Abstract
Purpose
The prognosis for older adolescents and young adults with acute lymphoblastic leukemia (ALL) has been historically much worse than that for younger patients. We reviewed the outcome of older adolescents (age 15 to 18 years) treated in four consecutive Total Therapy studies to determine if recent improved treatment extended to this high-risk group.
Patients and Methods
Between 1991 and 2007, 963 pediatric patients, including 89 older adolescents, were enrolled on Total Therapy studies XIIIA, XIIIB, XIV, and XV. In the first three studies, treatment selection was based on presenting clinical features and leukemic cell genetics. In study XV, the level of residual disease was used to guide treatment, which featured intensive methotrexate, glucocorticoid, vincristine, and asparaginase, as well as early triple intrathecal therapy for higher-risk ALL.
Results
The 89 older adolescents were significantly more likely to have T-cell ALL, the t(4;11)(MLL-AF4), and detectable minimal residual disease during or at the end of remission induction; they were less likely to have the t(12;21)(ETV6-RUNX1) compared with younger patients. In the first three studies, the 44 older adolescents had significantly poorer event-free survival and overall survival than the 403 younger patients. This gap in prognosis was abolished in study XV: event-free survival rates at 5 years were 86.4% ± 5.2% (standard error) for the 45 older adolescents and 87.4% ± 1.7% for the 453 younger patients; overall survival rates were 87.9% ± 5.1% versus 94.1% ± 1.2%, respectively.
Conclusion
Most older adolescents with ALL can be cured with risk-adjusted intensive chemotherapy without stem-cell transplantation.
INTRODUCTION
Contemporary clinical trials for acute lymphoblastic leukemia (ALL) have produced 5-year survival rates of 83% to 94% for children and 27% to 54% for adults.1–14 Specific treatment outcome data for older adolescents age 15 to 19 years are limited not only because ALL is relatively uncommon in this age group but also because such patients are treated by either adult or pediatric oncologists, depending on referral patterns. Historically, older adolescents have had a much worse prognosis than younger patients, which can be explained, at least in part, by an increased prevalence of high-risk leukemia and a poorer tolerance and adherence to therapy.15–18 Older adolescents with ALL treated in pediatric clinical trials have consistently fared better than those enrolled on adult trials, perhaps because of the more intensive treatment and the more stringent compliance as a result of parental involvement associated with pediatric trials.17–20 The poor treatment outcome obtained with adult regimens has led some oncologists to recommend matched-sibling allogeneic transplantation in first remission for older adolescents.21 In this report, we show that, with effective risk-directed chemotherapy, older adolescents can achieve an excellent treatment outcome, similar to the best results reported to date for younger children with ALL.1–3,7
PATIENTS AND METHODS
Patients
Four hundred sixty-five patients (including 44 adolescents age 15 to 18 years old) with newly diagnosed childhood ALL were enrolled on the Total Therapy studies XIIIA, XIIIB, and XIV9 at St Jude Children's Research Hospital from 1991 to 1999, whereas 498 (including 45 older adolescents) were enrolled on the Total Therapy study XV3 from 2000 to 2007. All protocols were approved by the institutional review boards, and study XV was registered at ClinicalTrials.gov. Signed informed consent was obtained from the parents or guardians, with assent from the patients as appropriate.
The risk classification system used in studies XIIIA, XIIIB and XIV was based on presenting clinical features and genetic abnormalities of the leukemic cells, as described previously.9 In study XV, risk classification was based mainly on treatment response.3 Patients with B-cell precursor disease who were between 1 and 10 years of age and who had leukocyte counts less than 50 × 109/L, DNA index ≥ 1.16, or the t(12;21)(ETV6-RUNX1) were provisionally classified as having low-risk ALL. Patients with the t(9;22)(BCR-ABL1) were considered to have high-risk ALL, whereas the remaining patients, including all those with T-cell ALL, were provisionally classified to have standard-risk (ie, intermediate) ALL. The final risk status was determined by the level of minimal residual disease (MRD), as measured by flow cytometry and/or the polymerase chain reaction.22 Any patient with ≥ 1% bone marrow MRD on day 19 of remission induction, or 0.1% to 0.99% MRD after completion of induction therapy was considered to have standard-risk ALL. The inability to achieve morphologic remission, the presence of MRD ≥ 1% after completion of induction therapy, and the persistence of MRD ≥ 0.1% beyond week 7 of continuation treatment denoted high-risk ALL and were indications for allogeneic stem-cell transplantation.
Treatment in Studies XIIIA, XIIIB, and XIV
Details of the treatment regimens of studies XIIIA, XIIIB, and XIV have been reported.9 In brief, a reinduction phase was first introduced in study XIIIA; double reinductions were administered, and dexamethasone was substituted for prednisone in study XIIIB; methotrexate was given at a higher dose for two courses during consolidation therapy for patients with higher-risk leukemia in study XIV. Antimetabolites and epipodophyllotoxins (for patients with higher-risk ALL) together with glucocorticoid plus vincristine pulses formed the backbone of continuation treatment for all three studies.
Treatment in Study XV With Remission Induction and Consolidation
After an optional 4-day treatment with methotrexate, remission-induction therapy began with prednisone, vincristine, daunorubicin, and asparaginase (Appendix Table A1, online only). Patients with ≥ 1% MRD on day 19 received three additional doses of asparaginase. Subsequent remission-induction therapy included cyclophosphamide, mercaptopurine, and cytarabine. On hematopoietic recovery (between days 43 and 46), consolidation therapy (Appendix Table A2, online only) with high-dose methotrexate, mercaptopurine, and triple intrathecal treatment began, and the dose of methotrexate was based on risk classification.
Continuation Therapy
During initial continuation therapy (Appendix Table A3, online only), patients with low-risk disease received daily mercaptopurine and weekly methotrexate with pulses of mercaptopurine, dexamethasone, and vincristine. Two reinduction treatments were given between weeks 7 to 9 and weeks 17 to 19. Patients with standard-risk disease received weekly asparaginase and daily mercaptopurine with pulses of doxorubicin plus vincristine plus dexamethasone. They also received two reinduction treatments between weeks 7 to 9 and weeks 17 to 20.
For the remaining part of continuation therapy, patients with low-risk disease received mercaptopurine and methotrexate, with pulses of dexamethasone, vincristine, and mercaptopurine, and patients with standard-risk disease received three rotating drug pairs (mercaptopurine plus methotrexate, cyclophosphamide plus cytarabine, and dexamethasone plus vincristine). Dosages of mercaptopurine and methotrexate were adjusted according to tolerance, thiopurine methyltransferase phenotype, and genotypes.3 Total scheduled dosages of anthracyclines and cyclophosphamide were limited to 110 mg/m2 and 1 g/m2 for patients with low-risk disease and to 230 mg/m2 and 4.6 g/m2 for patients with standard-risk disease. Continuation treatment lasted 120 weeks for girls and 146 weeks for boys.
CNS-Directed Therapy
Intrathecal cytarabine was instilled after diagnostic lumbar puncture, and triple intrathecal chemotherapy was given for all subsequent treatments (Appendix Table A1). Depending on the presenting features and the CNS status, patients with low-risk disease received 13 to 18 intrathecal treatments, and patients with standard-risk disease received 16 to 25 intrathecal treatments. According to the protocol design, none of the patients received prophylactic cranial irradiation.
Allogeneic Hematopoietic Stem-Cell Transplantation
This procedure was an option for patients with high-risk leukemia (whose early treatment was identical to that for patients with standard-risk disease). Reintensification therapy was given to maximize MRD reduction before transplantation.3
Statistical Analysis
The exact χ2 and Fisher's exact tests were used to compare differences in the distribution of presenting features between the two age groups (ie, 1 to 14 years and 15 to 18 years). Event-free survival and overall survival distributions were estimated by the method of Kaplan and Meier and were compared with the Mantel-Haenszel test; 95% CIs were calculated by the method of Kalbfleisch and Prentice. The cumulative risk of adverse events was calculated by the method of Kalbfleisch and Prentice and was compared with Gray's test. Because the overall treatment results for studies XIIIA, XIIIB, and XIV were similar, patients treated in these three studies were combined for the outcome analyses.
The database frozen on July 9, 2010, was used for the analysis; 80% of survivors in the three earlier studies had been seen within the last 2 years; 92% of survivors in study XV, within the last year. The median follow-up time was 12 years (range, 8.3 to 17.7 years) for survivors treated in the three earlier studies and 5.2 years (range, 1.2 to 9.7 years) for survivors in study XV. All reported P values are two sided and not adjusted for multiple tests.
RESULTS
Clinical and Biologic Features
The presenting characteristics of the 945 patients (excluding 18 infants) separated by age group are listed in Table 1. As expected, the older adolescents were more likely to have standard- or high-risk leukemia, T-cell ALL, and the t(4;11)(MLL-AF4), and they were less likely to have the t(12;21)(ETV6-RUNX1).
Table 1.
Comparison of Clinical and Biologic Variables According to Age Group
| Variable | Age Group |
P | |
|---|---|---|---|
| 1-14 Years (n = 856) | 15-18 Years (n = 89) | ||
| Risk group | |||
| Low | 377 | 11 | < .001 |
| Standard/high | 478 | 78 | |
| NCI risk group | |||
| B-cell precursor ALL | |||
| Standard | 462 | 0 | < .001 |
| High | 270 | 61 | |
| T-cell ALL | |||
| Standard | 24 | 0 | .008 |
| High | 100 | 28 | |
| Sex | |||
| Male | 464 | 65 | < .001 |
| Female | 392 | 24 | |
| Leukocyte count | |||
| < 10 × 109/L | 385 | 46 | .10 |
| 10 to 49 × 109/L | 255 | 15 | |
| 50 to 99 × 109/L | 103 | 14 | |
| 100 to 300 × 109/L | 76 | 11 | |
| ≥ 300 × 109/L | 37 | 3 | |
| Ethnicity | |||
| White | 607 | 68 | .61 |
| African American | 142 | 14 | |
| Hispanic | 56 | 4 | |
| Asian or other | 51 | 3 | |
| Immunophenotype | |||
| B-cell precursor | 732 | 61 | < .001 |
| T-cell | 124 | 28 | |
| CNS status* | |||
| CNS-1 | 581 | 60 | .91 |
| CNS-2 | 205 | 20 | |
| Traumatic with blasts | 51 | 6 | |
| CNS-3 | 19 | 3 | |
| DNA index† | |||
| ≥ 1.16 | 205 | 16 | .24 |
| < 1.16 | 651 | 73 | |
| t(9;22)( BCR-ABL1) | |||
| Present | 21 | 3 | .49 |
| Absent | 835 | 86 | |
| t(1;19)(TCF3-PBX1) | |||
| Present | 45 | 3 | .61 |
| Absent | 811 | 86 | |
| t(12;21)(ETV6-RUNX1) | |||
| Present | 181 | 3 | < .001 |
| Absent | 675 | 86 | |
| t(4;11)(MLL-AF4) | |||
| Present | 5 | 3 | .03 |
| Absent | 851 | 86 | |
Abbreviation: NCI, National Cancer Institute.
CNS-1, no detectable blast cells in cerebrospinal fluid sample; CNS-2, < 5 leukocytes/μL with blast cells in an atraumatic sample; CNS-3, ≥ 5 leukocytes/μL with blast cells in an atraumatic sample or the presence of a cranial nerve palsy; and traumatic lumbar puncture with blasts (≥ 10 erythrocytes/μL with blasts).
Ratio of DNA content as measured by flow cytometry in leukemic cells versus normal diploid G0/G1 cells.
Early Treatment Response
In studies XIIIA, XIIIB, and XIV, older adolescents had higher MRD levels on day 19 of remission induction compared with the younger patients (Table 2). In study XV, older adolescents also had an inferior early treatment response compared with that in younger patients, as indicated by higher levels of MRD on day 19 and at the end of remission induction. Because of initial remission induction failure or MRD level ≥ 1% at the end of remission induction, six of the 45 older adolescents in study XV underwent allogeneic transplantation compared with 28 of 453 younger patients (P = .11).
Table 2.
Comparison of MRD Levels During Remission Induction Therapy According to Age Group and Study
| Variable | Age Group |
P | |
|---|---|---|---|
| 1-14 Years | 15-18 Years | ||
| Studies XIIIA, XIIIB, and XIV | |||
| Minimal residual disease on day 19, % | |||
| < 0.01 | 72 | 6 | .05 |
| 0.01 to 0.99 | 47 | 3 | |
| 1 to 4.99 | 14 | 2 | |
| ≥ 5 | 13 | 5 | |
| Minimal residual disease on day 46, % | |||
| < 0.01 | 121 | 11 | .25 |
| 0.01 to 0.99 | 29 | 4 | |
| 1 to 4.99 | 5 | 1 | |
| ≥ 5 | 1 | 1 | |
| Study XV | |||
| Minimal residual disease on day 19, % | |||
| < 0.01 | 184 | 14 | .08 |
| 0.01 to 0.99 | 174 | 18 | |
| 1 to 4.99 | 40 | 3 | |
| ≥ 5 | 45 | 10 | |
| Minimal residual disease on day 46, % | |||
| < 0.01 | 362 | 30 | .02 |
| 0.01 to 0.99 | 66 | 11 | |
| 1 to 4.99 | 14 | 0 | |
| ≥ 5 | 6 | 3 | |
NOTE. Data missing for some patients.
Treatment Outcome
The 44 adolescents enrolled on studies XIIIA, XIIIB, and XIV had a 5-year event-free survival rate of 59.1% (95% CI, 43% to 72%) and a 5-year overall survival rate of 59.1% (95% CI, 43% to 72%), which were strikingly inferior to the 82.6% (95% CI, 78.5% to 86%; P < .001) event-free survival and 88.3% (95% CI, 84.7% to 91.1%; P < .001) overall survival rates for the 403 younger patients enrolled on the same studies.
In study XV, the 5-year event-free survival and overall survival estimates (± standard error [SE]) for the entire cohort of 498 patients were 87.2% ± 2.0% (95% CI, 83.7% to 90%) and 93.6% ± 1.1% (95% CI, 91% to 95.5%), respectively. Complete remission was achieved in 44 (97.8%) of the 45 older adolescents and 448 (98.9%) of the 453 younger patients (P = .44). Treatment failures among adolescents consisted of one induction failure, two hematologic relapses (one after transplantation), and three deaths from infection during postremission chemotherapy (one each during consolidation, continuation, and reinduction treatment). Forty adolescents, including the one with induction failure, remain alive in first remission 1 to 9 years from diagnosis (median, 4.3 years).
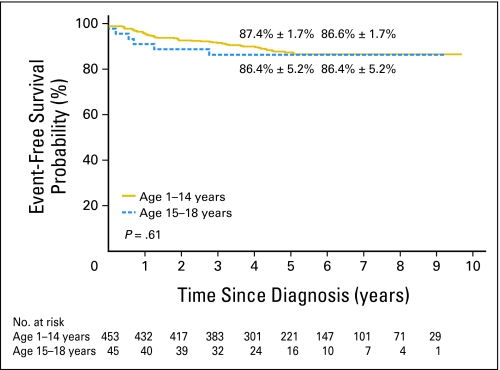
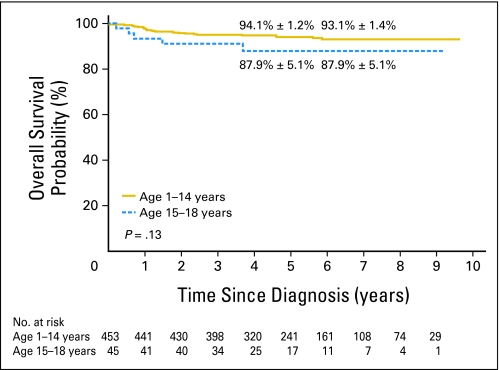
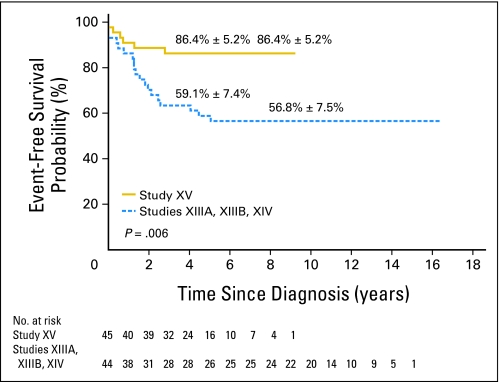
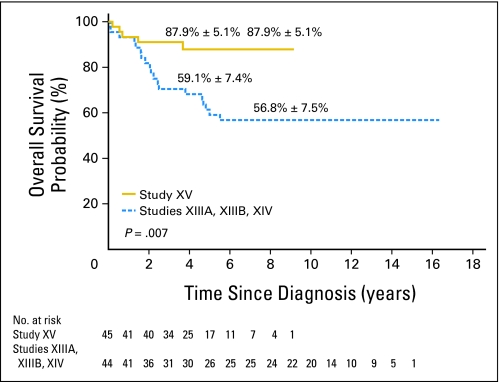
The 5-year event-free survival probabilities (± SE) for older adolescents versus younger patients in study XV were not significantly different: 86.4% ± 5.2% (95% CI, 72.1% to 93.6%) and 87.4% ± 1.7% (95% CI, 83.7% to 90.3%; P = .61; Fig 1). Although there was no significant difference in the 5-year overall survival between the two groups: 87.9% ± 5.1% (95% CI, 73.1% to 94.9%) and 94.1% ± 1.2% (95% CI, 91.4% to 96% P = .13; Fig 2), older adolescents appeared to have lower survival rate, perhaps because of their relatively high rate of toxic death. When compared with the older adolescents treated in studies XIIIA, XIIIB, and XIV, older adolescents in study XV had markedly superior event-free survival (P = .006; Appendix Fig A1, online only) and overall survival (P = .007; Appendix Fig A2, online only).
Fig 1.
Kaplan-Meier estimates of event-free survival in study XV according to two age groups (ages 1 to 14 years and ages 15 to 18 years). Event-free survival rates at 5 and 8 years, respectively, are reported as means ± standard errors.
Fig 2.
Kaplan-Meier estimates of overall survival in study XV according to two age groups (ages 1 to 14 years and ages 15 to 18 years). Survival rates at 5 and 8 years, respectively, are reported as means ± standard errors.
The improved prognosis for older adolescents treated in study XV was achieved without undue increases in toxicity. Cumulative rates of seizures, disseminated fungal infections, and allergic reactions to asparaginase were similar between younger patients and older adolescents, but the latter showed higher rates of severe infection, osteonecrosis, thrombosis, and hyperglycemia (Table 3).
Table 3.
Summary of Selected Toxicities by Age Group in Study XV
| Toxicity | 3-Year Cumulative Risk by Age Group |
||||
|---|---|---|---|---|---|
| 1-14 Years (n = 453) |
15-18 Years (n = 45) |
P | |||
| % | SE* | % | SE* | ||
| Seizures, grade 2, 3, or 4 | 4.6 | 1.0 | 4.4 | 3.1 | .89 |
| Severe infection, grade 4 or 5† | 3.9 | 0.9 | 11.7 | 5.0 | .03 |
| Disseminated fungal infection | 5.8 | 1.1 | 0 | 0 | .12 |
| Allergic reactions to asparaginase, grade 2, 3, or 4 | 41.9 | 2.4 | 32.8 | 7.3 | .49 |
| Osteonecrosis, grade 3 or 4‡ | 5.8 | 1.2 | 32.9 | 8.5 | < .001 |
| Thrombosis, grade 2, 3, or 4 | 6.0 | 1.1 | 23.8 | 6.7 | < .001 |
| Hyperglycemia, grade 3 or 4 | 6.6 | 1.2 | 27.7 | 6.9 | < .001 |
Abbreviation: SE, standard error.
SE data are ± relative risk data.
Three older adolescents died as a result of toxicity during postremission chemotherapy, whereas four toxic deaths (two during induction and two during reinduction) occurred in younger patients.
Eleven older adolescents and 23 younger patients developed grade 3 or 4 osteonecrosis. Six older adolescents underwent core decompression (with subsequent joint resurfacing procedure and arthroplasty in one patient and joint resurfacing procedure in another), and one each had arthroplasty and a joint resurfacing procedure. By contrast, only six patients in the younger age group underwent core decompression (with subsequent arthroplasty in two patients, and joint resurfacing procedure in one), and one had arthroplasty.
DISCUSSION
The results of this study demonstrate that high cure rate, comparable to the best reported results for younger children, can be achieved for older adolescents with ALL without prophylactic cranial irradiation or routine stem-cell transplantation. The 5-year event-free survival rate of 86.4% observed in the adolescent cohort enrolled on study XV is outstanding and is superior to that of older adolescents treated in three earlier Total Therapy studies (59%) and to those enrolled on adult clinical trials (34% to 41%)17,19,20; the rate compares favorably to results achieved in the recent pediatric clinical trials (60% to 78%).18–20,23,24 In fact, it surpasses those of patients with childhood ALL overall treated in other contemporary clinical trials (72.1% to 81.6%).1,2,4–12 Likewise, the 5-year overall survival rate observed in our study (87.9%) compares favorably with those achieved in both adult (38% to 46%)17,19,20 and pediatric (67% to 81%)18–20,23,24 trials and with the rate reported recently by the US Surveillance, Epidemiology, and End Results Program for patients age 15 to 19 years of age treated between 2000 and 2004 (61.1%).16
Hematologic relapse developed in only two adolescents treated on study XV: one had early T-cell precursor ALL, and the other severe hypodiploid ALL (DNA index of 0.75), leukemia subtypes associated with an exceptionally poor prognosis.25,26 It should be stressed that none of the adolescents on study XV developed CNS relapse, despite complete exclusion of prophylactic cranial irradiation from the protocol. Triple intrathecal therapy (ie, methotrexate, hydrocortisone, and cytarabine), which proved more effective than intrathecal methotrexate for CNS control,27 was used in study XV. Intrathecal treatment was administered immediately after the diagnostic lumbar puncture and was intensified during early remission induction and continuation treatment in those with high-risk features for CNS relapse. Special precautions were taken to decrease the rate of traumatic lumbar punctures and to optimize the administration of intrathecal therapy.3,28 Absence of cranial irradiation and limited use of anthracyclines, epipodophyllotoxins, and alkylating agents should help to reduce serious late sequelae, especially secondary cancer, and to improve the overall quality of life.
Several factors likely contributed to the improved outcome that we observed. In study XV, we used intensive dexamethasone, vincristine, and asparaginase, as well as early intrathecal therapy, treatment components which have been associated with improved outcome in adolescents and young adults with ALL.17–20,23,24,26,29 For patients with hypersensitivity reactions to native Escherichia coli asparaginase, we substituted Erwinia asparaginase at high and frequent doses, because an inadequate dose of this drug can lead to an inferior outcome.30 High-dose dexamethasone was used postremission for patients with standard- or high-risk ALL, because leukemia control is positively related to the dose-intensity of corticosteroids.31 Not surprisingly, the leukemic blast cells from our adolescent patients were significantly more resistant in vitro to dexamethasone and prednisone than those from our younger patients (data not shown). The use of high-dose dexamethasone in study XV might have overcome the relative drug resistance of adolescent ALL.
Treatment intensity in study XV was guided by risk classification that was based on MRD findings, which allowed us to precisely identify patients with a poor early treatment response who might otherwise not have been recognized by conventional morphologic bone marrow examination.14,22 This strategy was likely beneficial to adolescent patients, who tend to have a higher prevalence of measurable MRD than younger patients. Indeed, there was a trend that a higher proportion of older adolescents than younger patients on study XV underwent transplantation for poor early response, and five of the six older adolescent patients who underwent transplantation remain alive in first remission.
Antimetabolite treatment in study XV was adjusted on the basis of pharmacodynamics of the blast cells and pharmacogenetics of the patients. This is the first St Jude Total Therapy study that included consolidation treatment with four 24-hour infusions (given every other week) of high-dose methotrexate with leucovorin rescue. We targeted doses of high-dose methotrexate individually, a strategy that improved outcome in one of our previous trials.32 We used higher doses of high-dose methotrexate (ie, steady-state serum concentration of 65 μmol/L with an average dose of approximately 5 g/m2) in T-cell and t(1;19)(TCF3-PBX1) ALL, because these blast cells accumulate methotrexate polyglutamates less avidly than do other cell subtypes33 and because high-dose methotrexate (5 g/m2 per dose) has improved outcome in T-cell ALL.34 During continuation treatment, dosages of mercaptopurine and methotrexate were adjusted to the limits of tolerance, but judiciously, to avoid undue interruptions of therapy.14,35 Because we used a relatively high-dose of mercaptopurine (75 mg/m2 per day), we prospectively identified patients with inherited deficiency of thiopurine-S-methyltransferase, and we lowered mercaptopurine dosage accordingly to reduce the risk of acute myelosuppression and the late development of therapy-related acute myeloid leukemia.36,37 Finally, we routinely monitored levels of thioguanine nucleotides to assess mercaptopurine treatment, and we administered methotrexate intravenously to ensure compliance.
The higher rates of severe infection, osteonecrosis, thrombosis, and hyperglycemia observed in older adolescents could be partly related to a slower clearance of dexamethasone in this age group.38 More vigilance in supportive care could additionally improve the cure rate of older adolescents, because three deaths from infection occurred in 45 of our patients and accounted for half of the failures in this age group. By contrast, among the 453 younger patients treated in the same protocol, only four suffered from toxic deaths. Recognizing the high risk of osteonecrosis, especially in the older age group,39 we prospectively performed bilateral hip and knee magnetic resonance imaging examinations after each of the two reinduction treatments in our patients for early detection and therapeutic interventions (eg, dose reduction or discontinuation of dexamethasone) to reduce the severity of the complication. We also gave dexamethasone on an interrupted schedule during reinduction (ie, days 1 to 8 and days 15 through 21) to reduce the risk and severity of this complication. Thus, even though we encountered a high rate of osteonecrosis in our older adolescent patients, only three of them required arthroplasty, and another five had core decompression and/or joint resurfacing procedures. Nachman et al18 reported no statistical difference in treatment outcome between older adolescent patients with a rapid early response who were randomly assigned to receive either one or two courses of postinduction intensification therapy, a result also reported for younger patients enrolled on the same study.40 Thus, it will be of interest to test whether dexamethasone treatment can be omitted earlier during postremission treatment without compromising clinical outcome in this age group, or perhaps it can be adjusted individually on the basis of pharmacokinetic parameters to reduce toxicity. In summary, the treatment approach used in Total Therapy study XV abolished the adverse prognostic impact of older age in childhood ALL. We suggest that this strategy be tested in young adults with ALL.
Acknowledgment
We thank Julie Groff for assistance with the figure; Jeana Cromer, Emily Baum, and Linda Holloway for data management; Sheila Shurtleff, PhD, for molecular analysis; and the many patients and parents who participated in the research program.
Appendix
Table A1.
Remission Induction
| Agent | Induction |
|
|---|---|---|
| Dosage | Days of Administration | |
| Methotrexate | 1 g/m2 IV over 4 or 24 hours | 1 |
| Prednisone | 40 mg/m2/d | 5-32 |
| Vincristine | 1.5 mg/m2/wk | 5, 12, 19, 26 |
| Daunorubicin | 25 mg/m2/wk | 5, 12 |
| Asparaginase* | 10,000 U/m2 IM (three times weekly) | 6, 8, 10, 12, 14, 16, (19, 21, 23)† |
| Cyclophosphamide | 1000 mg/m2 IV | 26 |
| Cytarabine | 75 mg/m2/d IV | 27-30, 34-37 |
| Mercaptopurine | 60 mg/m2 per night | 26-39 |
| Intrathecal cytarabine | Age dependent | 1 |
| Triple intrathecal | Age dependent | 19 (8, 26)‡ |
Abbreviations: IV, intravenous; IM, intramuscular.
Elspar (Merck, West Point, PA).
Extra asparaginase on days 19, 21, and 23 for patients with ≥ 1% residual leukemia cells in the bone marrow on day 19.
Extra triple intrathecal treatment on days 8 and 26 for patients with high-risk features of CNS relapse: any amount of blasts in cerebrospinal fluid, T-cell acute lymphoblastic leukemia with leukocyte count > 50 × 109/L, B-cell precursor acute lymphoblastic leukemia with leukocyte count > 100 × 109/L, or the presence of t(9;22)(BCR-ABL1), MLL rearrangement, or hypodiploidy < 45 chromosomes.
Table A2.
Consolidation Therapy
| Agent | Consolidation |
|
|---|---|---|
| Dosage | Day(s) of Administration | |
| High-dose methotrexate* | 33 μmol/L (low risk) or 65 μmol/L (standard or high risk) | 1, 15, 29, and 43 |
| Mercaptopurine | 50 mg/m2 per night | 1 to 56 |
| Triple intrathecal | Age dependent | 1, 15, 29, and 43 |
Dosage was targeted to achieve a steady-state concentration of 33 μmol/L (corresponding to an average dose of 2.5 g/m2) in low-risk patients and 65 μmol/L (average 5 g/m2) in standard-risk patients.
Table A3.
Early Continuation/Reinduction Therapy
| Week | Therapy by Risk Level |
|
|---|---|---|
| Low-Risk Patients | Standard- or High-Risk Patients | |
| 1 | Mercaptopurine + dexamethasone + vincristine | Asparaginase + mercaptopurine + dexamethasone + vincristine + doxorubicin |
| 2 | Mercaptopurine + methotrexate | Asparaginase + mercaptopurine |
| 3 | Mercaptopurine + methotrexate | Asparaginase + mercaptopurine |
| 4 | Mercaptopurine + dexamethasone + vincristine | Asparaginase + mercaptopurine + dexamethasone + vincristine + doxorubicin |
| 5 | Mercaptopurine + methotrexate | Asparaginase + mercaptopurine |
| 6 | Mercaptopurine + methotrexate | Asparaginase + mercaptopurine |
| 7 | Dexamethasone + vincristine + asparaginase + doxorubicin | Asparaginase + dexamethasone + vincristine + doxorubicin |
| 8 | Vincristine + asparaginase | Asparaginase + vincristine + doxorubicin |
| 9 | Dexamethasone + vincristine + asparaginase | Asparaginase + dexamethasone + vincristine |
| 10 | Mercaptopurine + methotrexate | Asparaginase + mercaptopurine |
| 11 | Mercaptopurine + methotrexate | Asparaginase + mercaptopurine + vincristine + doxorubicin |
| 12 | Mercaptopurine + methotrexate | Asparaginase + mercaptopurine |
| 13 | Mercaptopurine + methotrexate | Asparaginase + mercaptopurine |
| 14 | Mercaptopurine + dexamethasone + vincristine | Asparaginase + mercaptopurine + dexamethasone + vincristine + doxorubicin |
| 15 | Mercaptopurine + methotrexate | Asparaginase + mercaptopurine |
| 16 | Mercaptopurine + methotrexate | Asparaginase + mercaptopurine |
| 17 | Dexamethasone + vincristine + asparaginase + doxorubicin | Asparaginase + dexamethasone + vincristine |
| 18 | Vincristine + asparaginase | Asparaginase + vincristine |
| 19 | Dexamethasone + vincristine + asparaginase | Asparaginase + vincristine + dexamethasone + high-dose cytarabine |
| 20 | Mercaptopurine + methotrexate | |
| 21 | Mercaptopurine + methotrexate | Mercaptopurine + methotrexate |
| 22 | Mercaptopurine + methotrexate | Mercaptopurine + methotrexate |
| 23 | Mercaptopurine + methotrexate | Cyclophosphamide + cytarabine |
| 24 | Mercaptopurine + dexamethasone + vincristine | dexamethasone + vincristine |
NOTE. Dosage regimens are as follows: mercaptopurine 75 mg/m2 orally every evening for 7 days for low-risk group; 50 mg/m2 in the first 16 weeks, and 75 mg/m2 thereafter for the standard- and high-risk groups. The starting dose for patients with heterozygous deficiency of thiopurine methyltransferase was 60 mg/m2 instead of 75 mg/m2.
Dexamethasone 8 mg/m2 orally per day in three divided doses for 5 days for low-risk group and 12 mg/m2 for standard-risk group; 8 mg/m2 on days 1 to 8 and 15 to 21 during reinduction I (weeks 7 to 9) and reinduction II (weeks 17 to 19) for both groups.
Asparaginase 10,000 units/m2 IM three times a week for 3 weeks (nine doses) during each reinduction for low-risk group, and 25,000 units/m2 IM weekly for 19 doses for the standard- and high-risk groups; in patients with allergic reactions to E. coli asparaginase, Erwinia asparaginase 20,000 units/m2 thrice weekly during reinduction treatment for the low-risk group, and 25,000 units/m2 twice weekly in standard-risk group; in patients with allergic reactions to both E. coli and Erwinia asparaginase, or in those for whom Erwinia asparaginase was not available, polyethylene glycol asparaginase (Oncospar; Sigma-Tau Pharmaceuticals, Gaithersburg, MD) 2,500 units/m2/wk.
Vincristine 2 mg/m2 IV, except for weeks 7-9 and 17-19 when given at 1.5 mg/m2; methotrexate 40 mg/m2 IV or IM; doxorubicin 30 mg/m2 IV; high-dose cytarabine 2 g/m2 IV every 12 hours for four doses; cyclophosphamide 300 mg/m2 IV; cytarabine 300 mg/m2 IV.
Triple intrathecal therapy: patients with low-risk features with CNS-1 status: weeks 7, 12, 17, 24, 32, 40, and 48; patients with low-risk features with CNS-2, traumatic lumbar punctures with blasts or leukocyte count ≥ 100 × 109/L: weeks 7, 12, 17, 24, 28, 32, 36, 40, 44, and 48; patients with standard-risk features: weeks 7, 12, 17, 24, 28, 32, 36, 40, 44, and 48; patients with high-risk features for CNS relapse: weeks 3, 7, 12, 17, 24, 28, 32, 36, 40, 44, 48, 56, 64, 72, 80, 88, and 96.
*Abbreviations: IV, intravenously; IM, intramuscularly.
Fig A1.
Comparison of event-free survival between older adolescents ages 15 to 18 years treated in study XV and those in studies XIIIA, XIIIB, and XIV. The event-free survival rates at 5 and 8 years, respectively, are reported as means ± standard errors.
Fig A2.
Comparison of overall survival between older adolescents ages 15 to 18 years treated in study XV and those in studies XIIIA, XIIIB, and XIV. The survival rates at 5 and 8 years, respectively, are reported as means ± standard errors.
Footnotes
Supported by grants No. CA21765, CA60419, CA78224, CA36401, and GM92666 from the National Institutes of Health; by American Cancer Society F.M. Kirby Clinical Research Professorship; and by the American Lebanese Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00137111.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Ching-Hon Pui, EUSA Pharma, Enzon Pharmaceuticals, sanofi-aventis Research Funding: Sima Jeha, Genzyme, sanofi-aventis, EUSA Pharma; Cheng Cheng, Enzon Pharmaceuticals; Mary V. Relling, Enzon Pharmaceuticals, Sigma-Tau Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Ching-Hon Pui, Dario Campana, William E. Evans, Mary V. Relling
Financial support: Ching-Hon Pui, James R. Downing, William E. Evans, Mary V. Relling
Administrative support: Ching-Hon Pui, Dario Campana, James R. Downing, William E. Evans, Mary V. Relling
Provision of study materials or patients: Ching-Hon Pui, W. Paul Bowman, John T. Sandlund, Sue C. Kaste, Raul C. Ribeiro, Jeffrey E. Rubnitz, Elaine Coustan-Smith, Sima Jeha, Monika L. Metzger, Deepa Bhojwani, Scott C. Howard
Collection and assembly of data: Ching-Hon Pui, Dario Campana, W. Paul Bowman, John T. Sandlund, Sue C. Kaste, Raul C. Ribeiro, Jeffrey E. Rubnitz, Elaine Coustan-Smith, Sima Jeha, Monika L. Metzger, Deepa Bhojwani, Hiroto Inaba, Susana C. Raimondi, Mihaela Onciu, Scott C. Howard, Wing Leung, William E. Evans, Mary V. Relling
Data analysis and interpretation: Ching-Hon Pui, Deqing Pei, Dario Campana, Sue C. Kaste, Elaine Coustan-Smith, Cheng Cheng, Hiroto Inaba, Susana C. Raimondi, Mihaela Onciu, Wing Leung, William E. Evans, Mary V. Relling
Manuscript writing: Ching-Hon Pui, Deqing Pei, Dario Campana, W. Paul Bowman, John T. Sandlund, Sue C. Kaste, Raul C. Ribeiro, Jeffrey E. Rubnitz, Elaine Coustan-Smith, Sima Jeha, Cheng Cheng, Monika L. Metzger, Deepa Bhojwani, Hiroto Inaba, Susana C. Raimondi, Mihaela Onciu, Scott C. Howard, Wing Leung, James R. Downing, William E. Evans, Mary V. Relling
Final approval of manuscript: Ching-Hon Pui, Deqing Pei, Dario Campana, W. Paul Bowman, John T. Sandlund, Sue C. Kaste, Raul C. Ribeiro, Jeffrey E. Rubnitz, Elaine Coustan-Smith, Sima Jeha, Cheng Cheng, Monika L. Metzger, Deepa Bhojwani, Hiroto Inaba, Susana C. Raimondi, Mihaela Onciu, Scott C. Howard, Wing Leung, James R. Downing, William E. Evans, Mary V. Relling
REFERENCES
- 1.Möricke A, Reiter A, Zimmermann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: Treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 96. Blood. 2008;111:4477–4489. doi: 10.1182/blood-2007-09-112920. [DOI] [PubMed] [Google Scholar]
- 2.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conter V, Aricò M, Basso G, et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:255–264. doi: 10.1038/leu.2009.250. [DOI] [PubMed] [Google Scholar]
- 5.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: A Children's Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escherich G, Horstmann MA, Zimmermann M, et al. Cooperative study group for childhood acute lymphoblastic leukaemia (COALL): Long-term results of trials 82,85,89,92 and 97. Leukemia. 2010;24:298–308. doi: 10.1038/leu.2009.249. [DOI] [PubMed] [Google Scholar]
- 7.Veerman AJ, Kamps WA, van den Berg H, et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: Results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997–2004) Lancet Oncol. 2009;10:957–966. doi: 10.1016/S1470-2045(09)70228-1. [DOI] [PubMed] [Google Scholar]
- 8.Schmiegelow K, Forestier E, Hellebostad M, et al. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010;24:345–354. doi: 10.1038/leu.2009.251. [DOI] [PubMed] [Google Scholar]
- 9.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–382. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang DC, Yang CP, Lin DT, et al. Long-term results of Taiwan Pediatric Oncology Group studies 1997 and 2002 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:397–405. doi: 10.1038/leu.2009.248. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell C, Richards S, Harrison CJ, et al. Long-term follow-up of the United Kingdom medical research council protocols for childhood acute lymphoblastic leukaemia, 1980-2001. Leukemia. 2010;24:406–418. doi: 10.1038/leu.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pui CH, Carroll WL, Meshinchi S, et al. Biology, risk stratification, and therapy of pediatric acute leukemias: An update. J Clin Oncol. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed]
- 13.Gökbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Semin Hematol. 2009;46:64–75. doi: 10.1053/j.seminhematol.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 15.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 16.Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009;113:1408–1411. doi: 10.1182/blood-2008-06-164863. [DOI] [PubMed] [Google Scholar]
- 17.Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nachman JB, La MK, Hunger SP, et al. Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: A report from the Children's Oncology Group. J Clin Oncol. 2009;27:5189–5194. doi: 10.1200/JCO.2008.20.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boissel N, Auclerc MF, Lhéritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 20.de Bont JM, Holt B, Dekker AW, et al. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia. 2004;18:2032–2035. doi: 10.1038/sj.leu.2403538. [DOI] [PubMed] [Google Scholar]
- 21.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: Final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 22.Campana D. Minimal residual disease in acute lymphoblastic leukemia. Semin Hematol. 2009;46:100–106. doi: 10.1053/j.seminhematol.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry E, DeAngelo DJ, Neuberg D, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol. 2007;25:813–819. doi: 10.1200/JCO.2006.08.6397. [DOI] [PubMed] [Google Scholar]
- 24.Ribera JM, Oriol A, Sanz MA, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol. 2008;26:1843–1849. doi: 10.1200/JCO.2007.13.7265. [DOI] [PubMed] [Google Scholar]
- 25.Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: A subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nachman JB, Heerema NA, Sather H, et al. Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood. 2007;110:1112–1115. doi: 10.1182/blood-2006-07-038299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matloub Y, Lindemulder S, Gaynon PS, et al. Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared to intrathecal methotrexate: Results of the Children's Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia—A report from the Children's Oncology Group. Blood. 2006;108:1165–1173. doi: 10.1182/blood-2005-12-011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:257–268. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]
- 29.Pui CH. Toward a total cure for acute lymphoblastic leukemia. J Clin Oncol. 2009;27:5121–5123. doi: 10.1200/JCO.2009.24.8518. [DOI] [PubMed] [Google Scholar]
- 30.Duval M, Suciu S, Ferster A, et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: Results of a randomized European Organization for Research and Treatment of Cancer—Children's Leukemia Group phase 3 trial. Blood. 2002;99:2734–2739. doi: 10.1182/blood.v99.8.2734. [DOI] [PubMed] [Google Scholar]
- 31.Inaba H, Pui CH. Glucocorticoid use in acute lymphoblastic leukemia: Comparison of prednisone and dexamethasone. Lancet Oncol. 2010;11:1096–1106. doi: 10.1016/S1470-2045(10)70114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans WE, Relling MV, Rodman JH, et al. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med. 1998;338:499–505. doi: 10.1056/NEJM199802193380803. [DOI] [PubMed] [Google Scholar]
- 33.Kager L, Cheok M, Yang W, et al. Folate pathway gene expression differs in subtypes of acute lymphoblastic leukemia and influences methotrexate pharmacodynamics. J Clin Invest. 2005;115:110–117. doi: 10.1172/JCI22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrappe M, Reiter A, Ludwig WD, et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: Results of trial ALL-BFM 90. Blood. 2000;95:3310–3322. [PubMed] [Google Scholar]
- 35.Relling MV, Hancock ML, Boyett JM, et al. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. 1999;93:2817–2823. [PubMed] [Google Scholar]
- 36.Relling MV, Pui CH, Cheng C, et al. Thiopurine methyltransferase in acute lymphoblastic leukemia. Blood. 2006;107:843–844. doi: 10.1182/blood-2005-08-3379. [DOI] [PubMed] [Google Scholar]
- 37.Schmiegelow K, Heyman M, Gustafsson G, et al. The degree of myelosuppression during maintenance therapy of adolescents with B-lineage intermediate risk acute lymphoblastic leukemia predicts risk of relapse. Leukemia. 2010;24:715–720. doi: 10.1038/leu.2009.303. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Panetta JC, Cai X, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol. 2008;26:1932–1939. doi: 10.1200/JCO.2007.13.8404. [DOI] [PubMed] [Google Scholar]
- 39.Relling MV, Yang W, Das S, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J Clin Oncol. 2004;22:3930–3936. doi: 10.1200/JCO.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 40.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: A report from the Children's Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]