Abstract
Purpose
Older men are more likely to be diagnosed with high-risk prostate cancer and to have lower overall survival. As a result, age often plays a role in treatment choice. However, the relationships among age, disease risk, and prostate cancer–specific survival have not been well established.
Patients and Methods
We studied men in the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) database with complete risk, treatment, and follow-up information. High-risk patients were identified by using the validated Cancer of the Prostate Risk Assessment (CAPRA) score. Competing risks regression was used to identify the independent impact of age on cancer-specific survival. We also analyzed the effect of local treatment on survival among older men with high-risk disease.
Results
In all, 26% of men age ≥ 75 years presented with high-risk disease (CAPRA score 6 to 10). Treatment varied markedly with age across risk strata; older men were more likely to receive androgen deprivation monotherapy. Controlling for treatment modality alone, or for treatment and risk, age did not independently predict cancer-specific survival. Furthermore, controlling for age, comorbidity, and risk, older men with high-risk tumors receiving local therapy had a 46% reduction in mortality compared with those treated conservatively.
Conclusion
Older patients are more likely to have high-risk prostate cancer at diagnosis and less likely to receive local therapy. Indeed, underuse of potentially curative local therapy among older men with high-risk disease may in part explain observed differences in cancer-specific survival across age strata. These findings support making decisions regarding treatment on the basis of disease risk and life expectancy rather than on chronologic age.
INTRODUCTION
By 2030, almost 20% of men in the United States will be older than age 65 years compared with 12% in 2000.1 Overall life expectancy continues to improve, with the average life expectancy of a 70-year-old man now reaching 13 years.2 Prostate cancer incidence increases with advancing age; thus, it can be anticipated that the prevalence of prostate cancer in men older than age 65 years will continue to increase. Prostate cancer already is the most common malignancy among older men3; 64% of new prostate cancer cases in the United States were diagnosed in men older than age 65 years, and 23% in men older than age 75 years.4
Despite the predicted increase in prevalence of disease in the elderly, most studies investigating optimal treatment regimens have focused on men younger than 75 years of age. Ongoing prospective studies investigating the utility of prostate cancer screening exclude patients older than age 75 years.5,6 Likewise, the US Preventive Services Task Force (USPSTF) explicitly recommends against screening men age 75 years or older—yet this statement is based in large part on extrapolations from studies of patients younger than age 75 years and does not account for health status or comorbidities.7 Furthermore, nomograms predicting outcomes after prostatectomy are principally derived from datasets with a median age < 65 years.8 Reports of treatment trends have found high prevalence of both overtreatment9 and undertreatment10 of older men diagnosed with prostate cancer. Patient age is thus a frequent source of disparity both in treatment practice and in clinical trial recruitment and participation.11,12
Older men in the United States are more likely to be diagnosed with high-risk prostate cancer and to have lower overall and cancer-specific survival.8,12–14 However, the evolution and progression of cancer of a given grade and stage should be expected to occur independent of chronologic age. Therefore, variation in lead-time at diagnosis and in management may explain, at least in part, observed differences in cancer-specific survival. Indeed, patient age is known to strongly influence treatment decision making, with older men less likely to receive potentially curative therapy.15 However, the independent impact of age on prostate cancer–specific survival has not been well established.
Recent studies8,16 have shown that with careful patient selection, older men up to age 75 years with Gleason score 5 to 7 prostate cancer and up to age 80 years with Gleason score 8 to 10 disease who undergo radical prostatectomy or radiotherapy have gains in life expectancy and quality-adjusted life expectancy comparable with those of younger men, raising the question of whether age per se drives outcomes and, in turn, whether it should drive treatment selection. We therefore sought to understand whether age plays a role in disease risk and prostate cancer–specific survival in a large, prospective patient cohort. We hypothesized that with adequate control for disease risk characteristics and treatment, the impact of age on cancer-specific survival may attenuate, and furthermore that variation in treatment driven by age rather than risk may explain observed survival differences.
PATIENTS AND METHODS
Disease Registry
Data for analysis were abstracted from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE), a longitudinal, observational disease registry of men with biopsy-proven prostate cancer, recruited from 40 community-based and academic urology practices throughout the United States. The database includes demographic, clinical, treatment, and follow-up outcome data on 13,805 patients as of July 2008. Comorbidities were recorded at baseline and follow-up, and a comorbidity score based on the Charlson index was recorded.17 Informed consent was obtained from each patient under institutional review board approval, and patients were followed until death or withdrawal from the study. Clinicians reported mortality events, copies of state death certificates were obtained, and the National Death Index was queried annually to identify date and cause of death for men lost to follow-up or whose certificates were not available. Detailed descriptions of the CaPSURE project methods can be found elsewhere.18–20
Study Design
Patients included in the analysis had localized disease (clinical stage up to T3aN0M0); complete risk, treatment, and age data available; comorbidity and ethnicity information available; and at least 6 months of follow-up after treatment. To determine whether age impacts disease severity, patient age was categorized as ≤ 55, 56 to 65, 66 to 75, and > 75 years at the time of diagnosis. Risk was determined by the Kattan preoperative nomogram21 and the University of California-San Francisco Cancer of the Prostate Risk Assessment (CAPRA) score.22 CAPRA scores of 0 to 2, 3 to 5, and 6 to 10 defined low-, intermediate-, and high-risk groups, respectively, as previously validated.22 Treatment modalities included radical prostatectomy (RP), cryotherapy, brachytherapy, electron beam radiation therapy (EBRT), primary androgen deprivation therapy (PADT), and watchful waiting/active surveillance (WW/AS).
Statistical Analysis
Logistic regression was used to determine the likelihood of receiving any local treatment (RP, EBRT, brachytherapy, or cryotherapy) by age at diagnosis, controlling for CAPRA score and year of treatment. The Pearson χ2 test was used to compare risk stratification and treatment patterns among men of various age groups. We assessed the relationships between age and both cancer-specific survival and overall survival via univariate Kaplan-Meier survival analysis. Fine and Gray's competing risks regression23 was performed to identify independent predictors of prostate cancer–specific survival while adjusting for age, year of treatment, treatment modality, and risk as determined by the CAPRA or Kattan score. Subhazard ratios (SHRs) were calculated with their 95% CIs.
We further analyzed the impact of local treatment on cancer-specific survival and overall survival among older men (defined as either ≥ 70 or ≥ 75 years old) with high-risk disease (CAPRA score of 6 to 10). We used competing risks regression for cancer-specific survival and standard Cox proportional hazards analysis for overall survival. Both analyses were adjusted for prostate-specific antigen (PSA), Gleason score, percent of biopsy cores positive, clinical stage, and year of treatment; the all-cause mortality analysis was additionally adjusted for comorbidity. All statistical tests were two-sided, and analyses were performed by using STATA version 11 (STATA, College Station, TX).
RESULTS
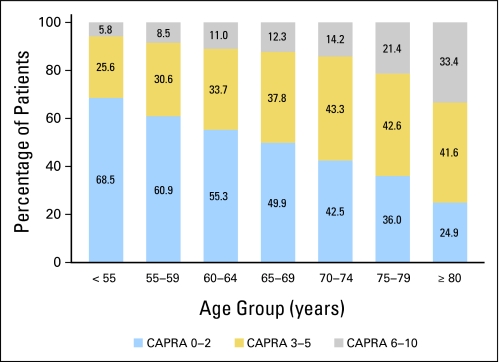
In our analysis, of the 13,805 men in CaPSURE, 12,286 had known primary treatment and at least 6 months of subsequent follow-up; of these, 496 had nonlocalized disease and were excluded, leaving 11,790. Baseline characteristics of the study sample are provided in Table 1. There was no consistent association between age and likelihood of missing data (11.4%, 20.0%, 10.1%, and 11.9% for men age ≤ 55, 56 to 65, 66 to 75, and > 75 years, respectively). Mean ± standard deviation age in the overall cohort was 66.2 ± 8.6 years, and median age was 66 years. Of these men 1,411 (12.0%) were age ≤ 55 years at the time of treatment, 4,005 (34.0%) were age 56 to 65, 4,667 (39.6%) were age 66 to 75, and 1,707 (14.5%) were older than age 75 years. The likelihood of high-risk disease by CAPRA classification increased significantly with increasing age cohort (P < .001 by Mantel-Haenszel χ2; Fig 1).
Table 1.
Baseline Study Sample Characteristics
| Characteristic | Age Group (years) |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤ 55 |
56-65 |
66-75 |
> 75 |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Race/ethnicity | ||||||||||
| Latino | 26 | 2 | 74 | 2 | 82 | 2 | 13 | 1 | 195 | 2 |
| African American | 200 | 14 | 508 | 13 | 429 | 9 | 79 | 5 | 1,216 | 10 |
| White | 1,158 | 82 | 3,336 | 83 | 4,083 | 87 | 1,585 | 93 | 10,162 | 86 |
| Other | 27 | 2 | 87 | 2 | 73 | 2 | 30 | 2 | 217 | 2 |
| Comorbidities | ||||||||||
| 0 | 308 | 22 | 646 | 16 | 528 | 11 | 142 | 8 | 1,624 | 14 |
| 1 | 352 | 25 | 895 | 22 | 931 | 20 | 264 | 15 | 2,442 | 21 |
| 2 | 220 | 16 | 740 | 18 | 995 | 21 | 362 | 21 | 2,317 | 20 |
| 3 | 102 | 7 | 437 | 11 | 708 | 15 | 295 | 17 | 1,542 | 13 |
| > 3 | 51 | 4 | 340 | 8 | 574 | 12 | 276 | 16 | 1,241 | 11 |
| Missing | 378 | 27 | 947 | 24 | 931 | 20 | 368 | 22 | 2,624 | 22 |
| PSA at diagnosis, ng/mL | ||||||||||
| 0-10 | 1,121 | 79 | 2,908 | 73 | 3,056 | 65 | 864 | 51 | 7,949 | 67 |
| > 10-20 | 136 | 10 | 513 | 13 | 818 | 18 | 425 | 25 | 1,892 | 16 |
| > 20-30 | 19 | 1 | 105 | 3 | 209 | 4 | 113 | 7 | 446 | 4 |
| > 30 | 44 | 3 | 178 | 4 | 243 | 5 | 177 | 10 | 642 | 5 |
| Missing | 91 | 6 | 301 | 7 | 341 | 7 | 128 | 8 | 861 | 7 |
| Gleason score | ||||||||||
| 2-6 | 1,011 | 72 | 2,623 | 65 | 2,813 | 60 | 805 | 47 | 7,252 | 62 |
| 3 + 4 | 213 | 15 | 613 | 15 | 732 | 16 | 318 | 19 | 1,876 | 16 |
| 4 + 3 | 71 | 5 | 278 | 7 | 406 | 9 | 188 | 11 | 943 | 8 |
| 8-10 | 67 | 5 | 272 | 7 | 413 | 9 | 262 | 15 | 1,014 | 9 |
| Missing | 49 | 3 | 219 | 5 | 303 | 6 | 134 | 8 | 705 | 6 |
| Clinical stage | ||||||||||
| T1 | 744 | 53 | 1,959 | 49 | 2,034 | 44 | 630 | 37 | 5,367 | 46 |
| T2a/b | 357 | 25 | 1,090 | 27 | 1,404 | 30 | 536 | 31 | 3,387 | 29 |
| T2c | 231 | 16 | 673 | 17 | 857 | 18 | 406 | 24 | 2,167 | 18 |
| T3a | 16 | 1 | 69 | 2 | 132 | 3 | 60 | 4 | 277 | 2 |
| Missing | 64 | 4 | 214 | 5 | 240 | 5 | 75 | 4 | 592 | 5 |
| Percent positive biopsy | ||||||||||
| ≤ 10 | 156 | 11 | 424 | 11 | 514 | 11 | 159 | 9 | 1,253 | 11 |
| 11-33 | 514 | 36 | 1,364 | 34 | 1,534 | 33 | 494 | 29 | 3,906 | 33 |
| 34-50 | 386 | 27 | 980 | 24 | 1,111 | 24 | 411 | 24 | 2,888 | 25 |
| 51-75 | 114 | 8 | 368 | 9 | 418 | 9 | 155 | 9 | 1,055 | 9 |
| > 75 | 128 | 9 | 432 | 11 | 504 | 11 | 220 | 13 | 1,284 | 11 |
| Missing | 113 | 8 | 437 | 11 | 586 | 13 | 268 | 16 | 1,404 | 12 |
| Treatment | ||||||||||
| RP | 1,197 | 85 | 2,801 | 70 | 1,810 | 39 | 47 | 3 | 5,855 | 50 |
| Cryotherapy | 15 | 1 | 129 | 3 | 263 | 6 | 51 | 3 | 458 | 4 |
| Brachytherapy | 81 | 6 | 441 | 11 | 789 | 17 | 241 | 14 | 1,552 | 13 |
| EBRT | 45 | 3 | 290 | 7 | 804 | 17 | 350 | 21 | 1,489 | 13 |
| PADT | 49 | 3 | 232 | 6 | 661 | 14 | 696 | 41 | 1,638 | 14 |
| WW | 24 | 2 | 112 | 3 | 340 | 7 | 322 | 19 | 798 | 7 |
| Total | 1,411 | 4,005 | 4,667 | 1,707 | 11,790 | |||||
Abbreviations: PSA, prostate-specific antigen; RP, radical prostatectomy; EBRT, external-beam radiation therapy; PADT, primary androgen deprivation therapy; WW, watchful waiting.
Fig 1.
Distribution of disease risk by age at diagnosis. Proportion of patients in each risk category are given by age stratum; risk is defined by validated groupings of the Cancer of the Prostate Risk Assessment (CAPRA) scores 0 to 2, 3 to 5, or 6 to 10.
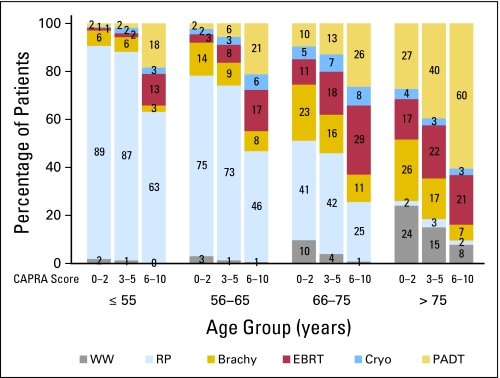
We observed substantial variation in treatment based on patient age and disease risk (Fig 2); in general, treatment varied to a greater extent with age at diagnosis than with cancer risk. Older men in particular were more likely to be treated with PADT than their younger counterparts, regardless of their risk score. Furthermore, men with high-risk disease were more likely to receive PADT than their age-matched low-risk counterparts. Men with increasing age were less likely to receive local therapy in general, and surgical treatment in particular, regardless of disease risk. Compared with men age ≤ 55 years, and adjusting for CAPRA score and year of treatment, the odds ratios for receiving local treatment were 0.59 (95% CI, 0.43 to 0.81), 0.21 (95% CI, 0.15 to 0.28), and 0.04 (95% CI, 0.03 to 0.06) for men age 56 to 65, 66 to 75, and > 75 years, respectively.
Fig 2.
Distribution of treatment modality by Cancer of the Prostate Risk Assessment (CAPRA) risk score and by age cohort. For patients in each age stratum within each risk group (defined by CAPRA scores 0 to 2, 3 to 5, or 6 to 10), distribution among treatments is given. WW, watchful waiting; RP, radical prostatectomy; Brachy, brachytherapy; EBRT, external-beam radiation therapy; Cryo, cryotherapy; PADT, primary androgen deprivation therapy.
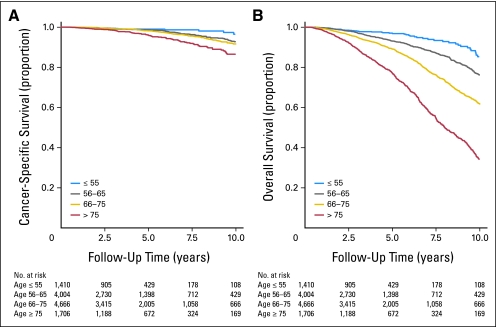
Age at diagnosis was a univariate predictor of both overall and prostate cancer–specific mortality; unadjusted Kaplan-Meier survival curves showed decreasing survival rates among men with increasing age (log-rank P < .001; Fig 3). On competing risks analysis controlling only for year of treatment, age as a continuous variable was a predictor of cancer-specific mortality (SHR, 1.02; 95% CI, 1.01 to 1.03; P < .01). When age was analyzed as a categorical variable, there was a consistent trend toward increased cancer-specific mortality with increasing age, but this did not reach statistical significance until men older than age 75 years were compared with those age ≤ 55 years (Table 2).
Fig 3.
Unadjusted Kaplan-Meier plots of cancer-specific and overall survival by age stratum.
Table 2.
Prostate Cancer–Specific Mortality by Age: Univariate Analysis
| Age (years) | Competing Risks Regression |
||
|---|---|---|---|
| Hazard Ratio | 95% CI | P | |
| ≤ 55 | Reference | ||
| 56-65 | 1.36 | 0.86 to 2.13 | .19 |
| 66-75 | 1.43 | 0.92 to 2.21 | .11 |
| > 75 | 1.86 | 1.17 to 2.97 | .009 |
On multivariable competing risks analysis, however, controlling either for treatment modality alone or for treatment modality and disease risk, age was no longer a predictor of prostate cancer–specific survival (Table 3). When controlling for treatment modality alone, all four age cohorts shared equal outcomes in terms of survival despite differences among specific treatments. When further adjusting for risk as well as year and treatment, the impact of age on prostate cancer–specific survival was further reduced. Of note, both CAPRA and Kattan risk scores were continuous predictors of mortality (hazard ratio [HR], 1.39; 95% CI, 1.32 to 1.47 and HR, 0.98; 95% CI, 0.97 to 0.98, respectively; both P < .001).
Table 3.
Multivariate Competing Risks Regression Analysis Controlling for Treatment Modality Alone or Treatment Modality and Cancer Risk by Age Cohort (CAPRA or Kattan)
| Age (years) | Age, Year |
Age, Year, Treatment |
Age, Year, Treatment,CAPRA Score |
Age, Year, Treatment,Kattan Score |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SHR | 95% CI | P | SHR | 95% CI | P | SHR | 95% CI | P | SHR | 95% CI | P | |
| ≤ 55 | Ref | Ref | Ref | Ref | ||||||||
| 56-65 | 1.36 | 0.86 to 2.13 | .19 | 1.29 | 0.82 to 2.03 | .27 | 1.19 | 0.66 to 2.14 | .57 | 0.99 | 0.96 to 1.02 | .50 |
| 66-75 | 1.44 | 0.93 to 2.23 | .10 | 1.10 | 0.69 to 1.74 | .70 | 0.93 | 0.51 to 1.71 | .82 | 1.06 | 0.58 to 1.95 | .85 |
| > 75 | 1.91 | 1.20 to 3.05 | .01 | 1.01 | 0.60 to 1.70 | .98 | 0.81 | 0.41 to 1.62 | .56 | 1.03 | 0.56 to 1.91 | .91 |
Abbreviations: CAPRA, Cancer of the Prostate Risk Assessment; SHR, subhazard ratio; Ref, reference.
Among 629 men ≥ 70 years old with high-risk (CAPRA score 6 to 10) disease, 275 (44%) died during follow-up at a median of 5.7 years; 57 (21% of decedents) died of prostate cancer. Among 392 men ≥ 75 years old with high-risk disease, 183 (47%) died during follow-up at a median of 5.3 years; 36 (20% of decedents) died of prostate cancer. Receipt of local therapy among older men with high-risk tumors was strongly associated with decreased mortality, although the association was not statistically significant for cancer-specific mortality among men older than age 75 years. After controlling for tumor risk and year of treatment, the SHR for cancer-specific mortality was 0.54 (95% CI, 0.32 to 0.92) for men age ≥ 70 years and 0.61 (95% CI, 0.30 to 1.25) for men age ≥ 75 years. After controlling for these variables in addition to comorbidity, the HR for all-cause mortality after local therapy was 0.54 (95% CI, 0.41 to 0.72) for men age ≥ 70 years and 0.58 (95% CI, 0.40 to 0.84) for men age ≥ 75 years. There were no differences in survival between men receiving PADT and those receiving WW/AS in any analysis.
DISCUSSION
With increasing age, men are significantly more likely to have high-risk prostate cancer. There is common use of PADT and WW/AS among these older men and less local therapy, particularly RP, compared with younger men. On univariate analysis, age predicted overall and prostate cancer–specific survival. However, when controlling for either treatment modality alone or treatment modality and risk, age was not an independent predictor of mortality from prostate cancer. These findings suggest that under-use of potentially curative local therapy among older men with high-risk disease may in part explain higher cancer-specific mortality rates observed with increasing age.
Overall survival is of course lower in patients of increasing age, reflecting the impact of other variables including comorbidities, increased susceptibility to major illness, and decreased immune response. However, our data support the hypothesis that prostate cancer evolution, and hence mortality, occurs not on the basis of patient age but instead on the basis of cellular mechanisms whose malignant potential can be categorized by established disease risk features. Therefore, older patients with clinically localized, high-risk disease, and a noncancer life expectancy of > 10 years should be considered for surgical treatment and/or radiation therapy. Recent studies have shown that with careful patient selection, gains in life expectancy in men older than age 70 years following radical or laparoscopic prostatectomy are comparable to those in younger men.8,16 Indeed, comorbidity has been found to be a more important predictor of surgical complications than age.24 Another recent report20 found that among men with higher-risk disease, mortality outcomes are improved following RP with either EBRT or PADT, findings that held with adjustment for age.
Our findings suggest a serious discrepancy among older patients between actual treatment practices and optimal treatment. Optimal treatment should be based primarily on disease risk and other clinical factors rather than chronologic age, yet actual treatment practices appear to reflect variation driven rather by physician practice style and patient age. In our analysis, the relatively few older men with high-risk disease who received local therapy had nearly a 50% reduction in risk-adjusted mortality compared with those in the same age cohort who received PADT or WW/AS. However, most older men were more likely to receive PADT, regardless of disease risk category, although the benefit of hormonal therapy in localized cancer is unclear4,25 and PADT is associated with potential musculoskeletal, cardiovascular, and other adverse effects.26,27
Multiple previous studies have similarly reported that older men were less likely to undergo the potentially curative treatments of RP or radiotherapy, regardless of disease risk and comorbidities.12,28–30 Men younger than 60 years of age are 25 times more likely to receive RP than men older than 70 years of age, likely due in part to physician tendencies to avoid surgery in older men.31,32 Indeed, individual physician preferences have been shown to play a significant role in determining whether patients receive PADT or any potentially life-prolonging treatment.31–34 Prior reports found that more than 15% of men older than age 75 years with high-risk disease were undertreated, and a majority never received curative therapy for their prostate cancer.10,35 Our results further suggest that prostate cancer survival differences across age strata are influenced by treatment decisions, which are themselves driven by age, not disease risk.
An earlier analysis12 of more than 2,000 men older than age 75 years in CaPSURE found that patient comorbidities and tumor-risk characteristics did not play a substantial role in treatment decision making. Similarly, in a study of PSA screening rates in US Veterans Affairs patients,36 among men older than age 85 years, those in the best health were screened less frequently than their counterparts in the worst health. The USPSTF recommendation against screening all men older than age 75 years also fails to consider comorbidity; such a broad statement could potentially harm older men in otherwise good health by missing treatable high-risk tumors.37 We found that within 5 years of follow-up of high-grade disease among men older than age 75 years, cancer-specific mortality reaches 20%; similar findings from other databases have been reported elsewhere.38
The estimation of life expectancy remains a difficult task, with both individual clinicians and life-table nomograms suffering from suboptimal accuracy.39,40 However, comorbidity and risk assessment remain critical to determining appropriate disease management to minimize both overtreatment of low-risk disease and undertreatment of high-risk disease. Overestimation of life expectancy can lead to overtreatment of lower-risk prostate cancer, especially in elderly men.14,34 Conversely, our data suggest that undertreatment, or unnecessary treatment with suboptimal therapy, is an equally important problem. The use of PADT increases dramatically with age despite a lack of evidence to support its efficacy as a monotherapy for localized disease.4,9 Developing more stringent criteria to help guide treatment in elderly men may enable a more uniform standard of care that could decrease morbidity and prevent unnecessary therapy.
The limitations of our study deserve mention. Comorbidity reporting is based on self-report and may not completely reflect the impact of comorbid illness on overall survival for each patient. However, the concordance of the proportional hazards and competing risks analyses is reassuring in this regard. Identifying cancer-specific mortality as determined by review of death certificates is limited by the thoroughness of information documented on the certificates, which in turn relies on the individual physician's knowledge of each patient's history. As a result, the database may underestimate mortality from prostate cancer because of the presence of other comorbidities and/or toxicities of treatment. It is important to consider why a given tumor was detected relatively late in life: a tumor detected in the context of a slowly rising PSA and prior negative biopsies may be quite different biologically from one detected in an older man who had never been screened previously. However, the focus of the CaPSURE registry is on management, not screening, so the database includes a variable depth of information on prediagnosis PSA values. Therefore, the impact of the intensity of prior screening on disease risk at diagnosis cannot be ascertained by using this database.
CaPSURE comprises data from community-based and academic urology practice sites across the country, but these sites were not chosen at random and the population cannot be assumed to represent a statistically valid sample of the US prostate cancer population. African Americans, for example, are moderately represented in the database, but other ethnic groups are underrepresented compared with the larger population. In addition, CaPSURE patients tend to have a higher socioeconomic status on average compared with the overall population.22 A total of 1,519 patients (11%) were excluded because of incomplete treatment and/or follow-up data. These gaps in the data are attributable to variability among the large number of physicians at multiple sites submitting data to the registry. There is no indication that the missing data are nonrandomly distributed across the age strata.
When diagnosed with prostate cancer, older men are more likely to have high-risk disease and are more likely to be treated with PADT rather than potentially curative local therapy. Older men also have lower overall survival. However, when controlling for treatment modality alone, or treatment modality and cancer risk category, age is not an independent predictor of prostate cancer–specific survival. These results may be due in part to differences in treatment by age group; older men are more likely to be treated by PADT or WW/AS. Those with high-risk tumors who receive local therapy have a 46% reduction in risk-adjusted mortality compared with men managed conservatively. These findings highlight the importance of making treatment decisions guided by disease risk and life expectancy rather than chronologic age. Most older men with low-risk disease are candidates for active surveillance, but selected patients with more aggressive tumors should not be denied the opportunity for potentially curative local therapy.
Footnotes
CaPSURE is supported by Abbott Laboratories, Chicago, IL, and by Grant No. P50CA89520 from the National Institutes of Health/National Cancer Institute University of California-San Francisco Special Program of Research Excellence.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Peter R. Carroll, Takeda Pharmaceuticals; Matthew R. Cooperberg, Takeda Pharmaceuticals, Abbott Laboratories Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Peter R. Carroll, Matthew R. Cooperberg
Financial support: Peter R. Carroll
Administrative support: Peter R. Carroll
Provision of study materials or patients: Peter R. Carroll
Data analysis and interpretation: Seth K. Bechis, Matthew R. Cooperberg
Manuscript writing: Seth K. Bechis, Peter R. Carroll, Matthew R. Cooperberg
Final approval of manuscript: Seth K. Bechis, Peter R. Carroll, Matthew R. Cooperberg
REFERENCES
- 1.Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62:3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Minino AM, Smith BL. National Vital Statistics Reports, Vol 49, No. 12. Hyattsville, MD: National Center for Health Statistics; 2001. Deaths: Preliminary data for 2000; pp. 1–40. http://www.cdc.gov/nchs/data/nvsr/nvsr49/nvsr49_12.pdf. [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Heinzer H, Steuber T. Prostate cancer in the elderly. Urol Oncol. 2009;27:668–672. doi: 10.1016/j.urolonc.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 6.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Preventive Services Task Force: Screening for prostate cancer. U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–191. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 8.Richstone L, Bianco FJ, Shah HH, et al. Radical prostatectomy in men aged > or = 70 years: Effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 2008;101:541–546. doi: 10.1111/j.1464-410X.2007.07410.x. [DOI] [PubMed] [Google Scholar]
- 9.Cooperberg MR, Lubeck DP, Meng MV, et al. The changing face of low-risk prostate cancer: Trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz KL, Alibhai SM, Tomlinson G, et al. Continued undertreatment of older men with localized prostate cancer. Urology. 2003;62:860–865. doi: 10.1016/s0090-4295(03)00690-3. [DOI] [PubMed] [Google Scholar]
- 11.Townsley C, Pond GR, Peloza B, et al. Analysis of treatment practices for elderly cancer patients in Ontario, Canada. J Clin Oncol. 2005;23:3802–3810. doi: 10.1200/JCO.2005.06.742. [DOI] [PubMed] [Google Scholar]
- 12.Konety BR, Cowan JE, Carroll PR. Patterns of primary and secondary therapy for prostate cancer in elderly men: Analysis of data from CaPSURE. J Urol. 2008;179:1797–1803. doi: 10.1016/j.juro.2008.01.044. discussion 1803. [DOI] [PubMed] [Google Scholar]
- 13.Ketchandji M, Kuo YF, Shahinian VB, et al. Cause of death in older men after the diagnosis of prostate cancer. J Am Geriatr Soc. 2009;57:24–30. doi: 10.1111/j.1532-5415.2008.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manton KG, Vaupel JW. Survival after the age of 80 in the United States, Sweden, France, England, and Japan. N Engl J Med. 1995;333:1232–1235. doi: 10.1056/NEJM199511023331824. [DOI] [PubMed] [Google Scholar]
- 15.Stangelberger A, Waldert M, Djavan B. Prostate cancer in elderly men. Rev Urol. 2008;10:111–119. [PMC free article] [PubMed] [Google Scholar]
- 16.Greco KA, Meeks JJ, Wu S, et al. Robot-assisted radical prostatectomy in men aged > or =70 years. BJU Int. 2009;104:1492–1495. doi: 10.1111/j.1464-410X.2009.08718.x. [DOI] [PubMed] [Google Scholar]
- 17.Marr PL, Elkin EP, Arredondo SA, et al. Comorbidity and primary treatment for localized prostate cancer: Data from CaPSURE. J Urol. 2006;175:1326–1331. doi: 10.1016/S0022-5347(05)00647-6. [DOI] [PubMed] [Google Scholar]
- 18.Lubeck DP, Litwin MS, Henning JM, et al. The CaPSURE database: A methodology for clinical practice and research in prostate cancer—CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1996;48:773–777. doi: 10.1016/s0090-4295(96)00226-9. [DOI] [PubMed] [Google Scholar]
- 19.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: Lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urol. 2004;171:1393–1401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 20.Cooperberg MR, Vickers AJ, Broering JM, et al. Comparative risk-adjusted mortality outcomes following primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer. 2010;116:5226–5234. doi: 10.1002/cncr.25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 22.Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009;101:878–887. doi: 10.1093/jnci/djp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of competing risk. J Am Statist Assoc. 1999;94:496–509. [Google Scholar]
- 24.Alibhai SM, Leach M, Tomlinson G, et al. 30-day mortality and major complications after radical prostatectomy: Influence of age and comorbidity. J Natl Cancer Inst. 2005;97:1525–1532. doi: 10.1093/jnci/dji313. [DOI] [PubMed] [Google Scholar]
- 25.Middleton RG, Thompson IM, Austenfeld MS, et al. Prostate Cancer Clinical Guidelines Panel Summary report on the management of clinically localized prostate cancer: The American Urological Association. J Urol. 1995;154:2144–2148. [PubMed] [Google Scholar]
- 26.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 27.Kumar RJ, Barqawi A, Crawford ED. Adverse events associated with hormonal therapy for prostate cancer. Rev Urol. 2005;7(suppl 5):S37–S43. [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzpatrick JM. Management of localized prostate cancer in senior adults: The crucial role of comorbidity. BJU Int. 2008;101(suppl 2):16–22. doi: 10.1111/j.1464-410X.2007.07487.x. [DOI] [PubMed] [Google Scholar]
- 29.Alibhai SM, Naglie G, Nam R, et al. Do older men benefit from curative therapy of localized prostate cancer? J Clin Oncol. 2003;21:3318–3327. doi: 10.1200/JCO.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 30.Jang TL, Bekelman JE, Liu Y, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. 2010;170:440–450. doi: 10.1001/archinternmed.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Y, Carvalhal GF, Catalona WJ, et al. Primary treatment choices for men with clinically localized prostate carcinoma detected by screening. Cancer. 2000;88:1122–1130. [PubMed] [Google Scholar]
- 32.Fleshner N, Rakovitch E, Klotz L. Differences between urologists in the United States and Canada in the approach to prostate cancer. J Urol. 2000;163:1461–1466. [PubMed] [Google Scholar]
- 33.Shahinian VB, Kuo YF, Freeman JL, et al. Determinants of androgen deprivation therapy use for prostate cancer: Role of the urologist. J Natl Cancer Inst. 2006;98:839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bubolz T, Wasson JH, Lu-Yao G, et al. Treatments for prostate cancer in older men: 1984-1997. Urology. 2001;58:977–982. doi: 10.1016/s0090-4295(01)01434-0. [DOI] [PubMed] [Google Scholar]
- 36.Walter LC, Bertenthal D, Lindquist K, et al. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296:2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 37.Konety BR, Cooperberg MR, Carroll PR. Are age-based criteria the best way to determine eligibility for prostate cancer screening? Ann Intern Med. 2009;150:220–221. doi: 10.7326/0003-4819-150-3-200902030-00018. author reply 221-222. [DOI] [PubMed] [Google Scholar]
- 38.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walz J, Gallina A, Perrotte P, et al. Clinicians are poor raters of life-expectancy before radical prostatectomy or definitive radiotherapy for localized prostate cancer. BJU Int. 2007;100:1254–1258. doi: 10.1111/j.1464-410X.2007.07130.x. [DOI] [PubMed] [Google Scholar]
- 40.Walz J, Suardi N, Shariat SF, et al. Accuracy of life tables in predicting overall survival in patients after radical prostatectomy. BJU Int. 2008;102:33–38. doi: 10.1111/j.1464-410X.2008.07614.x. [DOI] [PubMed] [Google Scholar]