblast culture was established from foreskins of healthy newborns from the Medical University of South Carolina Hospital in compliance with the Institutional Review Board for Human Studies.
Adenoviral constructs
Adenoviral vectors expressing CTGF and green fluorescent protein (GFP) were generated using published protocol (21) as previously described (17). An adenovirus expressing GFP alone (AdGFP) was generated via the same method for use as a control vector.
siRNA CTGF target sequences were as following: CCAAGCCTATCAAGTTTGA (787 in CDS), CCCAGACCCAACTATGATT (567 in CDS). Annealed double stranded oligonucleotides were cloned into Mlu1 and Xho 1 sites of pRNAT-H1.1 shuttle vector (Genescript). The shuttle vector with target siRNA sequence was linearized with Pme1 and then electroporated into BJ5183-AD-1 (Stratagene) to generate recombinant AdenoEasy vector. The recombinant Adenoeasy plasmid after linearization with Pac1 enzyme was transfected into QBI-293 cells using Transfectin (Bio-Rad) for generation of adenovirus. The shuttle vector plasmid and AdenoEasy vector plasmid were sequenced to confirm the cloning. The primary adenoviral stock was then amplified and concentrated by Cesium chloride density gradient centrifugation. The sequence of Scrambled siRNA used as a non-silencing control was as following: 5′ TTCTCCGAACGTGTCACGT 3′ and was prepared in the same manner (40).
To generate N- and C-terminal fragments of CTGF, the full-length CTGF cDNA cloned into pRC-CMV vector was released using ApaI and Hind III enzymes. The cDNA was then digested with Afl III enzyme. The N terminal and C terminal fragments was blunted with Klenow enzyme and then cloned into EcoRV site of pCDNA 3.1 Vector. The following primers were used to clone a signal sequence in frame upstream of C-terminal fragment by PCR from the full length CTGF cDNA - For 5′ TTGGATCCCGCAGTGCCAACCATGACC 3′ & Rev 5′ CATGAATCTTCTGGCCGACGGCCGG 3′. The signal sequence was then ligated into Kpn 1 and Eco R1 sites of the pcDNA vector carrying the C terminal fragment using. The stop codon in the C terminal region was modified using Quick change site directed mutagenesis kit (Qiagen) using the following primers – Forward 5′ CGGAGACATGGCATCGAGCCAGAGAGTGAGAG 3′ and Reverse 5′ CTCTCACTCTCTGGCTCGATGCCATGTCTCCG 3′. A Myc Tag was cloned inframe down stream of N-terminal or C-terminal sequences into the Xho 1 and Xba I sites of the vector. The N and C-terminal vectors were sequenced to check the integrity of sequence and in frame alignment of the Myc tag. To generate adenovirus for N and C terminal fragments, the vectors were digested using the enzymes Kpn I and Xba I to release the CTGF fragments along with the Myc tag from the pcDNA 3.1 and were then cloned into the pAdtrack shuttle vector. Adenovirus was then generated as described earlier.
Quantitative real-time RT-PCR
Total RNA was isolated using the guanidinium thiocyanate-phenol-chloroform method (11). Real-time PCR assays were performed using MyiQTMSingle-Color Real-Time PCR Detection System (Bio-Rad iCycler). Briefly, 5μg of total RNA was reverse transcribed with random hexamers using Transcriptor First Strand cDNA Synthesis Kit (Roche) according to manufacturer's protocol. Amplification mixture (20μl) contained 0.125mg of cDNA, 0.25mM of primers and 10 ml of iQTMSYBR Green Supermix (Bio-Rad). Amplification was for 95°C for 3 min, followed by a 40 cycles of 95°C for 30s, 58°C for 1 min 30s and 55°C for 30s. All samples were analyzed for B2MG expression in parallel in the same run. Results of real-time PCR data were represented as Ct values, where Ct was defined as the threshold cycle of PCR at which amplified product was first detected. To compare the different samples in an experiment, RNA expression in samples were compared to that of the control in each experiment. The following primers were used:
CTGF-F 5′-TTGCGAAGCTGACCTGGAAGAGAA-3′,
CTGF-R-5′-AGCTCGGTATGTCTTCATGCTGGT-3′
B2MG-F 5′-GCCGTGTGAACCATGTGACTTT-3′
B2MG-R 5′-CCAAATGCGGCATCTTCAAA-3′
PCR was performed as a triplicate for each sample.
Western blot analysis
Confluent HDMECs were lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris HCl [pH 8.0], 150 mM NaCl, 0.02% sodium azide, 0.1% sodium dodecyl sulfate [SDS], 1% Nonidet P40, 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride). Protein concentration was quantified using the BCA Protein Assay kit (Pierce, Rockford, IL). Twenty five micrograms of protein was separated via SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA) which was then blocked at room temperature using 2% gelatine/Tris buffered saline-Tween (TBST) for 1 hour. The blots were probed overnight with a 1:1,000 dilution of primary antibody directed against the CTGF, MMP1, MMP9 (Santa Cruz Biotechnology. Inc), or anti-Myc (Sigma) in 1% gelatin/TBST at RT. Following washes with TBST, blots were incubated with appropriate horseradish peroxidase-conjugated secondary antibody and developed with ECL kit (Pierce). As a control for equal protein loading, membranes were stripped and reprobed for β-actin using a monoclonal antibody to β-actin (Sigma).
Three-dimensional (3D) cord formation assay
3D cord formation assay was performed as we previously described (50) with few modifications. Confluent monolayer of HDMECs (80%) was transduced with AdCTGF, AdC-terminal CTGF, and AdN-terminal CTGF at MOI 20. Control cells were transduced with adenoviral vector carrying GFP cassette only (Go). Next day cells were overlaid with bovine collagen I solution (PureCol, Inamed). Gel overlay was prepared as follows: 1 ml chilled collagen was mixed with 125μl 10xPBS, 125 μl 0.1 NaOH and 25 μl of 0.1 HCl. Then, the collagen I neutralized solution was mixed with Growth factor reduced Matrigel (BD Biosciences) with ratio 3:1. After gel polymerization (an average thickness 1mm), 5×103 skin fibroblasts were mixed with the neutralized collagen I gel solution and plated over the acellular 1mm layer followed by incubation for the next 24 to 48 hr in the EBM2 medium containing 0.5% serum with or without the addition of S1P (Avanti Polar Lipids, Inc.). Capillary like-structure formation was examined microscopically. The endothelial cells were visualized under fluorescence microscope since they were transduced with adenoviruses expressing green fluorescence protein (GFP). The total length of the cords visible on five different photographs from one well was measured by tracing the paths of each capillary-like cords using Spot Advanced Image software. The nuclei of cells were stained with DAPI and number of cells was counted.
Cell migration wound healing (“scratch”) assay
HDMECs were plated on collagen type I coated six-well plates. After transduction with adenoviruses (Go, full length CTGF, N-terminus CTGF, C-terminus CTGF) or treated with rhCTGF (EMP Genetech, Germany) cells were cultured to confluence in EBM-2 medium containing 5% serum. 8 hours later scratches were made and floating cells were removed by PBS washing. Incubation was continued for 24-72 hr in EBM2 medium containing 0.5% serum with addition of Mitomycin C (10μg/ml) (Sigma) to prevent cell proliferation. Migration rate was calculated by counting cells that cross into “scratch” area and data represent a percentage of total cells on identical “nonscratch” areas (100%).
Transwell assay
24-multiwell plate with cell culture HTS FluoroBlok Inserts (BD Falcon,Inc) were used in this assay. At 90% confluence dermal fibroblasts seeded on the bottom wells (4×104 cells per well) were transduced with AdGo, AdCTGF, Ad-terminal CTGF, AdC-terminal CTGF or treated with S1P in 5% DMEM. Next day, the medium was removed and replaced with EBM2 medium containing 0.5% serum and 2×104 HDMECs were plated on the cell culture inserts. 24 hr later inserts were removed and the top surface was cleaned with swab; the migrated cells attached to the bottom surface of the membrane were stained with DAPI for 5 min at RT and counted in five fields of view under the microscope.
Cell proliferation assay
Cell proliferation analysis was performed using Cell Proliferation
BrdU incorporation assay (Millipore, INC), according to the manufacturer's protocol. Briefly, HDMECs (2×104/well) were cultured in 96-well plates in EBM2 supplemented with 5% serum. After 12 hours the medium was changed to EBM2 supplemented with 0.5% serum and the cells were transduced with AdGo, AdCTGF, AdCT-CTGF and AdNT-CTGF adenoviruses. After 12 hours, BrdU reagent was added and cells were incubated for another 5 hours under standard culture condition. Then the cells were fixed, washed 3 times and incubated with anti-BrdU antibody followed by addition of goat anti-mouse IgG-peroxidase conjugated secondary antibody. After additional washing TMB peroxidase substrate, stop solution was added and the amount of BrdU was determined at 450nm directly from 96-well assay plates.
Statistical analysis
Results were compared using Students' unpaired t test. Asterisk symbol indicate statistically significant values: * p<0.05.
RESULTS
S1P induces CTGF in HDMECs
Previous studies have shown that S1P up-regulates CTGF in several cell types, including endothelial cells from large vessels (38). Treatment of HDMECs with S1P resulted in a dose dependent increase in CTGF mRNA and protein levels (Fig. 1A and B) with maximal stimulation obtained with 500nM S1P (2.3 fold in mRNA level) in comparison to untreated control cells. At the protein level, CTGF expression was also increased with higher dose of S1P (500nM) (Fig. 1B andC) and this amount was comparable to CTGF expression level in cells transduced with AdCTGF at MOI 5. To study the role of CTGF in S1P effects in HDMECs, we have generated adenoviral vector carrying CTGF-specific silencing siRNA. Transduction of HDMECs with 10 and 20 MOI of AdCTGF siRNA resulted in dose dependent decrease (80-85%) of CTGF mRNA basal level (Fig. 1D) compared to non-silencing siRNA (NS) control. Furthermore, the increase in CTGF mRNA after S1P treatment was also reduced by the CTGF siRNA. Similar results were observed at the protein level. S1P induced CTGF expression decreased to the basal level upon AdCTGF siRNA treatment (Fig. 1E and F).
Figure 1. S1P induces CTGF mRNA and protein levels in HDMECs.
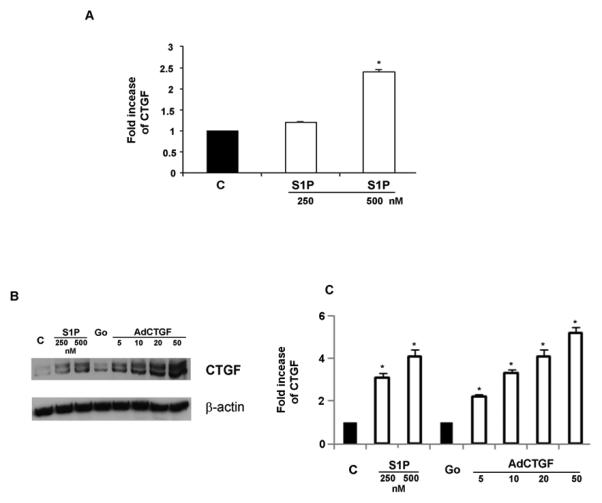
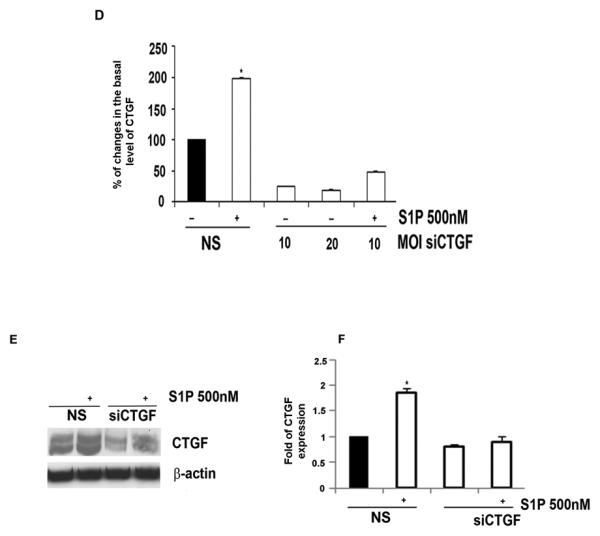
(A) Total RNA was isolated from control and S1P treated HDMECs and analyzed by Real-Time PCR. Values represent the mean ± SEM of three independent experiments done in duplicate. (B) HDMECs were treated with two different doses of S1P or transduced with a different MOI of AdCTGF and control AdGo for 24 hrs. Western blot analysis showed increased protein level of CTGF parallel with increased concentration of S1P as compared to untreated cells. β-actin was used as a control for equal loading. (C) Graphical representation of CTGF expression presented in (B). (D) Endogenous CTGF was inhibited by transduction of HDMECs with AdCTGF siRNA for 24 hrs with or without addition of S1P. Graph represents the percentage of changes in the basal level of CTGF under various experimental conditions. (E) HDMECs were transduced with non-silencing (NS) or AdCTGF siRNA for 24 hrs without or with addition of S1P. CTGF protein level was determined by Western blot analysis. (F) Graphical representation of CTGF protein expression determined by Western blot analysis presented in (E). The values represent the mean ± SEM of three independent experiment done in duplicate.* Significant values at p<0.05
C-terminal of CTGF induces migration and proliferation of HDMECs
Formation of new vessels from preexisting one is conditioned by obligatory remodeling mediated by MMPs such as MMP1 and MMP9. We therefore, in the present study investigated the effect of CTGF on MMPs expression. As shown in Fig. 2A, B CTGF significantly induced the expression levels of MMP1 whereas there was no significant effect on MMP9 levels. In addition, when C-terminal domain of CTGF was overexpressed, we observed an increase in MMP1 levels whereas we observed suppression of MMP1 levels upon CTGF N-terminal overexpression. Furthermore, we did not observe any change in MMP9 levels when any CTGF domain was overexpressed (Fig.2A, B). Taken together these results suggest that CTGF may contribute to angiogenesis by inducing MMP1 levels via its C-terminal domain.
Figure 2. Full length and C-terminal domain of CTGF induce migration and proliferation of HDMECs.
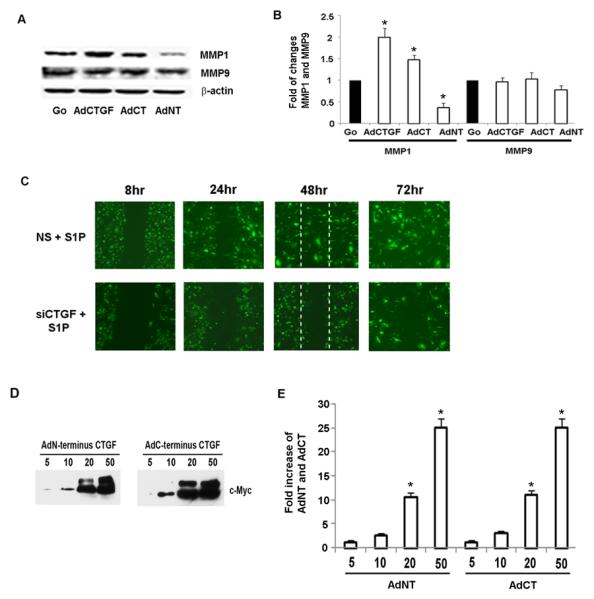
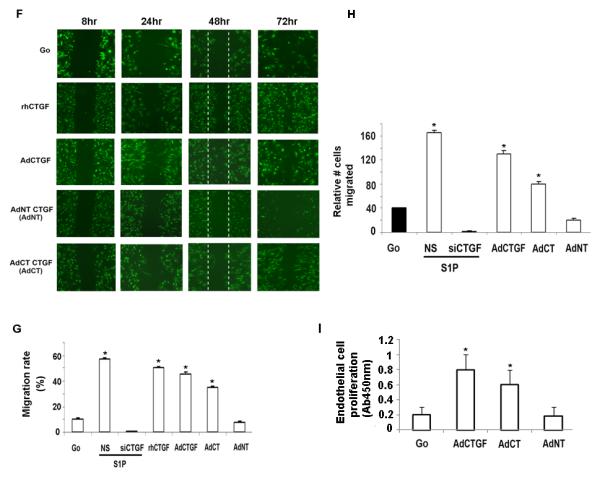
(A) HDMECs were transduced with adenoviruses Go, CTGF,CT and NT of CTGF at MOI10 for 24hrs; 25 μg of total proteins were separated via SDS-PAGE for MMP1, MMP9 and transferred to a nitrocellulose membrane. The blots were probed overnight with primary Abs at 4°C. As a control for equal protein loading, membranes were stripped and reprobed for β-actin. (B) Graphical representation of MMP1 and MMP9 protein expression determined by Western blot analysis presented in (A). The values represent the mean ± SEM of three independent experiment done in duplicate. *Significant values at p<0.05 (C) HDMECs were transduced with non silencing (NS) or AdCTGF siRNA with addition of S1P. Blocking endogenous CTGF inhibited S1P stimulated cells migration. (D) HDMECs were transduced with increasing doses of AdN-terminal (AdNT) and AdC-terminal (AdCT) domain of CTGF and expression level was determined by Western Blot analysis. (E) Graphical presentation of AdNT and AdCT expression level presented in (D). (F) HDMECs were cultured to 80% confluence in full medium followed by transduction with adenoviruses (AdGo, AdCTGF, AdNT and AdCT) or treated with rhCTGF. After 8 hrs cells were mechanically “wounded” by scraping with a Fisher-brand ready-tip. For the next 24-72 hrs cells were kept in EBM2 medium containing 0.5%serum. Representative images are presented. (G) The bar graph represents migration rate expressed as percentage of cells that crossed into the “scratch” area in comparison to identical “nonscratch” area. Cell counting was performed 48 hr after injury. The experiments were repeated three times in duplicate. (H) Graphical presentation of the transwell co-culture migration assay. Briefly, dermal fibroblasts were transduced with indicated adenoviruses. Next day, the medium was changed and HDMECs were plated on the cell culture inserts. 24 hr later the inserts were removed and cells were counted as described in the Methods section. (I) Bar graph presents endothelial cell proliferation increased after cells transduction with AdCTGF and AdCT. The values represent the mean ± SEM of three independent experiment done in duplicate.
* Significant values at p<0.05
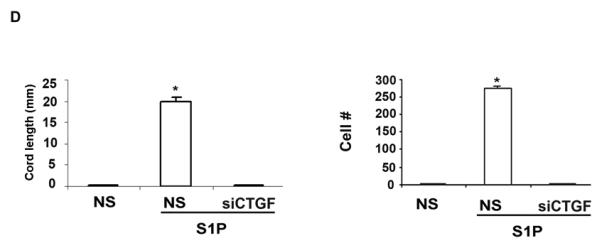
Furthermore, we sought to determine whether CTGF is involved in mediating migration of HDMECs in response to S1P using wound healing “scratch” assay. This assay measures cells migration toward the injured sites resulting in the closure of the wound/scratch. Confluent monolayers of endothelial cells were transduced with AdCTGF siRNA or control (NS) adenoviruses and treated with S1P. Pictures were taken at the indicated time points and representative images are shown in Fig. 2C. We observed that blockade of endogenous CTGF abrogated S1P-induced endothelial cell migration after 48 hours. These results suggest that endogenous CTGF is required for the promigratory effects of S1P in HDMECs.
It has been previously shown that CTGF promotes endothelial cell migration and proliferation (7), but the role of the individual domains in these processes had not been reported. Therefore, to further investigate the role of CTGF in the process of angiogenesis in vitro, we have generated adenoviral vectors expressing Myc tagged N- and C-terminal domains of CTGF. Figure 2D shows the expression of N and C-terminal domains of CTGF after transduction with adenovirus at increasing MOI. The optimal dose 20 MOI of virus was used in the next series of experiments. Figure 2E is a graphical presentation of Western blot data from Fig.2D. Furthermore, we compared the effects of full-length CTGF with the effects of the individual domains on HDMEC migration in “scratch” assay. Pictures were taken at the indicated time points and representative images are shown in Fig. 2F. We observed that rhCTGF treated cells or cells transduced with either AdCTGF, or AdCT-CTGF started to migrate into the scratch ~ 24 hr after the scratch was created (Fig. 2F). The migration rate of HDMECs at 48 hr reached 50% and 47% after treatment with rhCTGF or transduction with AdCTGF, respectively (Fig. 2G). AdCT-CTGF also significantly increased the migration rate (35%) as compared to the cells transduced with AdN-CTGF (7%) (Fig.2G). These results suggest that the production of CTGF facilitates HDMEC migration and that the C-terminal domain of CTGF may be primarily responsible for this effect.
It is known that CTGF and its fragments are present in biological fluids, therefore we examined the effects of exogenously produced CTGF and its domains on HDMEC migration using transwell assay as described in the Methods section. Relative number of migrated cells is presented in Fig. 2H. Interestingly, in fibroblasts treated with S1P, CTGF siRNA completely inhibited promigratory effect suggesting that CTGF produced by fibroblasts in response to S1P is a primary chemoattractant for endothelial cells. In comparison to full-length CTGF, CT-CTGF stimulated cell migration with less potency, whereas NT-CTGF had no effect. These results suggest that CTGF and its C-terminal domain stimulate endothelial cell migration through autocrine and paracrine mechanisms. Furthermore, we examine endothelial cell proliferation after transduction with AdCTGF, AdCT-CTGF or AdNT-CTGF. We observed that full- length CTGF as well as C-terminal of CTGF significantly increased cell proliferation ~4 and ~3 fold respectively within 24hours. N-terminal alone had no effect on endothelial cell proliferation (Fig.2I).
CTGF mediates S1P-induced capillary-like cord formation
Previous reports showed that S1P stimulates formation of capillary-like cords in two-dimensional matrigel angiogenesis model in endothelial cells (34). In the present study we adopted a three-dimensional cord like-formation assay to study the effects of S1P and CTGF on capillary-like structure formation in HDMECs. As shown in Fig. 3A and quantified in Fig. 3B in the presence of S1P, HDMECs formed truncated capillary-like cords, which were multicellular (270 ± 13 cells/5fields, p<0.05), while the untreated cells deteriorated at this time. The results were similar in the presence of VEGF (298 ± 15 cells/5fields, p<0.05), a known inducer of cords in endothelial cells. The cord length was also comparable between S1P and VEGF (22 ± 2 vs 27 ± 1.1 mm/5 fieds, p<0.05 ). We next examined whether CTGF is involved in the S1P induced capillary-like cord formation. Endogenous CTGF was depleted in HDMECs using AdCTGF siRNA followed by treatment with S1P. We found that suppression of CTGF abrogated S1P-induced cord formation (Fig. 3C and D) suggesting that CTGF is required for the cord formation function of S1P.
Figure 3. AdCTGF siRNA abrogates S1P-induced cord formation.
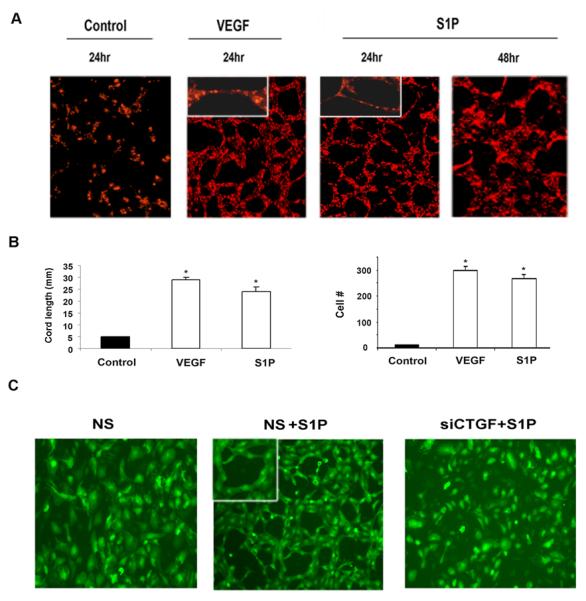
(A) At 80% confluency, HDMECs were overlaid with collagen and incubated in 0.5% EBM2 and, where indicated, with addition of S1P (500nM) or VEGF for 24-48 hrs (endothelial cells were labeled with lipophilic live-stain CM Dil (1μg/ml) [Molecular Probes]. In the presence of VEGF and S1P capillary like cords were formed 24 hrs later while endothelial cells in SFM did not form a cords. The experiments were repeated three times. (B) Graphical representation of cord length and cell number presented in (A). (C) HDMECs were transduced with non silencing (NS) or AdCTGF siRNA for 24 hrs without or with addition of S1P. (D) Quantification of cord formation observed in (C) performed as described in Methods section. The values represent the mean ± SEM of three independent experiment done in duplicate.* Significant values at p<0.05
CTGF domains are sufficient to induce capillary cord formation
To examine the influence of full length CTGF or CTGF N-terminus/C-terminus separately on cord formation, HDMECs were transduced with respective adenoviruses. After transducing cells with the virus for 24 hr, 3D co-culture assay was employed as described in the Methods section. Robust capillary like-cords formation was observed 24-48 hr later in HDMECs transduced with AdCTGF. Formation of the capillary-like structures was also detected in the wells transduced with CTGF domains, but not in cells transduced with control AdGo (Fig.4A). Cord length in cells transduced with AdCTGF was 20 ± 1.1 mm/5 fields, AdNT-CTGF 8 ± 2 mm/5 fields, and AdCT-CTGF 14 ± 1.2 mm/5 fields (Fig.4B). Endothelial cell number was 260 ± 10, 560 ± 15, 380 ± 12 /5 fields, respectively. The capillary like structures were truncated, short and multicellular suggesting that the cords are immature. This data demonstrate that CTGF, as well as its C-terminal domain have the ability to induce capillary-like cord formation, with the N-terminal domain also producing a small effect.
Figure 4. Elevated N-terminal and C-terminal expression of CTGF induces capillary-cord formation.
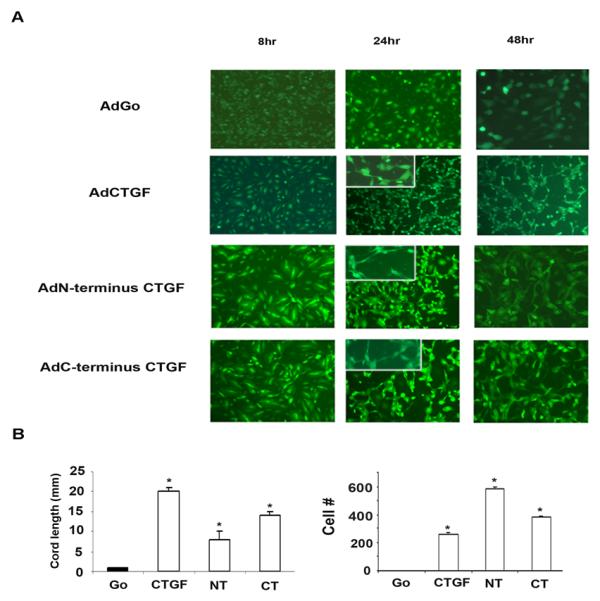
(A) HDMECs were transduced with AdCTGF, AdNT or AdCT or control AdGo. The following day 3D co-culture was established as described in Methods section. Cells were incubated in 0.5% EBM2 for 24-48 hr. The experiments were repeated three times. (B) Graphical representation of tube length and cell number presented in (A). The values represent the mean ± SEM of three independent experiment done in duplicate.* Significant values at p<0.05
DISCUSSION
Formation of new blood vessels is a complex tightly controlled process that is central to physiological wound healing, while dysregulated angiogenesis is associated with tumor development, diabetic nephropathy, rheumatoid arthritis and other pathological conditions (25, 30, 41, 54). Accumulating evidence suggests that sphingolipid metabolite, S1P, is an important regulator of cell viability, differentiation, and migration in various cell types, including endothelial cells. S1P exerts its pleiotropic effects through the activation of GPCRs and an extensive cross-talk with other cellular signaling pathways downstream from tyrosine kinase receptors (33). In this study, we report that secreted matricellular protein CTGF, is required for S1P induced migration of microvascular endothelial cells. We show that, S1P induces CTGF both at the mRNA and protein levels in HDMECs. Depletion of the endogenous CTGF abrogated S1P-induced endothelial cell migration in in vitro wound healing assay and prevented capillary-like cord formation in 3D collagen assays. Furthermore, we show that expression of CTGF alone at the levels corresponding to those induced by S1P, was sufficient to induce similar cell responses in these assays. Previous studies, which investigated the mechanisms underlying endothelial cell migration in response to S1P focused on cytoskeletal changes and signal transduction pathways. It was shown that S1P migration of HUVECs on vitronectin substrate was mediated via activation of αvβ3 integrin and colocalization with FAK and α-actinin (52). Also, it was shown that S1P induced lung capillary tube formation by regulating early cytoskeletal rearrangement (34). A recent report linked S1P-induced migration of human pulmonary artery endothelial cells to activation of phospholipase D2, protein kinase C-ζ (PKC-ζ), and Rac1 (18). The above studies suggest a cross-talk between S1P and a growth factor with the ability to acivate integrin signaling. Our study suggests that CTGF, which signals through integrin receptors, including αvβ3, is a likely candidate to mediate those responses in endothelial cells. Further studies are needed to delineate the specific contribution of CTGF to activation of intracellular signaling molecules required for endothelial cell migration in response to S1P. Relevant to our findings, it was reported that CTGF mediates migration of breast cancer cells in response to activation of another GPCR, ER GRP30 (43).
Increasing evidence suggest a universal role for CTGF as a promoter of cell migration. Pro-migratory effects of CTGF were reported in several experimental models, including mesangial cells, smooth muscle cells, hepatic stellate cells, and corneal epithelial cells (14). While the mechanistic details of the role of CTGF in cell migration are not fully elucidated, it is of interest that CTGF-induced mesangial cell migration was associated with translocation of PKC-ζ, to the leading edge of migrating cells (13). Furthermore, a recent study of human lung fibroblasts has linked pro-migratory function of CTGF to upregulation of IQGAP, a scaffolding protein controlling cell migration (6). CTGF was also shown to stimulate formation of capillary-like tubules upon addition to a monolayer of bovine arterial endothelial cells (48) or HUVECs (26). In addition, Luo and collaborators described similar pro-migratory effect in HUVECs after CTGF transfection (36). Our study demonstrated that CTGF induced cell migration and proliferation in monolayers of HDMECs. In addition, CTGF was effective in inducing capillary-like cord formation in 3D collagen gels. Importantly, our study provides the evidence that CTGF is required for the pro-migratory effects of S1P examined in both assays.
In this study, we also sought to determine the domain specific effects of CTGF. Previous studies have demonstrated that C- and N-terminal fragments of CTGF correspond to the same fragments produced by proteolysis. Also, it has been shown that C- and N-terminal domains have distinct roles in fibroblast proliferation and collagen deposition. It was reported that C-terminal domain stimulates fibroblasts proliferation, while N-terminal domain stimulates collagen synthesis (19). In contrast, C-terminal domain was shown to promote collagen deposition (22). Another study reported that module 3 which contains thrombospondin (TSP-1) motif induced p42/44MAPK phosphorylation and fibronectin expression in hepatic stellate cells (49). Module 3 was also required for adhesion of hepatic stellate cells either in the presence of lipoprotein-related receptor or integrin α6β1 (16, 49). On the other hand, module 4 (known as a CT module) promoted adhesion in bovine aortic endothelial cells (4). Our study has examined the role of C- and N-terminal domain in endothelial cell migration and proliferation. C-terminal domain induced cell migration and proliferation of monolayer cultures, as well as cord formation in 3D collagen gels with a potency similar to that observed with full length CTGF. In contrast, N-terminal domain was not effective in these assays.
In conclusion this study shows, for the first time, that CTGF is required for the angiogenic responses of microvascular endothelial cells induced by S1P. S1P is present at high levels in circulation mostly bound to HDL and albumin with relatively lower levels in the tissues (24). However, during injury or in pathological conditions characterized by leaky vessels, such as tumor angiogenesis, S1P can be leaked into surrounding tissues and exert its effects on various cell types. S1P upregulates expression of CTGF in smooth muscle cells (12) and other cell types (28), suggesting that a cross-talk between these pathways may also contribute to responses of those cells. The present study also suggests that C-terminal and N-terminal domains of CTGF may play distinct roles in endothelial cell migration and proliferation. Further investigations are required to explore the precise mechanism of these events and to investigate whether CTGF has a role in mediating other S1P regulated responses in endothelial cells.
ACKNOWLEDGMENTS
This work was supported by grants from Scleroderma Fundation (MM) and National Institute of Health PO1-CA78582 (MT).
REFERENCES
- 1.Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- 2.Argraves KM, Wilkerson BA, Argraves WS, Fleming PA, Obeid LM, Drake CJ. Sphingosine-1-phosphate signaling promotes critical migratory events in vasculogenesis. J Biol Chem. 2004;279:50580–50590. doi: 10.1074/jbc.M404432200. [DOI] [PubMed] [Google Scholar]
- 3.Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball DK, Rachfal AW, Kemper SA, Brigstock DR. The heparin-binding 10 kDa fragment of connective tissue growth factor (CTGF) containing module 4 alone stimulates cell adhesion. J Endocrinol. 2003;176:R1–7. doi: 10.1677/joe.0.176r001. [DOI] [PubMed] [Google Scholar]
- 5.Bayless KJ, Davis GE. Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. Biochem Biophys Res Commun. 2003;312:903–913. doi: 10.1016/j.bbrc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Bogatkevich GS, Ludwicka-Bradley A, Singleton CB, Bethard JR, Silver RM. Proteomic analysis of CTGF-activated lung fibroblasts: identification of IQGAP1 as a key player in lung fibroblast migration. Am J Physiol Lung Cell Mol Physiol. 2008;295:L603–611. doi: 10.1152/ajplung.00530.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61) Angiogenesis. 2002;5:153–165. doi: 10.1023/a:1023823803510. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P, Collen D. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann N Y Acad Sci. 2000;902:249–262. doi: 10.1111/j.1749-6632.2000.tb06320.x. discussion 262-244. [DOI] [PubMed] [Google Scholar]
- 9.Chae SS, Paik JH, Furneaux H, Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest. 2004;114:1082–1089. doi: 10.1172/JCI22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chintalapudi MR, Markiewicz M, Kose N, Dammai V, Champion KJ, Hoda RS, Trojanowska M, Hsu T. Cyr61/CCN1 and CTGF/CCN2 mediate the proangiogenic activity of VHL-mutant renal carcinoma cells. Carcinogenesis. 2008;29:696–703. doi: 10.1093/carcin/bgn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury I, Chaqour B. Regulation of connective tissue growth factor (CTGF/CCN2) gene transcription and mRNA stability in smooth muscle cells. Involvement of RhoA GTPase and p38 MAP kinase and sensitivity to actin dynamics. Eur J Biochem. 2004;271:4436–4450. doi: 10.1111/j.1432-1033.2004.04382.x. [DOI] [PubMed] [Google Scholar]
- 13.Crean JK, Furlong F, Finlay D, Mitchell D, Murphy M, Conway B, Brady HR, Godson C, Martin F. Connective tissue growth factor [CTGF]/CCN2 stimulates mesangial cell migration through integrated dissolution of focal adhesion complexes and activation of cell polarization. FASEB J. 2004;18:1541–1543. doi: 10.1096/fj.04-1546fje. [DOI] [PubMed] [Google Scholar]
- 14.de Winter P, Leoni P, Abraham D. Connective tissue growth factor: structure-function relationships of a mosaic, multifunctional protein. Growth Factors. 2008;26:80–91. doi: 10.1080/08977190802025602. [DOI] [PubMed] [Google Scholar]
- 15.Dziadzio M, Usinger W, Leask A, Abraham D, Black CM, Denton C, Stratton R. N-terminal connective tissue growth factor is a marker of the fibrotic phenotype in scleroderma. QJM. 2005;98:485–492. doi: 10.1093/qjmed/hci078. [DOI] [PubMed] [Google Scholar]
- 16.Gao R, Brigstock DR. Low density lipoprotein receptor-related protein (LRP) is a heparin-dependent adhesion receptor for connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatol Res. 2003;27:214–220. doi: 10.1016/s1386-6346(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 17.Gore-Hyer E, Shegogue D, Markiewicz M, Lo S, Hazen-Martin D, Greene EL, Grotendorst G, Trojanowska M. TGF-beta and CTGF have overlapping and distinct fibrogenic effects on human renal cells. Am J Physiol Renal Physiol. 2002;283:F707–716. doi: 10.1152/ajprenal.00007.2002. [DOI] [PubMed] [Google Scholar]
- 18.Gorshkova I, He D, Berdyshev E, Usatuyk P, Burns M, Kalari S, Zhao Y, Pendyala S, Garcia JG, Pyne NJ, Brindley DN, Natarajan V. Protein kinase C-epsilon regulates sphingosine 1-phosphate-mediated migration of human lung endothelial cells through activation of phospholipase D2, protein kinase C-zeta, and Rac1. J Biol Chem. 2008;283:11794–11806. doi: 10.1074/jbc.M800250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grotendorst GR, Duncan MR. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005;19:729–738. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- 20.He S, Jin ML, Worpel V, Hinton DR. A role for connective tissue growth factor in the pathogenesis of choroidal neovascularization. Arch Ophthalmol. 2003;121:1283–1288. doi: 10.1001/archopht.121.9.1283. [DOI] [PubMed] [Google Scholar]
- 21.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heng EC, Huang Y, Black SA, Jr., Trackman PC. CCN2, connective tissue growth factor, stimulates collagen deposition by gingival fibroblasts via module 3 and alpha6- and beta1 integrins. J Cell Biochem. 2006;98:409–420. doi: 10.1002/jcb.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinton DR, Spee C, He S, Weitz S, Usinger W, LaBree L, Oliver N, Lim JI. Accumulation of NH2-terminal fragment of connective tissue growth factor in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Care. 2004;27:758–764. doi: 10.2337/diacare.27.3.758. [DOI] [PubMed] [Google Scholar]
- 24.Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochim Biophys Acta. 2008;1781:477–482. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes CC. Endothelial-stromal interactions in angiogenesis. Curr Opin Hematol. 2008;15:204–209. doi: 10.1097/MOH.0b013e3282f97dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- 27.Jang C, Koh YJ, Lim NK, Kang HJ, Kim DH, Park SK, Lee GM, Jeon CJ, Koh GY. Angiopoietin-2 Exocytosis Is Stimulated by Sphingosine-1-Phosphate in Human Blood and Lymphatic Endothelial Cells. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.172676. [DOI] [PubMed] [Google Scholar]
- 28.Katsuma S, Ruike Y, Yano T, Kimura M, Hirasawa A, Tsujimoto G. Transcriptional regulation of connective tissue growth factor by sphingosine 1-phosphate in rat cultured mesangial cells. FEBS Lett. 2005;579:2576–2582. doi: 10.1016/j.febslet.2005.03.073. [DOI] [PubMed] [Google Scholar]
- 29.Kimura T, Sato K, Malchinkhuu E, Tomura H, Tamama K, Kuwabara A, Murakami M, Okajima F. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler Thromb Vasc Biol. 2003;23:1283–1288. doi: 10.1161/01.ATV.0000079011.67194.5A. [DOI] [PubMed] [Google Scholar]
- 30.Koch AE, Distler O. Vasculopathy and disordered angiogenesis in selected rheumatic diseases: rheumatoid arthritis and systemic sclerosis. Arthritis Res Ther. 2007;9(Suppl 2):S3. doi: 10.1186/ar2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubota S, Eguchi T, Shimo T, Nishida T, Hattori T, Kondo S, Nakanishi T, Takigawa M. Novel mode of processing and secretion of connective tissue growth factor/ecogenin (CTGF/Hcs24) in chondrocytic HCS-2/8 cells. Bone. 2001;29:155–161. doi: 10.1016/s8756-3282(01)00492-6. [DOI] [PubMed] [Google Scholar]
- 32.Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 33.Lebman DA, Spiegel S. Cross-talk at the crossroads of sphingosine-1-phosphate, growth factors, and cytokine signaling. J Lipid Res. 2008;49:1388–1394. doi: 10.1194/jlr.R800008-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linz-McGillem LA, Moitra J, Garcia JG. Cytoskeletal rearrangement and caspase activation in sphingosine 1-phosphate-induced lung capillary tube formation. Stem Cells Dev. 2004;13:496–508. doi: 10.1089/scd.2004.13.496. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo Z, Li H, Zhang J, Zhang H, Xiu R. Effects of human connective tissue growth factor gene transfection on migration of human umbilical vein endothelial cell. Clin Hemorheol Microcirc. 2006;34:185–192. [PubMed] [Google Scholar]
- 37.Miura S, Tanigawa H, Matsuo Y, Fujino M, Kawamura A, Saku K. Ras/Raf1-dependent signal in sphingosine-1-phosphate-induced tube formation in human coronary artery endothelial cells. Biochem Biophys Res Commun. 2003;306:924–929. doi: 10.1016/s0006-291x(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 38.Muehlich S, Schneider N, Hinkmann F, Garlichs CD, Goppelt-Struebe M. Induction of connective tissue growth factor (CTGF) in human endothelial cells by lysophosphatidic acid, sphingosine-1-phosphate, and platelets. Atherosclerosis. 2004;175:261–268. doi: 10.1016/j.atherosclerosis.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Panetti TS. Differential effects of sphingosine 1-phosphate and lysophosphatidic acid on endothelial cells. Biochim Biophys Acta. 2002;1582:190–196. doi: 10.1016/s1388-1981(02)00155-5. [DOI] [PubMed] [Google Scholar]
- 40.Pannu J, Nakerakanti S, Smith E, ten Dijke P, Trojanowska M. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 2007;282:10405–10413. doi: 10.1074/jbc.M611742200. [DOI] [PubMed] [Google Scholar]
- 41.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 42.Payne SG, Milstien S, Spiegel S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett. 2002;531:54–57. doi: 10.1016/s0014-5793(02)03480-4. [DOI] [PubMed] [Google Scholar]
- 43.Prakash Pandey D, Lappano R, Albanito L, Madeo A, Maggiolini M, Picard D. Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. EMBO J. 2009 doi: 10.1038/emboj.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- 45.Richard L, Velasco P, Detmar M. A simple immunomagnetic protocol for the selective isolation and long-term culture of human dermal microvascular endothelial cells. Exp Cell Res. 1998;240:1–6. doi: 10.1006/excr.1998.3936. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz BM, Hong G, Morrison BH, Wu W, Baudhuin LM, Xiao YJ, Mok SC, Xu Y. Lysophospholipids increase interleukin-8 expression in ovarian cancer cells. Gynecol Oncol. 2001;81:291–300. doi: 10.1006/gyno.2001.6124. [DOI] [PubMed] [Google Scholar]
- 47.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- 49.Tong ZY, Brigstock DR. Intrinsic biological activity of the thrombospondin structural homology repeat in connective tissue growth factor. J Endocrinol. 2006;188:R1–8. doi: 10.1677/joe.1.06719. [DOI] [PubMed] [Google Scholar]
- 50.Velazquez OC, Snyder R, Liu ZJ, Fairman RM, Herlyn M. Fibroblast-dependent differentiation of human microvascular endothelial cells into capillary-like 3-dimensional networks. FASEB J. 2002;16:1316–1318. doi: 10.1096/fj.01-1011fje. [DOI] [PubMed] [Google Scholar]
- 51.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Lee JF, Lin CY, Lee MJ. Rho GTPases mediated integrin alpha v beta 3 activation in sphingosine-1-phosphate stimulated chemotaxis of endothelial cells. Histochem Cell Biol. 2008;129:579–588. doi: 10.1007/s00418-008-0389-8. [DOI] [PubMed] [Google Scholar]
- 53.Zarrinkalam KH, Stanley JM, Gray J, Oliver N, Faull RJ. Connective tissue growth factor and its regulation in the peritoneal cavity of peritoneal dialysis patients. Kidney Int. 2003;64:331–338. doi: 10.1046/j.1523-1755.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- 54.Zent R, Pozzi A. Angiogenesis in diabetic nephropathy. Semin Nephrol. 2007;27:161–171. doi: 10.1016/j.semnephrol.2007.01.007. [DOI] [PubMed] [Google Scholar]


