Abstract
Purpose
Active surveillance (AS) is an option for the initial management of early-stage prostate cancer. Current risk stratification schema identify patients with low-risk disease who are presumed to be most suitable for AS. However, some men with higher risk disease also elect AS; outcomes for such men have not been widely reported.
Patients and Methods
Men managed with AS at University of California, San Francisco, were classified as low- or intermediate-risk based on serum prostate-specific antigen (PSA), Gleason grade, extent of biopsy involvement, and T stage. Clinical and demographic characteristics, and progression in terms of Gleason score, PSA kinetics, and active treatment were compared between men with low- and intermediate-risk tumors.
Results
Compared to men with low-risk tumors, those with intermediate-risk tumors were older (mean, 64.9 v 62.3 years) with higher mean PSA values (10.9 v 5.1 ng/mL), and more tumor involvement (mean, 20.4% v 15.3% positive biopsy cores; all P < .01). Within 4 years of the first positive biopsy, the clinical risk group did not differ in terms of the proportions experiencing progression-free survival, (low [54%] v intermediate [61%]; log-rank P = .22) or the proportions who underwent active treatment (low [30%] v intermediate [35%]; log-rank P = .88). Among men undergoing surgery, none were node positive and none had biochemical recurrence within 3 years.
Conclusion
Selected men with intermediate-risk features be appropriate candidates for AS, and are not necessarily more likely to progress. AS for these men may provide an opportunity to further reduce overtreatment of disease that is unlikely to progress to advanced cancer.
INTRODUCTION
While prostate cancer remains a leading cause of cancer mortality among American men,1 management is challenging given the variable and often indolent natural history of the disease. As screening with prostate-specific antigen (PSA) has become increasingly commonplace, more cases of early-stage, low-risk prostate cancer have been identified.2 Many low-risk cancers may represent clinically insignificant lesions that if not detected would remain indolent and never progress to symptomatic or lethal disease.3 A substantial majority of men with low-risk disease in fact die of other causes, even under a conservative management strategy.4
Therefore, in light of growing concerns regarding overdiagnosis and subsequent overtreatment of low-risk disease,2 a major goal of recent research efforts has been to identify patients who would benefit from active surveillance (AS) with selective delayed intervention rather than immediate active treatment.5,6 Several groups of investigators have evaluated the appropriateness of AS in patients with established low-risk disease and demonstrated favorable outcomes with intermediate-term follow-up.7,8
However, some men with higher risk disease characteristics may also elect AS, and series reported to date have not determined whether expanding criteria for surveillance to include such men could be viable or safe. At our institution, a large cohort of men is managed with AS. Most patients meet strict criteria for low-risk disease but some have intermediate-risk features (based on Gleason score and/or a multivariable risk prediction score9,10)—these are men who elect AS despite counseling that they may face a greater likelihood of disease progression.7 We sought to determine the extent to which low- and intermediate-risk men differ in terms of risk factors for progression, and whether the rate of cancer progression is in fact higher among the men at intermediate risk.
PATIENTS AND METHODS
As of June 2010, 640 men at University of California, San Francisco (UCSF), have been followed on AS, defined as no active treatment within 6 months after first positive biopsy. Of these, 540 men consented to participate in the prospectively accrued Urologic Oncology Database under supervision of the UCSF institutional review board. Patients were followed on AS with digital rectal examination and PSA measurements at approximately 3-month intervals, transrectal ultrasonography every 6 to 12 months, and follow-up biopsies every 12 to 24 months.7 Staging and grading were based on at least a sextant biopsy (median, 12 cores; interquartile range, 10 to 16) in all patients. Biopsies performed outside UCSF were routinely reviewed by UCSF pathologists.
The UCSF Cancer of the Prostate Risk Assessment (CAPRA) score was calculated based on clinical features at diagnosis: PSA, biopsy Gleason score, age, clinical T stage, and percent of biopsy cores positive.9,10 Validated CAPRA risk groups are 0 to 2 (low), 3 to 5 (intermediate), and 6 to 10 (high).10 Patients in this study were classified as low risk if they had a Gleason sum 2 to 6 and CAPRA score 0 to 2. A CAPRA score of 2 could represent, for example, Gleason 3+3 disease in multiple cores or with PSA in the 6 to 10 ng/mL range. Men considered to have intermediate risk had a Gleason sum 7 or CAPRA score of 3 to 5. Tumors with CAPRA scores in this range may be higher-volume Gleason 3+4 tumors, and/or associated with higher PSAs. Both criteria were applied because a man with low-volume Gleason 3+4 disease and a low PSA level may be considered low risk by CAPRA score, but would frequently be excluded from surveillance cohorts; conversely, a man with low-grade disease but with, for example, a PSA of 9 ng/mL and 40% of cores involved, would meet criteria for inclusion in some surveillance cohorts,5 but would have a higher risk of progression by most multivariable assessments including CAPRA.11 Those with a CAPRA score 6 to 10, Gleason sum 8 to 10, and/or cT3 disease were excluded.
Of the 540 potential participants, 85% had all component data to compute a CAPRA score. The remaining men were missing percentage of positive cores, which was replaced using multiple imputation. All patients were required to have a minimum follow-up of 1 year—unless they underwent active treatment at or after 6 months—and at least one follow-up biopsy or PSA value 6 to 18 months after diagnosis, yielding a sample size of 466. For each participant, the time between the first and last PSA for calculation of kinetics was at least 18 months. We compared the demographic and disease characteristics at diagnosis of low versus intermediate-risk AS patients using χ2 and t-tests, as appropriate.
Our primary outcome was cancer progression, defined as Gleason upgrade to any pattern ≥ 4 on repeat biopsy for those with Gleason score ≤ 6 at diagnosis, or to Gleason ≥ 4+3 for those with Gleason 3+4 at diagnosis; PSA doubling time (PSADT) ≤ 2 years or ≤ 3 years12; or active treatment (ie, surgery, radiation, and/or androgen deprivation therapy). For each risk group, we used Kaplan-Meier analysis to estimate progression-free survival. We compared the two risk groups with respect to each of the three definitions of progression. While the median number of follow-up assessments was similar for both low- and intermediate-risk patients, the number of repeat biopsies varied by risk group. Therefore, we used Poisson regression to estimate the Gleason upgrade incidence rate per group, adjusted for the log of number of biopsies; we adjusted this model for diagnostic age and year. PSADT was calculated as the time after the first measurement until the patient's log(PSA) increased by a factor of 2.12 PSA velocity was also calculated using linear regression to determine the slope of PSA values between 18 months before and 4 years after diagnosis.13
In a supplementary analysis, we assessed the AS patients who subsequently underwent radical prostatectomy (RP; ie, the AS + RP cohort) and compared them with a 2:1 matched cohort of men who underwent RP at UCSF within 6 months after diagnosis. Patients were matched by age and CAPRA score at diagnosis. We compared these groups with respect to incidence of positive margins, stage ≥ T3 disease, and upgrading (in terms of total or primary grade) on surgical pathology from most recent biopsy. All statistical analyses were performed with SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Three hundred seventy-six men had Gleason 2 to 6 and CAPRA 0 to 2 (low risk), and 90 had Gleason 7 and/or CAPRA 3 to 5 (intermediate risk). Two percent of the CAPRA 0 to 2 men had Gleason 3+4 tumors, and 74% of CAPRA 3 to 5 men had Gleason ≤ 6 tumors. Mean age at diagnosis was 62.8 ± 8.1 years (standard deviation). Eighty-two percent of the participants were white. Intermediate risk men were older (64.9 v 62.3 years; P < .01) and had higher PSA values (10.9 v 5.1 ng/mL; P < .01) and greater tumor involvement (20.4% v 15.3% positive biopsy cores; P < .01) than low-risk men. Other clinical characteristics by risk group are described in Table 1. Median follow-up since diagnosis was 47 months (range, 12 to 182 months) for low-risk men and 51 months (range, 14 to 140 months) for intermediate-risk men.
Table 1.
Patient Characteristics at Time of Diagnosis
| Characteristic at Diagnosis | Risk |
P* | |||
|---|---|---|---|---|---|
| Low (n = 376) |
Intermediate (n = 90) |
||||
| No. | % | No. | % | ||
| Median year | 2005 | 2004 | .08 | ||
| Range | 1995-2010 | 1995-2010 | |||
| Median age, years | 62 | 65 | < .01 | ||
| Range | 40-84 | 46-86 | |||
| Race | |||||
| White | 304 | 81 | 77 | 86 | .44 |
| Other | 30 | 8 | 7 | 8 | — |
| Unknown | 42 | 11 | 6 | 7 | — |
| Median biopsy cores taken | 12 | 12 | .02 | ||
| IQR | 10-16 | 9-18 | |||
| Median biopsy cores positive, % | 11 | 13 | < .01 | ||
| IQR | 8-20 | 6-30 | |||
| Gleason grade | |||||
| 2-6 | 376 | 100 | 61 | 68 | < .01 |
| 7 (3 + 4) | — | 27 | 30 | — | |
| 7 (4 + 3) | — | 2 | 2 | — | |
| Biopsy source | |||||
| UCSF biopsy | 84 | 22 | 25 | 28 | .44 |
| Outside biopsy | 290 | 77 | 65 | 72 | — |
| TURP | 2 | 1 | 0 | 0 | — |
| Clinical T stage | |||||
| T1 | 247 | 66 | 56 | 62 | .54 |
| T2 | 129 | 34 | 34 | 38 | — |
| Median PSA, ng/ml | 4.99 | 10.30 | |||
| Range | 0.30-11.00 | 3.14-37.91 | < .01 | ||
| CAPRA score | |||||
| Low (0-2) | 376 | 100 | 7 | 8 | |
| Intermediate (3-5) | — | 83 | 92 | . | |
Abbreviations: IQR, interquartile range; UCSF, University of California, San Francisco; TURP, transurethral resection of the prostate; PSA, prostate-specific antigen; CAPRA, Cancer of the Prostate Risk Assessment.
T-test P for continuous variables; χ2 P for categorical variables.
Twenty-four percent of men underwent diagnostic biopsy at UCSF and 76% at outside institutions; follow-up biopsies were performed at UCSF. Biopsy surveillance of the intermediate group was somewhat less complete: at least one repeat biopsy was performed in 313 low-risk men (83%), with 111 (35%) upgraded to Gleason 7 or higher. In contrast, 63 intermediate-risk patients (70%) underwent rebiopsy, of whom 19 (30%) were upgraded and none were downgraded (Table 2). PSA velocity appeared higher among intermediate- versus low-risk men (mean, 0.32 v 0.14 ng/mL/yr), but this was not statistically significant, nor were differences in the proportions of patients experiencing PSADT ≤ 2 years (7% v 5%; P = .54) or ≤ 3 years (10% v 11%; P = .80). Thirty-percent of low-risk and 35% of intermediate-risk men underwent active treatment within 4 years of diagnosis (log-rank P = .88). Selection of different treatment types did not vary significantly between risk groups (Table 2).
Table 2.
4-Year Disease Progression During Active Surveillance
| Progression | Risk |
P* |
|||
|---|---|---|---|---|---|
| Low (n = 376) |
Intermediate (n = 90) |
||||
| No. | % | No. | % | ||
| Gleason upgrade | |||||
| No | 202 | 65 | 44 | 70 | .42 |
| Yes | 111 | 35 | 19 | 30 | — |
| No repeat biopsy | 63 | 27 | |||
| PSADT | |||||
| Within 24 months | |||||
| No | 357 | 95 | 84 | 93 | .54 |
| Yes | 19 | 5 | 6 | 7 | — |
| Within 36 months | |||||
| No | 335 | 89 | 81 | 90 | .80 |
| Yes | 41 | 11 | 9 | 10 | |
| Active treatment type | |||||
| None | 257 | 68 | 61 | 68 | .92 |
| Radical prostatectomy | 58 | 15 | 16 | 18 | — |
| Radiation | 46 | 12 | 9 | 10 | — |
| Androgen deprivation | 14 | 4 | 4 | 4 | — |
| Chemotherapy/ketoconazole | 1 | < 1 | 0 | 0 | — |
| Upstage at surgery | |||||
| No | 42 | 72 | 8 | 50 | .09 |
| Yes | 16 | 28 | 8 | 50 | — |
| Cumulative Incidence by Time to Event | Low-Risk Cumulative Rate |
Intermediate-Risk Cumulative Rate |
Log-Rank P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Any progression (upgrade/PSADT ≤ 2 years/treatment/pT3), years | |||||
| 1 | 50 | 13 | 6 | 7 | .22 |
| 2 | 104 | 29 | 21 | 24 | — |
| 3 | 136 | 39 | 24 | 28 | |
| 4 | 152 | 46 | 30 | 39 | |
| Any progression (upgrade/PSADT ≤ 3 years/treatment/pT3), years | |||||
| 1 | 51 | 14 | 6 | 7 | .13 |
| 2 | 106 | 29 | 21 | 24 | — |
| 3 | 143 | 41 | 24 | 28 | |
| 4 | 159 | 48 | 30 | 39 | |
Abbreviations: PSADT, prostate-specific antigen doubling time; pT3, pathological stage T3.
T-test P for continuous variables; χ2 P for categorical variables; log-rank P for cumulative incidence.
The number of clinical assessments in follow-up did not vary between the low- and intermediate-risk groups: median assessments were nine (range, three to 32) and nine (range, three to 17), respectively (P = .92). Number of PSAs were likewise similar: median of seven (range, two to 30) and seven (range, two to 13), respectively (P = .55), as were number of biopsies: median of two (range, one to seven) and two (range, one to six), respectively; and duration of follow-up: median of 47 (range, 12 to 182) and 51 months (range, 14 to 140), respectively (P = .59). However low-risk patients were more likely to have at least two repeat biopsies (47%) compared with intermediate-risk patients (37%; P = .01). Median time interval between surveillance biopsies did not vary significantly between the low-risk (13 months) and intermediate-risk groups (14.5 months; P = .2).
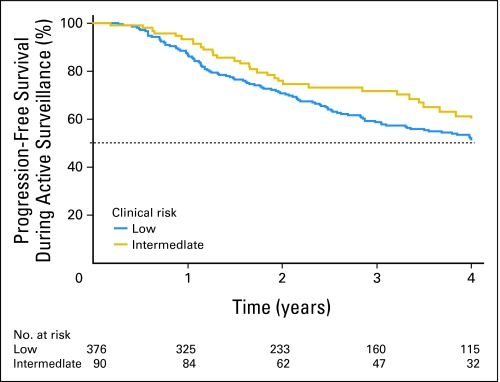
Progression-free survival, defined as no upgrade, no PSADT ≤ 2 years, and no active treatment, did not differ by clinical risk group: 54% of low-risk and 61% of intermediate-risk men were progression free at 4 years (log-rank P = .22; Fig 1). When progression was redefined using the threshold of PSADT ≤ 3 years, 52% of low-risk versus 61% of intermediate-risk men were progression free at 4 years (log-rank P = .13). No association between risk group and progression (using either PSADT definition) was found using Poisson regression modeling adjusted for number of biopsies.
Fig 1.
Kaplan-Meier curves illustrating time to progression for patients with low- and intermediate-risk prostate cancer initially electing active surveillance.
Among 74 men on AS ultimately electing to undergo RP, 58 were low-risk and 16 intermediate-risk. Fifty two (70%) upgraded at repeat biopsy and/or at surgery and 11 (15%) had positive margins (Table 3). Sixteen (28%) low-risk and eight (50%) intermediate-risk had pT3 disease (P = .09). The rate of upgrading from last biopsy to surgical pathology was somewhat higher among patients in the risk-matched immediate RP cohort (42%) compared with the AS + RP cohort (31%; P = .10), while the rate of upstaging to pT3 tended to be lower in the immediate RP cohort (23%) compared to the AS + RP cohort (32%; P = .13). None of the AS + RP cohort patients was node positive. No AS + RP patient, and two immediate RP patient experienced biochemical recurrence by 3 years.
Table 3.
Surgical Pathology Among Men Who Underwent Immediate Surgery (RP) Versus Those Who Underwent Surgery After a Period of AS(AS + RP)
| Surgical Pathology | % |
P* | |
|---|---|---|---|
| AS + RP (n = 74) | RP (n = 148) | ||
| Median months from diagnosis to surgery | 19.5 | 3 | |
| IQR | 14-36 | 3-4 | < .01 |
| Median months of follow-up after start of AS or primary RP | 37.5 | 35.5 | |
| IQR | 27-60 | 23-56 | .25 |
| Gleason grade | |||
| 2-6 | 31 | 49 | .07 |
| 7 (3 + 4) | 55 | 40 | |
| 7 (4 + 3) | 11 | 7 | |
| 8-10 | 3 | 3 | |
| Pathologic T stage, T3 | 32 | 23 | .13 |
| Pathologic N stage, N0 | 22 | 23 | .82 |
| Positive margins, yes | 15 | 9 | .23 |
| Extracapsular extension, yes | 27 | 19 | .17 |
| Seminal vesicle invasion, yes | 7 | 5 | .69 |
Abbreviations: RP, radical prostatectomy; AS, active surveillance; IQR, interquartile range.
T-test P for continuous variables; χ2 P for catagoric variables.
DISCUSSION
Data from the national CaPSURE registry indicated that the percentage of prostate cancers with low-risk characteristics increased from approximately 27% in 1990 to 1994, to 45% in 2004 to 2006.2 A recent analysis based on Surveillance, Epidemiology, and End Results data demonstrated a similar trend.14 During this period, prostate cancer mortality rates at the US population level have fallen by roughly 40%,1 but this gain has come at the cost of overtreatment of many tumors.4 Therefore, with careful risk stratification, a growing subset of men diagnosed with prostate cancer are recognized to be candidates for at least a trial of AS.5,7,15
To date, a number of studies using AS have reported favorable short- to intermediate-term outcomes among men with low-risk disease, including cohorts from the University of Toronto,8 Johns Hopkins University,16 Memorial Sloan-Kettering Cancer Center,17 the Royal Marsden Hospital,18 and UCSF.7 Results from these series have established the viability of AS protocols for selected men, though specific inclusion criteria vary across the institutions. At 3- to 5-year follow-up, men in these studies have generally done well, with 20% to 35% moving to active treatment on the basis of disease progression and/or patient preference. Nonetheless, relatively few men with eligible low-risk disease elect AS, a trend which holds even among older men.2,19–21
The University of Toronto cohort was recently updated with a report on 450 men, including 14% with PSA higher than 10 ng/mL, 17% with Gleason sum 7, and 3% with both risk factors. Just under 9% had clinical stage ≥ T2b. Nineteen percent were intermediate-risk based on PSA higher than 15 ng/mL, Gleason 7, and/or clinical stage T3. Clinical stage, PSA, and Gleason score, as well as PSADT all predicted intervention among those starting with AS. However, the outcomes were not explicitly stratified between low- and intermediate-risk groups.8
At UCSF, the ideal criteria for AS are relatively strict, including PSA lower than 10 ng/mL, no Gleason pattern higher than 3, clinical stage T1-2a, and ≤ 33% of biopsy cores positive. Moreover, at least a sextant biopsy is required, and men with less than a 10-core biopsy at diagnosis are recommended to undergo an immediate extended-template biopsy at UCSF.7 Less stringent criteria tend to be associated with higher rates of upgrading and/or upstaging among those later undergoing surgery.22 However, many men come to our institution interested in AS who do not meet the strict criteria. Such men are advised that their risk of progression may be higher, and indeed, younger men with higher-risk disease (generally high-volume Gleason 3+4 tumors) are explicitly recommended to undergo treatment. If they are strongly motivated, however, and are willing to accept these risks they are offered a trial of AS. Many participate in the Prostate Active Surveillance Study sponsored by the Canary Foundation and the National Cancer Institute's Early Detection Research Network,6 under which clinical data and biospecimens are collected prospectively for future identification of better markers of progression.23
In this study, we defined as intermediate-risk men with CAPRA 3 to 5 prostate cancer—a validated definition of intermediate risk—as well as men with low CAPRA scores, but with Gleason 3+4 disease.7 Early outcomes were favorable for men with both low- and intermediate-risk disease whether measured by upgrading on rebiopsy, PSADT, or incidence of active treatment. Men at intermediate risk had higher rates of adverse pathologic findings after RP. Additional follow-up is needed to determine whether biochemical outcomes are comparable to those among low-risk men.
While high-risk prostate cancer is frequently lethal even among older men, intermediate-risk disease may be marked by a more indolent course, depending in part on how risk is defined. Competing sources of mortality far outweigh prostate cancer in determining outcomes for men with prostate cancer with Gleason score ≤ 7 managed conservatively.4 Data from the Prostate Cancer Prevention Trial demonstrated that 2.3% of the enrolled population of men with PSA levels lower than 4.0 ng/mL harbored prostate cancer with Gleason score ≥ 7.24 The population risk of prostate cancer mortality is 2.8%25; thus many men clearly harbor Gleason 7 histologic tumors which never progress.
Many men with low-volume Gleason 3+4 cancer, particularly those with comorbid conditions, may be appropriate candidates for AS; others are highly motivated to avoid treatment even in the face of higher-risk factors. Patients electing AS, particularly those with intermediate- rather than low-risk disease, are counseled that in many cases AS denotes delayed rather than avoided treatment. During this interval quality of life is presumed to be maintained—though this has not yet been proved prospectively—and patients may benefit from advances in medical care that occur in the interim, including both clinicians' improvement along their learning curves26 and development and dissemination of improved treatments. Conversely, some men experience increasing anxiety on AS even in the absence of objective evidence of progression,27 and the risks of serial prostate biopsy are increasingly recognized.28,29
The most important risk, however, is the likelihood of disease progression during a period of AS. Contemporary studies of AS have found significant rates of upgrading and upstaging among men undergoing RP who meet various sets of criteria for surveillance,22,30 with the rates varying predictably with the specific criteria examined. However, PSA screening identifies cancers during what is typically a years-long lead-time before clinical progression, and the as-yet unanswered question is whether cancer actually progresses during AS or is undersampled by the diagnostic biopsy.31 The latter is presumed to be the more common scenario, highlighting the importance of a high-quality, extended-template biopsy before initiating AS.32
In the Toronto cohort, five men died of prostate cancer, but only one of these had been on AS for more than 2 years, suggesting that in most such cases aggressive tumor biology drives the outcome regardless of timing of intervention.8 A recent analysis from the Swedish cohort of the European Randomized Study of Screening for Prostate Cancer found that men who underwent RP after a mean 2.6-year delay had statistically similar pathologic and biochemical outcomes as those undergoing immediate surgery. The analysis corrected for clinical risk variables but was not randomized and may be subject to acknowledged selection bias.33 Such data suggest—though they do not prove—that a window of opportunity for cure is not commonly missed during a period of AS.
We found that in the short-term, AS may be a viable option for carefully selected men with intermediate-risk prostate cancer, assuming they are counseled regarding their possibly increased risk of progression. Important caveats to this analysis include, most critically, the definitions of progression used. It is more likely for a man in the low-risk group to upgrade to Gleason 3+4 due to resampling than for an intermediate-risk Gleason 3+4 tumor to be further upgraded to 4+3 or higher. Moreover, PSA kinetic measures and the other end points assayed are not uniformly predictive of clinical progression, and the decision for treatment may reflect anxiety and other factors rather than true progression. These definitions reflect the best available for AS cohorts, but we fully acknowledge that they remain arbitrary, and more work must be done to determine which definitions are optimal.34
Gleason grading standards have changed over time; most commonly, cases originally graded in the early 1990s would be upgraded if read to contemporary standards.35 Very few of the cases in this series were diagnosed so long ago, so we do not anticipate that this potential artifact affects our findings. Our database does not include consistent information on the number of prior negative biopsies before prostate cancer diagnosis. It would be expected that men with multiple negative prior biopsies might be less likely to upgrade during early surveillance, but we cannot verify that presumption. Among the small cohort of AS + RP men with intermediate-risk disease, upstaging was relatively common, though early biochemical outcomes in this cohort are reassuring.
Longer-term follow-up of these men beyond the timeframe of this study is required. Equally important, future research must establish better markers of both presumed indolence at diagnosis and of disease progression.6 Ultimately, with improved imaging and/or tissue-based biomarkers, a subset of prostate tumors may be determined to be indolent with sufficient confidence that they will not be called cancer.3 Furthermore, focal rather than radical therapy may become an acceptable option for growing numbers of patients with low-risk cancer, and may alleviate both the morbidity of radical therapy and—at least to an extent—the uncertainty and anxiety associated with surveillance.36 We would hope and anticipate that with better risk assessment through emerging biomarkers and better integration of clinical data,37 together with appropriately selected treatment, the ongoing controversy regarding prostate cancer screening will eventually fade, and men with high-risk disease will not miss the opportunity for early diagnosis and treatment for fear of overtreatment of indolent disease.
Footnotes
Supported by University of California-San Francisco Special Program of Research Excellence Grant No. P50CA89520 from the National Institutes of Health/National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: Kirsten L. Greene, Intuitive Surgical, American Medical Systems Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Matthew R. Cooperberg, Maxwell V. Meng, Peter R. Carroll
Financial support: Peter R. Carroll
Administrative support: Maxwell V. Meng, Peter R. Carroll
Provision of study materials or patients: Matthew R. Cooperberg, Katsuto Shinohara, Maxwell V. Meng, Kirsten L. Greene, Peter R. Carroll
Collection and assembly of data: Janet E. Cowan, Adam C. Reese, Harras B. Zaid, Sima P. Porten, Katsuto Shinohara, Maxwell V. Meng, Kirsten L. Greene, Peter R. Carroll
Data analysis and interpretation: Matthew R. Cooperberg, Janet E. Cowan, Joan F. Hilton, Adam C. Reese, Peter R. Carroll
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Kantoff PW, et al. Contemporary trends in low risk prostate cancer: Risk assessment and treatment. J Urol. 2007;178:S14–19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685–1692. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 4.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dall'Era MA, Cooperberg MR, Chan JM, et al. Active surveillance for early-stage prostate cancer: Review of the current literature. Cancer. 2008;112:1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 6.Newcomb LF, Brooks JD, Carroll PR, et al. Canary Prostate Active Surveillance Study: Design of a multi-institutional active surveillance cohort and biorepository. Urology. 2010;75:407–413. doi: 10.1016/j.urology.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dall'Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112:2664–2670. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 8.Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 9.Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: A straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005;173:1938–1942. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009;101:878–887. doi: 10.1093/jnci/djp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shariat SF, Karakiewicz PI, Roehrborn CG, et al. An updated catalog of prostate cancer predictive tools. Cancer. 2008;113:3075–3099. doi: 10.1002/cncr.23908. [DOI] [PubMed] [Google Scholar]
- 12.Klotz L. Active surveillance with selective delayed intervention using PSA doubling time for good risk prostate cancer. Eur Urol. 2005;47:16–21. doi: 10.1016/j.eururo.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Nam RK, Klotz LH, Jewett MA, et al. Prostate specific antigen velocity as a measure of the natural history of prostate cancer: Defining a ‘rapid riser’ subset. Br J Urol. 1998;81:100–104. doi: 10.1046/j.1464-410x.1998.00523.x. [DOI] [PubMed] [Google Scholar]
- 14.Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101:1280–1283. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastian PJ, Carter BH, Bjartell A, et al. Insignificant prostate cancer and active surveillance: From definition to clinical implications. Eur Urol. 2009;55:1321–1330. doi: 10.1016/j.eururo.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Carter HB, Walsh PC, Landis P, et al. Expectant management of nonpalpable prostate cancer with curative intent: Preliminary results. J Urol. 2002;167:1231–1234. [PubMed] [Google Scholar]
- 17.Patel MI, DeConcini DT, Lopez-Corona E, et al. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol. 2004;171:1520–1524. doi: 10.1097/01.ju.0000118224.54949.78. [DOI] [PubMed] [Google Scholar]
- 18.Ulmert D, Serio AM, O'Brien MF, et al. Long-term prediction of prostate cancer: Prostate-specific antigen (PSA) velocity is predictive but does not improve the predictive accuracy of a single PSA measurement 15 years or more before cancer diagnosis in a large, representative, unscreened population. J Clin Oncol. 2008;26:835–841. doi: 10.1200/JCO.2007.13.1490. [DOI] [PubMed] [Google Scholar]
- 19.Miller DC, Gruber SB, Hollenbeck BK, et al. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98:1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 20.Harlan SR, Cooperberg MR, Elkin EP, et al. Time trends and characteristics of men choosing watchful waiting for initial treatment of localized prostate cancer: Results from CaPSURE. J Urol. 2003;170:1804–1807. doi: 10.1097/01.ju.0000091641.34674.11. [DOI] [PubMed] [Google Scholar]
- 21.Barocas DA, Cowan JE, Smith JA, Jr, et al. What percentage of patients with newly diagnosed carcinoma of the prostate are candidates for surveillance? An analysis of the CaPSURE database. J Urol. 2008;180:1330–1334. doi: 10.1016/j.juro.2008.06.019. discussion 180:1334-1335, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Conti SL, Dall'era M, Fradet V, et al. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181:1628–1633. doi: 10.1016/j.juro.2008.11.107. discussion 181:1633-1634, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Pepe MS, Feng Z, Janes H, et al. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: Standards for study design. J Natl Cancer Inst. 2008;100:1432–1438. doi: 10.1093/jnci/djn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 25.Altekruse S, Kosary C, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2010. SEER Cancer Statistics Review, 1975-2007. [Google Scholar]
- 26.Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171–1177. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 27.Latini DM, Elkin EP, Cooperberg MR, et al. Differences in clinical characteristics and disease-free survival for Latino, African American, and non-Latino white men with localized prostate cancer: Data from CaPSURE. Cancer. 2006;106:789–795. doi: 10.1002/cncr.21675. [DOI] [PubMed] [Google Scholar]
- 28.Fujita K, Landis P, McNeil BK, et al. Serial prostate biopsies are associated with an increased risk of erectile dysfunction in men with prostate cancer on active surveillance. J Urol. 2009;182:2664–2669. doi: 10.1016/j.juro.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 29.Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010;183:963–968. doi: 10.1016/j.juro.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 30.Smaldone MC, Cowan JE, Carroll PR, et al. Eligibility for active surveillance and pathological outcomes for men undergoing radical prostatectomy in a large, community based cohort. J Urol. 2010;183:138–143. doi: 10.1016/j.juro.2009.08.152. [DOI] [PubMed] [Google Scholar]
- 31.Suardi N, Capitanio U, Chun FK, et al. Currently used criteria for active surveillance in men with low-risk prostate cancer: An analysis of pathologic features. Cancer. 2008;113:2068–2072. doi: 10.1002/cncr.23827. [DOI] [PubMed] [Google Scholar]
- 32.San Francisco IF, DeWolf WC, Rosen S, et al. Extended prostate needle biopsy improves concordance of Gleason grading between prostate needle biopsy and radical prostatectomy. J Urol. 2003;169:136–140. doi: 10.1016/S0022-5347(05)64053-0. [DOI] [PubMed] [Google Scholar]
- 33.van den Bergh RC, Steyerberg EW, Khatami A, et al. Is delayed radical prostatectomy in men with low-risk screen-detected prostate cancer associated with a higher risk of unfavorable outcomes? Cancer. 2010;116:1281–1290. doi: 10.1002/cncr.24882. [DOI] [PubMed] [Google Scholar]
- 34.Whitson JM, Carroll PR. Active surveillance for early-stage prostate cancer: Defining the triggers for intervention. J Clin Oncol. 2010;28:2807–2809. doi: 10.1200/JCO.2010.28.5817. [DOI] [PubMed] [Google Scholar]
- 35.Smith EB, Frierson HF, Jr, Mills SE, et al. Gleason scores of prostate biopsy and radical prostatectomy specimens over the past 10 years: Is there evidence for systematic upgrading? Cancer. 2002;94:2282–2287. doi: 10.1002/cncr.10457. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed HU, Emberton M. Active surveillance and radical therapy in prostate cancer: Can focal therapy offer the middle way? World J Urol. 2008;26:457–467. doi: 10.1007/s00345-008-0317-5. [DOI] [PubMed] [Google Scholar]
- 37.Chun FK, Haese A, Ahyai SA, et al. Critical assessment of tools to predict clinically insignificant prostate cancer at radical prostatectomy in contemporary men. Cancer. 2008;113:701–709. doi: 10.1002/cncr.23610. [DOI] [PubMed] [Google Scholar]