Abstract
Purpose
This European Organisation for Research and Treatment of Cancer phase III trial assesses whether adjuvant whole-brain radiotherapy (WBRT) increases the duration of functional independence after surgery or radiosurgery of brain metastases.
Patients and Methods
Patients with one to three brain metastases of solid tumors (small-cell lung cancer excluded) with stable systemic disease or asymptomatic primary tumors and WHO performance status (PS) of 0 to 2 were treated with complete surgery or radiosurgery and randomly assigned to adjuvant WBRT (30 Gy in 10 fractions) or observation (OBS). The primary end point was time to WHO PS deterioration to more than 2.
Results
Of 359 patients, 199 underwent radiosurgery, and 160 underwent surgery. In the radiosurgery group, 100 patients were allocated to OBS, and 99 were allocated to WBRT. After surgery, 79 patients were allocated to OBS, and 81 were allocated to adjuvant WBRT. The median time to WHO PS more than 2 was 10.0 months (95% CI, 8.1 to 11.7 months) after OBS and 9.5 months (95% CI, 7.8 to 11.9 months) after WBRT (P = .71). Overall survival was similar in the WBRT and OBS arms (median, 10.9 v 10.7 months, respectively; P = .89). WBRT reduced the 2-year relapse rate both at initial sites (surgery: 59% to 27%, P < .001; radiosurgery: 31% to 19%, P = .040) and at new sites (surgery: 42% to 23%, P = .008; radiosurgery: 48% to 33%, P = .023). Salvage therapies were used more frequently after OBS than after WBRT. Intracranial progression caused death in 78 (44%) of 179 patients in the OBS arm and in 50 (28%) of 180 patients in the WBRT arm.
Conclusion
After radiosurgery or surgery of a limited number of brain metastases, adjuvant WBRT reduces intracranial relapses and neurologic deaths but fails to improve the duration of functional independence and overall survival.
INTRODUCTION
Brain metastases are a frequent cause of morbidity and mortality in patients suffering from a variety of solid tumors. Surgery, radiosurgery, and whole-brain radiotherapy (WBRT) are the main treatment options.1–8 A point of controversy is whether adjuvant WBRT, the rationale for which is destroying microscopic disease at the original tumor site or at distant intracranial locations, is necessary after complete surgical resection or radiosurgery. The risk of long-term neurotoxicity and availability of effective salvage treatments9,10 are the main arguments against adjuvant WBRT, whereas the negative impact of CNS progression on neurologic and neurocognitive function when omitting initial WBRT and the uncertainty regarding the value of salvage treatments in reversing neurologic symptoms are arguments in favor of WBRT.11
Two randomized trials12,13 reported that the omission of WBRT in patients with newly diagnosed brain metastases after either surgery or stereotactic radiosurgery results in a significantly worse local and distant control, although it does not affect overall and functionally independent survival. These studies included patients with both stable and progressive systemic cancer.
This article reports the results of a randomized phase III trial of the European Organisation for Research and Treatment of Cancer (EORTC; 22952-26001) that investigated the role of adjuvant WBRT after either surgery or radiosurgery of a limited number of brain metastases from solid tumors, focusing on patients in good condition with stable systemic cancer. It was hypothesized that these patients would have the greatest benefit from the expected increase in intracranial tumor control after WBRT and would thus keep their functional independence for a longer time period.
PATIENTS AND METHODS
Eligibility Criteria
Patients with one to three brain metastases in good condition (WHO performance status [PS] of 0 to 2) and with stable systemic disease or asymptomatic synchronous primary tumor were included. For the diagnosis of brain metastases, magnetic resonance imaging (MRI) not older than 6 weeks was required. Patients with progressive systemic disease were excluded. Eligibility criteria are listed in Table 1. All patients gave written informed consent according to International Conference on Harmonisation/Good Clinical Practice and national regulations. The protocol was reviewed and approved by the ethics committee in each institution. The study was conducted according to the Declaration of Helsinki.14
Table 1.
Eligibility Criteria
| Eligibility Criteria |
|---|
| Inclusion criteria |
| Age ≥ 18 years |
| WHO performance status ≤ 2 |
| 1-3 brain metastases |
| Radiosurgery: single metastasis ≤ 3.5 cm, multiple metastases ≤ 2.5 cm in diameter |
| Surgery: complete surgical resection |
| Radiosurgery: histologic confirmation of primary tumor or other metastases ≤ 4 years ago, stereotactic biopsy of the brain metastasis otherwise |
| Stable systemic cancer for ≥ 3 months and/or asymptomatic synchronous primary tumor without metastases outside the CNS or unknown primary tumor |
| Exclusion criteria |
| Brain metastasis of small-cell lung cancer, lymphoma, leukemia, myeloma, germ cell tumors |
| Brain stem metastases |
| Leptomeningeal metastases |
| Recurrent brain metastases after surgery and/or radiosurgery and/or brain irradiation |
| Inability to interrupt chemotherapy during whole-brain radiotherapy |
Radiosurgery
Both linear accelerators and gamma-knife devices were allowed. The planning target volume consisted of the gross tumor volumes of all (up to three) metastases surrounded by a margin of 1 to 2 mm around each metastasis. A dose of 25 Gy was prescribed to the center of each metastasis. The minimum dose at the surface of each planning target volume had to be 20 Gy. For the gamma-knife, a peripheral dose of 20 Gy to the 50% isodose was allowed. Size limits were 35 mm (maximal diameter) for singular metastases and 25 mm for multiple metastases. Dose limits for organs at risk were as follows: brainstem, 8 Gy; optic chiasm or optic nerves, 8 Gy; other cranial nerves, 12 Gy; and sensorimotor cortical areas, 18 Gy.
Surgery
The main prerequisite for entering patients onto the study was the complete resection of the brain metastases, judged either by the surgeon's impression or early (≤ 24 hours) postoperative contrast-enhanced computed tomography and/or MRI. There were no limitations regarding size of the metastases.
Random Assignment to WBRT or Observation
For the radiosurgery patients, measurement of WHO PS and random assignment to WBRT or observation (OBS) were performed before radiosurgery. For the surgery patients, measurement of WHO PS and random assignment were performed after surgery. WBRT was applied using standard techniques. The prescribed dose was 30 Gy in 10 fractions of 3 Gy at the midline, five fractions per week. A maximum of 6 weeks between radiosurgery/surgery and WBRT was allowed.
Follow-Up, Evaluation of Toxicity, and Quality of Life
Follow-up visits with clinical (including WHO PS) and neurologic examination and MRI scans were scheduled every 3 months. Acute and late toxicity was assessed using the Late Effects of Normal Tissues–Subjective, Objective, Management, Analytic scales,15,16 and health-related quality of life was assessed using the EORTC Quality of Life Questionnaire C3017 and brain tumor module.18 All intra- and extracranial progressions had to be reported.
Statistical Methods
Within 4 weeks after surgery or within 2 weeks before radiosurgery, patients were allocated to WBRT or OBS by a minimization algorithm19 stratifying for institution, brain metastases (one v > one metastasis), local treatment (radiosurgery v surgery), macroscopic tumor outside the brain (present v absent), and initial WHO PS (0 to 1 v 2). The trial primary end point (duration of functional independence) was measured from the day of random assignment to the first report of deterioration to a WHO PS of more than 2. The trial was designed to detect a difference of 11% in the proportion of patients alive with PS ≤ 2 at 6 months, from 50% on OBS to 61% with WBRT (hazard ratio [HR], 0.714), with 80% power and a two-sided 5% significance level. To achieve this, 280 events (death or PS > 2) were needed, and 340 patients were planned to be recruited.
Secondary end points were frequency of intracranial relapse at initially treated and at new sites, progression-free and overall survival, late toxicities, and quality of life. The analysis was performed by intention to treat. Survival (until WHO PS > 2) was estimated using the Kaplan-Meier method20 and compared using the log-rank test stratified for prior treatment.21 Sensitivity analyses were conducted by Cox model adjusted for the stratification factors except institution and by location of the primary tumor (lung v other).22 Cumulative incidence curves and the Gray test were used to compare the intracranial or extracranial relapse rates accounting for death as competing risk.23 All analyses were conducted in the intent-to-treat population. Quality of life will be reported separately.
RESULTS
Patients Characteristics
In total, 359 patients were recruited from November 1996 to November 2007. In patients who were alive, the minimum follow-up was 4 months, except for three patients who had 1 day, 1 month, and 2 months of follow-up. In the radiosurgery group, 100 patients were assigned to OBS, and 99 were assigned to WBRT. In the surgery group, 79 patients were assigned to OBS, and 81 patients were assigned to adjuvant WBRT. The patient and lesion characteristics are listed in Tables 2 and 3 and were well balanced between the random assignment arms. Patients who entered after surgery more often had a single metastasis with a larger diameter (up to 70 mm), and lesions were more frequently located in the posterior fossa.
Table 2.
Patient Demographics and Clinical Characteristics
| Characteristic | Observation (n = 179) |
WBRT (n = 180) |
Total (N = 359) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Age, years | ||||||
| Median | 61 | 60 | 60 | |||
| Range | 37-80 | 26-81 | 26-81 | |||
| Sex | ||||||
| Male | 122 | 68 | 113 | 63 | 235 | 65 |
| Female | 57 | 32 | 67 | 37 | 124 | 35 |
| WHO performance status | ||||||
| 0 | 82 | 46 | 75 | 42 | 157 | 44 |
| 1 | 78 | 44 | 84 | 47 | 162 | 45 |
| 2 | 19 | 11 | 21 | 12 | 40 | 11 |
| Neurologic status | ||||||
| No deficit | 97 | 54 | 97 | 54 | 194 | 54 |
| Some deficit, useful work | 52 | 29 | 54 | 30 | 106 | 30 |
| Moderate impairment | 29 | 16 | 29 | 16 | 58 | 16 |
| Major impairment | 1 | 1 | 0 | 0 | 1 | 0 |
| Localization of primary tumor | ||||||
| Lung (NSCLC) | 93 | 52 | 97 | 54 | 190 | 53 |
| Breast | 20 | 11 | 22 | 12 | 42 | 12 |
| Kidney | 13 | 7 | 16 | 9 | 29 | 8 |
| Colorectal | 16 | 9 | 14 | 8 | 30 | 8 |
| Melanoma | 8 | 5 | 10 | 6 | 18 | 5 |
| Other | 15 | 8 | 12 | 7 | 27 | 8 |
| CUP | 14 | 8 | 9 | 5 | 23 | 6 |
| Macroscopic tumor outside the brain | ||||||
| Absent | 89 | 50 | 79 | 44 | 168 | 47 |
| Present | 82 | 46 | 91 | 51 | 173 | 48 |
| Unknown | 8 | 4 | 10 | 6 | 18 | 5 |
Abbreviations: WBRT, whole-brain radiotherapy; NSCLC, non–small-cell lung cancer; CUP, cancer of unknown primary tumor.
Table 3.
Lesion Characteristics
| Characteristic | RS/Observation (n = 90)* |
S/Observation (n = 81)† |
RS/WBRT (n = 95)* |
S/WBRT (n = 81) |
Total (N = 347) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Size of largest lesion, mm | ||||||||||
| Median | 20 | 30 | 18 | 30 | ||||||
| Range | 4-40 | 10-60 | 5-34 | 11-70 | ||||||
| No. of lesions | ||||||||||
| 1 | 61 | 68 | 76 | 94 | 63 | 66 | 79 | 98 | 279 | 81 |
| 2 | 20 | 22 | 5 | 6 | 23 | 24 | 2 | 2 | 50 | 14 |
| 3 | 9 | 10 | 0 | 0 | 9 | 10 | 0 | 0 | 18 | 5 |
| Location of largest lesion | ||||||||||
| Supratentorial lobes | 73 | 81 | 59 | 73 | 84 | 88 | 59 | 73 | 275 | 79 |
| Posterior fossa | 9 | 10 | 17 | 21 | 7 | 7 | 20 | 25 | 53 | 15 |
| Other/unknown | 8 | 9 | 5 | 6 | 4 | 4 | 2 | 2 | 19 | 6 |
Abbreviations: RS, radiosurgery; S, surgery; WBRT, whole-brain radiotherapy.
Only patients who received RS were shown.
Two patients who received S instead of RS were shown.
Compliance With Eligibility Criteria and Protocol Treatment
The CONSORT diagram is shown in Figure 1.24 Only six patients were ineligible. For five patients in the radiosurgery group, the reasons of ineligibility were as follows: primary treatment before random assignment (n = 3), lymphoma (n = 1), and too large brain metastasis (n = 1). One patient in the surgery group had an incomplete surgery.
Fig 1.
CONSORT diagram showing compliance to eligibility criteria and protocol treatment. WBRT, whole-brain radiotherapy.
In the radiosurgery group, 185 (93%) of 199 patients underwent radiosurgery for primary treatment. The median target dose to all lesions was 25 Gy (range, 19 to 37 Gy), and the median surface dose was 20 Gy (range, 14 to 25 Gy). The treatment device was a linear accelerator in 132 patients (71%) and a gamma knife in 53 patients (29%). Two patients had resection and one patient had seed implantation instead of radiosurgery because the lesion grew beyond the limits before initiation of radiosurgery. Eleven patients did not receive radiosurgery because of rapid progression (n = 5), early death (n = 4), refusal (n = 1), or inadequate tumor type (lymphoma; n = 1).
All 160 patients in the surgery group (and two patients in the radiosurgery group) had their lesion(s) removed. The resection was assumed to be complete in 161 (99%) of 162 patients. Assessment of completeness was based on the surgeon's impression in 42 (26%) of 162 patients, on the postoperative computed tomography/MRI in 14 (9%) of 162 patients, and on both criteria in 105 (65%) of 162 patients.
WBRT (30 Gy) was applied to 88 (89%) of 99 patients randomly assigned to radiosurgery/WBRT. The reasons for not receiving WBRT after radiosurgery were as follows: progression (n = 4), refusal by the patient (n = 4) or oncologist (n = 1), infection (n = 1), and unknown (n = 1). In four patients, treatment was stopped after 9 to 27 Gy as a result of extracranial progression (n = 2), hemorrhage into the metastasis (n = 1), or worsening because of anemia (n = 1). In the surgery/WBRT group, 78 (96%) of 81 patients received WBRT, and three patients (4%) refused it. In two patients, WBRT was stopped after 9 to 20 Gy because of leptomeningeal tumor spread (n = 1) or general deterioration (n = 1).
In the 100 patients treated with radiosurgery and randomly assigned to OBS alone, three patients had adjuvant WBRT in opposition to the random assignment as a result of rapid growth of the lesion (n = 1), early progression with intratumoral hemorrhage (n = 1), and patient refusal of OBS (n = 1). In patients treated by surgery and randomly assigned to OBS alone, one patient refused OBS and received WBRT.
Acute Toxicity
Acute toxicity of WBRT was mild. Regarding the skin, 11% of the treated patients (11 of 88 patients in radiosurgery/WBRT arm and eight of 78 patients in surgery/WBRT arm) had severe erythema and 2% had dry desquamation. Otitis developed in 5% of patients, including 1% who required the help of an otolaryngologist. Moderate vomiting was seen in 9% of patients, and severe vomiting was seen in 1%. Mild headache was observed in 28% of patients, and moderate to severe headache was observed in 4%. Other mild acute reactions occurred in 27% of patients, mainly asthenia and fatigue.
Serious Adverse Events and Late Toxicity
Late effects were reported on the Late Effects of Normal Tissues–Subjective, Objective, Management, Analytic brain toxicity scales. In summary, 2% to 22% of the patients had grade 3 late effects, and 1% to 4% had grade 4 adverse effects without clear differences between the OBS and WBRT arms (Table 4).
Table 4.
Objective and Subjective Late Toxicities According to the LENT/SOMA Scales
| Toxicity | Observation |
WBRT |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 0 |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 0 |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
|||||||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Objective | ||||||||||||||||||||
| Neurologic deficit | 68 | 38 | 32 | 18 | 23 | 13 | 25 | 14 | 7 | 4 | 73 | 41 | 39 | 22 | 15 | 8 | 24 | 13 | 7 | 4 |
| Cognitive functions | 85 | 48 | 50 | 28 | 11 | 6 | 9 | 5 | 0 | 0 | 78 | 43 | 50 | 28 | 12 | 7 | 17 | 9 | 0 | 0 |
| Mood and personality | 86 | 48 | 43 | 24 | 19 | 11 | 4 | 2 | 3 | 2 | 92 | 51 | 38 | 21 | 11 | 6 | 16 | 9 | 1 | 1 |
| Seizures | 109 | 61 | 23 | 13 | 14 | 8 | 10 | 6 | 23 | 13 | 117 | 65 | 17 | 9 | 10 | 6 | 14 | 8 | 22 | 12 |
| Subjective | ||||||||||||||||||||
| Headache | 58 | 32 | 61 | 34 | 26 | 15 | 10 | 6 | 1 | 1 | 73 | 41 | 54 | 30 | 22 | 12 | 7 | 4 | 2 | 1 |
| Somnolence | 74 | 41 | 42 | 24 | 26 | 15 | 12 | 7 | 2 | 1 | 60 | 33 | 48 | 27 | 30 | 17 | 16 | 9 | 4 | 2 |
| Intellectual deficit | 99 | 55 | 33 | 18 | 13 | 7 | 10 | 6 | 1 | 1 | 92 | 51 | 36 | 20 | 15 | 8 | 14 | 8 | 1 | 1 |
| Functional competence | 76 | 43 | 30 | 17 | 31 | 17 | 18 | 10 | 1 | 1 | 69 | 38 | 45 | 25 | 19 | 11 | 22 | 12 | 3 | 2 |
| Memory | 78 | 44 | 56 | 31 | 13 | 7 | 7 | 4 | 2 | 1 | 68 | 38 | 66 | 37 | 8 | 4 | 15 | 8 | 1 | 1 |
Abbreviations: LENT/SOMA, Late Effects of Normal Tissues–Subjective, Objective, Management, Analytic scales; WBRT, whole-brain radiotherapy.
Serious acute toxicities related to surgery and radiosurgery were evaluated by serious adverse event forms. In total, 16 serious adverse events were reported, 13 in the WBRT arm (epileptic seizures, n = 3; radionecrosis, of which one patient probably died, n = 2; infection, n = 2; hemorrhage into brain metastasis, n = 1; hospitalization as a result of intracranial progression, n = 2; general deterioration, n = 1; stroke, n = 1; and erythema multiforme, n = 1) and three in the OBS arm (radiation necrosis, n = 1; leukoencephalopathy with memory loss after salvage WBRT, n = 1; and hydrocephalus as a result of cerebellar tumor dissemination, n = 1). Besides the reported incidents of symptomatic radionecrosis, contrast-enhancing lesions suspicious of radiation-induced breakdown of the blood-brain barrier were observed in seven (8%) of 90 patients after radiosurgery and in 12 (13%) of 95 patients after radiosurgery/WBRT.
Progression Status
At the time of final analysis, 292 (81%) of 359 patients had died, and 67 (19%) of 359 patients were alive. The median follow-up time of the surviving patients was 49 months in the WBRT arm and 40 months in the OBS arm (P = .17).
Extracranial progressions were reported in 115 (64%) of 179 patients in the OBS arm and 119 (66%) of 180 in the WBRT arm. The cumulative incidence rates of extracranial progression (with death as competing risk) at 6 months were 37% (95% CI, 30% to 44%) in the OBS arm and 38% (95% CI, 31% to 45%) in the WBRT arm. At 2 years, the rates were 63% (95% CI, 56% to 70%) in the OBS arm and 65% (95% CI, 58% to 72%) in the WBRT arm (P = .73, Gray test).
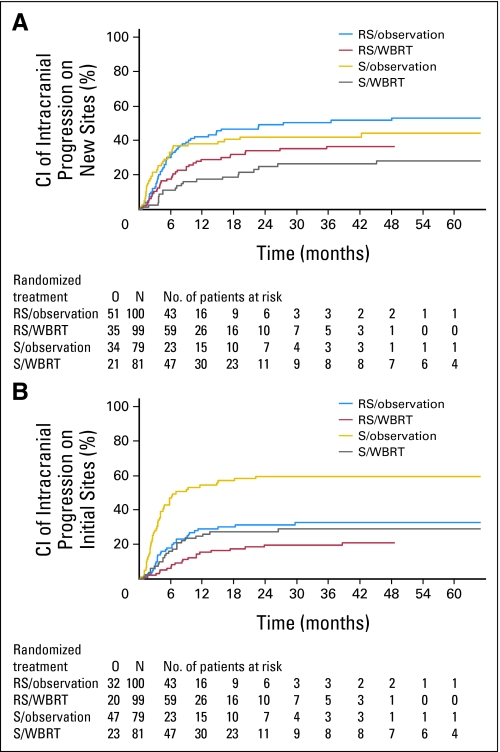
Progression at intracranial sites occurred both at the sites treated primarily by radiosurgery or surgery (initial sites) and at new sites not treated before. Overall, intracranial progression was significantly more frequent in the OBS arm (139 of 179 patients, 78%) than in the WBRT arm (87 of 180 patients, 48%; P < .001, Gray test). After surgery, at 2 years, WBRT reduced the probability of relapse at initial sites from 59% (95% CI, 48% to 71%) to 27% (95% CI, 17% to 37%; P < .001) and at new sites from 42% (95% CI, 31% to 53%) to 23% (95% CI, 14% to 33%; P = .008). After radiosurgery, at 2 years, WBRT reduced the probability of relapse at initial sites from 31% (95% CI, 22% to 40%) to 19% (95% CI, 11% to 27%; P = .040) and at new sites from 48% (95% CI, 38% to 58%) to 33% (95% CI, 24% to 43%; P = .023; Figs 2A and 2B).
Fig 2.
Time to intracranial progression at (A) new sites and (B) initial sites in patients treated initially by radiosurgery (RS) or surgery (S) after observation or adjuvant whole-brain radiotherapy (WBRT). Patients who died before the event (competing risk) were censored. CI, cumulative incidence; O, number of events; N, number of patients.
Salvage Therapy
The protocol allowed any type of salvage therapy for both intracranial and extracranial relapses. Salvage therapies for intracranial relapses were more frequently used in patients after OBS (92 of 179 patients, 51%) than in those who received adjuvant WBRT (29 of 180 patients, 16%). Salvage WBRT was used in 31% of patients in the OBS arm but in only 3% of patients in the WBRT arm (Table 5).
Table 5.
Intracranial Progression and Salvage Therapy
| Progression and Salvage Therapy | Observation |
WBRT |
||||
|---|---|---|---|---|---|---|
| No. of Patients | % of Patients Who Experienced Progression (n = 139) | % of Total Patients (n = 179) | No. of Patients | % of Patients Who Experienced Progression (n = 87) | % of Total Patients (n = 180) | |
| Site of intracranial progression | ||||||
| New sites | 60 | 43 | 34 | 44 | 51 | 24 |
| Initial sites | 54 | 39 | 30 | 31 | 36 | 17 |
| Both | 19 | 14 | 11 | 7 | 8 | 4 |
| Unknown | 6 | 4 | 3 | 5 | 6 | 3 |
| Salvage treatment | ||||||
| WBRT | 56 | 40 | 31 | 6 | 7 | 3 |
| Radiosurgery | 21 | 15 | 12 | 20 | 23 | 11 |
| Surgery | 11 | 8 | 6 | 3 | 3 | 2 |
| Radiosurgery + WBRT | 1 | 1 | 1 | |||
| Surgery + WBRT | 3 | 2 | 2 | |||
Abbreviation: WBRT, whole-brain radiotherapy.
Survival With Functional Independence (Time to WHO PS > 2)
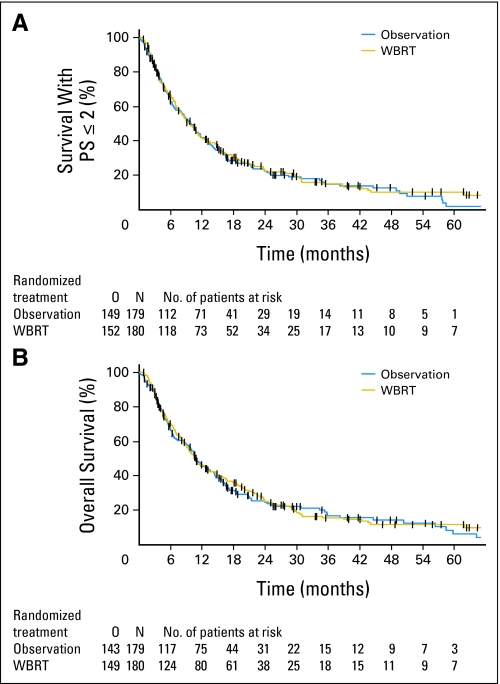
For the main end point of the study, no difference was found between the two random assignment arms. The median time to WHO PS more than 2 was 10.0 months (95% CI, 8.1 to 11.7 months) in the OBS arm and 9.5 months (95% CI, 7.8 to 11.9 months) in the WBRT arm (P = .71; HR = 0.96; 95% CI, 0.76 to 1.20; Fig 3A). At 2 years, 22.3% and 22.6% of the patients were alive and functionally independent in the OBS and WBRT arms, respectively.
Fig 3.
(A) Survival with WHO performance score ≤ 2 and (B) overall survival after observation or adjuvant whole-brain radiotherapy (WBRT). O, number of events; N, number of patients.
In a multivariate analysis, the only factors with a significant impact on survival with WHO PS ≤ 2 were the initial WHO PS (0 v 2, P = .004) and the presence of macroscopic tumor outside the brain (absent v present P < .001). Treatment was not statistically significant (P = .53). Heterogeneity of treatment effects in subgroups defined by the following factors was considered: prior treatment (radiosurgery v surgery), initial WHO PS (0 v 1 v 2), number of brain metastases (single v multiple), localization of the primary tumor (lung v other), and macroscopic tumor outside the brain (absent v present); however, no heterogeneity and no treatment effect for the primary end point in any subset was observed.
Progression-Free Survival
Median progression-free survival was slightly longer in patients receiving WBRT (4.6 months; 95% CI, 3.9 to 6.1 months) compared with those on OBS alone (3.4 months; 95% CI, 3.1 to 3.9 months; P = .020, Wald test).
Overall Survival
Overall survival did not differ (HR = 0.98; 95% CI, 0.78 to 1.24; P = .89) between the two arms, with a median survival of 10.9 months (95% CI, 9.5 to 14.2 months) in the OBS arm and 10.7 months (95% CI, 9.0 to 14.4 months) in the WBRT arm (Fig 3B). Malignant disease was the dominant cause of death in both arms (OBS, 129 of 143 patients; WBRT, 127 of 149 patients). In the radiosurgery/WBRT group, one patient probably died of toxicity (radionecrosis; see Serious Adverse Events and Late Toxicity). Neurologic death, defined as intracranial failure as a component of cause of death, was more frequent in the OBS arm (78 of 179 patients, 44%; 95% CI, 36% to 51%) than in the WBRT arm (50 of 180 patients, 28%; 95% CI, 21% to 34%; P < .002 χ2 test). The subgroup analysis for the previously mentioned factors did not detect any significant survival advantage for adjuvant WBRT in any of the subgroups.
DISCUSSION
This study shows that after radiosurgery or surgery of a limited number of brain metastases (one to three metastases) in patients with stable or asymptomatic solid tumor outside the brain, standard adjuvant WBRT reduces the probability of intracranial relapses from nearly 80% to approximately 50%. This effect is most pronounced after surgery, where the frequency of recurrence in the resection bed is reduced from 60% to less than 30%. Although it translated into a modest increase in progression-free survival, the increased intracranial tumor control did not translate into a prolonged survival time with functional independence or into a prolonged overall survival time.
The issue of adjuvant WBRT after resection of brain metastases has been addressed in several retrospective studies.25–28 It seemed that adjuvant WBRT could reduce intracranial relapses and improve survival in some patients. The only randomized study so far has been published by Patchell et al.12 This study randomly assigned 95 patients with a single brain metastasis to receive surgery plus adjuvant WBRT or surgery alone. Patients were observed closely by MRI. Relapses at the resection site were reduced by WBRT from 46% to 10%, and new intracranial metastases developed in only 14% of patients compared with 37% of patients without postoperative WBRT. A significant reduction in neurologic death by WBRT was also observed. As in the present study, median survival was approximately 10 months, with no difference between the two arms.
Several retrospective studies were undertaken to explore the role of adjuvant WBRT after radiosurgery, suggesting an increase in tumor control at the radiosurgical sites and a prevention of new brain metastases.29–32 This observation was confirmed in a phase III trial from Japan13 that randomly assigned 132 patients with one to four metastases to radiosurgery alone (18 to 25 Gy) or to radiosurgery plus WBRT (30 Gy in 3-Gy fractions); patients were observed by repeated MRI. At 2 years, adjuvant WBRT resulted in an increased control rate at the radiosurgical sites of 80%, versus 50% for radiosurgery alone, and a decreased risk for new brain metastases of 50%, versus 75% for radiosurgery alone. No significant reduction in neurologic deaths was observed. Overall survival (8 months) was not prolonged. Similar results were found in a three-armed phase III trial and in a small trial of the Trans-Tasman Radiation Oncology Group that was closed early.33,34
From the present study, it seems that although the risk for intracranial relapse is significantly reduced by adjuvant WBRT, the time period with functional independence is not increased. In addition, in the study of Aoyama et al,13 the proportion of patients with a Karnofsky performance score ≥ 70 at 12 months was also not significantly increased by WBRT added to radiosurgery (27% for radiosurgery alone v 34% for WBRT and radiosurgery).
Three factors probably explain the lack of efficacy of WBRT on both functionally independent and overall survival. First, WHO PS is subject to some interpretive variability, which makes it a soft end point. Second, if follow-up imaging is regularly performed, brain recurrences are often detected by MRI before becoming symptomatic. Approximately 30% to 40% of patients without initial WBRT will receive salvage WBRT at recurrence, and in addition, salvage radiosurgery can be used for patients with a limited number of recurrent metastases independently of initial treatment. Thus, irreversible neurologic deterioration will not develop. Third, patients with brain metastases have a high risk for systemic progression requiring treatments that causes deterioration of the functional status independent of the type of initial treatment of the brain disease. The situation looks different in trials that studied the role of surgery or radiosurgery added to WBRT,2,3,35 where functional competence,35 survival in subgroups,35 and overall survival2,3 were increased.
In the present study, severe acute toxicity was slightly more frequent in the WBRT arm, but the long-term effects of WBRT are of more concern because it has been shown that WBRT may significantly impair learning and memory function.36 A serial monitoring of cognitive functions was not performed, so future trials will have to gather information on the risk of late neurotoxicity from WBRT in long-term survivors.
In conclusion, it seems that in well-performing patients with otherwise stable systemic disease and a limited number of brain metastases (one to three metastases), who are initially treated with either radiosurgery or surgery, WBRT can be withheld if serial imaging for follow-up is performed. Regarding the patients undergoing resection of a single lesion, because adjuvant irradiation substantially reduces the risk of recurrence in the tumor bed, postoperative local irradiation37,38 should be an option that is investigated.
Acknowledgment
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL; 15th Congress of the European Cancer Organisation and 34th Congress of the European Society for Medical Oncology, September 20-24, 2009, Berlin, Germany; 51st Annual Meeting of the American Society of Radiation Oncology, November 1-5, 2009, Chicago, IL; Joint Meeting of the Society for Neuro-Oncology and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Section on Tumors, October 22-24, 2009, New Orleans, LA; and 3rd Quadrennial Meeting of the World Federation of Neuro-Oncology, May 11-14, 2009, Yokohama, Japan.
Written on behalf of the European Organisation for Research and Treatment of Cancer (EORTC) Radiation Oncology and Brain Tumor Groups.
Appendix
List of contributors: Total number of patients, 359 (100.0%); M.U. Abacioglu Marmara University Hospital, Istanbul, Turkey 72 (20.1%); S. Villa, Hospital Germans Trias i Pujol, ICO Badalona, Spain 58 (16.2%); F. Fauchon, Centre Haute Energie, Nice, France 50 (13.9%); M. Frenay, Centre Antoine Lacassagne, Nice, France; R. Soffietti, Universita di Torino, Torino, Italy 29 (8.1%); M. Kocher, R.-P. Müller, Universitätsklinikum Koeln, Germany 29 (8.1%); B.G. Baumert, Academisch Ziekenhuis Maastricht, the Netherlands 26 (7.2%); L. Fariselli, Istituto Nazionale Neurologico Carlo Besto, Milano, Italy 19 (5.3%); T. Tzuk-Shina, Rambam Medical Center, Haïfa, Israel 15 (4.2%); R.-D. Kortmann, Universitätsklinikum Leipzig, Germany 8 (2.2%); C. Carrie, Centre Leon Bérard, Lyon, France 6 (1.7%); M. Ben Hassel, Centre Eugène Marquis-Rennes, Rennes, France 5 (1.4%); M. Kouri, Helsinki University Central Hospital, Helsinki, Finland 5 (1.4%); E. Valeinis, Pauls Stradins Clinical University Hospital, Riga, Latvia 5 (1.4%); D. Van den Berge, Univesitair Ziekenhuis Brussel, Brussel, Belgium 5 (1.4%); R. Weytens, Allgemein Ziekenhuis Sint-Augustinus, Wilrijk, Belgium 4; F. Mascarenhas, Hospital Santa Maria, Lisboa, Portugal 4; D. A.L. Morgan, Nottingham General City Hospital, Nottingham, United Kingdom 4; M. Riva, Ospedale Niguarda Ca Granda, Milano, Italy 4; R. Enting, University Medical Center Groningen, Groningen, the Netherlands 4; W. Hinkelbein, Charite, Universitätsmedizin Berlin, Berlin, Germany 2; M.-H. Baron-Maillot, Centre Hopsitalier Régional Unversitaire de Besançon, Besançon, France 1; P.Y. Bondiau, Centre Antoine Lacassagne, Nice, France 1; F. Laigle-Donadey, Centre Hospitalier Universitaire Pitie-Salpetrière, Paris, France 1; J. Menten, Unversitair Ziekenhuis Gastruisberg, Leuven, Belgium 1; T. Wendt, Universitätsklinikum Jena, Jena, Germany 1.
Writing Committee: For the Radiation Oncology Group: R.-P. Müller, M. Kocher; V. Sturm, H. Treuer; W. Schlegel; G. Storme, D. van den Berge; for the Brain Tumor Group: R. Soffietti; H.S. Poulsen; R.-D. Kortmann; Ph. Cornu.
Footnotes
See accompanying editorial on page 121
Supported by Grants No. 2U10 CA11488-25 through 5U10 CA011488-40 from the National Cancer Institute (Bethesda, MD) and by a donation from the Deutsche Krebshilfe from Germany through the EORTC Charitable Trust.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00002899.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Martin Kocher, Riccardo Soffietti, Rolf-Dieter Kortmann, Dirk van den Berge, Laurence Collette, Rolf-Peter Mueller
Provision of study materials or patients: Martin Kocher, Riccardo Soffietti, Ufuk Abacioglu, Salvador Villà, Francois Fauchon, Brigitta G. Baumert, Laura Fariselli, Tzahala Tzuk-Shina, Rolf-Dieter Kortmann, Christian Carrie, Mohamed Ben Hassel, Mauri Kouri, Egils Valeinis, Dirk van den Berge, Rolf-Peter Mueller
Collection and assembly of data: Martin Kocher, Riccardo Soffietti, Ufuk Abacioglu, Salvador Villà, Francois Fauchon, Brigitta G. Baumert, Laura Fariselli, Tzahala Tzuk-Shina, Rolf-Dieter Kortmann, Christian Carrie, Mohamed Ben Hassel, Mauri Kouri, Egils Valeinis, Dirk van den Berge
Data analysis and interpretation: Martin Kocher, Riccardo Soffietti, Sandra Collette, Laurence Collette, Rolf-Peter Mueller
Manuscript writing: Martin Kocher, Riccardo Soffietti, Ufuk Abacioglu, Salvador Villà, Francois Fauchon, Brigitta G. Baumert, Laura Fariselli, Tzahala Tzuk-Shina, Rolf-Dieter Kortmann, Christian Carrie, Mohamed Ben Hassel, Mauri Kouri, Egils Valeinis, Dirk van den Berge, Sandra Collette, Laurence Collette, Rolf-Peter Mueller
Final approval of manuscript: Martin Kocher, Riccardo Soffietti, Ufuk Abacioglu, Salvador Villà, Francois Fauchon, Brigitta G. Baumert, Laura Fariselli, Tzahala Tzuk-Shina, Rolf-Dieter Kortmann, Christian Carrie, Mohamed Ben Hassel, Mauri Kouri, Egils Valeinis, Dirk van den Berge, Sandra Collette, Laurence Collette, Rolf-Peter Mueller
REFERENCES
- 1.Soffietti R, Rudà R, Trevisan E. Brain metastases: Current management and new developments. Curr Opin Oncol. 2008;20:676–684. doi: 10.1097/CCO.0b013e32831186fe. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 3.Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29:711–717. doi: 10.1016/0360-3016(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 4.Borgelt B, Gelber R, Kramer S. The palliation of brain metastases: Final results of the first two studies by the RTOG. Int J Radiat Oncol Biol Phys. 1980;6:1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 5.Sturm V, Kober B, Höver KH, et al. Stereotactic percutaneous single dose irradiation of brain metastases with a linear accelerator. Int J Radiat Oncol Biol Phys. 1987;13:279–282. doi: 10.1016/0360-3016(87)90140-4. [DOI] [PubMed] [Google Scholar]
- 6.Mehta MP, Khuntia D. Current strategies in whole-brain radiation therapy for brain metastases. Neurosurgery. 2005;57:S33–s44. doi: 10.1227/01.neu.0000182742.40978.e7. [DOI] [PubMed] [Google Scholar]
- 7.Auchter RM, Lamond JP, Alexander E, III, et al. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys. 1996;35:27–35. doi: 10.1016/s0360-3016(96)85008-5. [DOI] [PubMed] [Google Scholar]
- 8.Kocher M, Maarouf M, Bendel M, et al. Linac radiosurgery versus whole brain radiotherapy for brain metastases: A survival comparison based on the RTOG recursive partitioning analysis. Strahlenther Onkol. 2004;180:263–267. doi: 10.1007/s00066-004-1180-y. [DOI] [PubMed] [Google Scholar]
- 9.Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: Is whole brain irradiation necessary? Int J Radiat Oncol Biol Phys. 1999;43:549–558. doi: 10.1016/s0360-3016(98)00447-7. [DOI] [PubMed] [Google Scholar]
- 10.Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53:519–526. doi: 10.1016/s0360-3016(02)02770-0. [DOI] [PubMed] [Google Scholar]
- 11.Regine WF, Huhn JL, Patchell RA, et al. Risk of symptomatic brain tumor recurrence and neurologic deficit after radiosurgery alone in patients with newly diagnosed brain metastases: Results and implications. Int J Radiat Oncol Biol Phys. 2002;52:333–338. doi: 10.1016/s0360-3016(01)02645-1. [DOI] [PubMed] [Google Scholar]
- 12.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 13.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association. World Medical Association. France: Ferney-Voltaire; 1996. World Medical Association Declaration of Helsinki: Recommendations Guiding Medical Doctors in Biomedical Research Involving Human Subjects (4th revision) [Google Scholar]
- 15.Bartelink H, Overgaard J. Special issue: Late Effects of Normal Tissues (LENT) consensus conference. Radiother Oncol. 1995;35:1–60. [Google Scholar]
- 16.Rubin P, Constine LS, Fajardo LF, et al. Overview: Late Effects of Normal Tissues (LENT) scoring system. Int J Radiat Oncol Biol Phys. 1995;31:1041–1042. doi: 10.1016/0360-3016(95)00057-6. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 18.Osoba D, Aaronson NK, Muller M, et al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5:139–150. doi: 10.1007/BF00435979. [DOI] [PubMed] [Google Scholar]
- 19.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data (ed 2) New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 22.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 23.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 24.Moher D, Schulz KF, Altman DG. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–1194. [PubMed] [Google Scholar]
- 25.DeAngelis LM, Mandell LR, Thaler HT, et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. 1989;24:798–805. doi: 10.1227/00006123-198906000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Smalley SR, Schray MF, Laws ER, Jr, et al. Adjuvant radiation therapy after surgical resection of solitary brain metastasis: Association with pattern of failure and survival. Int J Radiat Oncol Biol Phys. 1987;13:1611–1616. doi: 10.1016/0360-3016(87)90154-4. [DOI] [PubMed] [Google Scholar]
- 27.Smalley SR, Laws ER, Jr, O'Fallon JR, et al. Resection for solitary brain metastasis: Role of adjuvant radiation and prognostic variables in 229 patients. J Neurosurg. 1992;77:531–540. doi: 10.3171/jns.1992.77.4.0531. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong JG, Wronski M, Galicich J, et al. Postoperative radiation for lung cancer metastatic to the brain. J Clin Oncol. 1994;12:2340–2344. doi: 10.1200/JCO.1994.12.11.2340. [DOI] [PubMed] [Google Scholar]
- 29.Fuller BG, Kaplan ID, Adler J, et al. Stereotactic radiosurgery for brain metastases: The importance of adjuvant whole brain irradiation. Int J Radiat Oncol Biol Phys. 1992;23:413–418. doi: 10.1016/0360-3016(92)90762-7. [DOI] [PubMed] [Google Scholar]
- 30.Pirzkall A, Debus J, Lohr F, et al. Radiosurgery alone or in combination with whole-brain radiotherapy for brain metastases. J Clin Oncol. 1998;16:3563–3569. doi: 10.1200/JCO.1998.16.11.3563. [DOI] [PubMed] [Google Scholar]
- 31.Chidel MA, Suh JH, Reddy CA, et al. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:993–999. doi: 10.1016/s0360-3016(00)00527-7. [DOI] [PubMed] [Google Scholar]
- 32.Shehata MK, Young B, Reid B, et al. Stereotactic radiosurgery of 468 brain metastases < or = 2 cm: Implications for SRS dose and whole brain radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:87–93. doi: 10.1016/j.ijrobp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Chougule PB, Burton-Williams M, Saris S, et al. Randomized treatment of brain metastasis with gamma knife radiosurgery, whole brain radiotherapy or both. Int J Radiat Oncol Biol Phys. 2000;48:114a. abstr. [Google Scholar]
- 34.Roos DE, Wirth A, Burmeister BH, et al. Whole brain irradiation following surgery or radiosurgery for solitary brain metastases: Mature results of a prematurely closed randomized Trans-Tasman Radiation Oncology Group trial (TROG 98.05) Radiother Oncol. 2006;80:318–322. doi: 10.1016/j.radonc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 36.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 37.Ueki K, Matsutani M, Nakamura O, et al. Comparison of whole brain radiation therapy and locally limited radiation therapy in the treatment of solitary brain metastases from non-small cell lung cancer. Neurol Med Chir (Tokyo) 1996;36:364–369. doi: 10.2176/nmc.36.364. [DOI] [PubMed] [Google Scholar]
- 38.Soltys SG, Adler JR, Lipani JD, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:187–193. doi: 10.1016/j.ijrobp.2007.06.068. [DOI] [PubMed] [Google Scholar]