Abstract
Objective
Prior studies investigating the association between APOE alleles ε2 / ε4 and risk of Intracerebral Hemorrhage (ICH) have been inconsistent, limited to small sample sizes and did not account for confounding by population stratification or determine which genetic risk model was best applied.
Methods
We performed a large-scale genetic association study of 2,189 ICH cases and 4,041 controls from seven cohorts, which were analyzed using additive models for ε2 and ε4. Results were subsequently meta-analyzed using a random effects model. A proportion of the individuals (322 cases and 357 controls) had available genome-wide data to adjust for population stratification.
Results
ε2 and ε4 were associated with lobar ICH at genome-wide significance levels (Odds Ratio (OR) = 1.82, 95% Confidence Interval (CI) 1.50 – 2.23, p = 6.6 × 10−10 and OR = 2.20, 95%CI 1.85 – 2.63, p = 2.4 × 10−11 respectively). Restriction of analysis to definite / probable CAA ICH uncovered a stronger effect. ε4 was also associated with increased risk for deep ICH (OR = 1.21, 95% CI 1.08 – 1.36, p = 2.6 × 10−4). Risk prediction evaluation identified the additive model as best for describing the effect of APOE genotypes.
Interpretation
APOE ε2 and ε4 are independent risk factors for lobar ICH, consistent with their known associations with amyloid biology. In addition, we present preliminary findings on a novel association between APOE ε4 and deep ICH. Finally, we demonstrate that an additive model for these APOE variants is superior to other forms of genetic risk modeling previously applied.
INTRODUCTION
Intracerebral hemorrhage (ICH) accounts for approximately 15% of acute strokes in the United States 1 and carries the worst prognosis of all acute cerebrovascular diseases. Even with state-of-the-art medical care, ICH results in death or severe disability in more than 50% of cases 2,3.
The ε2 and ε4 alleles of Apolipoprotein E (APOE) have been reported to be associated with risk of ICH in several small studies and meta-analyses 4,5, but results thus far have been inconsistent 7-9. In a recent meta-analysis of the role of APOE in ICH 5, the largest study included 333 ICH cases and the smallest contributed 48. Furthermore, previous reviews compiled data from published reports rather than perform meta-analysis of individual-level data.
Previous results suggest that the degree of association between APOE and ICH might depend on hemorrhage location: most studies have shown associations between ε2 / ε4 and lobar ICH, while results for non-lobar ICH have been contradictory 4-6. Despite these observations of location-specific effects, only four cohorts in the latest meta-analysis 5 provided association results by ICH location for APOE variants (244 Lobar ICH cases, 437 Non-lobar ICH cases).
Possible confounding for reported associations between APOE and ICH has not been extensively explored. Population stratification (the phenomenon by which genetic ancestry imbalance between cases and controls generates a false positive association) is a particularly concerning potential confounder, given the variation in APOE minor allele frequencies (MAF) worldwide 10. Previous results could also have been distorted by inappropriate genetic modeling. Published studies have consistently applied a dominant genetic model to all analyses 4,5, despite limited data for correspondence between this genetic model and the biological effects of APOE.
We performed a large-scale multi-center genetic association study to clarify these issues, capitalizing on the resources and infrastructure available to investigators within the International Stroke Genetics Consortium (ISGC). We pooled cases (n = 2,189) and controls (n = 4,041) with neuroimaging-confirmed hemorrhage location for analysis and used genome-wide genetic data available for 322 cases and 357 controls to investigate and rule out population stratification as a possible source of confounding. Finally, we tested various genetic models to clarify the influence of ε2 and ε4 alleles on ICH risk.
METHODS
Participating Studies
Genotype and phenotype data for ICH cases and controls were provided by ISGC investigators from the following studies: North American (USA) multi-center Genetics Of Cerebral Hemorrhage on Anticoagulation (GOCHA) Study11, Genetic and Environmental Risk Factors for Hemorrhagic Stroke (GERFHS) at the University of Cincinnati (Cincinnati, OH, USA) 12, the Hospital del Mar (Barcelona, Spain) ICH study (HM-ICH) 13, Jagiellonian University (Krakow, Poland) Hemorrhagic Stroke Study (JUHSS) 14, Lund University (Lund, Sweden) Hemorrhagic Stroke Study (LUHSS) 15, Medical University of Graz (Graz, Austria) ICH study (MUG-ICH) 16, and the Vall d’Hebron Hospital (Barcelona, Spain) ICH Study (VHH-ICH) 17. All studies were approved by the Institutional Review Boards (IRB) or Ethics Committee (EC) of participating institutions, and all participating subjects provided informed consent for participation in this study, including APOE and genome-wide genotyping.
Subjects
Subjects enrolled in each study included primary acute ICH cases aged > 55 years presenting to the emergency departments of participating Institutions (all accredited stroke centers). Eligibility for study participation required neuroimaging (CT or MRI) confirmation of hemorrhagic stroke (Table 1). Exclusion criteria included the presence of trauma, brain tumor, hemorrhagic transformation of a cerebral infarction, vascular malformation, or any other perceived cause of secondary ICH. Only individuals of self-described European or European-American ancestry were included for analysis in each study. Individuals of African-American ancestry (63 Lobar ICH cases, 110 Deep ICH Cases and 297 controls) enrolled in GOCHA and GERFHS were analyzed as a separate cohort (US-AA) for replication purposes, with additional adjustment for recruitment site (GOCHA vs. GERFHS).
Table 1. European ancestry individuals enrolled in participating studies.
| GOCHA | GERFHS | JUHSS | MUG-ICH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobar ICH |
Deep ICH |
Controls | Lobar ICH |
Deep ICH |
Controls | Lobar ICH |
Deep ICH |
Controls | Lobar ICH |
Deep ICH |
Controls | |
| No. of Subjects |
398 | 312 | 555 | 203 | 337 | 1304 | 102 | 130 | 429 | 77 | 114 | 1023 |
| Age - Mean (SD) |
73.4 (10.3) |
70.2 (12.4) |
73.1 (8.03) |
64.3 (17.1) |
64.0 (15.5) |
60.8 (15.2) |
63.2 (13.3) |
67.5 (14.1) |
63.6 (13.0) |
70.2 (13.2) |
68.1 (13.6) |
65.2 (8.0) |
| Gender (% Female) |
46 | 43 | 45 | 45 | 51 | 47 | 48 | 48 | 53 | 45 | 48 | 43 |
| Hypertension (%) |
77 | 86 | 72 | 52 | 74 | 48 | 73 | 81 | 48 | 60 | 67 | 50 |
| APOE ε2 (MAF) |
0.11 | 0.07 | 0.07 | 0.15 | 0.10 | 0.10 | 0.13 | 0.09 | 0.08 | 0.09 | 0.07 | 0.07 |
| APOE ε4 (MAF) |
0.21 | 0.15 | 0.12 | 0.21 | 0.16 | 0.15 | 0.13 | 0.11 | 0.08 | 0.13 | 0.11 | 0.10 |
| HM-ICH | LUHSS | VHH-ICH | US-AA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobar ICH |
Deep ICH |
Controls | Lobar ICH |
Deep ICH |
Controls | Lobar ICH |
Deep ICH |
Controls | Lobar ICH |
Deep ICH |
Controls | |
| No. of Subjects |
66 | 103 | 185 | 42 | 89 | 161 | 43 | - | 87 | 63 | 110 | 297 |
| Age - Mean (SD) |
76.8 (10.0) |
70.7 (12.6) |
69.3 (7.1) |
74.5 (9.4) |
74.9 (10.2) |
74.3 (9.6) |
72.6 (6.5) |
- | 70.8 (6.7) |
63.4 (17.5) |
59.8 (13.6) |
55.5 (14.6) |
| Gender (% Female) |
46 | 48 | 51 | 47 | 48 | 43 | 42 | - | 40 | 50 | 46 | 45 |
| Hypertension (%) |
48 | 59 | 42 | 51 | 62 | 42 | 55 | - | 42 | 55 | 58 | 52 |
| APOE ε2 (MAF) |
0.11 | 0.09 | 0.08 | 0.11 | 0.09 | 0.09 | 0.10 | - | 0.08 | 0.15 | 0.12 | 0.10 |
| APOE ε4 (MAF) |
0.13 | 0.11 | 0.09 | 0.20 | 0.18 | 0.16 | 0.12 | - | 0.09 | 0.24 | 0.20 | 0.19 |
GERFHS = Genetic and Environmental Risk Factors for Hemorrhagic Stroke Study at the University of Cincinnati (Cincinnati, OH, USA), GOCHA = Multi-center North-American (USA) Genetics of Cerebral Hemorrhage on Anticoagulation Study, HM-ICH = Hospital del Mar (Barcelona, Spain) ICH Study, JUHSS = Jagiellonian University (Krakow, Poland) Hemorrhagic Stroke Study, LUHSS = Lund University (Lund, Sweden) Hemorrhagic Stroke Study, MUG-ICH = Medical University of Graz (Graz, Austria) ICH Study, US-AA: African-American subjects recruited in the USA (Boston and Cincinnati) as part of the GOCHA and GERFHS studies, VHH-ICH = Val d’Hebron Hospital (Barcelona, Spain) ICH Study. ICH = Intracerebral Hemorrhage, MAF = Minor Allele Frequency, SD = Standard Deviation
ICH location was assigned based on admission CT scan by stroke neurologists at each participating site. ICH isolated to the cortex (with or without involvement of subcortical white matter) was defined as lobar, while ICH selectively involving the thalamus, basal ganglia or brainstem was defined as deep (non-lobar) ICH. Multiple concurrent bleeds involving deep and lobar territories were defined as Mixed ICH and represented an exclusion criterion. Similarly, subjects presenting with evidence of prior bleeds in a different location than the index (enrollment) ICH were excluded from analysis. Cerebellar hemorrhages were also not analyzed in the present study. Individuals with CT scans of insufficient quality for location determination were excluded from all analyses. When ICH location assignment was not clear, the scan was reviewed by a group of study neurologists and neuroradiologists for consensus. Scans lacking a consensus location were excluded from analysis. All readers interpreting neuroimaging data were blinded to clinical and APOE genotype information.
Recorded clinical characteristics included history of hypertension (clinical diagnosis of hypertension or history of antihypertensive drug use), pre-ICH exposure to warfarin, antiplatelet agents and statins, first-degree relative history of ICH, alcohol and tobacco use.
Controls were enrolled from the same population as the cases at each participating institution, and included only individuals aged > 55 years at time of enrollment. Controls were confirmed to have no medical history of ICH, Alzheimer’s disease or pre-enrollment dementia by means of interview and review of medical records. Recorded clinical characteristics were identical to ICH cases.
Cerebral Amyloid Angiopathy-related ICH
In order to determine the specificity of APOE alleles for ICH related to cerebral amyloid angiopathy (CAA), we separately analyzed definite and/or probable CAA ICH cases and possible CAA cases for association with ε2 or ε4. 223 lobar ICH cases from the GOCHA cohort had pathology and/or MRI gradient-echo (GRE) data available for analysis. Microbleed presence and location was assessed for these individuals according to validated protocols 18,19. Briefly, MRI with GRE images (TR 750/TE 50/5 to 6-mm slice thickness/1 mm interslice gap) was performed using a 1.5-T magnet. Cortical (lobar) and deep hemorrhages were classified as microbleeds according to their size (<5 mm in diameter). All MRI analyses were performed and recorded without knowledge of clinical or genetic information. Only MRI scans obtained within 90 days from the index ICH were considered for analysis.
Definite / probable CAA was defined as lobar ICH in the presence of confirmed CAA pathology 20 and/or microbleeds confined to the lobar brain region (n = 82) 21. Possible CAA included all remaining lobar ICH cases lacking CAA pathology and lobar microbleeds (n = 141). Each group was matched with separate hemorrhage-free controls based on age (within five years of the age of the index ICH case), gender, and hypertension status in a 1:2 case:control ratio.
Genotyping
All DNA samples were isolated from fresh or frozen blood, quantified using a Quantification Kit and normalized to a concentration of 30 ng/ul. Two genotype-determining variants in APOE, rs7412 and rs429358, were independently genotyped using two separate assays 21. The allelic reads from the two assays were then translated to APOE genotypes (ε3ε3, ε3ε4, ε4ε4, ε3ε2, ε2ε2 and ε2ε4). All genotyping personnel were blinded to clinical and neuroimaging data. Genotype and phenotype data were subsequently submitted to the Coordinating Center (Massachusetts General Hospital) for analysis. All case and control groups were found to be in Hardy-Weinberg equilibrium for APOE genotypes. Genome-wide genotyping was performed on a subset of the GOCHA samples (322 cases, 357 controls) using the Illumina 610-Quad array. Genotypes were called using BeadStudio v 3.2.
Statistical Analysis
Individual Studies
Single-study level data were initially analyzed by logistic regression under independent additive genetic models. Our multivariate model included the following variables: age, gender, pre-ICH history of hypertension, number of ε4 alleles (0, 1, or 2) and number of ε2 alleles (0, 1, or 2). Subsequent analyses also adjusted for warfarin or antiplatelet agent exposure at time of ICH, smoking history (ever smoker), alcohol use (> 1 drink/week), family history of ICH, pre-ICH history of ischemic stroke and pre-ICH history of hyperlipidemia or statin exposure. None of the additional covariates modified the results from the initial regression model (data not shown). We therefore extracted results from the previously described model (adjusting for age, gender and pre-ICH hypertension) for subsequent meta-analysis (see below). Differences in effect sizes comparing lobar vs. deep ICH and definite / probable CAA vs. possible CAA were assessed using the Breslow-Day test.
Meta-analysis
Results from multivariate models for individual studies were combined using a conservative inverse variance random effects model (DerSimonian-Laird). Results from individuals with genome-wide data were entered separately as an independent study. This allowed direct comparison of results from studies controlling for population stratification with those without control. Meta-analysis heterogeneity was quantified by computing Cochrane’s Q and corresponding p-value and I2 (percent of effect size attributable to heterogeneity). Heterogeneity was considered to be significant for heterogeneity p-value < 0.10 (due to the conservative nature of Cochrane’s test) or I2 > 0.20. We decided to set the threshold for significance in the initial meta-analysis at genome-wide level (p < 5 × 10−8). This threshold is equivalent to the estimated Bonferroni correction for all independently testable common variants (minor allele frequency > 0.01) in the human genome (i.e. not correlated by linkage disequilibrium on the basis of Hapmap and sequencing data) 22. All analyses were performed using the R statistical software v 2.10.0 (http://www.r-project.org).
Genetic modeling
We re-analyzed all available data under dominant and recessive models, and compared predictive power for disease status to the initial results from the additive model. Comparison of predictive power for different genetic models was carried out using both a likelihood ratio test (LRT) based method and by analyzing Receiver Operator Characteristics (ROC) curves for disease status prediction. Both analyses returned very similar results.
Population Stratification
To determine whether the frequency of APOE alleles varies across different populations, a finding that could lead to confounding due to population stratification, we extracted minor allele frequency (MAF) data for European control individuals from all genetic studies of APOE listed in PubMed (www.pubmed.gov) as of December 1st 2010 (Table S1). These data were subsequently correlated with latitude and longitude of their geographic position in Europe using a linear regression method. This analysis included size of the cohort and number of studies performed in each region as covariates.
We were able to control for population stratification in samples with available genome-wide data (322 cases, 357 controls) using PLINK v. 1.07 (http://pngu.mgh.harvard.edu/~purcell/plink) to perform Principal Component Analysis (PCA) in accordance with previously published methods 23. Principal components 1 and 2 were extracted from PCA results and entered as additional covariates in logistic regression analysis for these samples.
RESULTS
Lobar ICH
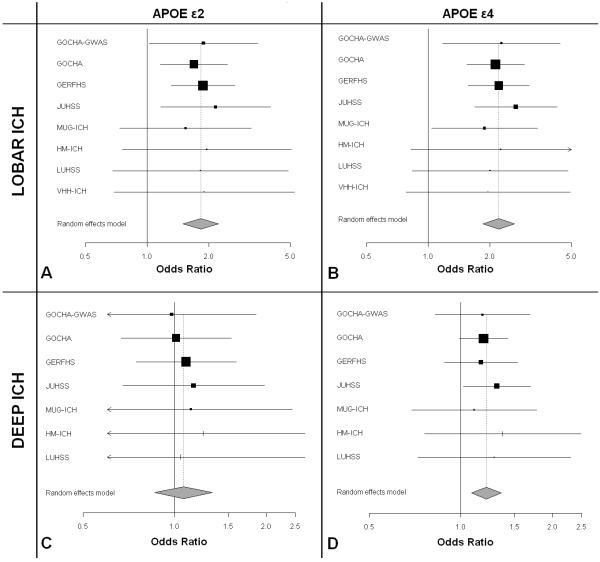
We meta-analyzed 931 lobar ICH cases and 3,744 controls from seven studies, and found genome-wide significant association between lobar ICH risk and ε2 (OR = 1.82, p = 6.6 × 10−10) and ε4 (OR = 2.20, p = 2.4 × 10−11) (Figure 1, A-B). We identified no evidence of heterogeneity among studies (Table 2).
Figure 1. Forest plots of meta-analysis of APOE in Lobar and Deep ICH.
GERFHS = Genetic and Environmental Risk Factors for Hemorrhagic Stroke Study at the University of Cincinnati (Cincinnati, OH, USA), GOCHA = Multi-center North American (USA) Genetics of Cerebral Hemorrhage on Anticoagulation Study, HM-ICH = Hospital del Mar (Barcelona, Spain) ICH Study, JUHSS = Jagiellonian University (Krakow, Poland) Hemorrhagic Stroke Study, LUHSS = Lund University (Lund, Sweden) Hemorrhagic Stroke Study, MUG-ICH = Medical University of Graz (Graz, Austria) ICH Study, VHH-ICH = Val d’Hebron Hospital (Barcelona, Spain) ICH Study.
ICH = Intracerebral Hemorrhage
Table 2. Meta-analysis: Association of APOE alleles with Lobar and Deep ICH.
| LOBAR ICH | |||||||
|---|---|---|---|---|---|---|---|
| Allele | Cases | Controls | OR | 95% CI OR | p-value | Heterogeneity p-value |
I2 (95% I2 CI) |
| ε2 | 931 | 3,744 | 1.82 | 1.50 – 2.23 | 6.6 × 10−10 | 0.98 | 0.00 (0.00 – 0.00) |
| ε4 | 931 | 3,744 | 2.20 | 1.85 – 2.63 | 2.4 × 10−11 | 0.99 | 0.00 (0.00 – 0.00) |
| DEEP ICH | |||||||
|---|---|---|---|---|---|---|---|
| Allele | Cases | Controls | OR | 95% CI OR | p-value | Heterogeneity p-value |
I2 (95% I2 CI) |
| ε2 | 1,085 | 3,657 | 1.07 | 0.86 – 1.33 | 0.54 | 0.95 | 0.00 (0.00 – 0.00) |
| ε4 | 1,085 | 3,657 | 1.21 | 1.08 – 1.36 | 2.6 × 10−4 | 0.97 | 0.00 (0.00 – 0.00) |
CI = Confidence Interval, ICH = Intracerebral Hemorrhage, I2 = Percentage of meta-analysis effect size due to heterogeneity, OR = Odds Ratio.
We separately analyzed definite / probable CAA ICH cases (n = 82) and possible CAA ICH cases (n= 141) samples in the subset of the GOCHA lobar ICH cases with available pathology and/or MRI data (n = 223). We then compared effect sizes in order to determine the specificity of the APOE association to definite / probable CAA (Table 3). Definite / probable CAA was associated with both ε4 (OR = 3.08, p < 0.001) and ε2 (OR = 2.89, p < 0.001), while no association was evident for possible CAA (ε4: OR = 1.21, p = 0.46 and ε2: OR = 1.02, p = 0.57). Effect size point-estimates and 95% CIs were significantly larger for definite / probable CAA ICH compared to possible CAA ICH for both ε4 (p = 0.012) and ε2 (p = 0.032).
Table 3. Association of APOE alleles with CAA-related ICH 12.
| Definite / Probable CAA ICH | |||||||
|---|---|---|---|---|---|---|---|
| Allele | Cases | Controls | MAF (Cases) |
MAF (Controls) |
OR | 95% CI OR | p-value |
| ε2 | 82 | 164 | 0.18 | 0.07 | 2.89 | 1.57 – 5.33 | 5.2 × 10−4 |
| ε4 | 82 | 164 | 0.25 | 0.12 | 3.08 | 1.68 – 5.63 | 4.6 × 10−4 |
| Possible CAA ICH | |||||||
|---|---|---|---|---|---|---|---|
| Allele | Cases | Controls | MAF (Cases) |
MAF (Controls) |
OR | 95% CI OR | p-value |
| ε2 | 141 | 282 | 0.09 | 0.07 | 1.02 | 0.63 – 1.65 | 0.57 |
| ε4 | 141 | 282 | 0.16 | 0.12 | 1.21 | 0.74 – 1.99 | 0.46 |
CAA = Cerebral Amyloid Angiopathy, CI = Confidence Interval, ICH = Intracerebral Hemorrhage, MAF = Minor Allele Frequency, OR = Odds Ratio.
Deep ICH
We meta-analyzed 1,085 deep ICH cases and 3,657 controls from six studies, and found an association between deep ICH risk and ε4 (OR = 1.21, 95% CI: 1.08 – 1.36). This association failed to surpass the pre-defined genome-wide significance threshold (p = 2.6 × 10−4). No association was identified for ε2 (OR = 1.07, 95% CI: 00.86 – 1.33, p = 0.54) (Figure 1, C-D). We identified no evidence of meta-analysis heterogeneity (Table 2). To explore whether the inclusion of misclassified lobar ICH cases in the group of deep ICH category might have generated a spurious association for ε4, we re-analyzed brainstem ICH cases (less likely to represent misdiagnosed lobar ICH due to the anatomic location and smaller average ICH volume) separately from the rest of the deep ICH cases. We then compared effect sizes and looked for meta-analysis heterogeneity that might indicate differential effects due to misclassification bias. The odds ratio for ε4 in brainstem ICH (OR = 1.21) was identical to our meta-analysis estimate for deep ICH, and we identified no evidence of heterogeneity between studies (heterogeneity p-value = 0.99, I2 = 0.00, 95% CI: 0.00 – 0.00). Comparison of effect sizes for ε4 in lobar vs. deep ICH resulted in a statistical significant difference (p < 0.001).
Replication in African-American individuals
We attempted replication of observed associations in 63 Lobar ICH cases, 110 Deep ICH Cases and 297 controls of US African-American ancestry (US-AA) enrolled in GOCHA and GERFHS. We observed replication of associations between lobar ICH and both ε2 (OR = 1.99, 95% CI: 1.10 – 3.61, p = 0.036) and ε4 (OR = 2.10, 95% CI: 1.09 – 4.03, p = 0.012). Inclusion of US-AA samples in meta-analysis with European ancestry samples did not introduce significant heterogeneity (p = 0.99, I2 = 0.0). While we did not replicate the association between ε4 and Deep ICH (p = 0.21), the effect size estimate (OR = 1.15) was consistent with that observed in the European ancestry samples. Inclusion of US-AA samples in the Deep ICH meta-analysis did not introduce significant heterogeneity (p = 0.99, I2 = 0.0) and increased the level of significance of the observed association (p-value for all individuals = 1.0 × 10−4 vs. p-value for Europeans only = 2.6 × 10−4)
Genetic model specification
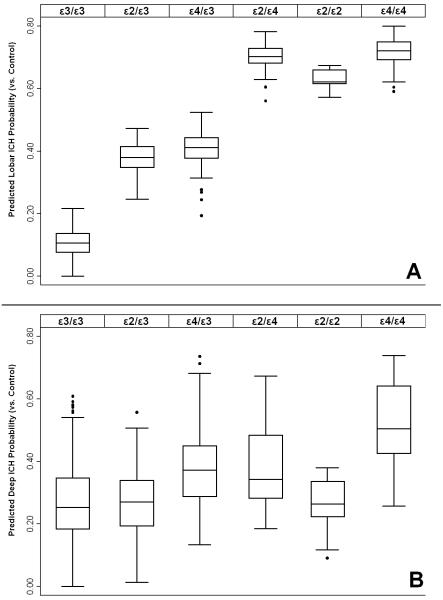
We repeated all analyses for Lobar ICH under dominant and recessive genetic models and compared predictive performance with the additive model based on individual genotypes. Significance was assessed using both likelihood ratio testing (LRT) and comparing Receiver Operator Characteristics (ROC) curves. Disease status (lobar ICH case vs. control) prediction was significantly more accurate for the additive model compared to the dominant model (LRT p < 0.0001, ROC p < 0.0001) or the recessive model (LRT p = 0.0002, ROC p = 0.0001). This was reflected in the predicted disease risk by APOE genotype, showing an increased risk for the ε4/ε4, ε4/ε2 and ε2/ε2 over the ε3 heterozygote genotypes (Figure 2, A). We performed an identical analysis for Deep ICH: results obtained under different models revealed superior predictive performance for the additive model over dominant (LRT p = 0.001, ROC p = 0.003) or recessive (LRT p = 0.0002, ROC p = 0.0001) models (Figure 2, B).
Figure 2. Effect of APOE genotype on predicted probability of ICH status vs. Control.
A: Lobar ICH probability, B: Deep ICH Probability. Box plots display the median (solid line), interquartile range (box) and total range (whiskers) of probability distribution for each genotype. Disease status probability based on meta-analysis of logistic regression analyses from individual studies under the assumption of the additive model, including adjustment for age, gender, hypertension and principal components (where available).
Population Stratification at the APOE locus
The APOE locus demonstrated significant population stratification across the European continent in our review of previously published reports. ε2 was associated with both latitude (p = 0.025) and longitude (p = 0.001) across the European continent, while ε4 was associated with latitude (p < 0.001). Observed MAFs ranged from 0.01 (Siberia) to 0.15 (UK) for ε2 and from 0.06 (Southern Italy) to 0.27 (Finland) for ε4 (Figure S1).
We therefore re-analyzed lobar and deep ICH GOCHA individuals with GWAS data (GOCHA-GWAS), comparing results before and after inclusion of principal components. For lobar ICH, result for GOCHA-GWAS (181 cases and 357 controls) were very similar before (ε2: OR = 1.89, p = 0.012 and ε4: OR = 2.28, p = 0.010) and after (ε2: OR = 1.88, p = 0.010 and ε4: OR = 2.28, p = 0.009) inclusion of principal components. No difference in results was evident for deep ICH (141 cases and 357 controls) comparing unadjusted (ε2: OR = 0.99, p = 0.67 and ε4: OR = 1.19, p = 0.14) and principal components-adjusted analyses (ε2: OR = 0.98, p = 0.54 and ε4: OR = 1.18, p =0.15).
DISCUSSION
Our analyses show strong associations between APOE variants and lobar ICH, providing the first evidence of association between sequence variants and intracerebral hemorrhage that surpass the genome-wide significance threshold. Furthermore, we have demonstrated that previously adopted genetic models of APOE and ICH (dominant and recessive) do not provide the best possible description of the increase in ICH risk associated with the ε2 and ε4 alleles. This additional finding is important for follow-up studies of the APOE locus, as it supports the existence of a dose-response relationship between the biological effect of APOE and lobar ICH risk, which is poorly understood at present. Finally, although APOE MAF clearly varies across populations, we were able to rule out population stratification as a possible source of confounding.
We have also found that the effect of ε2 and ε4 in lobar ICH appears to be predominantly associated with CAA-related ICH. The increase in effect size observed when analysis is restricted to definite/probable CAA suggests that different mechanisms account for hemorrhagic stroke in the presence or absence of pathological and neuroimaging markers of amyloid angiopathy 24. Of note, effect sizes associated with definite/probable CAA-related ICH are in line with those observed for ε4 in Alzheimer’s disease 25, consistent with the existence of shared biological pathways between the two conditions that do not necessarily extend to lobar ICH as a whole.
We found an association between ε4 APOE and deep ICH, although it did not achieve genome-wide significance. Previous findings in the PROGRESS trial implicated APOE variants in deep ICH, particularly in subjects of Asian ancestry 6. Our data extend this association to European-ancestry individuals. We are not able to rule out the possibility that lobar or CAA-related hemorrhages mis-classified as deep hemorrhage might have generated a spurious association with ε4. However, our observation that ε4 is associated with brainstem ICH, with an effect size identical to that observed in the deep ICH cohort as a whole, supports the presence of a more fundamental mechanism linking ε4 and non-CAA-related ICH. APOE plays a critical role in redistributing lipids among central nervous system cells for normal lipid homeostasis 26,27, repairing injured neurons 28, maintaining synaptodendritic connections 29, neurite outgrowth 30, synaptic plasticity 31, mitochondrial resistance to oxidative stress 32 and glucose use by neurons and glial cells 33-35 In multiple pathways affecting neuropathology, APOE ε4 acts directly or in concert with age, head injury, oxidative stress, ischemia and inflammation to alter disease onset, progression, and prognosis 36. Mechanisms such as these could be involved in determining individual responses to ICH-associated oxidative and ischemic stress, driving the increased frequency of ε4 in deep ICH cases. Indeed, these biological phenomena could potentially play a role in both lobar and deep ICH. Future studies, however, will be required to clarify the biological implications of our findings.
Our review of publicly available data on APOE allele frequencies in Europeans confirmed an association between geography and ε2 / ε4 genotype. This observation raises the possibility of confounding due to population stratification in our analyses. We were able to conclusively rule out population stratification only in the GOCHA-GWAS dataset via principal component analysis. However, effect size estimates within the GOCHA-GWAS data are entirely in line with those observed in the cohorts without population stratification control. This observation is inconsistent with the hypothesis that observed associations for APOE are due to confounding by population stratification. Furthermore, we provide evidence of replication in African Americans, where minor allele frequencies for ε2 and ε4 are different from those in European-ancestry cohorts (Table 1). In light of these results, confounding due to population stratification is theoretically possible but unlikely in our analyses.
Prior meta-analyses of the effect of APOE alleles on ICH risk failed to identify genome-wide significant associations with lobar ICH or any role for ε4 in deep ICH 4,5. However, all studies included in prior meta-analyses had substantial limitations. Sample sizes were smaller compared to the present study, and the vast majority of individuals did not have ICH location information available for analysis, which likely resulted in loss of statistical power given the divergent effect sizes for both APOE alleles in deep and lobar ICH. Furthermore, prior studies and meta-analyses applied the dominant genetic model in their description of the effects of APOE alleles on ICH risk. Our own data demonstrate that the additive model is superior to the dominant model in the description of genetic risk at APOE. Model misspecification in prior studies likely further eroded statistical power. Finally, previous meta-analyses did not have direct access to individual-level data, thus limiting the harmonization in statistical methods that we employed in our study.
Our study has limitations. Despite the large number of cases and controls available for analysis, the association between ε4 and deep ICH did not achieve genome-wide significance. This result therefore must be considered preliminary. Similarly, while we were able to observe a significant difference in effect size for ε2 and ε4 when comparing definite / probable vs. possible CAA, we do not have sufficient power to rule out any effect in the latter. Indeed, the estimated odds ratio for ε4 in possible CAA-related ICH is very close to the one observed for deep ICH, thereby raising the possibility of shared mechanism between non CAA-related effects in both locations.
In summary, we have identified genome-wide significant associations between APOE ε2 and ε4 and lobar ICH. Additionally, we report preliminary findings on a novel association between ε4 and deep ICH. Future studies will be required to clarify the functional mechanisms underlying the effect of APOE variants on ICH.
Supplementary Material
Minor allele frequency distribution for APOE alleles ε2 and ε4 in healthy subjects across Europe
Minor Allele Frequencies (MAFs) for APOE alleles across Europe are displayed as circles with diameter proportional to the observed MAFs. Data obtained from control subjects in medical genetics studies of APOE index in Pubmed (as of 01/01/2010). Correlation with geographical coordinates (p-values shown) was determined via linear regression, adjusting for sample size and number of studies at each location.
ACKNOWLEDGEMENTS
The authors would like to thank Tammy Gillis, PhD and Marcy McDonald, PhD, for technical assistance in genotyping APOE variants and Elisa Cuadrado-Godia, MD, Angel Ois, MD and Ana Rodriguez-Campello, MD for valuable support in patient recruitment and clinical data collection.
FUNDING
GOCHA: This study was funded by NIH-NINDS grants K23NS042695, R01NS059727 and 5R01NS042147, by the Deane Institute for Integrative Research in Atrial Fibrillation and Stroke, by the University of Michigan General Clinical Research Center (M01 RR000042) and by a grant from the National Center for Research Resources. Drs. Biffi and Anderson were supported in part by the American Heart Association/Bugher Foundation Centers for Stroke Prevention Research (0775010N).
GERFHS: This study was supported by NIH grants NS36695 (Genetic and Environmental Risk Factors for Hemorrhagic Stroke) and NS30678 (Hemorrhagic and Ischemic Stroke among Blacks and Whites) and by the Greater Cincinnati Foundation Grant (Cincinnati Control Cohort)
HM-ICH: Ministerio de Sanidad y Consumo de España, Instituto de Salud Carlos III with the grants: “Registro BASICMAR” Funding for Research in Health (PI051737); Contract for Research Training for Professionals with Specialty (CM06100067); “Ramon y Cajal” Postdoctoral Contract and Grant from Spanish Research Networks “Red HERACLES” (RD06/ 0009).
JUHSS: This study is supported by a grant funded by the Polish Ministry of Education (N N402 083934).
LUHSS: Lund University, Region Skåne and the Swedish Medical Research Council (K2007-61X -20378-01-3). Biobank services and genotyping were performed at Region Skåne Competence Centre (RSKC Malmö), Skåne university hospital, Malmö, Sweden
MUG-ICH: Controls of the MUG-ICH study are from the Austrian Stroke Prevention Study (ASPS), a population-based study funded by the Austrian Science Fond (FWF) grant number P20545-P05 and P13180. The Medical University of Graz supports the databank of the ASPS.
VHH-ICH: This study was funded by grants by the Spanish government (Geno-tPA project-FIS PJ060586 and GRECAS project EC08/00137)
REFERENCES
- 1.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 2.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38(6):2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 3.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Archives of Internal Medicine. 2004;164(8):880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 4.Sudlow C, Martínez González NA, Kim J, Clark C. Does apolipoprotein E genotype influence the risk of ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage? Systematic review and meta-analyses of 31 studies among 5961 cases and 17,965 controls. Stroke. 2006;37(2):364–70. doi: 10.1161/01.STR.0000199065.12908.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peck G, Smeeth L, Whittaker J, Casas, et al. The genetics of primary haemorrhagic stroke, subarachnoid haemorrhage and ruptured intracranial aneurysms in adults. PLoS One. 2008;3(11):e3691. doi: 10.1371/journal.pone.0003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzourio C, Arima H, Harrap S, et al. APOE genotype, ethnicity, and the risk of cerebral hemorrhage. Neurology. 2008;70(16):1322–8. doi: 10.1212/01.wnl.0000308819.43401.87. [DOI] [PubMed] [Google Scholar]
- 7.Duzenli S, Pirim I, Gepdiremen A, Deniz O. Apolipoprotein E polymorphism and stroke in a population from eastern Turkey. J Neurogenet. 2004;18(1):365–375. doi: 10.1080/01677060490500294. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury AH, Yokoyama T, Kokubo Y, et al. Apolipoprotein E genetic polymorphism and stroke subtypes in a Bangladeshi hospital-based study. J Epidemiol. 2001;11(3):131–138. doi: 10.2188/jea.11.131. [DOI] [PubMed] [Google Scholar]
- 9.Catto AJ, McCormack LJ, Mansfield MW, et al. Apolipoprotein E polymorphism in cerebrovascular disease. Acta Neurol Scand. 2000;101(6):399–404. doi: 10.1034/j.1600-0404.2000.90308a.x. [DOI] [PubMed] [Google Scholar]
- 10.Singh PP, Singh M, Mastana SS. APOE distribution in world populations with new data from India and the UK. Ann Hum Biol. 2006;33(3):279–308. doi: 10.1080/03014460600594513. [DOI] [PubMed] [Google Scholar]
- 11.Genes for Cerebral Hemorrhage on Anticoagulation (GOCHA) Collaborative Group Exploiting common genetic variation to make anticoagulation safer. Stroke. 2009;40:S64–6. doi: 10.1161/STROKEAHA.108.533190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo D, Sauerbeck LR, Kissela BM, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33:1190–1195. doi: 10.1161/01.str.0000014774.88027.22. [DOI] [PubMed] [Google Scholar]
- 13.Gomis M, Ois A, Rodríguez-Campello A, et al. Outcome of intracerebral haemorrhage patients pre-treated with statins. Eur J Neurol. 2010;17:443–448. doi: 10.1111/j.1468-1331.2009.02838.x. [DOI] [PubMed] [Google Scholar]
- 14.Pera J, Slowik A, Dziedzic T, Pulyk R, Wloch D, Szczudlik A. Glutathione peroxidase 1 C593T polymorphism is associated with lobar intracerebral hemorrhage. Cerebrovasc Dis. 2008;25:445–449. doi: 10.1159/000126918. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson OG, Lindgren A, Brandt L, Säveland H. Prediction of death in patients with primary intracerebral hemorrhage: a prospective study of a defined population. J Neurosurg. 2002;97:531–536. doi: 10.3171/jns.2002.97.3.0531. [DOI] [PubMed] [Google Scholar]
- 16.Seifert T, Lechner A, Flooh E, Schmidt H, et al. Lack of association of lobar intracerebral hemorrhage with apolipoprotein E genotype in an unselected population. Cerebrovasc Dis. 2006;21:266–270. doi: 10.1159/000091225. [DOI] [PubMed] [Google Scholar]
- 17.Domingues-Montanari S, Hernandez-Guillamon M, Fernandez-Cadenas I, et al. ACE variants and risk of intracerebral hemorrhage recurrence in amyloid angiopathy. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.019. In press. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg SM, Eng JA, Ning M, et al. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35(6):1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56(4):537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997;28(7):1418–22. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg SM, Rebeck GW, Vonsattel JP, et al. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 22.Hoggart CJ, Clark TG, De Iorio M, et al. Genome-wide significance for dense SNP and resequencing data. Genet Epidemiol. 2008;32(2):179–85. doi: 10.1002/gepi.20292. [DOI] [PubMed] [Google Scholar]
- 23.Biffi A, Anderson CD, Desikan RS, et al. Genetic Variation and Neuroimaging Measures in Alzheimer’s Disease. Arch. Neurol. 2010 doi: 10.1001/archneurol.2010.108. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarron MO, Nicoll JA. Apolipoprotein E genotype and cerebral amyloid angiopathy-related hemorrhage. Ann N Y Acad Sci. 2000;903:176–179. doi: 10.1111/j.1749-6632.2000.tb06366.x. [DOI] [PubMed] [Google Scholar]
- 25.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 26.Gong JS, Kobayashi M, Hayashi H, et al. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J Biol Chem. 2002;277(33):29919–26. doi: 10.1074/jbc.M203934200. [DOI] [PubMed] [Google Scholar]
- 27.Fagan AM, Holtzman DM, Munson G, et al. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (−/−), and human apoE transgenic mice. J Biol Chem. 1999;274(42):30001–7. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- 28.Buttini M, Orth M, Bellosta S, et al. Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: isoform-specific effects on neurodegeneration. J Neurosci. 1999;19(12):4867–80. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan BP, Bellosta S, Sanan DA, et al. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264(5160):850–2. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- 30.Bellosta S, Nathan BP, Orth M, et al. Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem. 1995;270(45):27063–71. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- 31.Trommer BL, Shah C, Yun SH, et al. ApoE isoform affects LTP in human targeted replacement mice. Neuroreport. 2004;15(17):2655–8. doi: 10.1097/00001756-200412030-00020. [DOI] [PubMed] [Google Scholar]
- 32.Gibson GE, Haroutunian V, Zhang H, et al. Mitochondrial damage in Alzheimer’s disease varies with apolipoprotein E genotype. Ann Neurol. 2000;48(3):297–303. [PubMed] [Google Scholar]
- 33.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–9. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiman EM, Caselli RJ, Chen K, et al. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98(6):3334–9. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Small GW, Mazziotta JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273(12):942–7. [PubMed] [Google Scholar]
- 36.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103(15):5644–51. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Minor allele frequency distribution for APOE alleles ε2 and ε4 in healthy subjects across Europe
Minor Allele Frequencies (MAFs) for APOE alleles across Europe are displayed as circles with diameter proportional to the observed MAFs. Data obtained from control subjects in medical genetics studies of APOE index in Pubmed (as of 01/01/2010). Correlation with geographical coordinates (p-values shown) was determined via linear regression, adjusting for sample size and number of studies at each location.