Abstract
Purpose
Estimates of prostate cancer–specific mortality (PCSM) were similar for men randomly assigned to intervention compared with usual care on the Prostate, Lung, Colorectal and Ovarian PC screening study. However, results analyzed by comorbidity strata remain unknown.
Patients and Methods
Between 1993 and 2001, of 76,693 men who were randomly assigned to usual care or intervention at 10 US centers, 73,378 (96%) completed a questionnaire that inquired about comorbidity and prostate-specific antigen (PSA) testing before random assignment. Fine and Gray's multivariable analysis was performed to assess whether the randomized screening arm was associated with the risk of PCSM in men with no or minimal versus at least one significant comorbidity, adjusting for age and prerandomization PSA testing.
Results
After 10 years of follow-up, 9,565 deaths occurred, 164 from PC. A significant decrease in the risk of PCSM (22 v 38 deaths; adjusted hazard ratio [AHR], 0.56; 95% CI, 0.33 to 0.95; P = .03) was observed in men with no or minimal comorbidity randomly assigned to intervention versus usual care, and the additional number needed to treat to prevent one PC death at 10 years was five. Among men with at least one significant comorbidity, those randomly assigned to intervention versus usual care did not have a decreased risk of PCSM (62 v 42 deaths; AHR, 1.43; 95% CI, 0.96 to 2.11; P = .08).
Conclusion
Selective use of PSA screening for men in good health appears to reduce the risk of PCSM with minimal overtreatment.
INTRODUCTION
Prostate cancer (PC) in the United States is usually diagnosed through serial prostate-specific antigen (PSA) testing1 and is treated by using radical prostatectomy (RP) or radiation therapy (RT) with or without hormonal therapy (HT). Although these treatments are often curative, the additional number of men who need to be treated (NNT) by using PSA testing compared with usual care to eliminate one death from PC at 9 years has been estimated from early follow-up of the European randomized PC screening study2 at 48. Why is this number so large?
About one third of men treated for screen-detected PC will recur.3 However, many recurrences will not progress to death from PC.4 The reason for this is twofold. First, most men are diagnosed with PC later in life during the sixth decade, and most PSA recurrences have long (> 9 months) PSA doubling times in which the median time to prostate cancer–specific mortality (PCSM) following PSA failure is in excess of 15 years, which may exceed the patient's life expectancy.5 Second, at the time of recurrence, which can be several years following initial therapy,6 the patient may have prior or acquired competing risks of mortality which lead to death before the PC has the opportunity to progress. Both of these factors increase the additional NNT to eliminate one PC death with PSA screening compared with usual care in the general population. However, the additional NNT may be less for men in good health, as suggested by the NNT of 12 in the Göteborg randomized population-based PC screening trial7 in which the men were younger (median age, 56 years) than in the US1 and European2 studies and likely were healthier.
Given that estimates of PCSM were not analyzed by comorbidity strata in the European PC screening study,2 it is possible that the additional NNT in men with no or minimal comorbidity may be < 48. Moreover, it is possible that while the Prostate, Lung, Colorectal and Ovarian (PLCO) PC screening study1 did not show a reduction in the risk of PCSM with both serial PSA testing and digital rectal examination (DRE) compared with usual care overall, such a reduction may be observed in men with no or minimal comorbidity. Conversely, diagnosing PC earlier in men with more significant comorbidity may not decrease the risk of PCSM because of competing risks. Therefore, the purpose of this study was to evaluate whether an association existed between the extent of pre-existing comorbidity and the risk of PCSM in men undergoing intervention compared with usual care in the randomized PLCO PC screening trial.
PATIENTS AND METHODS
Patient Population and Randomized Screening Arms
From 1993 through 2001, 76,693 men between the ages of 55 and 74 years were enrolled at 10 study centers across the United States and were randomly assigned to intervention versus usual care. Intervention consisted of being offered annual PSA testing for 6 years and annual DRE for 4 years with prostate biopsy recommended for a PSA > 4 ng/mL and/or an abnormal DRE. Despite these guidelines, the usual care arm was estimated to have a 52% contamination rate due to PSA screening by year 6.1
Comorbidity Assessment and Assignment
Of 76,693 men who were randomly assigned to usual care versus intervention, 73,378 (96%) were available for mortality analysis by 10 years, and they completed a baseline questionnaire that inquired in detail about comorbidities and prior PSA testing. Two comorbidity strata were defined: (1) no or minimal comorbidity, and (2) having at least one significant comorbidity. A significant comorbidity was defined as one that could increase the risk of dying from one of the two leading causes of death in the United States for men between the ages of 55 and 74 years (ie, cardiovascular disease or cancer) published in the most recent publication of the National Vital Statistics Report by the US National Center for Health Statistics.8 For cardiovascular disease, the specific comorbidities included known coronary heart disease and/or myocardial infarction and risk factors9 such as hypertension, diabetes, stroke, and obesity defined as a body mass index > 30 kg/m2. For cancer, specific medical conditions that increase the risk of developing a non-PC were included, and these were ulcerative colitis, Crohn's disease, familial polyposis syndrome/Gardner's syndrome, diverticulitis, diverticulosis, gallbladder stones/inflammation, colorectal polyps, bronchitis, emphysema, cirrhosis of the liver, and hepatitis. Minimal comorbidities were defined as those that would be unlikely to increase the risk of dying from one of the leading causes of death8 for men between the ages of 55 and 74 years and included arthritis and osteoporosis.
Follow-Up and Determination of the Cause of Death
Follow-up started on the day of random assignment and concluded on the date the patient was last observed or the date of death through August 31, 2009, whichever came first. All diagnosed cancers and all deaths occurring during the trial were ascertained, primarily by means of a mailed annual questionnaire that asked about the type of cancer and the date of diagnosis in the previous year. Death certificates were obtained to confirm death and to provisionally determine the underlying cause. Since the true underlying cause may not always be evident or accurately recorded on the death certificate, the trial used a special end point adjudication process to assign the cause of death in a uniform and unbiased manner. All deaths from causes that were potentially related to PC were reviewed, including any cause of death in which the patient had PC or a possible metastasis from PC and all deaths of unknown or uncertain cause. Reviewers of these deaths were unaware of study-group assignments for deceased patients.
For men diagnosed with PC, clinical stage was determined by using the fifth edition of the American Joint Committee on Cancer (AJCC) Staging Manual.10 Gleason scoring11 was used to grade the cancers. Advanced PC in this analysis was defined as either Gleason score 7 to 10 or AJCC category10 T3 or T4 node-positive or metastatic PC.
Statistical Methods
Description of the study cohort at random assignment and at the time of PC diagnosis stratified by comorbidity category.
A Mantel-Haenszel χ2 metric12 was used to compare the distribution of men by randomized screening arm, by age in years at random assignment, and by the presence of prerandomization PSA testing across the two comorbidity strata. A Wilcoxon two-sample test13 was used to compare the distribution of the median age in years at random assignment among men in the two comorbidity cohorts.
Men who were diagnosed with PC and the treatment they received were enumerated and stratified by randomized screening arm and by comorbidity strata. A Mantel-Haenszel χ2 metric12 was used to compare the distribution of men and the treatment they received stratified by the presence or absence of advanced PC and comorbidity strata across randomized screening arms.
PC incidence and death stratified by randomized screening arm and comorbidity category.
The cumulative incidence14 method was used to estimate and characterize PC incidence and PCSM stratified by the randomized screening arm for each comorbidity subgroup. Comparisons of these estimates were performed by using a k-sample P value.15 For the purpose of illustration, unadjusted cumulative incidence estimates of PC incidence and PCSM stratified by screening arm are displayed for men with no or minimal and at least one significant comorbidity. A two-sided P < .05 was considered statistically significant with a Bonferroni correction made for multiple comparisons lowering the level for significance to .05 divided by the number of comparisons.16
Calculation of additional NNT to prevent one PC death by 10 years.
We first calculated the numerical difference in the cumulative incidence estimates of PCSM at 10 years between usual care and intervention arms. To prevent one PC death during a 10-year period, the number of additional men who would need to be screened would be one divided by difference in the 10-year estimates of PCSM.
Next, we computed the numerical difference in the cumulative incidence estimates of PC diagnoses at 10 years in men randomly assigned to intervention compared with usual care. To prevent one PC death during a 10-year period, the additional NNT17 would equal the numerical difference in the cumulative incidence estimates of PC incidence at 10 years in men randomly assigned to intervention compared with the usual care arm multiplied by the additional number of men who would need to be screened.
Competing risks regression and assessment for interaction between comorbidity and randomized screening arm.
The primary end point of this study was the risk of PCSM. A multivariable Fine and Gray's competing risks regression analysis18 was used to evaluate whether randomized screening arm was significantly associated with the risk of PCSM within each comorbidity subgroup, adjusting for age at random assignment and the use of prerandomization PSA testing. Men who reported undergoing PSA testing before random assignment or if this was unknown were placed into separate categories. The baseline category for this three-level categorical covariate was no prerandomization PSA testing. Age in years at random assignment was treated as a continuous covariate.
To assess whether a significant interaction18 existed between comorbidity and randomized screening arm, arm × comorbidity was evaluated in the multivariable model. Unadjusted hazard ratios and adjusted hazard ratios (AHRs) for PCSM with associated 95% CIs and P values were calculated for each covariate from the Fine and Gray regression model. Schoenfeld residuals were computed for each covariate, and residuals were plotted against time in years. A nonzero slope of Schoenfeld residuals against time is an indication of a violation of the proportional hazard assumption, which we did not observe. Therefore, no evidence was found against proportional hazards assumption. R version 2.6.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for all calculations pertaining to Gray's k-sample P value and Fine and Gray regression. SAS version 9.2 (SAS Institute, Cary, NC) was used for all remaining analyses.
RESULTS
Description of the Study Cohort at Randomization and PC Diagnosis Stratified by the Two Comorbidity Cohorts
Men with no or minimal comorbidity represented 35.7% (26,175 of 73,378) of the study cohort and were significantly younger (median age, 61 v 63 years; P < .001; Table 1) when compared with men with at least one significant comorbidity. The distribution of the randomized screening arm across the two comorbidity cohorts was not significantly different (P = .60).
Table 1.
Distribution of the Clinical Characteristics of 73,378 Men at Baseline Stratified by the Extent of Comorbidity
| Clinical Characteristic | No or Minimal Comorbidity(n = 26,175) |
At Least One Significant Comorbidity (n = 47,203) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age at random assignment, years | < .001 | ||||
| 55-59 | 9,847 | 37.6 | 13,615 | 28.8 | |
| 60-64 | 8,334 | 31.8 | 14,671 | 31.1 | |
| 65-69 | 5,360 | 20.5 | 11,766 | 24.9 | |
| 70+ | 2,634 | 10.1 | 7,151 | 15.2 | |
| Median | 61 | 63 | < .001 | ||
| IQR | 57-66 | 59-67 | |||
| Randomized arm | |||||
| Usual care | 12,909 | 49.3 | 23,184 | 49.1 | .60 |
| Intervention | 13,266 | 50.7 | 24,019 | 50.9 | |
| PSA testing prior to random assignment | < .001 | ||||
| No | 12,989 | 49.6 | 20,467 | 43.4 | |
| Yes | 11,373 | 43.5 | 22,130 | 46.9 | |
| Unknown | 1,813 | 6.9 | 4,606 | 9.8 | |
NOTE. Percentages may not sum to 100 due to rounding.
Abbreviations: IQR, interquartile range; PSA, prostate-specific antigen.
Table 2 illustrates that among men diagnosed with PC who were randomly assigned to intervention compared with usual care, there were more men with less advanced PC in both the no or minimal comorbidity cohort (62% v 68%; P = .002) and at least one significant comorbidity cohort (61% v 67%; P < .001). While no significant differences existed in the distribution of PC treatments among men randomly assigned to intervention compared with usual care, men with at least one significant comorbidity compared with those with no or minimal comorbidity were less likely to receive curative treatment (RP, RT, and RT and HT) and more likely to be treated with noncurative or HT (P < .001).
Table 2.
Distribution of Treatment Received in the 6,245 Men Diagnosed With Prostate Cancer Stratified by the Comorbidity Subgroup, Randomized Screening Arm, and Prostate Cancer Aggressiveness
| Treatment | No or Minimal Comorbidity(n = 2,271) |
At Least One Significant Comorbidity(n = 3,974) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual Care(n = 1,077) |
Intervention(n = 1,194) |
Usual Care(n = 1,778) |
Intervention(n = 2,196) |
|||||||||||||
| Less Advanced PC*(n = 670; 62%) |
Advanced PC†(n = 407; 38%) |
Less Advanced PC*(n = 816; 68%) |
Advanced PC†(n = 378; 32%) |
Less Advanced PC*(n = 1,091; 61%) |
Advanced PC†(n = 687; 39%) |
Less Advanced PC*(n = 1,464; 67%) |
Advanced PC†(n = 732; 33%) |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| RP | 300 | 45 | 181 | 44 | 424 | 52 | 172 | 46 | 413 | 38 | 215 | 31 | 603 | 41 | 267 | 36 |
| RT | 157 | 23 | 54 | 13 | 174 | 21 | 50 | 13 | 260 | 24 | 113 | 16 | 360 | 25 | 129 | 18 |
| RT + HT | 96 | 14 | 112 | 28 | 98 | 12 | 99 | 26 | 178 | 16 | 227 | 33 | 206 | 14 | 198 | 27 |
| HT | 26 | 4 | 43 | 11 | 21 | 3 | 40 | 11 | 70 | 6 | 95 | 14 | 68 | 5 | 93 | 13 |
| Other | 5 | 1 | 4 | 1 | 2 | 0.3 | 2 | 0.5 | 10 | 1 | 6 | 1 | 9 | 1 | 9 | 1 |
| Noncurative | 85 | 13 | 12 | 3 | 93 | 11 | 15 | 4 | 149 | 14 | 30 | 4 | 212 | 14 | 33 | 5 |
| Unknown | 1 | 0.2 | 1 | 0.3 | 4 | 0.5 | 0 | 0 | 11 | 1 | 1 | 0.2 | 6 | 0.4 | 3 | 0.4 |
NOTE. Percentages may not sum to 100 due to rounding. All P values calculated using the Mantel-Haenszel χ2 metric.12 Bonferroni adjustment16 for a significant P value was < .05 ÷ 7 or .007. For no or minimal comorbidity: P = .002 for comparing distribution of prostate cancer (PC) aggressiveness across randomized screening arms; P = .07 for comparing distribution of treatment across randomized screening arms for less advanced PC; and P = .87 for comparing distribution of treatment across randomized screening arms for advanced PC. For at least one significant comorbidity: P < .001 for comparing distribution of PC aggressiveness across randomized screening arms; P= .11 for comparing distribution of treatment across randomized screening arms for less advanced PC; and P= .19 for comparing distribution of treatment across randomized screening arms for advanced PC.
Abbreviations: PC, prostate cancer; RP, radical prostatectomy; RT, radiation therapy; HT, hormonal therapy.
Less advanced includes men with American Joint Commission on Cancer (AJCC)10 category T1 or T2 and Gleason score 6 or lower prostate cancer.
Advanced includes men with Gleason score 7 to 10, AJCC10 category T3 or T4 node positive or metastatic prostate cancer.
Estimates of PC Incidence Stratified by Randomized Screening Arm for Men in the Two Comorbidity Subgroups
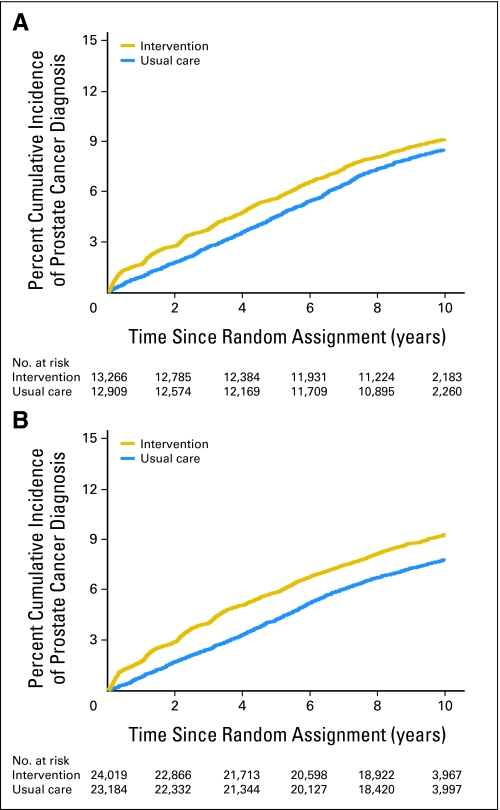
Men with no or minimal as well as those with more extensive comorbidity had estimates of PC incidence that were higher than those randomly assigned to intervention compared with the usual care arm (P = .04 and P < .001, respectively) as shown in Figures 1A and 1B, respectively. Ten-year estimates of PC incidence were 9.12% (95% CI, 8.64% to 9.63%) and 8.46% (95% CI, 7.99% to 8.96%) for men with no or minimal comorbidity randomly assigned to intervention and usual care arms, respectively. These respective estimates were 9.28% (95% CI, 8.91% to 9.66%) and 7.79% (95% CI, 7.44% to 8.14%) for men with at least one significant comorbid illness.
Fig 1.
(A) Unadjusted cumulative incidence estimates14 of prostate cancer incidence in men with no or minimal comorbidity randomly assigned to usual care or intervention. k-sample P = .04. (B) Unadjusted cumulative incidence estimates14 of prostate cancer incidence in men with at least one significant comorbidity randomly assigned to usual care or intervention. k-sample P < .001.
Risk of PCSM and Assessment for Interaction Between Comorbidity and Randomized Screening Arm
After 10 years of follow-up (interquartile range, 9.9 to 10), 9,565 deaths have occurred, 164 from PC. A significant interaction was noted between comorbidity extent and randomized screening arm (AHR, 2.53; 95% CI, 1.31 to 4.86; P = .006) as depicted in Table 3. Specifically, a significant decrease in the risk of PCSM (22 v 38 deaths; AHR, 0.56; 95% CI, 0.33 to 0.95; P = .03) was observed in men with no or minimal comorbidity randomly assigned to intervention versus usual care, and the additional numbers needed to screen and treat to prevent one PC death were 723 and 5, respectively, at 10 years. However, among men with at least one significant comorbidity, those randomly assigned to intervention versus usual care did not have a decreased risk of PCSM (62 v 42 deaths; AHR, 1.43; 95% CI, 0.96 to 2.11; P = .08). Prerandomization PSA testing (AHR, 0.55; 95% CI, 0.39 to 0.76; P = .001) was associated with a decreased risk of PCSM, whereas increasing age at randomization was associated with an increased risk (AHR, 1.11; 95% CI, 1.08 to 1.14; P < .001) of PCSM.
Table 3.
Hazard Ratio for Prostate Cancer-Specific Mortality for Patient and Clinical Characteristics at Random Assignment From the Univariable and Multivariable Fine and Gray's Competing Risks Interaction Model18 for the 73,378 Men in the Study Cohort
| Covariate | No. of Men | No. of Events | Univariable Analysis |
Multivariable Analysis |
||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Adjusted Hazard Ratio | 95% CI | P | |||
| No or minimal comorbidity | ||||||||
| Usual care arm | 12,909 | 38 | 1.000 Ref | — | 1.000 Ref | — | ||
| Intervention arm | 13,266 | 22 | 0.56 | 0.33 to 0.95 | .03 | 0.56 | 0.33 to 0.95 | .03 |
| At least one significant comorbidity | ||||||||
| Usual care arm | 23,184 | 42 | 1.000 Ref | — | 1.000 Ref | — | ||
| Intervention arm | 24,019 | 62 | 1.43 | 0.96 to 2.11 | .08 | 1.43 | 0.96 to 2.11 | .08 |
| Usual care arm | ||||||||
| No or minimal comorbidity | 12,909 | 38 | 1.000 Ref | — | 1.000 Ref | — | ||
| At least one significant comorbidity | 23,184 | 42 | 0.61 | 0.40 to 0.95 | .03 | 0.55 | 0.35 to 0.86 | .009 |
| Intervention arm | ||||||||
| No or minimal comorbidity | 13,266 | 22 | 1.000 Ref | — | 1.000 Ref | — | ||
| At least one significant comorbidity | 24,019 | 62 | 1.56 | 0.96 to 2.53 | .07 | 1.39 | 0.85 to 2.28 | .19 |
| Interaction term between screening arm and comorbidity | 73,378 | 164 | 2.532 | 1.315 to 4.875 | .005 | 2.526 | 1.312 to 4.863 | .006 |
| Age, years | 73,378 | 164 | 1.10 | 1.07 to 1.13 | < .001 | 1.11 | 1.08 to 1.14 | < .001 |
| PSA testing prior to random assignment | ||||||||
| No | 33,456 | 91 | 1.000 Ref | — | 1.000 Ref | — | ||
| Yes | 33,503 | 56 | 0.61 | 0.44 to 0.85 | .004 | 0.55 | 0.39 to 0.76 | < .001 |
| Unknown | 6,419 | 17 | 0.97 | 0.58 to 1.63 | .91 | 0.91 | 0.54 to 1.54 | .73 |
Abbreviation: PSA, prostate-specific antigen; Ref, reference value.
Estimates of PCSM Stratified by Randomized Screening Arm for Men in the Two Comorbidity Subgroups
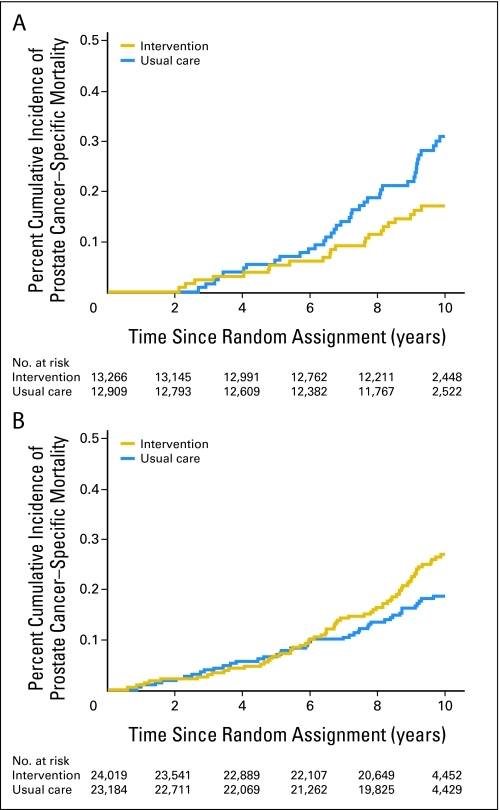
For men with no or minimal comorbidity, estimates of PCSM were lower (P = .03) in those randomly assigned to the intervention compared with the usual care arm as shown in Figure 2A. However, as shown in Figure 2B, a significant reduction in estimates of PCSM was not observed (P = .07) in men with at least one significant comorbidity. Ten-year estimates of PCSM were 0.17% (95% CI, 0.11% to 0.25%) and 0.31% (95% CI, 0.22% to 0.42%) for men with no or minimal comorbidity randomly assigned to intervention or usual care arm, respectively. These respective estimates were 0.27% (95% CI, 0.21% to 0.34%) and 0.19% (95% CI, 0.14% to 0.25%) for men with at least one significant comorbidity.
Fig 2.
(A) Unadjusted cumulative incidence estimates14 of prostate cancer–specific mortality in men with no or minimal comorbidity randomly assigned to usual care or intervention. k-sample P = .03. (B) Unadjusted cumulative incidence estimates14 of prostate cancer–specific mortality in men with at least one significant comorbidity randomly assigned to usual care or intervention. k-sample P = .07. Bonferroni adjustment16 for a significant P value was < .05 ÷ 2 or .025.
Being randomly assigned to intervention compared with usual care was associated with a reduction in all-cause mortality (AHR, 0.93; 95% CI, 0.86 to 1.02) in men with no or minimal comorbidity, but because of the small proportion (< 5%) that PCSM represented in all deaths, this reduction was not significant (P = .11). Specifically, 10-year estimates of non-PCSM were 8.2% (95% CI, 7.8% to 8.5%) and 16.1% (95% CI, 15.7% to 16.4%) in men with no or minimal comorbidity compared with significant comorbidity, respectively.
DISCUSSION
This study observed a significant interaction between the randomized screening arm and the extent of comorbidity at random assignment on the primary end point: the risk of PCSM. Specifically, intervention with annual PSA screening compared with usual care in men with no or minimal comorbidity was associated with the more frequent diagnosis of less advanced PC and a significant reduction in the risk of PCSM requiring the treatment of five additional men to eliminate one PC death at 10 years. However, a reduction in the risk of PCSM was not observed in men who had at least one significant comorbidity, despite the more frequent diagnosis of less advanced PC in these men on the intervention arm compared with those on the usual care arm.
The clinical implication of these findings is that annual PSA testing by using a PSA cut point of 4 ng/mL and/or an abnormal DRE to recommend prostate biopsy leads to the more frequent diagnosis of less advanced PC, and in healthy men with no or minimal competing risks, following curative treatment for PC, a significant reduction in the risk of PCSM may be achieved. Conversely, in men with more significant comorbidity, a reduction in the risk of PCSM was not observed. Why might this be? First, given the competing risks due to the significant comorbidity, the patient may die of another cause before any possible benefit of PSA testing, early PC detection, and curative treatment have the opportunity to manifest. This is supported by the two-fold increase in the 10-year estimates of non-PCSM in men with significant comorbidity compared with no or minimal comorbidity. Second, similar to a prior report,19 in this study, men with significant comorbidity compared with no or minimal comorbidity were significantly less likely to undergo curative therapy and more likely to be treated with primary HT or other noncurative therapy. Yet, less advanced and potentially curable PC was diagnosed significantly more often in men randomly assigned to the intervention arm (67%) compared with the usual care arm (61%). Therefore, it is possible that if the comorbidity present at random assignment was subsequently addressed, then the increased use of noncurative therapies in men withless advanced PC could lead to PC progression and subsequent PCSM, further reducing the likelihood of seeing any benefit to PSA testing in these men. Finally, men with more extensive comorbidity are more likely to experience treatment interruptions during RT, leading to increased PC recurrence rates,20 and the 30-day treatment-related mortality rates are higher in men with significant comorbidity compared with no or minimal comorbidity following RP21 and RT.22
Three points require further discussion. First, the association between a significant reduction in the risk of PCSM in men with no or minimal comorbidity as defined in this study and randomly assigned to intervention compared with the usual care arm is hypothesis generating, given that this was a postrandomization analysis. However, the estimated 52% contamination rate by year 6 in the usual care arm1 and the 15% nonattendance rate in the intervention arm1 add support to the association because these issues23,24 should reduce the likelihood of observing a significant reduction in the risk of PCSM between the two randomized screening arms. Second, people who participate in screening studies are generally more healthy and more health conscious than nonattendees,25 meaning that the 35.7% estimate of men with no or minimal comorbidity in this study may be an overestimate when applied to the general population. Finally, a perfect screening test would have an additional NNT of 1. Therefore, both overdetection and overtreatment can still exist.
Despite these considerations, given the uncertainty about the possible benefit of PSA testing in the general population and concerns regarding overtreatment, these data serve to raise awareness that the selective use of PSA screening for men in good health appears to reduce the risk of PCSM with minimal overtreatment.
Footnotes
Supported by the National Cancer Institute and Grants No. N01-CN-25514, University of Colorado; N01-CN-25522, Georgetown University; N01-CN-25515, Pacific Health Research Institute; N01-CN-25512, Henry Ford Health System; N01-CN-25513, University of Minnesota; N01-CN-25516, Washington University; N01-CN-25511, University of Pittsburgh Medical Center; N01-CN-25524, University of Utah; N01-CN-25518, Marshfield Clinic Research Foundation; N01-CN-75022, University of Alabama at Birmingham; N01-CN-25476, Westat; and N01-CN-25404, University of California at Los Angeles Immunogenetics Center.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00002540
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: E. David Crawford, GlaxoSmithKline (C), Ferring Pharmaceuticals (C); Gerald L. Andriole Jr, Amgen (C), Caris Life Sciences (C), EMD Serono (C), Ferring Pharmaceuticals (C), The France Foundation (C), Gen-Probe (C), GlaxoSmithKline (C), Johnson & Johnson (C), Myriad Genetics (C), Nema Steba (C), Onconome (C), Ortho Clinical Diagnostics (C) Stock Ownership: Gerald L. Andriole Jr, Cambridge Endoscopic Devices, Envisioneering, Viking Medical Technologies Honoraria: E. David Crawford, AstraZeneca, Ferring Pharmaceuticals, GlaxoSmithKline; Gerald L. Andriole Jr, GlaxoSmithKline Research Funding: Robert Grubb III, GlaxoSmithKline; Gerald L. Andriole Jr, Aeterna Zentaris, Antigenics, Ferring Pharmaceuticals, Veridex Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Anthony V. D'Amico
Administrative support: Robert Grubb III, Amanda Black, Gerald L. Andriole Jr, Christine D. Berg, Anthony V. D'Amico
Provision of study materials or patients: E. David Crawford, Robert Grubb III, Amanda Black, Gerald L. Andriole Jr, Christine D. Berg
Collection and assembly of data: Amanda Black, Ming-Hui Chen, Grant Izmirlian, Christine D. Berg
Data analysis and interpretation: E. David Crawford, Amanda Black, Ming-Hui Chen, Grant Izmirlian, Anthony V. D'Amico
Manuscript writing: E. David Crawford, Robert Grubb III, Amanda Black, Gerald L. Andriole Jr, Ming-Hui Chen, Grant Izmirlian, Christine D. Berg, Anthony V. D'Amico
Final approval of manuscript: E. David Crawford, Robert Grubb III, Amanda Black, Gerald L. Andriole Jr, Ming-Hui Chen, Grant Izmirlian, Christine D. Berg, Anthony V. D'Amico
REFERENCES
- 1.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European Study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.D'Amico AV, Chen MH, Roehl KA, et al. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125–135. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 4.Roberts WB, Han M. Clinical significance and treatment of biochemical recurrence after definitive therapy for localized prostate cancer. Surg Oncol. 2009;18:268–274. doi: 10.1016/j.suronc.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedland SJ, Humphreys EB, Mangold LA, et al. Death in patients with recurrent prostate cancer after radical prostatectomy: Prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25:1765–1771. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 6.Buyyounouski MK, Hanlon AL, Horwitz EM, et al. Interval to biochemical failure highly prognostic for distant metastasis and prostate cancer-specific mortality after radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:59–66. doi: 10.1016/j.ijrobp.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. National Center for Health Statistics. National Vital Statistics Report (NVSR) Deaths: Final Data for 2006. 2009 Apr 24;Vol. 57 No. 14. [PubMed] [Google Scholar]
- 9.Framingham Heart Study: General Cardiovascular Disease (10-year risk) www.framinghamheartstudy.org/risk/gencardio.html.
- 10.Fleming ID, Cooper JS, Henson DE, et al., editors. Philadelphia, PA: Lippincott-Raven; 1997. AJCC Cancer Staging Manual (ed 5) [Google Scholar]
- 11.Gleason DF, Veterans Administration Cooperative Urological Research Group . Histologic grading and staging of prostatic carcinoma. In: Tannenbaum M, editor. Urologic Pathology. Philadelphia, PA: Lea & Febiger; 1977. pp. 171–187. [Google Scholar]
- 12.Agresti A, editor. New York, NY: John Wiley & Sons; 2002. An Introduction to Categorical Data Analysis (ed 2) pp. 16–52. [Google Scholar]
- 13.Hollander M, Wolfe DA, editors. New York, NY: John Wiley & Sons; 1999. Nonparametric Statistical Methods (ed 2) pp. 189–269. [Google Scholar]
- 14.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: Examples from clinical oncology data. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 15.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 16.Neter J, Wassermann W, Kutner M, editors. Homewood, IL: Richard D. Irwin; 1983. Simultaneous inferences and other topics in regression analyses-1, in Applied Linear Regression Models; pp. 150–153. [Google Scholar]
- 17.Hildebrandt M, Vervölgyi E, Bender R. Calculation of NNTs in RCTs with time-to-event outcomes: A literature review. BMC Med Res Methodol. 2009;9:21. doi: 10.1186/1471-2288-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Marr PL, Elkin EP, Arredondo SA, et al. Comorbidity and primary treatment for localized prostate cancer: Data from CaPSURE. J Urol. 2006;175:1326–1331. doi: 10.1016/S0022-5347(05)00647-6. [DOI] [PubMed] [Google Scholar]
- 20.D'Ambrosio DJ, Li T, Horwitz EM, et al. Does treatment duration affect outcome after radiotherapy for prostate cancer? Int J Radiat Oncol Biol Phys. 2008;72:1402–1407. doi: 10.1016/j.ijrobp.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alibhai SM, Leach M, Tomlinson G, et al. 30-day mortality and major complications after radical prostatectomy: Influence of age and comorbidity. J Natl Cancer Inst. 2005;97:1525–1532. doi: 10.1093/jnci/dji313. [DOI] [PubMed] [Google Scholar]
- 22.Alibhai SM, Leach M, Warde P. Major 30-day complications after radical radiotherapy: A population-based analysis and comparison with surgery. Cancer. 2009;115:293–302. doi: 10.1002/cncr.24008. [DOI] [PubMed] [Google Scholar]
- 23.Cuzick J, Edwards R, Segnan N. Adjusting for non-compliance and contamination in randomized clinical trials. Stat Med. 1997;16:1017–1029. doi: 10.1002/(sici)1097-0258(19970515)16:9<1017::aid-sim508>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Bergdahl AG, Aus G, Lilja H, et al. Risk of dying from prostate cancer in men randomized to screening: Differences between attendees and nonattendees. Cancer. 2009;115:5672–5679. doi: 10.1002/cncr.24680. [DOI] [PubMed] [Google Scholar]
- 25.Weiss NS, Rossing MA. Healthy screened bias in epidemiologic studies of cancer incidence. Epidemiology. 1996;7:319–322. [PubMed] [Google Scholar]