Abstract
Purpose
To examine underlying etiologic factors that may explain the racial disparity in non-Hodgkin's lymphoma (NHL) incidence patterns.
Patients and Methods
We assessed immune-related conditions and risk of developing NHL among more than 4 million hospitalized US veterans including 9,496 patients with NHL (7,999 white patients and 1,497 black patients) with up to 26 years of follow-up. We used time-dependent Poisson regression to estimate rate ratios (RRs) and 95% CIs for NHL risk among patients with a history of specific autoimmune diseases, infections, and allergies compared with patients without such history, adjusting for attained age, calendar year, race, number of hospital visits, and time between study entry and exit.
Results
Patients with infectious conditions had an increased risk of developing NHL (RR, 1.2; 95% CI, 1.1 to 1.2), particularly for gastrohepatic, genital, and systemic infectious conditions. Patients with autoimmune disease were generally more likely to develop NHL than patients without autoimmune disease, especially for conditions that typically present with detectable autoantibodies with systemic involvement (RR, 2.0; 95% CI, 1.8 to 2.2). Allergies were also associated with increased risk (RR, 1.4; 95% CI, 1.3 to 1.5). Although the risk of NHL was lower for blacks than whites (RR, 0.87; 95% CI, 0.82 to 0.92), blacks had a slightly higher risk of NHL associated with infections than whites (likelihood ratio test, P = .002) and a tendency toward higher risk associated with allergies (likelihood ratio test, P = .05). Risks associated with autoimmune conditions were similar by race (likelihood ratio test, P = .5).
Conclusion
The observed difference in NHL risk by race supports a role for race-related differences in genes regulating immune/inflammatory response.
INTRODUCTION
In 2010, non-Hodgkin's lymphoma (NHL) will account for an estimated 65,540 new cancer cases and 20,210 deaths in the United States.1 NHL has been associated with broadly categorized immune-related conditions,2,3 including immunodeficiency,4 autoimmune diseases,5,6 infections,7 and allergies.8,9 We previously found that the association of infectious conditions with risk of Waldenström macroglobulinemia, a rare B-cell subtype of NHL, was consistently higher for blacks than whites.10
Although the incidence of NHL is somewhat lower among blacks compared with whites,2 blacks have higher rates of the extranodal NHL subtypes peripheral T-cell lymphoma and mycosis fungoides.11 These disparities, coupled with findings that associations between immune system–related gene polymorphisms and NHL vary by race,12 suggest that genetic predisposition may play a role in immune-related NHL. Immune status has been associated with race, with blacks having higher levels of immunoglobulin A and CD8 cell counts than whites.13 Furthermore, several studies observed that whites and blacks living in the same geographical region had different levels of γ-globulin, a marker of immune disruption.14,15
Previous epidemiologic studies on NHL generally have not included many blacks or were limited by small sample size. These studies often adjusted for race in their analyses3 and rarely compared results across racial/ethnic groups. To further examine the role of immune-related conditions in lymphomagenesis and potential differences by race, we investigated the relation between immune-related conditions and risk of developing NHL among a large cohort of more than 4 million adult male military veterans, including more than 800,000 black veterans, admitted to the US Veterans Affairs (VA) hospitals.
PATIENTS AND METHODS
Patients, Outcome, and Exposures
With more than 150 hospitals, the VA medical care system has been used to create a database of inpatient records from more than 4 million US veterans hospitalized between July 1, 1969, and September 30, 1996.16,17 These patients originated from approximately 30 million US veterans eligible for admission to VA hospitals during the study period.18 White or black men age 18 to 100 years who were hospitalized at least once during the study period were included if they were cancer free during the first year of follow-up and survived at least 1 year after the initial visit. Females and males of other ethnicities and ages were excluded because they formed a small proportion of hospitalized veterans. This study was exempt from institutional review board review and informed consent as per the National Institutes of Health Office of Human Subjects Research because it analyzed existing data stripped of personal identifiers and there was no patient contact.
NHL diagnoses were identified using the eighth and ninth revisions of the International Classification of Diseases codes (200, 202) as were the specific autoimmune, infectious, and allergic conditions listed in Tables 2 through 4. Combined categories of bacterial, viral, parasitic, organ site–specific, and total infections also were analyzed, as were autoimmune diseases that typically present with detectable autoantibodies (with either systemic involvement or organ-specific involvement) and those that do not have detectable autoantibodies.
Statistical Analysis
Person-time at risk began 1 year after the first hospital discharge and ended at diagnosis of NHL. Individuals not diagnosed with NHL were censored at first malignancy, death, or the end of follow-up (September 30, 1996), whichever came first. Records were linked to Social Security Administration Death Master File Records to determine dates of death.19 Exposed person-time was calculated for chronic immune stimulatory conditions (autoimmunity, infections, and allergies) by subtracting the date of the first hospital discharge diagnosis for an immune stimulatory condition from the date of first hospital admission for NHL.
Time-dependent Poisson regression20 (AMFIT module in Epicure version 1.4; HiroSoft International, Seattle, WA) was used to calculate rate ratios (RRs) and two-sided 95% CIs for the risk of NHL associated with race and with immune-related conditions in black and white patients. RRs were adjusted for attained age (< 40, 40 to 49, 50 to 59, 60 to 69, 70 to 79, or ≥ 80 years), attained calendar year (1969 to 1974, 1975 to 1979, 1980 to 1984, 1985 to 1989, or 1990 to 1996), race (black or white), number of hospital visits (one to two, three to four, or ≥ five visits), and latency between study entry and exit (2 to 3, 4 to 5, 6 to 9, 10 to 14, or ≥ 15 years). Because estimates based on small numbers are unstable, we present RRs when there are five or more patients with NHL. The likelihood ratio test (LRT) for multiplicative interaction was used to formally test whether race modified the RRs for these conditions.
To assess whether undetected NHL might cause the immune-related conditions (ie, reverse causality), models for infectious, autoimmune, and allergic conditions were stratified by latency (time in the cohort) of 2 to 5 years and more than 5 years. To evaluate whether changes in the definition of NHL over time might affect the results, models were stratified by median calendar time (< 1980 and ≥ 1980 at first visit). We also stratified by age at first visit (< and ≥ 50 years).
RESULTS
We identified 9,496 patients with NHL (7,999 white patients and 1,497 black patients) with a mean follow-up time of 7.9 years (range, 1 to 26 years; Table 1). Overall, blacks had a lower risk of NHL than whites (RR, 0.87; 95% CI, 0.82 to 0.92). Patients with NHL tended to be older at study entry and to have slightly more hospital visits than patients without NHL, and blacks were younger than whites (Table 1).
Table 1.
Demographics and Clinical Characteristics of the Study Cohort: White and Black Male US Veterans With at Least One Hospital Admission Between July 1, 1969, and September 30, 1996, Who Were Observed More Than 1 Year
| Characteristic | Whites |
Blacks |
||
|---|---|---|---|---|
| No NHL | NHL | No NHL | NHL | |
| No. of persons | 3661,245 | 7,999 | 830,837 | 1,497 |
| Mean age at study entry, years* | 52.1 | 55.8 | 47.7 | 49.6 |
| Mean years of follow-up† | 11.7 | 7.8 | 11.9 | 8.6 |
| Person-years at risk† | 42,702,434 | 62,254 | 9876,557 | 12,935 |
| Median No. of hospital visits | 3 | 4‡ | 3 | 4‡ |
Abbreviation: NHL, non-Hodgkin's lymphoma.
Age at first discharge record for inpatient hospitalization at Veterans Affairs hospitals between July 1, 1969, and September 30, 1996.
Follow-up started 1 year after the first hospital visit.
Includes visits up to the exit date.
Infections and Risk of NHL
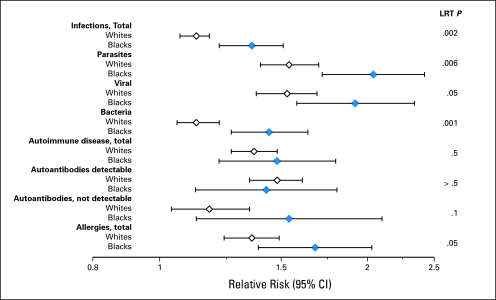
Patients with infectious conditions had an increased risk of developing NHL (RR, 1.2; 95% CI, 1.1 to 1.2; Table 2). The risk was somewhat higher for viral (RR, 1.6; 95% CI, 1.5 to 1.8) and parasitic infections (RR, 1.7; 95% CI, 1.5 to 1.8) than for bacterial infections (RR, 1.2; 95% CI, 1.1 to 1.3). Gastrohepatic, genital, systemic, and arthropod-borne infectious conditions were particularly likely to increase risk. Among the conditions in the other category, herpes simplex, herpes zoster, infectious mononucleosis, and toxoplasmosis were associated with two- to eight-fold increased risk. Both blacks and whites had an increased risk of NHL associated with infections, but the risk was slightly higher for blacks than whites (Fig 1). This pattern was similar for systemic, reproductive, and lower airway infections (RR, 4.0; 95% CI, 3.3 to 4.9; RR, 1.7; 95% CI, 1.2 to 2.3; and RR, 1.3; 95% CI, 1.2 to 1.6, respectively, in blacks and RR, 2.7; 95% CI, 2.4 to 3.0; RR, 1.1; 95% CI, 0.89 to 1.3; and RR, 1.1; 95% CI, 1.0 to 1.2, respectively, in whites; LRT, P ≤ .001, .01, and .03, respectively). In contrast to other systemic infections, the RR for HIV was higher in whites (RR, 46.2; 95% CI, 38.8 to 55.0) than blacks (RR, 22.3; 95% CI, 17.1 to 29.0). Additional RRs for individual infection-related conditions with ≥ five exposed black and white patients are listed in Appendix Table A1 (online only). To ensure that the observed differences by race were not a result of HIV infection, we conducted analyses adjusting broad categories of infections for HIV; differences by race remained for all categories (LRT, P = .02 for total, bacterial, and parasitic infections) except viral infections (LRT, P = .2).
Table 2.
Infection-Related Conditions and Risk of NHL in US Male Veterans
| Category | No. of Veterans Exposed |
RR | 95% CI | |
|---|---|---|---|---|
| No NHL | NHL | |||
| Total infection-related conditions | 1,536,589 | 3,832 | 1.2* | 1.1 to 1.2 |
| Upper airway | 223,750 | 573 | 1.1* | 1.0 to 1.2 |
| Acute bronchitis/bronchiolitis | 89,435 | 191 | 0.94 | 0.81 to 1.1 |
| Chronic sinusitis | 74,701 | 243 | 1.3* | 1.2 to 1.5 |
| Laryngitis | 9,890 | 27 | 1.1 | 0.77 to 1.6 |
| Nasopharyngitis/pharyngitis | 54,430 | 125 | 1.0 | 0.86 to 1.2 |
| Otitis media/mastoiditis | 12,190 | 39 | 1.1 | 0.78 to 1.5 |
| Lower airway | 442,063 | 1,088 | 1.2* | 1.1 to 1.2 |
| Influenza | 22,172 | 69 | 1.2 | 0.94 to 1.6 |
| Pneumonia | 361,834 | 867 | 1.2* | 1.1 to 1.3 |
| Tuberculosis | 82,151 | 225 | 1.1 | 0.92 to 1.2 |
| Gastrohepatic | 145,366 | 415 | 1.4* | 1.2 to 1.5 |
| Cholangitis/cholecystitis | 32,844 | 86 | 0.98 | 0.78 to 1.2 |
| Hepatitis virus | 45,826 | 115 | 1.9* | 1.5 to 2.3 |
| Hepatitis A virus | 2,911 | 9 | 1.9 | 0.89 to 3.9 |
| Hepatitis B virus | 14,678 | 52 | 2.6* | 1.9 to 3.5 |
| Hepatitis C virus | 16,610 | 21 | 1.2 | 0.68 to 2.2 |
| Intestinal | 70,392 | 228 | 1.4* | 1.2 to 1.6 |
| Genital | 76,399 | 250 | 1.6* | 1.4 to 1.8 |
| Condyloma acuminatum | 11,436 | 30 | 1.5* | 1.0 to 2.1 |
| Genital herpes | 3,234 | 34 | 6.9* | 4.7 to 10.1 |
| Gonorrhea | 9,387 | 33 | 1.7* | 1.2 to 2.4 |
| Syphilis | 49,161 | 156 | 1.5* | 1.2 to 1.7 |
| Trichomoniasis | 4,851 | 9 | 0.95 | 0.49 to 1.8 |
| Reproductive | 66,319 | 204 | 1.2* | 1.0 to 1.4 |
| Orchitis and epididymitis | 48,829 | 155 | 1.2* | 1.0 to 1.4 |
| Chronic prostatitis | 18,620 | 51 | 0.98 | 0.73 to 1.3 |
| Urinary | 27,583 | 72 | 1.2 | 0.91 to 1.5 |
| Chronic cystitis | 4,049 | 8 | 0.98 | 0.49 to 2.0 |
| Chronic pyelonephritis | 10,317 | 30 | 1.3 | 0.88 to 1.9 |
| Urethritis | 13,726 | 34 | 1.1 | 0.76 to 1.5 |
| Cardiovascular† | 111,164 | 233 | 1.0 | 0.89 to 1.2 |
| Acute pericarditis | 6,714 | 15 | 1.1 | 0.68 to 1.9 |
| Endocarditis | 104,554 | 216 | 1.0 | 0.87 to 1.2 |
| Systemic | 96,770 | 508 | 2.9* | 2.7 to 3.3 |
| HIV | 14,410 | 329 | 35.8* | 30.9 to 41.5 |
| Meningitis | 13,827 | 74 | 2.4* | 1.9 to 3.1 |
| Septicemia | 72,643 | 193 | 1.3* | 1.1 to 1.6 |
| Arthropod-borne diseases‡ | 20,720 | 55 | 1.5* | 1.1 to 1.9 |
| Acariasis | 7,522 | 17 | 1.2 | 0.73 to 1.9 |
| Malaria | 4,656 | 7 | 1.2 | 0.54 to 2.7 |
| Pediculosis and Phthirus infestation | 6,157 | 14 | 1.2 | 0.72 to 2.1 |
| Rickettsia | 815 | 7 | 3.7* | 1.7 to 8.2 |
| Viral arthropod-borne diseases | 1,147 | 6 | 2.2* | 1.0 to 5.0 |
| Other | ||||
| Conjunctivitis | 16,790 | 42 | 0.96 | 0.70 to 1.3 |
| Gingivitis and periodontitis | 478,113 | 1,142 | 0.93* | 0.87 to 0.99 |
| Helminthiases | 4,251 | 17 | 1.6 | 0.98 to 2.7 |
| Herpes simplex | 13,441 | 110 | 3.8* | 3.0 to 4.7 |
| Herpes zoster | 18,913 | 106 | 2.2* | 1.8 to 2.7 |
| Infectious mononucleosis | 2,330 | 11 | 2.7* | 1.4 to 5.5 |
| Infective arthritis | 8,079 | 28 | 1.8* | 1.2 to 2.7 |
| Mycoses | 174,953 | 599 | 1.7* | 1.5 to 1.8 |
| Osteomyelitis | 57,655 | 112 | 0.86 | 0.71 to 1.1 |
| Skin and soft tissue infections | 296,539 | 757 | 1.2* | 1.1 to 1.3 |
| Toxoplasmosis | 1,509 | 40 | 7.8* | 4.8 to 12.7 |
| Zoonoses | 1,630 | 5 | 1.5 | 0.61 to 3.5 |
NOTE. All analyses were adjusted for age, calendar time, race, latency, and number of hospital visits.
Abbreviations: NHL, non-Hodgkin's lymphoma; RR, rate ratio.
Significant result.
Includes acute myocarditis, for which only two veterans had NHL.
Includes Lyme disease and babesiosis, for which no veterans had NHL, and other bacterial and protozoan arthropod-borne disease, for which only four veterans had NHL.
Fig 1.
Forest plots of the rate ratios (RRs) and 95% CIs for infectious, autoimmune, and allergic conditions (left) and subsequent non-Hodgkin's lymphoma among blacks and whites. The likelihood ratio test (LRT) provides a formal evaluation of the difference in RRs by race. All analyses were adjusted for age, calendar time, latency, and number of hospital visits.
Autoimmune Disease and Risk of NHL
Patients with autoimmune disease had a 1.4-fold increased risk of NHL (95% CI, 1.3- to 1.5-fold), although there was considerable variability across individual conditions (Table 3). The strongest associations tended to be for autoimmune diseases in which autoantibodies are detectable (RR, 1.5; 95% CI, 1.3 to 1.6), especially for those with systemic involvement (RR, 2.0; 95% CI, 1.8 to 2.2). Autoimmune diseases with organ involvement had a much lower increased risk (RR, 1.2; 95% CI, 1.1 to 1.4), although some conditions, such as autoimmune hemolytic anemia (RR, 5.0; 95% CI, 3.4 to 7.3), immune thrombocytopenic purpura (RR, 3.7; 95% CI, 2.2 to 6.4), discoid lupus erythematosus (RR, 2.2; 95% CI, 1.5 to 3.4), and celiac disease (RR, 2.6; 95% CI, 1.5 to 4.5), showed notable elevations. Interestingly, risk of NHL was decreased in patients with multiple sclerosis (RR, 0.66; 95% CI, 0.45 to 0.96). Patients with autoimmune conditions that generally do not have detectable autoantibodies had a slightly increased risk of NHL (RR, 1.2; 95% CI, 1.1 to 1.4), especially for sarcoidosis (RR, 1.6; 95% CI, 1.0 to 2.4), Crohn's disease (RR, 1.5; 95% CI, 1.0 to 2.1), psoriasis (RR, 1.3; 95% CI, 1.1 to 1.6), and Wegener's granulomatosis (RR, 3.8; 95% CI, 1.4 to 10.3). Risks associated with autoimmune conditions were similar for black and white patients (Fig 1). Appendix Table A2 (online only) provides the distribution of individual autoimmune conditions by race.
Table 3.
Autoimmune Conditions and Risk of NHL in US Male Veterans
| Category | No. of Veterans Exposed |
RR | 95% CI | |
|---|---|---|---|---|
| No NHL | NHL | |||
| Total autoimmune conditions | 288,874 | 918 | 1.4* | 1.3 to 1.5 |
| Autoantibodies detectable | 199,426 | 667 | 1.5* | 1.3 to 1.6 |
| Systemic involvement | 66,005 | 317 | 2.0* | 1.8 to 2.2 |
| Rheumatoid arthritis | 59,208 | 280 | 1.9* | 1.7 to 2.2 |
| Sjögren's syndrome | 1,875 | 18 | 3.3* | 2.0 to 5.5 |
| Systemic sclerosis | 2,667 | 7 | 1.5 | 0.70 to 3.1 |
| Systemic lupus | 3,798 | 24 | 3.0* | 1.9 to 4.6 |
| Organ involvement† | 139,475 | 382 | 1.2* | 1.1 to 1.4 |
| Addison's disease | 4,192 | 9 | 0.84 | 0.38 to 1.9 |
| Amyotrophic lateral sclerosis | 7,269 | 11 | 0.80 | 0.41 to 1.5 |
| Autoimmune hemolytic anemia | 3,046 | 32 | 5.0* | 3.4 to 7.3 |
| Celiac disease | 2,019 | 13 | 2.6* | 1.5 to 4.5 |
| Chronic rheumatic heart disease | 64,839 | 151 | 1.1 | 0.90 to 1.3 |
| Discoid lupus erythematosus | 5,076 | 27 | 2.2* | 1.5 to 3.4 |
| Graves' disease | 6,391 | 9 | 0.55 | 0.27 to 1.1 |
| Hashimoto's thyroiditis | 1,185 | 5 | 1.4 | 0.54 to 3.8 |
| Immune thrombocytopenic purpura | 9,160 | 47 | 2.8* | 2.1 to 3.8 |
| Multiple sclerosis | 19,341 | 27 | 0.66* | 0.45 to 0.96 |
| Myasthenia gravis | 2,429 | 8 | 1.2 | 0.59 to 2.6 |
| Pernicious anemia | 9,220 | 30 | 1.1 | 0.71 to 1.6 |
| Primary biliary cirrhosis | 7,807 | 15 | 0.98 | 0.59 to 1.6 |
| Autoantibodies not detectable‡ | 106,340 | 317 | 1.2* | 1.1 to 1.4 |
| Ankylosing spondylitis | 10,468 | 30 | 1.2 | 0.81 to 1.7 |
| Crohn's disease | 9,313 | 31 | 1.5* | 1.0 to 2.1 |
| Hemorrhagic proctitis | 19,896 | 40 | 0.84 | 0.59 to 1.2 |
| Polymyalgia rheumatica | 9,488 | 8 | 1.2 | 0.44 to 3.1 |
| Psoriasis | 40,540 | 133 | 1.3* | 1.1 to 1.6 |
| Reiter's disease | 3,628 | 12 | 1.5 | 0.80 to 2.8 |
| Rheumatic fever | 4,677 | 14 | 1.3 | 0.70 to 2.3 |
| Sarcoidosis | 8,264 | 26 | 1.6* | 1.0 to 2.4 |
| Ulcerative colitis | 11,464 | 24 | 0.82 | 0.54 to 1.3 |
| Wegener's granulomatosis | 9,488 | 8 | 3.8* | 1.4 to 10.3 |
NOTE. All analyses were adjusted for age, calendar time, race, latency, and number of hospital visits.
Abbreviations: NHL, non-Hodgkin's lymphoma; RR, rate ratio.
Significant result.
Includes polyarteritis and localized scleroderma, for which only two and four veterans had NHL, respectively.
Includes Behçet's syndrome, for which no veterans had NHL, and rheumatic chorea, for which only one veteran had NHL.
Allergies and Risk of NHL
Overall, risk of NHL was increased with previous diagnosis of an allergic condition (RR, 1.4; 95% CI, 1.3 to 1.5), but the association was not consistent across individual conditions (Table 4). Allergic alveolitis, dermatitis, and erythema were associated with approximately two- to four-fold increased risk (although the RR for allergic alveolitis was based on only five exposed patients with NHL), whereas prior asthma, rhinitis, and urticaria were not associated with NHL. The risk of NHL associated with allergies seemed slightly higher for blacks than whites (LRT, P = .05; Fig 1). Appendix Table A3 (online only) provides the distribution of individual allergic conditions by race.
Table 4.
Allergic Conditions and Risk of NHL in US Male Veterans
| Category | No. of Veterans Exposed |
RR | 95% CI | |
|---|---|---|---|---|
| Non-NHL | NHL | |||
| Total allergic conditions | 223,529 | 718 | 1.4* | 1.3 to 1.5 |
| Asthma | 86,099 | 175 | 0.90 | 0.77 to 1.1 |
| Allergic alveolitis | 633 | 5 | 4.2* | 1.8 to 10.1 |
| Dermatitis | 92,651 | 367 | 1.6* | 1.5 to 1.8 |
| Erythema | 29,202 | 189 | 2.7* | 2.3 to 3.2 |
| Rhinitis | 16,800 | 29 | 0.81 | 0.56 to 1.2 |
| Urticaria | 10,895 | 33 | 1.2 | 0.85 to 1.7 |
NOTE. All analyses were adjusted for age, calendar time, race, latency, and number of hospital visits.
Abbreviations: NHL, non-Hodgkin's lymphoma; RR, rate ratio.
Significant result.
Sensitivity Analyses
For all but infections of the reproductive system, the risk of NHL was more strongly elevated for infections that occurred 2 to 4 years before NHL than ≥ 5 years before NHL (Table 5). Infections also tended to be more strongly associated with NHL when they occurred after 1980. Nearly all of the increased risk associated with infections occurred among patients who were younger than 50 years old at their first visit. In contrast, we saw few differences for autoimmune conditions across latency, calendar year, or age (Table 5). Although the association between autoimmune conditions and NHL seemed somewhat stronger for diagnoses before 1980 versus 1980 or later, the RRs were fairly similar across calendar year for subgroups of autoimmune conditions. Autoimmune conditions with organ involvement had slightly stronger associations with NHL diagnosed before age 50 years (RR, 1.4; 95% CI, 1.2 to 1.7) than NHL diagnosed at or after age 50 years (RR, 1.2; 95% CI, 1.0 to 1.3; LRT, P = .03), but the RRs for total autoimmune diseases were the same across age groups. Allergies were more strongly associated when diagnosed 2 to 4 years before NHL than ≥ 5 years before NHL (LRT, P = .02), but the association did not vary by calendar year or age (Table 5).
Table 5.
Risk of NHL for Selected Conditions by Years of Follow-Up (latency), Year of First Visit, and Age at First Visit in US Male Veterans
| Condition | Follow-Up (Latency) |
Year of First Visit |
Age at First Visit |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-4 Years |
≥ 5 Years |
LRT P | < 1980 |
≥ 1980 |
LRT P | < 50 Years |
≥ 50 Years |
LRT P | |||||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||||
| Total infectious diseases | 1.4 | 1.3 to 1.5 | 1.1 | 1.0 to 1.1 | < .001* | 1.1 | 1.1 to 1.2 | 1.2 | 1.1 to 1.3 | .2 | 1.5 | 1.4 to 1.6 | 1.0 | 0.98 to 1.1 | < .001* |
| Upper airway | 1.3 | 1.1 to 1.5 | 1.1 | 0.95 to 1.2 | .1 | 1.1 | 0.99 to 1.2 | 1.1 | 0.93 to 1.3 | > .5 | 1.1 | 0.98 to 1.3 | 1.1 | 0.96 to 1.2 | > .5 |
| Lower airway | 1.4 | 1.2 to 1.6 | 1.1 | 1.0 to 1.2 | .003* | 1.1 | 1.0 to 1.2 | 1.3 | 1.2 to 1.5 | .02* | 1.6 | 1.4 to 1.8 | 1.0 | 0.91 to 1.1 | < .001* |
| Gastrohepatic | 1.7 | 1.4 to 2.1 | 1.3 | 1.1 to 1.5 | .3 | 1.3 | 1.1 to 1.5 | 1.6 | 1.4 to 2.0 | .03* | 1.9 | 1.7 to 2.2 | 1.0 | 0.88 to 1.2 | < .001* |
| Genital | 1.8 | 1.4 to 2.4 | 1.5 | 1.3 to 1.8 | .07 | 1.4 | 1.2 to 1.7 | 2.1 | 1.7 to 2.7 | .004* | 2.2 | 1.8 to 2.6 | 1.1 | 0.87 to 1.4 | < .001* |
| Reproductive | 0.9 | 0.58 to 1.3 | 1.2 | 1.1 to 1.5 | .04* | 1.3 | 1.1 to 1.5 | 1.0 | 0.72 to 1.3 | .07 | 1.3 | 1.0 to 1.7 | 1.1 | 0.91 to 1.3 | .2 |
| Urinary | 1.8 | 1.2 to 2.8 | 1.0 | 0.76 to 1.3 | .04* | 1.2 | 0.88 to 1.5 | 1.2 | 0.69 to 1.9 | > .5 | 0.9 | 0.6 to 1.4 | 1.3 | 0.99 to 1.8 | .2 |
| Cardiovascular | 1.1 | 0.88 to 1.5 | 1.0 | 0.76 to 1.3 | .3 | 0.9 | 0.76 to 1.2 | 1.1 | 0.90 to 1.3 | .4 | 1.1 | 0.8 to 1.4 | 1.0 | 0.85 to 1.2 | > .5 |
| Systemic | 5.6 | 4.8 to 6.5 | 2.1 | 1.9 to 2.4 | < .001* | 1.7 | 1.5 to 2.1 | 4.3 | 3.8 to 4.9 | < .001* | 6.5 | 5.7 to 7.4 | 1.3 | 1.1 to 1.5 | < .001* |
| Arthropod-borne diseases | 1.8 | 0.98 to 3.2 | 1.4 | 1.0 to 1.9 | .5 | 1.2 | 0.84 to 1.8 | 2.0 | 1.3 to 3.1 | .08 | 1.5 | 1.0 to 2.1 | 1.4 | 0.93 to 2.3 | > .5 |
| Total autoimmune diseases | 1.5 | 1.3 to 1.7 | 1.4 | 1.3 to 1.5 | .4 | 1.5 | 1.4 to 1.6 | 1.2 | 1.1 to 1.4 | .01* | 1.4 | 1.2 to 1.5 | 1.4 | 1.3 to 1.5 | > .5 |
| Autoantibodies detectable | 1.5 | 1.3 to 1.8 | 1.4 | 1.3 to 1.6 | > .5 | 1.5 | 1.4 to 1.7 | 1.3 | 1.1 to 1.5 | .08 | 1.5 | 1.3 to 1.8 | 1.5 | 1.3 to 1.6 | > .5 |
| Systemic involvement | 1.8 | 1.4 to 2.3 | 2.0 | 1.7 to 2.3 | .1 | 2.1 | 1.8 to 2.4 | 1.7 | 1.4 to 2.1 | .1 | 2.1 | 1.7 to 2.7 | 2.0 | 1.7 to 2.2 | .3 |
| Organ involvement | 1.5 | 1.2 to 1.8 | 1.1 | 0.99 to 1.3 | .3 | 1.3 | 1.1 to 1.4 | 1.1 | 0.93 to 1.4 | > .5 | 1.4 | 1.2 to 1.7 | 1.2 | 1.0 to 1.3 | .03* |
| Autoantibodies not detectable | 1.3 | 1.0 to 1.6 | 1.2 | 1.1 to 1.4 | > .5 | 1.3 | 1.2 to 1.5 | 1.1 | 0.88 to 1.3 | .2 | 1.2 | 0.99 to 1.5 | 1.2 | 1.1 to 1.4 | > .5 |
| Total allergies | 1.7 | 1.5 to 2.0 | 1.3 | 1.2 to 1.4 | .02* | 1.5 | 1.3 to 1.6 | 1.4 | 1.2 to 1.6 | > .5 | 1.4 | 1.2 to 1.6 | 1.5 | 1.3 to 1.6 | .4 |
Abbreviations: NHL, non-Hodgkin's lymphoma; RR, rate ratio; LRT, likelihood ratio test.
Significant result.
DISCUSSION
In this large study of more than 4 million US veterans, infectious, autoimmune, and allergic conditions were all associated with increased risk of NHL, but the risk of NHL associated with infections was slightly higher in blacks compared with whites. A notable exception was the risk associated with HIV, which was two-fold higher in whites. Allergies also tended to be more strongly associated with risk of NHL in blacks than in whites, whereas the risks associated with autoimmune conditions were generally similar by race.
Similar to previous studies,2,21 we found an increased risk of NHL in patients with autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus, Sjögren's syndrome, autoimmune hemolytic anemia, and celiac disease. We found that patients with multiple sclerosis had a reduced risk of NHL, in accord with previous studies,22,23 although the literature is mixed.21,24,25 Patients with multiple sclerosis have an innate immune profile skewed toward T helper 1–type immune activation, which may inhibit carcinogenesis.26–31 Previous studies have largely included white participants, preventing stratification by race.21 With more than 800,000 black patients, our study demonstrates that autoimmune conditions are associated with increased risk of NHL in both blacks and whites.
Results from previous studies of allergies and NHL are inconsistent. Many previous studies have found an inverse association with NHL,2,32 but prospective cohort studies tend to suggest that allergies are associated with an increased risk of NHL.33 Part of this inconsistency may be a result of the reliance on self-report of allergies,2 which is less objective than assessment based on discharge diagnoses. In addition, because NHL may reduce immunoglobulin reactivity, an apparent inverse association between allergies and NHL may be a result of reverse causality (ie, patients with undetected NHL may have an impaired immune response and thus reduced allergic responses).9,34 To our knowledge, only one previous study has evaluated allergies and risk of NHL by race and found some variation by race/ethnicity.35
Previous studies have fairly consistently reported an increased risk of NHL for a number of individual infectious agents, such as HIV, Epstein-Barr virus, human herpesvirus 8, and human T-cell lymphotropic virus.2 Similarly, we found that patients with infection-related conditions had an increased risk of NHL, most notably for systemic, genital, or arthropod-borne diseases. Although one recent study found no associations between genital infectious or sexual behavior and NHL,36 it had few exposed patients and controls and thus limited power.
Few studies have examined the association between infections and NHL by race. One case-only study found that Chinese patients with NHL were three times more likely to have Epstein-Barr virus–associated B-cell lymphoma than Malay patients with NHL.37 In a black South African population, HIV was associated with a six-fold increased risk of NHL, which is an order of magnitude lower than that observed in developed countries38 and implicates a racial disparity. In our study, the RR of NHL associated with HIV in blacks was approximately half as high as the RR in whites. Such racial differences in the incidence of HIV-associated NHL might be a result of racial/ethnic variation in stromal cell–derived factor 1-3′A chemokine allele frequency.39
The patterns of NHL and immune response in different ethnic groups also implicate genetic dissimilarities. For instance, the risk of NHL varies by ethnicity among second-generation immigrants to Sweden, and γ-globulin levels remain high in indigenous people who have migrated from tropical to temperate regions even several centuries after their migration.40–42 In addition, some evidence suggests that immune response to infections, such as Epstein-Barr virus, is depressed in African children compared with Chinese, Indian, and white children.43
A recent pooled analysis from the International Lymphoma Epidemiology Consortium found evidence for ethnic variability in the risk of NHL associated with polymorphisms in two immune system–related genes, tumor necrosis factor (TNF) and interleukin-10 (IL10).12 For example, the TNF-308A allele increased the risk of diffuse large B-cell lymphoma by 2.7-fold (95% CI, 1.3- to 5.6-fold) in blacks but only 1.3-fold (95% CI, 1.2- to 1.4-fold) in whites. Similarly, the IL10-1082G allele was associated with a 1.7-fold (95% CI, 1.1- to 2.6-fold) increased risk of NHL in Hispanic whites but no increased risk (odds ratio, 1.1; 95% CI, 0.98 to 1.2) in non-Hispanic whites. Thus, the differential distribution of immune gene polymorphisms by race might explain why infections, and possibly allergies, are more strongly associated with NHL in blacks than whites. At this time, we have no clear explanation for the observed similar associations for blacks and whites regarding autoimmune diseases and subsequent NHL risk.
Given that the development of NHL can alter immunity in and of itself,8 the potential for reverse causality (ie, that the observed associations are a result of developing disease, rather than a cause of the disease) is of concern. However, our cohort design allowed us to evaluate associations with NHL over time. The stability of RRs for autoimmune diseases across latency categories suggests that they are not explained by reverse causality. Although associations with infection-related conditions were generally stronger when they occurred closer to the diagnosis of NHL, many of the site-specific categories remained elevated with a latency of ≥ 5 years, suggesting that the observed associations are not entirely a result of undetected NHL. In addition, nearly all the increased risk of NHL occurred in patients younger than 50 years old, which is consistent with infection-related lymphomagenesis because infection-related cancer tends to occur at younger ages.44,45 Alternatively, this pattern could reflect minor immunologic defects, as suggested by the observation that children hospitalized for infection in the first year of life are at increased risk of developing NHL later in life.46 Allergies also were associated more strongly with NHL when diagnosed within 2 to 4 years of disease. Because the development of NHL is inversely associated with immunoglobulin reactivity,9,34 reverse causality would be expected to produce an inverse association. Instead, we observed a positive association, similar to other prospective studies.33,35
NHL was more strongly associated with several categories of infection-related conditions at or after 1980, possibly as a result of the discovery, and therefore increased diagnosis, of cancer-related infections. For example, the relation between human papillomavirus (HPV) and cervical cancer was not clearly elucidated until the development of sensitive polymerase chain reaction assays in the late 1980s to early 1990s.47 This improved understanding of cervical carcinogenesis may also have led to increased reporting of the HPV-associated condition condyloma acuminatum, even though genital warts are caused by noncarcinogenic HPV. Similarly, the discovery of hepatitis C virus in 1989 and HIV in 1983 might have influenced associations with gastrohepatic and systemic infections over time.
Our study included only hospitalized male US veterans, limiting generalizability. Also, less severe immune-related conditions, typically diagnosed in outpatient settings, may be under-represented. Clinical data, laboratory data, and medical records were not available to verify diagnoses. However, we expect any potential exposure misclassification to be nondifferential because immune-related conditions were identified similarly for patient cases and non–patient cases. Another limitation is the large number of tested immune-related conditions, which implies that one has to interpret detected associations with caution as a result of multiple comparisons. Some diagnoses may have been missed because veterans may choose either VA or civilian health care, although this potential bias may be limited because many patients who choose VA care lack other health insurance.48 In addition, we do not have data on potential confounders such as organ transplantation; chemotherapy/radiation; chemical exposure to benzene, solvents, herbicides, or pesticides; family history; or socioeconomic status. Within this study population, the potential for confounding by socioeconomic status may be limited because patients within the VA system typically have low socioeconomic status,48 and previous VA studies found similar health care utilization and outcomes for blacks and whites.49,50
This study has several strengths. First, the large sample size of more than 4 million veterans permitted us to evaluate risk of NHL separately in blacks and whites for infectious, autoimmune, and allergic conditions. These patients had access to standardized medical care without regard to socioeconomic status. The follow-up time was extensive. In addition, immune-related conditions were identified before the development of NHL using discharge diagnoses, thus eliminating recall bias.
In summary, we found that risk of NHL is associated with infectious, autoimmune, and allergic conditions. The increased risk of NHL associated with infection-related conditions, and possibly allergies, seemed to be stronger in blacks than in whites, whereas the risk related to autoimmune conditions was similar for both. These patterns could reflect underlying race-related genetic differences in immune response. Additional studies are needed to explore the pathologic mechanisms underlying these associations.
Acknowledgment
We thank the Medical Administration Service of the Veterans Health Services and Research Administration for providing the data on which this study is based and David Campbell and Eric Boyd, Information Management Services, Silver Spring, MD, for assistance with data preparation. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Appendix
Table A1.
RRs for Each of the Specific Infectious Conditions and Subsequent NHL Among Blacks and Whites
| Category | Whites |
Blacks |
LRT P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Veterans Exposed |
RR | 95% CI | No. of Veterans Exposed |
RR | 95% CI | ||||
| No NHL | NHL | No NHL | NHL | ||||||
| Total infection-related conditions | 1,213,610 | 3,086 | 1.1 | 1.1 to 1.2 | 322,979 | 746 | 1.4 | 1.2 to 1.5 | .002* |
| Upper airway | 186,795 | 489 | 1.1 | 0.99 to 1.2 | 36,955 | 84 | 1.1 | 0.87 to 1.4 | > .5 |
| Acute bronchitis/bronchiolitis | 77,337 | 169 | 0.94 | 0.80 to 1.1 | 12,098 | 22 | 0.97 | 0.62 to 1.5 | > .5 |
| Chronic sinusitis | 62,433 | 202 | 1.3 | 1.1 to 1.5 | 12,268 | 41 | 1.5 | 1.1 to 2.2 | .4 |
| Nasopharyngitis/pharyngitis | 41,906 | 103 | 1.1 | 0.88 to 1.3 | 12,524 | 22 | 0.88 | 0.56 to 1.4 | .4 |
| Lower airway | 346,387 | 845 | 1.1 | 1.0 to 1.2 | 95,676 | 243 | 1.3 | 1.2 to 1.6 | .02* |
| Influenza | 18,788 | 62 | 1.3 | 0.96 to 1.6 | 3,384 | 7 | 0.44 | 0.06 to 3.1 | .3 |
| Pneumonia | 289,391 | 676 | 1.1 | 1.0 to 1.2 | 72,443 | 191 | 1.4 | 1.2 to 1.7 | .008* |
| Tuberculosis | 55,462 | 149 | 1.0 | 1.0 to 1.2 | 26,689 | 76 | 1.2 | 0.90 to 1.5 | .4 |
| Gastrohepatic | 116,070 | 335 | 1.3 | 1.2 to 1.5 | 29,296 | 80 | 1.5 | 1.2 to 2.0 | .3 |
| Cholangitis/cholecystitis | 28,563 | 76 | 0.97 | 0.76 to 1.2 | 4,281 | 10 | 1.1 | 0.53 to 2.1 | > .5 |
| Hepatitis virus | 32,035 | 83 | 2.0 | 1.6 to 2.5 | 13,827 | 32 | 1.6 | 1.1 to 2.4 | .4 |
| Hepatitis B virus | 9,414 | 34 | 2.7 | 1.9 to 3.9 | 5,264 | 18 | 2.4 | 1.4 to 4.0 | > .5 |
| Hepatitis C virus | 10,835 | 15 | 1.4 | 0.70 to 2.8 | 5,775 | 6 | 0.95 | 0.30 to 3.0 | > .5 |
| Intestinal | 58,394 | 188 | 1.4 | 1.2 to 1.6 | 11,998 | 40 | 1.4 | 1.2 to 1.6 | > .5 |
| Genital | 37,432 | 127 | 1.5 | 1.3 to 1.8 | 38,967 | 123 | 1.6 | 1.4 to 2.0 | > .5 |
| Condyloma acuminatum | 8,007 | 23 | 1.6 | 1.0 to 2.4 | 3,429 | 7 | 1.2 | 0.58 to 2.6 | > .5 |
| Genital herpes | 2,078 | 14 | 4.2 | 2.3 to 7.6 | 1,156 | 20 | 12.4 | 7.5 to 20.5 | .006* |
| Gonorrhea | 4,155 | 18 | 2.1 | 1.3 to 3.3 | 5,232 | 15 | 1.3 | 0.8 to 2.3 | .2 |
| Syphilis | 22,198 | 73 | 1.3 | 1.1 to 1.7 | 26,963 | 83 | 1.6 | 1.3 to 2.0 | .4 |
| Reproductive | 52,638 | 155 | 1.1 | 0.89 to 1.3 | 13,681 | 49 | 1.7 | 1.2 to 2.2 | .01* |
| Orchitis and epididymitis | 37,794 | 111 | 1.1 | 0.88 to 1.3 | 11,035 | 44 | 1.8 | 1.3 to 2.5 | .006* |
| Chronic prostatitis | 15,790 | 46 | 1.0 | 0.74 to 1.4 | 2,830 | 5 | 0.79 | 0.30 to 2.1 | > .5 |
| Urinary | 20,323 | 60 | 1.2 | 0.96 to 1.6 | 7,260 | 12 | 0.86 | 0.48 to 1.5 | .2 |
| Urethritis | 9,255 | 26 | 1.1 | 0.77 to 1.7 | 4,471 | 8 | 0.89 | 0.44 to 1.8 | > .5 |
| Cardiovascular | 90,210 | 196 | 1.0 | 0.88 to 1.2 | 20,954 | 37 | 0.97 | 0.68 to 1.4 | > .5 |
| Endocarditis | 85,232 | 183 | 1.0 | 0.87 to 1.2 | 19,322 | 33 | 0.99 | 0.68 to 1.4 | > .5 |
| Systemic | 71,346 | 367 | 2.7 | 2.4 to 3.0 | 25,424 | 141 | 4.0 | 3.3 to 4.9 | < .001* |
| HIV | 7,589 | 225 | 46.2 | 38.8 to 55.0 | 6,821 | 104 | 22.3 | 17.1 to 29.0 | < .001* |
| Meningitis | 10,142 | 48 | 2.1 | 1.5 to 2.8 | 3,685 | 26 | 3.6 | 2.3 to 2.8 | .06 |
| Septicemia | 55,828 | 145 | 1.3 | 1.0 to 1.5 | 16,815 | 48 | 1.6 | 1.1 to 2.3 | .2 |
| Arthropod-borne diseases | 17,563 | 43 | 1.3 | 0.96 to 1.8 | 3,158 | 12 | 2.2 | 1.3 to 4.0 | .1 |
| Pediculosis and Phthirus infestation | 4,486 | 8 | 0.95 | 0.48 to 1.9 | 1,671 | 6 | 1.9 | 0.86 to 4.3 | .2 |
| Other | |||||||||
| Conjunctivitis | 13,763 | 31 | 0.86 | 0.60 to 1.2 | 3,027 | 11 | 1.5 | 0.80 to 2.8 | .1 |
| Gingivitis and periodontitis | 374,567 | 936 | 0.95 | 0.88 to 1.0 | 103,546 | 206 | 0.85 | 0.73 to 1.0 | .2 |
| Herpes simplex | 10,195 | 70 | 3.0 | 2.3 to 3.9 | 3,246 | 40 | 7.0 | 4.8 to 10.1 | < .001* |
| Herpes zoster | 16,226 | 86 | 2.0 | 1.6 to 2.5 | 2,687 | 20 | 4.0 | 2.4 to 6.7 | .02* |
| Infective arthritis | 5,990 | 23 | 1.9 | 1.2 to 2.9 | 7,976 | 5 | 1.7 | 0.70 to 4.0 | > .5 |
| Mycoses | 128,474 | 431 | 1.6 | 1.4 to 1.7 | 7,568 | 168 | 2.1 | 1.8 to 2.5 | .006* |
| Osteomyelitis | 45,899 | 93 | 0.85 | 0.68 to 1.1 | 11,756 | 19 | 0.90 | 0.57 to 1.4 | > .5 |
| Skin and soft tissue infections | 241,710 | 612 | 1.1 | 1.0 to 1.2 | 54,829 | 145 | 1.5 | 1.2 to 1.7 | .02* |
| Toxoplasmosis | 1,044 | 32 | 9.3 | 5.5 to 15.7 | 465 | 8 | 3.7 | 0.92 to 14.7 | .2 |
NOTE. Only conditions with five or more exposed black and white veterans are included. The LRT provides a formal evaluation of the difference in RRs by race. All analyses were adjusted for age, calendar time, latency, and number of hospital visits.
Abbreviations: RR, rate ratio; NHL, non-Hodgkin's lymphoma; LRT, likelihood ratio test.
Significant result.
Table A2.
RRs for Each of the Specific Autoimmune Conditions and Subsequent NHL Among Blacks and Whites
| Category | Whites |
Blacks |
LRT P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Veterans Exposed |
RR | 95% CI | No. of Veterans Exposed |
RR | 95% CI | ||||
| No NHL | NHL | No NHL | NHL | ||||||
| Total autoimmune conditions | 244,024 | 791 | 1.4 | 1.3 to 1.5 | 44,850 | 127 | 1.5 | 1.2 to 1.8 | .5 |
| Autoantibodies detectable | 168,711 | 584 | 1.5 | 1.4 to 1.6 | 30,715 | 83 | 1.4 | 1.1 to 1.8 | > .5 |
| Systemic involvement | 57,044 | 283 | 2.0 | 1.7 to 2.2 | 8,961 | 34 | 2.1 | 1.4 to 2.8 | > .5 |
| Rheumatoid arthritis | 51,736 | 253 | 1.9 | 1.7 to 2.2 | 7,472 | 27 | 1.8 | 1.2 to 2.6 | > .5 |
| Sjögren's syndrome | 1,583 | 13 | 2.5 | 1.4 to 4.7 | 292 | 5 | 8.7 | 3.6 to 21.0 | .04* |
| Organ involvement | 116,665 | 329 | 1.2 | 1.1 to 1.4 | 22,810 | 53 | 1.2 | 0.88 to 1.6 | .5 |
| Amyotrophic lateral sclerosis | 6,157 | 6 | 0.51 | 0.21 to 1.2 | 1,112 | 5 | 2.8 | 1.0 to 7.4 | .02* |
| Chronic rheumatic heart disease | 53,875 | 138 | 1.1 | 0.95 to 2.6 | 10,964 | 13 | 0.58 | 0.31 to 1.1 | .03* |
| Immune thrombocytopenic purpura | 7,660 | 39 | 2.7 | 2.0 to 3.8 | 1,500 | 8 | 3.1 | 2.1 to 3.8 | > .5 |
| Multiple sclerosis | 17,352 | 22 | 0.59 | 0.39 to 0.90 | 1,989 | 5 | 1.3 | 0.56 to 3.2 | .1 |
| Autoantibodies not detectable | 90,348 | 268 | 1.2 | 1.0 to 1.3 | 15,992 | 49 | 1.5 | 1.1 to 2.1 | .1 |
| Sarcoidosis | 2,923 | 14 | 1.9 | 1.0 to 3.6 | 5,341 | 12 | 1.3 | 0.74 to 2.4 | .4 |
| Reiter's disease | 2,906 | 7 | 1.1 | 0.50 to 2.5 | 722 | 5 | 2.9 | 1.1 to 7.8 | .2 |
| Psoriasis | 37,532 | 118 | 1.2 | 1.0 to 1.5 | 3,008 | 15 | 2.7 | 1.6 to 4.5 | .02* |
| Hemorrhagic proctitis | 16,371 | 33 | 0.83 | 0.57 to 1.2 | 3,525 | 7 | 0.86 | 0.36 to 2.1 | > .5 |
NOTE. Only conditions with five or more exposed black and white veterans are included. The LRT provides a formal evaluation of the difference in RRs by race. All analyses were adjusted for age, calendar time, latency, and number of hospital visits.
Abbreviations: RR, rate ratio; NHL, non-Hodgkin's lymphoma; LRT, likelihood ratio test.
Significant result.
Table A3.
RRs for Each of the Specific Allergic Conditions and Subsequent NHL Among Blacks and Whites
| Category | No. of Veterans Exposed |
RR | 95% CI | No. of Veterans Exposed |
RR | 95% CI | LRT P | ||
|---|---|---|---|---|---|---|---|---|---|
| No NHL | NHL | No NHL | NHL | ||||||
| Total allergic conditions | 182,246 | 584 | 1.4 | 1.2 to 1.5 | 41,283 | 134 | 1.7 | 1.4 to 2.0 | .05 |
| Asthma | 68,133 | 140 | 0.89 | 0.75 to 1.1 | 17,966 | 35 | 0.95 | 0.66 to 1.3 | > .5 |
| Dermatitis | 76,486 | 294 | 1.5 | 1.4 to 1.8 | 16,165 | 73 | 2.2 | 1.7 to 2.8 | .02* |
| Erythema | 24,575 | 154 | 2.6 | 2.2 to 3.0 | 4,627 | 35 | 3.7 | 2.6 to 5.4 | .08 |
| Rhinitis | 13,876 | 24 | 0.79 | 0.53 to 1.2 | 2,924 | 5 | 0.97 | 0.40 to 2.3 | > .5 |
NOTE. Only conditions with five or more exposed black and white veterans are included. The LRT provides a formal evaluation of the difference in RRs by race. All analyses were adjusted for age, calendar time, latency, and number of hospital visits.
Abbreviations: RR, rate ratio; NHL, non-Hodgkin's lymphoma; LRT, likelihood ratio test.
Significant result.
Footnotes
Supported by general funds from the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics; and the Cancer Prevention Fellowship Program, Office of Preventive Oncology, National Cancer Institute, Bethesda, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jill Koshiol, Gloria Gridley, David Check,Ola Landgren
Administrative support: Gloria Gridley, David Check
Collection and assembly of data: Jill Koshiol, Gloria Gridley,David Check
Data analysis and interpretation: Jill Koshiol, Tram Kim Lam, Gloria Gridley, David Check, Linda Morris Brown, Ola Landgren
Manuscript writing: Jill Koshiol, Tram Kim Lam, Gloria Gridley, David Check, Linda Morris Brown, Ola Landgren
Final approval of manuscript: Jill Koshiol, Tram Kim Lam, Gloria Gridley, David Check, Linda Morris Brown, Ola Landgren
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Alexander DD, Mink PJ, Adami HO, et al. The non-Hodgkin lymphomas: A review of the epidemiologic literature. Int J Cancer. 2007;120(suppl 12):1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- 3.Morton LM, Wang SS, Cozen W, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes. Blood. 2008;112:5150–5160. doi: 10.1182/blood-2008-01-133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little RF, Gutierrez M, Jaffe ES, et al. HIV-associated non-Hodgkin lymphoma: Incidence, presentation, and prognosis. JAMA. 2001;285:1880–1885. doi: 10.1001/jama.285.14.1880. [DOI] [PubMed] [Google Scholar]
- 5.Goldin LR, Landgren O. Autoimmunity and lymphomagenesis. Int J Cancer. 2009;124:1497–1502. doi: 10.1002/ijc.24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin DN, Mikhail IS, Landgren O. Autoimmunity and hematologic malignancies: Associations and mechanisms. Leuk Lymphoma. 2009;50:541–550. doi: 10.1080/10428190902780677. [DOI] [PubMed] [Google Scholar]
- 7.Hjalgrim H, Engels EA. Infectious aetiology of Hodgkin and non-Hodgkin lymphomas: A review of the epidemiological evidence. J Intern Med. 2008;264:537–548. doi: 10.1111/j.1365-2796.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 8.Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:405–408. doi: 10.1158/1055-9965.EPI-06-1070. [DOI] [PubMed] [Google Scholar]
- 9.Melbye M, Smedby KE, Lehtinen T, et al. Atopy and risk of non-Hodgkin lymphoma. J Natl Cancer Inst. 2007;99:158–166. doi: 10.1093/jnci/djk019. [DOI] [PubMed] [Google Scholar]
- 10.Koshiol J, Gridley G, Engels EA, et al. Chronic immune stimulation and subsequent Waldenström macroglobulinemia. Arch Intern Med. 2008;168:1903–1909. doi: 10.1001/archinternmed.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu XC, Andrews P, Chen VW, et al. Incidence of extranodal non-Hodgkin lymphomas among whites, blacks, and Asians/Pacific Islanders in the United States: Anatomic site and histology differences. Cancer Epidemiol. 2009;33:337–346. doi: 10.1016/j.canep.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Skibola CF, Bracci PM, Nieters A, et al. Tumor necrosis factor (TNF) and lymphotoxin-alpha (LTA) polymorphisms and risk of non-Hodgkin lymphoma in the InterLymph Consortium. Am J Epidemiol. 2010;171:267–276. doi: 10.1093/aje/kwp383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mili F, Flanders WD, Boring JR, et al. The associations of race, cigarette smoking, and smoking cessation to measures of the immune system in middle-aged men. Clin Immunol Immunopathol. 1991;59:187–200. doi: 10.1016/0090-1229(91)90017-5. [DOI] [PubMed] [Google Scholar]
- 14.Lichtman MA, Vaughan JH, Hames CG. The distribution of serum immunoglobulins, anti-gamma-G globulins (“rheumatoid factors”) and antinuclear antibodies in white and Negro subjects in Evans County, Georgia. Arthritis Rheum. 1967;10:204–215. doi: 10.1002/art.1780100306. [DOI] [PubMed] [Google Scholar]
- 15.Tollerud DJ, Brown LM, Blattner WA, et al. Racial differences in serum immunoglobulin levels: Relationship to cigarette smoking, T-cell subsets, and soluble interleukin-2 receptors. J Clin Lab Anal. 1995;9:37–41. doi: 10.1002/jcla.1860090107. [DOI] [PubMed] [Google Scholar]
- 16.Boyko EJ, Koepsell TD, Gaziano JM, et al. US Department of Veterans Affairs medical care system as a resource to epidemiologists. Am J Epidemiol. 2000;151:307–314. doi: 10.1093/oxfordjournals.aje.a010207. [DOI] [PubMed] [Google Scholar]
- 17.Landgren O, Gridley G, Turesson I, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107:904–906. doi: 10.1182/blood-2005-08-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson C, Waldrop J. Washington, DC: US Department of Commerce; 2003. Veterans: 2000— Census 2000 Brief. [Google Scholar]
- 19.Page WF, Mahan CM, Kang HK. Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration. Ann Epidemiol. 1996;6:102–109. doi: 10.1016/1047-2797(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 20.Breslow NE, Day NE. Statistical methods in cancer research: Volume II—The design and analysis of cohort studies. IARC Sci Publ. 1987;82:1–406. [PubMed] [Google Scholar]
- 21.Smedby KE, Askling J, Mariette X, et al. Autoimmune and inflammatory disorders and risk of malignant lymphomas: An update. J Intern Med. 2008;264:514–527. doi: 10.1111/j.1365-2796.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- 22.Bahmanyar S, Montgomery SM, Hillert J, et al. Cancer risk among patients with multiple sclerosis and their parents. Neurology. 2009;72:1170–1177. doi: 10.1212/01.wnl.0000345366.10455.62. [DOI] [PubMed] [Google Scholar]
- 23.Söderberg KC, Jonsson F, Winqvist O, et al. Autoimmune diseases, asthma and risk of haematological malignancies: A nationwide case-control study in Sweden. Eur J Cancer. 2006;42:3028–3033. doi: 10.1016/j.ejca.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen NM, Rostgaard K, Rasmussen S, et al. Cancer risk among patients with multiple sclerosis: A population-based register study. Int J Cancer. 2006;118:979–984. doi: 10.1002/ijc.21437. [DOI] [PubMed] [Google Scholar]
- 25.Vineis P, Crosignani P, Viganò C, et al. Lymphomas and multiple sclerosis in a multicenter case-control study. Epidemiology. 2001;12:134–135. doi: 10.1097/00001648-200101000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Gregory SG, Schmidt S, Seth P, et al. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat Genet. 2007;39:1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- 27.Hafler DA, Compston A, Sawcer S, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 28.Lundmark F, Duvefelt K, Iacobaeus E, et al. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat Genet. 2007;39:1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- 29.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sørensen TL, Tani M, Jensen J, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Windhagen A, Newcombe J, Dangond F, et al. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J Exp Med. 1995;182:1985–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vajdic CM, Falster MO, de Sanjose S, et al. Atopic disease and risk of non-Hodgkin lymphoma: An InterLymph pooled analysis. Cancer Res. 2009;69:6482–6489. doi: 10.1158/0008-5472.CAN-08-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Diepgen TL. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy. 2005;60:1098–1111. doi: 10.1111/j.1398-9995.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 34.Grulich AE, Vajdic CM, Riminton S, et al. Re: Atopy and risk of non-Hodgkin lymphoma. J Natl Cancer Inst. 2007;99:1417. doi: 10.1093/jnci/djm114. [DOI] [PubMed] [Google Scholar]
- 35.Erber E, Lim U, Maskarinec G, et al. Common immune-related risk factors and incident non-Hodgkin lymphoma: The multiethnic cohort. Int J Cancer. 2009;125:1440–1445. doi: 10.1002/ijc.24456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vajdic CM, Grulich AE, Kaldor JM, et al. Specific infections, infection-related behavior, and risk of non-Hodgkin lymphoma in adults. Cancer Epidemiol Biomarkers Prev. 2006;15:1102–1108. doi: 10.1158/1055-9965.EPI-06-0078. [DOI] [PubMed] [Google Scholar]
- 37.Peh SC. Host ethnicity influences non-Hodgkin's lymphoma subtype frequency and Epstein-Barr virus association rate: The experience of a multi-ethnic patient population in Malaysia. Histopathology. 2001;38:458–465. doi: 10.1046/j.1365-2559.2001.01104.x. [DOI] [PubMed] [Google Scholar]
- 38.Stein L, Urban MI, O'Connell D, et al. The spectrum of human immunodeficiency virus-associated cancers in a South African black population: Results from a case-control study, 1995-2004. Int J Cancer. 2008;122:2260–2265. doi: 10.1002/ijc.23391. [DOI] [PubMed] [Google Scholar]
- 39.Rabkin CS, Yang Q, Goedert JJ, et al. Chemokine and chemokine receptor gene variants and risk of non-Hodgkin's lymphoma in human immunodeficiency virus-1-infected individuals. Blood. 1999;93:1838–1842. [PubMed] [Google Scholar]
- 40.Hemminki K, Li X. Cancer risks in second-generation immigrants to Sweden. Int J Cancer. 2002;99:229–237. doi: 10.1002/ijc.10323. [DOI] [PubMed] [Google Scholar]
- 41.Albandar JM, DeNardin AM, Adesanya MR, et al. Associations of serum concentrations of IgG, IgA, IgM and interleukin-1beta with early-onset periodontitis classification and race. J Clin Periodontol. 2002;29:421–426. doi: 10.1034/j.1600-051x.2002.290506.x. [DOI] [PubMed] [Google Scholar]
- 42.Penny R. Paraprotein patterns in Australia. Australas Ann Med. 1969;18:251–257. doi: 10.1111/imj.1969.18.3.251. [DOI] [PubMed] [Google Scholar]
- 43.de-Thé G, Day NE, Geser A, et al. Sero-epidemiology of the Epstein-Barr virus: Preliminary analysis of an international study—A review. IARC Sci Publ. 1975;11:3–16. [PubMed] [Google Scholar]
- 44.American Cancer Society: Cancer facts and figures special section, 2005. Cancers linked to infectious diseases. http://www.cancer.org/acs/groups/content/@nho/documents/document/caff2005f4pwsecuredpdf.pdf.
- 45.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113:3036–3046. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paltiel O, Laniado DE, Yanetz R, et al. The risk of cancer following hospitalization for infection in infancy: A population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:1964–1968. doi: 10.1158/1055-9965.EPI-06-0313. [DOI] [PubMed] [Google Scholar]
- 47.zur Hausen H. Papillomaviruses in the causation of human cancers: A brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 48.Randall M, Kilpatrick KE, Pendergast JF, et al. Differences in patient characteristics between Veterans Administration and community hospitals: Implications for VA planning. Med Care. 1987;25:1099–1104. doi: 10.1097/00005650-198711000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Deswal A, Petersen NJ, Souchek J, et al. Impact of race on health care utilization and outcomes in veterans with congestive heart failure. J Am Coll Cardiol. 2004;43:778–784. doi: 10.1016/j.jacc.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 50.Giordano TP, Morgan RO, Kramer JR, et al. Is there a race-based disparity in the survival of veterans with HIV? J Gen Intern Med. 2006;21:613–617. doi: 10.1111/j.1525-1497.2006.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]