Abstract
Purpose
Numerous studies have examined the comorbidity of depression with cancer, and some have indicated that depression may be associated with cancer progression or survival. However, few studies have assessed whether changes in depression symptoms are associated with survival.
Methods
In a secondary analysis of a randomized trial of supportive-expressive group therapy, 125 women with metastatic breast cancer (MBC) completed a depression symptom measure (Center for Epidemiologic Studies–Depression Scale [CES-D]) at baseline and were randomly assigned to a treatment group or to a control group that received educational materials. At baseline and three follow-up points, 101 of 125 women completed a depression symptom measure. We used these data in a Cox proportional hazards analysis to examine whether decreasing depression symptoms over the first year of the study (the length of the intervention) would be associated with longer survival.
Results
Median survival time was 53.6 months for women with decreasing CES-D scores over 1 year and 25.1 months for women with increasing CES-D scores. There was a significant effect of change in CES-D over the first year on survival out to 14 years (P = .007) but no significant interaction between treatment condition and CES-D change on survival. Neither demographic nor medical variables explained this association.
Conclusion
Decreasing depression symptoms over the first year were associated with longer subsequent survival for women with MBC in this sample. Further research is necessary to confirm this hypothesis in other samples, and causation cannot be assumed based on this analysis.
INTRODUCTION
Can psychosocial factors such as depression influence cancer survival? Researchers have examined this intriguing question in studies testing associations between depression and cancer survival1 and in studies testing whether psychosocial interventions that reduce depression can prolong survival,2–6 although none of these studies was designed a priori to test these associations. Although differences across studies in cancer type, depression definitions, and measures limit definitive conclusions,7 an answer is emerging to the question of whether depression is associated with shorter survival. The answer seems to be yes.1
A recent meta-analysis of 31 prospective studies found a 25% higher mortality rate for patients with cancer with depressive symptoms and a 39% higher mortality rate for those with major depression, after adjusting for prognostic factors.1 This analysis clarifies earlier reviews of the role of depression in cancer progression.7–12 Results from intervention studies are less conclusive. The results of new intervention studies examining survival and efforts to replicate studies showing that psychosocial interventions prolonged cancer survival2,13 are mixed; one study has shown such an effect,4,6 but some studies show no difference in survival outcomes despite improvements in distress.3,5,14,15 The important question of whether treating depression can prolong survival in patients with cancer remains unanswered.
Strong research and clinical evidence suggests that depression and cancer co-occur, with bidirectional relationships potentially linking depression with cancer progression.16–21 Researchers have found prevalence rates of up to 38% for major depression and 58% for depression-spectrum syndromes, depending on the cancer site22 and stage.23 Cancer's multiple traumatic losses can lead to or restimulate depression.24–26 To examine whether treating depression can affect survival, researchers must test whether changes are associated with survival. If depression improves, will survival lengthen?
Studies investigating depression and survival from breast cancer yield divided results27–37 and highlight the impact of multiple measures on outcome. In a study of 24,696 older patients with breast cancer at any stage in the United States, patients diagnosed with depression within 2 years before cancer died sooner than patients without depression.31 In a population-based study of 20,593 patients with early- or late-stage breast cancer in Denmark, patients hospitalized for depression died sooner.35 Conversely, researchers found no association between depression and survival in a study of 49 patients with metastatic breast cancer (MBC),37 or in a study of 297 patients with primary breast cancer.28 In studies in which researchers demonstrated significant associations between depression and survival, participants more often reported depression multiple times, through multiple diagnoses,31 diagnoses of persistent depression,35 or multiple measurements during the study.34
Few survival studies assess changes in depression.2,7,20 Single measurements of depression may obscure results because chronic or major depression is associated more strongly with cancer survival.11,38,39 Depression that is present at a single time point and resolves in a timely manner might be an appropriate and adaptive response to diagnosis and not a risk factor for shorter cancer survival,7,40 a point reinforced by findings that repression of distress predicts poor cancer outcomes.41–43
Specific physiologic mechanisms and treatment nonadherence may link depression and cancer progression. Depression may promote cancer through dysregulation of respiratory sinus arrhythmia,19,44 effects on the hypothalamic-pituitary-adrenal (HPA) axis,45,46 immune suppression,9 or increases in inflammation.20,47 Depression reduces adherence to treatment recommendations, which can impact physiologic outcomes.4,48–50 Because people can improve deficits in respiratory sinus arrhythmia,51 inflammation,20 HPA axis function,52 adherence to treatment,53 and other features of depression through intervention, examining whether changes in depression improve survival is timely and essential.
In the current secondary analysis, we hypothesized that decreases in depressive symptoms in patients with MBC over the first year of a randomized clinical trial (RCT) would be associated significantly with subsequent survival. Because of possible bidirectional associations, tests were two-tailed.
METHODS
Sample
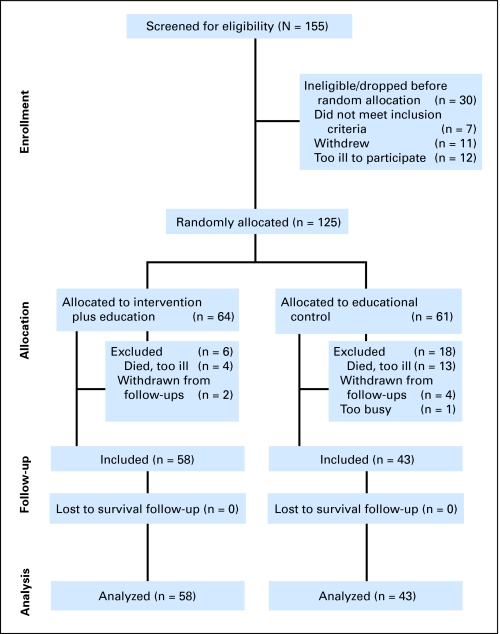
Between 1991 and 1996, we screened 155 women for eligibility for this RCT of supportive-expressive group therapy (SET) and excluded 30 women before random assignment (12 were excluded as a result of disease progression, seven were ineligible after medical record review, and 11 did not want to continue; Fig 1). Thus, 125 women provided written informed consent, approved by the Stanford Institutional Review Board. We included women with documented metastatic or recurrent (n = 3) breast cancer (recurrence in the same breast after lumpectomy and judged by our medical oncologist to have equivalent prognoses). We excluded women with a Karnofsky performance score of less than 70, so that all participants were engaging in normal activity. All participants lived in the Greater San Francisco Bay Area, spoke English, and could complete questionnaires. We excluded women who did not have metastasis beyond positive supraclavicular lymph nodes, had active cancers within 10 years (other than breast cancer, basal cell or squamous cell carcinomas of the skin, in situ cancer of the cervix, or melanoma with a Breslow depth < 0.76 mm), or had medical conditions that could affect short-term survival.
Fig 1.
CONSORT diagram.
All participants (N = 125) received educational materials and completed the Center for Epidemiologic Studies–Depression Scale (CES-D), among other measures, at baseline (before random assignment) and at 4, 8, and 12 months (for more complete information on this study, see Spiegel et al3 or Giese-Davis et al54). The treatment group received 1 year of SET (n = 64), which was not offered to the control group (n = 61).
In the primary analysis to test our hypothesis, we included all 101 of 125 women who provided at least one CES-D follow-up questionnaire so that the linear slope of change in depression symptoms over 1 year could be estimated. Change in depression score is estimated from the data and, therefore, has a certain error variance that is not considered in the analysis.
Of the 24 excluded women, 17 women had died or were too ill to complete the questionnaires, five women withdrew, and two women were too busy or no longer interested. Table 1 lists the demographic and medical information for both included and excluded women. Women providing follow-up data were significantly less depressed at baseline, less likely to be estrogen receptor negative, and had less often received chemotherapy and more often received hormone therapy, which are all indicators of a less advanced disease state.
Table 1.
Demographic, Medical, and Independent Variables Measured at Baseline for Patients With Metastatic Breast Cancer Included and Excluded From Primary Analyses (N = 125)
| Variable | Included Patients (n = 101) |
Excluded Patients (n = 24) |
Effect Size, SRD | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| CES-D baseline* | −0.25 | ||||
| Mean | 11.06 | 15.42 | |||
| SD | 9.11 | 10.48 | |||
| CES-D slope | |||||
| Mean | 0.05 | — | |||
| SD | 0.92 | ||||
| Age, years | 0.04 | ||||
| Mean | 53.53 | 51.79 | |||
| SD | 10.61 | 10.87 | |||
| Age at initial diagnosis, years | 0.01 | ||||
| Mean | 47.37 | 46.50 | |||
| SD | 10.11 | 10.80 | |||
| Age at metastatic diagnosis, years | 0.02 | ||||
| Mean | 51.24 | 50.54 | |||
| SD | 10.30 | 10.38 | |||
| Disease-free interval, months | −0.04 | ||||
| Mean | 45.77 | 47.66 | |||
| SD | 35.94 | 34.24 | |||
| Time from metastatic diagnosis to study entry, months | 0.16 | ||||
| Mean | 27.37 | 17.04 | |||
| SD | 40.59 | 29.34 | |||
| No. of years of education | 0.08 | ||||
| Mean | 16.09 | 16.21 | |||
| SD | 2.58 | 2.41 | |||
| Ethnicity | |||||
| Asian | 8 | 7.9 | 0 | 0.0 | 0.08 |
| Black | 1 | 1.0 | 0 | 0.0 | 0.01 |
| Hispanic | 1 | 1.0 | 2 | 8.3 | −0.07 |
| Native American | 2 | 2.0 | 0 | 0.0 | 0.02 |
| White | 87 | 86.1 | 22 | 91.7 | −0.06 |
| Other | 2 | 2.0 | 0 | 0.0 | 0.02 |
| Marital status | |||||
| Married | 59 | 58.4 | 12 | 50.0 | 0.08 |
| Never married | 10 | 9.9 | 1 | 4.2 | −0.06 |
| Separated | 2 | 2.0 | 1 | 4.2 | −0.02 |
| Divorced | 23 | 22.8 | 9 | 37.5 | −0.15 |
| Widowed | 6 | 5.9 | 1 | 4.2 | −0.02 |
| Other | 1 | 1.0 | 0 | 0.0 | −0.01 |
| Household income | −0.27 | ||||
| < $20,000 | 14 | 13.9 | 3 | 12.5 | |
| $20,000-$39,999 | 11 | 10.9 | 7 | 29.2 | |
| $40,000-$59,999 | 25 | 24.8 | 7 | 29.2 | |
| $60,000-$79,999 | 12 | 11.9 | 2 | 8.3 | |
| $80,000-$99,999 | 13 | 12.9 | 2 | 8.3 | |
| ≥ $100,000 | 25 | 24.8 | 3 | 12.5 | |
| Estrogen receptor status | |||||
| Negative† | 15 | 14.9 | 10 | 41.7 | −0.27 |
| Positive | 80 | 79.2 | 13 | 54.2 | |
| Treatment | |||||
| Chemotherapy* | 43 | 42.6 | 17 | 70.8 | −0.28 |
| Hormone therapy† | 84 | 83.2 | 13 | 54.2 | 0.29 |
| Site of metastasis | −0.01 | ||||
| Chest wall | 29 | 28.7 | 9 | 37.5 | |
| Bone | 44 | 43.6 | 6 | 25.0 | |
| Viscera | 28 | 27.7 | 9 | 37.5 | |
NOTE. Included sample includes every woman who had completed at least one follow-up CES-D questionnaire and for whom a slope of CES-D over 1 year could be created. Excluded sample includes every woman for whom no follow-up CES-D questionnaires were completed and for whom no slope could be created. Significance tests were two-tailed.
Abbreviations: SRD, success rate difference; CES-D, Center for Epidemiologic Studies–Depression Scale; SD, standard deviation.
P < .05.
P < .01.
Depression Symptoms
The CES-D55 is a self-report Likert-type scale rated for the past week from 0 (rarely) to 3 (most or all of the time) and includes 20 common affective symptoms (13 items) and somatic symptoms (seven items) of depression.56 Researchers designed this scale as an epidemiologic instrument for community samples. It does not provide a diagnostic criterion for depression, although a score of 16 or higher indicates clinically significant depression. Previous test-retest reliability coefficients for patients with breast cancer and healthy controls were adequate at 0.57 (P = .001) and 0.51 (P = .001), respectively, over 2.5 weeks.57 Cronbach's α was 0.89 at baseline.
Survival
Research staff obtained follow-up survival data from the participants, their families, and/or physicians or by consulting the Social Security Death Index. A death certificate confirmed all reported deaths. Breast cancer was the cause of death for 94.4% of the sample. Three patients died of cardiopulmonary causes, two died of neurologic disease, and one died of colon cancer. Cause of death was determined either by death certificate (82%) or medical records (18%).
Intervention: SET
Researchers designed this RCT to replicate a previous finding that women with MBC randomly assigned to SET lived significantly longer than women assigned to a control group.13 In the current trial, women randomly assigned to SET received weekly 1.5-hour group sessions led by cotherapy teams. Researchers encouraged women to attend SET sessions for at least a year, and many attended until just before death. Women randomly assigned to SET in this trial improved significantly with regard to trauma symptoms, mood disturbance,58 pain,59 and emotion regulation.54 However, we could not demonstrate an overall increase in survival time for the intervention group, although in a significant moderator analysis, estrogen receptor–negative women lived longer in the treatment group.3
Educational Control Group
We randomly assigned half of the women to a control group that received educational materials only. Thirty-two women (53%) in the control group and 35 women (55%) in the treatment group used these resources.
Analysis
In the primary a priori analysis, we used Cox proportional hazards to test for the effects of linear slope of change60 in CES-D depression symptoms over 1 year (as a continuous variable) on survival up to 14 years. The equation also included treatment condition (intervention or control), geographic site (San Francisco, San Jose, or Stanford), and all interactions (with all variables centered).61 For descriptive purposes only, we used Kaplan-Meier analysis with a split at zero CES-D change over 1 year (< zero = decreasing; ≥ zero = increasing) as the independent variable to illustrate the effect sizes and as a basis for reporting the median survival statistics.
In sensitivity analyses, the analysis structure was the same; however in the first analysis, we excluded all women who died in the first year, excluding nine additional women who had died (n = 92). In the second analysis, for all 101 women, we excluded all last CES-D follow-ups just before death (and recalculated the slope of change in CES-D), resulting in a sample of 93 women, because prior evidence suggests that a common spike (increase) in CES-D symptoms occurs at this last follow-up point.62 In the third analysis, we split the CES-D into Affective and Vegetative subscales based on prior work56,63 and reran the original analysis.
We also examined whether baseline demographic or medical variables were significantly associated with CES-D slope of change over 1 year to test whether CES-D slope was a proxy for another underlying prognostic variable. To test this association, we conducted a one-way analysis of variance with CES-D slope of change over 1 year as the dependent variable and the demographic or clinical variable (with all levels if categorical or median split if continuous), geographic site, treatment condition, and all interactions as independent variables.
RESULTS
Primary Analysis
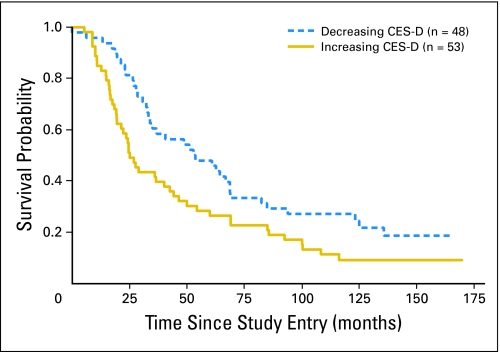
For women with decreasing CES-D scores over 1 year, overall median survival time was 53.6 months (n = 48), compared with 25.1 months for women with increasing scores (n = 53). A decrease in CES-D score over 1 year was significantly associated with longer survival over 14 years (n = 101; hazard ratio [HR], 1.68; 95% CI, 1.16 to 2.45; P = .007; Table 2, Fig 2), but we could not demonstrate any significant interaction effect of treatment condition by CES-D decrease on survival (HR, 1.48; 95% CI, 0.70 to 3.13; P = .30; Table 2).
Table 2.
Cox Regression Analysis on Survival for Change in Depression Symptoms (Slope) Over 1 Year by Treatment Group Versus Control Group by Site for Women With Metastatic Breast Cancer (n = 101)
| Factor | B | SE | Wald | df | P | Hazard Ratio | 95% CI |
|---|---|---|---|---|---|---|---|
| CES-D slope | 0.52 | 0.19 | 7.37 | 1 | .007 | 1.68 | 1.16 to 2.45 |
| Condition | 0.13 | 0.25 | 0.26 | 1 | .613 | 1.13 | 0.70 to 1.85 |
| Site, San Francisco | −0.10 | 0.34 | 0.09 | 1 | .770 | 0.91 | 0.47 to 1.75 |
| Site, Stanford | 0.00 | 0.30 | 0.00 | 1 | 1.000 | 1.00 | 0.55 to 1.81 |
| Condition × CES-D slope | 0.39 | 0.38 | 1.07 | 1 | .302 | 1.48 | 0.70 to 3.13 |
| Condition × San Francisco | −2.21 | 0.68 | 10.57 | 1 | .001 | 0.11 | 0.03 to 0.42 |
| Condition × Stanford | −0.79 | 0.61 | 1.69 | 1 | .194 | 0.45 | 0.14 to 1.49 |
| CES-D slope × San Francisco | −0.75 | 0.51 | 2.17 | 1 | .141 | 0.47 | 0.18 to 1.28 |
| CES-D slope × Stanford | −0.78 | 0.52 | 2.24 | 1 | .134 | 0.46 | 0.17 to 1.27 |
| Condition × CES-D slope × San Francisco | 0.23 | 1.01 | 0.05 | 1 | .820 | 1.26 | 0.18 to 9.01 |
| Condition × CES-D slope × Stanford | 0.57 | 1.04 | 0.30 | 1 | .582 | 1.77 | 0.23 to 13.43 |
NOTE. Cox regression analysis included condition, dummy variables for sites (San Francisco and Stanford), and the interaction between condition and sites. All variables were centered.
Fig 2.
Kaplan-Meier survival curve for increasing (solid gold line) versus decreasing (dashed blue line) Center for Epidemiologic Studies–Depression Scale (CES-D) symptoms during the initial intervention year in a randomized trial of supportive-expressive group therapy. Breast cancer was the cause of death for 94.4% of the patients.
Sensitivity Analyses
We challenged our results to investigate alternative explanations that would eliminate the finding. First, we removed all women from the sample who had died in the first year of the study, and we continued to find that a decrease in CES-D over 1 year was significantly associated with longer survival (n = 92; HR, 1.53; 95% CI, 1.04 to 2.26; P = .03).
Second, when we removed the last CES-D follow-up before death, we continued to find that a decrease in CES-D over 1 year was significantly associated with longer survival (n = 93; HR, 1.54; 95% CI, 1.05 to 2.26; P = .03). Thus, the result we found does not seem to be a result of early death or the biasing effects of the preterminal depression.
Third, when we split the CES-D into two subscales,56,63 we continued to find that a decrease in either subscale over 1 year was associated significantly with longer survival (Affective scale: n = 101; HR, 2.72; 95% CI, 1.36 to 5.47; P = .005; Vegetative scale: n = 101; HR, 2.65; 95% CI, 1.32 to 5.32; P = .006).
Effect of Baseline Demographic and Prognostic Variables on Change in CES-D
We could not demonstrate that age at random assignment, age at initial diagnosis (> and < 50 years), age at metastatic diagnosis, disease-free interval, time from metastasis to study entry, years of education, ethnicity, household income, estrogen receptor status, chemotherapy, hormone therapy, site of metastasis, Karnofsky performance score, dexamethasone use, and antidepressant use were related significantly to slope of change in CES-D over 1 year. However, a significant treatment condition by marital status interaction effect on CES-D change over 1 year (n = 101; F1,89 = 4.85; P = .03) indicated that married women in the control group became more depressed over time, whereas women who were not married became less depressed. The opposite was true for the treatment group. Having found this significant result, we added marital status to the main equation (and its interactions) to examine whether it was a proxy for change in CES-D and might eliminate the significant survival effect. We found that CES-D decrease over 1 year still significantly predicted longer survival with marital status in the equation (n = 101; HR, 1.80; 95% CI, 1.17 to 2.75; P = .007).
Impact of Baseline CES-D on Survival
Although our primary hypothesis was that a decrease in CES-D over 1 year would predict significantly longer survival, we examined the impact of baseline CES-D scores as a post hoc analysis. There was no significant effect of baseline CES-D on survival over 14 years (n = 125; HR, 0.98; 95% CI, 0.95 to 1.01; P = .11).
DISCUSSION
As hypothesized in this secondary analysis, we found that decreases in depression symptoms over the first year of an RCT predicted longer survival times over the ensuing 14 years for a sample of 101 women with metastatic or recurrent breast cancer. Women with improving depressive symptoms had longer median survival times (53.6 months) compared with women with worsening symptoms (25.1 months). The magnitude of this effect, the roughly doubling of survival time, is comparable to that observed in studies of depression and mortality from heart disease.64 We could not demonstrate that SET enhanced this effect significantly. Instead, for all women in the study, the more they decreased depression symptoms, the longer their survival, suggesting that any effective intervention may enhance this result. We did not find that antidepressant use was significantly associated with change in depression. Sensitivity analyses and examination of the Kaplan-Meier curve (Fig 1) indicate that the survival disadvantage is not a result of an increase in depression in the preterminal phase or primarily a result of vegetative symptoms, but rather reflects an effect of affective and somatic depression change over 1 year on mortality 2 to 14 years later.
The novelty of our study is that we found that a decrease in depression over the initial intervention year of this randomized intervention trial predicted survival many years later. This result extends past research demonstrating that multiple measurements of depression more often significantly predict survival.31,34,35,38 Multiple measurements make it possible to test whether an individual's depression symptoms have changed (eg, the measure reflects a state) or their response reflects a chronic style or trait.7 It is unfortunate that in prior studies with multiple measures of depression, researchers have not often used these additional data. Because of this limitation, our study is one of only a few studies to test the process of change in depression as it relates to cancer survival.2,20
Similar to cardiovascular disease,64,65 cancer researchers increasingly find significant associations between depression and endocrine dysregulation,45 heart rate variability,19 inflammatory markers,17,20,66 and mortality end points.1 Some hypothesize that these relationships represent a common mechanism of disease.44,66–71
We have previously reported greater cortisol dysregulation among patients with MBC than among controls72 and that dysregulation of diurnal cortisol predicts shorter survival for patients with breast cancer.73 Strong evidence exists that cortisol dysregulation is common in depression.19,74 Abnormal glucocorticoid levels may represent a failed response to the chronic inflammatory aspects of cancer, which depression may exacerbate.20 Tumor cells can co-opt certain mediators of inflammation such as nuclear factor-κB and growth-promoting cytokines and angiogenic factors to promote tumor progression and metastasis. Such chronic inflammation with relatively constant cytokine release into the circulation may trigger a glucocorticoid response that disrupts circadian variation in cortisol levels. This may induce a cycle of glucocorticoid resistance that disrupts negative feedback and glucocorticoid control,66 as we found in patients with MBC.75 Thus, there may be an inflammatory cytokine-mediated influence on diurnal cortisol that is associated with breast cancer and its progression. This effect would be worsened by the HPA axis dysregulation associated with depression, which is also connected to cytokines that trigger sickness behavior76–79 and is coupled with HPA axis hyperactivity.80,81 Dexamethasone, which is commonly given during chemotherapy,82 can also impact these physiologic systems. Dysregulation is also associated with sleep and other circadian system disruptions.73,83 Alleviating depression may reduce this inflammatory cycle,20 in addition to reducing sickness signs and symptoms.84
Correlation does not equate with causation even though the change in depression in our sample preceded the survival outcomes.64 A third proxy variable, that current research has not identified, could drive both outcomes. Caution interpreting these results is warranted because tumors themselves can induce depression-like behavior in rats21 and it is possible that the developing cancer has broad physiologic and psychological impact that is reported at the symptom level as depression.85
We did not measure treatment adherence. However, decreasing depression may accompany an improvement in both health behaviors and adherence53 and may mediate physiologic and survival outcomes.4,48
A possible clinical implication of our study is that although becoming depressed shortly after diagnosis may be a normal, necessary, and healthy experience of grieving and adjustment, if depression lingers, it may have toxic survival consequences.7,11 Future research needs to examine these processes of change in depression symptoms and their physiologic and survival correlates.
The details of these associations must await future research; however, here we have evidence that the course of depression over 1 year predicts subsequent survival time and that adjustment for prognostic variables does not alter significance. Treatment of depression, both psychotherapeutic and pharmacologic, is feasible and effective even in advanced cancer.86–89 Although we were unable to show that an intervention likely to decrease depression was associated with increased survival, we did demonstrate that decreasing depression, with or without formal intervention, may improve not only the quality but also the quantity of life for women with advanced breast cancer.
Acknowledgment
We thank Irvin Yalom, Jane Benson, Elaine Miller, Catherine Classen, Lisa D. Butler, Patricia Fobair, Cheryl Koopman, Sue DiMiceli, Robert Carlson, Susan Diamond Moore, Susan Weisberg, Meg Marnell, and the patients and their families who participated in the study.
Footnotes
Supported by National Institute of Mental Health Grant No. 5R01MH047226, with additional funding from the National Cancer Institute (RO1CA118567 and PO1AG018784), the National Institute on Aging (PO1AG018784), the American Cancer Society (Grant No. PF-4185), The John D. and Catherine T. MacArthur Foundation, and the Fetzer Institute.
Presented at the 159th Annual Meeting of the American Psychiatric Association, May 20-25, 2006, Toronto, Ontario, Canada; Department of Oncology Grand Rounds, April 18, 2007, Calgary, Alberta, Canada; and Department of Psychiatry Grand Rounds, April 11, 2007, Tucson, AZ.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00226928.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Janine Giese-Davis, Helena C. Kraemer,David Spiegel
Financial support: Janine Giese-Davis, David Spiegel
Administrative support: Kate M.S. Rancourt, David Spiegel
Provision of study materials or patients: David Spiegel
Collection and assembly of data: Janine Giese-Davis, Helena C. Kraemer, David Spiegel
Data analysis and interpretation: Janine Giese-Davis, Eric Neri, Helena C. Kraemer, David Spiegel
Manuscript writing: Janine Giese-Davis, Kate Collie, Kate M.S. Rancourt, Eric Neri, Helena C. Kraemer, David Spiegel
Final approval of manuscript: Janine Giese-Davis, Kate Collie, Kate M.S. Rancourt, Eric Neri, Helena C. Kraemer, David Spiegel
REFERENCES
- 1.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 2.Fawzy FI, Fawzy NW, Hyun CS, et al. Malignant melanoma: Effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry. 1993;50:681–689. doi: 10.1001/archpsyc.1993.01820210015002. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel D, Butler LD, Giese-Davis J, et al. Effects of supportive-expressive group therapy on survival of patients with metastatic breast cancer: A randomized prospective trial. Cancer. 2007;110:1130–1138. doi: 10.1002/cncr.22890. [DOI] [PubMed] [Google Scholar]
- 4.Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer. 2008;113:3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719–1726. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 6.Andersen BL, Thornton LM, Shapiro CL, et al. Biobehavioral, immune, and health benefits following recurrence for psychological intervention participants. Clin Cancer Res. 2010;16:3270–3278. doi: 10.1158/1078-0432.CCR-10-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giese-Davis J, Spiegel D. Emotional expression and cancer progression. In: Davidson RJ, Scherer KR, Hill Goldsmith H, editors. Handbook of Affective Sciences: Series in Affective Science. Oxford, United Kingdom: Oxford University Press; 2003. pp. 1053–1082. [Google Scholar]
- 8.Falagas ME, Zarkadoulia EA, Ioannidou EN, et al. The effect of psychosocial factors on breast cancer outcome: A systematic review. Breast Cancer Res. 2007;9:R44. doi: 10.1186/bcr1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel D, Giese-Davis J. Depression and cancer: Mechanisms and disease progression. Biol Psychiatry. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 10.Hilakivi-Clarke L, Rowland J, Clarke R, et al. Psychosocial factors in the development and progression of breast cancer. Breast Cancer Res Treat. 1994;29:141–160. doi: 10.1007/BF00665676. [DOI] [PubMed] [Google Scholar]
- 11.Armaiz-Pena GN, Lutgendorf SK, Cole SW, et al. Neuroendocrine modulation of cancer progression. Brain Behav Immun. 2009;23:10–15. doi: 10.1016/j.bbi.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petticrew M, Bell R, Hunter D. Influence of psychological coping on survival and recurrence in people with cancer: Systematic review. BMJ. 2002;325:1066. doi: 10.1136/bmj.325.7372.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiegel D, Bloom JR, Kraemer HC, et al. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2:888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- 14.Kissane DW, Love A, Hatton A, et al. Effect of cognitive-existential group therapy on survival in early-stage breast cancer. J Clin Oncol. 2004;22:4255–4260. doi: 10.1200/JCO.2004.12.129. [DOI] [PubMed] [Google Scholar]
- 15.Kissane DW, Grabsch B, Clarke DM, et al. Supportive-expressive group therapy for women with metastatic breast cancer: Survival and psychosocial outcome from a randomized controlled trial. Psychooncology. 2007;16:277–286. doi: 10.1002/pon.1185. [DOI] [PubMed] [Google Scholar]
- 16.Onitilo AA, Nietert PJ, Egede LE. Effect of depression on all-cause mortality in adults with cancer and differential effects by cancer site. Gen Hosp Psychiatry. 2006;28:396–402. doi: 10.1016/j.genhosppsych.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Lutgendorf SL, Lamkin DM, DeGeest K, et al. Depressed and anxious mood and T-cell cytokine expressing populations in ovarian cancer patients. Brain Behav Immun. 2008;22:890–900. doi: 10.1016/j.bbi.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutgendorf SK, Weinrib AZ, Penedo F, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–4827. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giese-Davis J, Wilhelm FH, Conrad A, et al. Depression and stress reactivity in metastatic breast cancer. Psychosom Med. 2006;68:675–683. doi: 10.1097/01.psy.0000238216.88515.e5. [DOI] [PubMed] [Google Scholar]
- 20.Thornton LM, Andersen BL, Schuler TA, et al. A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: Secondary analysis of a randomized controlled trial. Psychosom Med. 2009;71:715–724. doi: 10.1097/PSY.0b013e3181b0545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyter LM, Pineros V, Galang JA, et al. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc Natl Acad Sci U S A. 2009;106:9069–9074. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;32:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 23.Kissane DW, Grabsch B, Love A, et al. Psychiatric disorder in women with early stage and advanced breast cancer: A comparative analysis. Aust N Z J Psychiatry. 2004;38:320–326. doi: 10.1080/j.1440-1614.2004.01358.x. [DOI] [PubMed] [Google Scholar]
- 24.Breen S, Baravelli C, Schofield P, et al. Is symptom burden a predictor of anxiety and depression in patients with cancer about to commence chemotherapy? Med J Aust. 2009;190(suppl):S99–S104. doi: 10.5694/j.1326-5377.2009.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 25.Banou E, Hobfoll SE, Trochelman RD. Loss of resources as mediators between interpersonal trauma and traumatic and depressive symptoms among women with cancer. J Health Psychol. 2009;14:200–214. doi: 10.1177/1359105308100204. [DOI] [PubMed] [Google Scholar]
- 26.Gandubert C, CarrièreI, Escot C, et al. Onset and relapse of psychiatric disorders following early breast cancer: A case-control study. Psychooncology. 2009;18:1029–1037. doi: 10.1002/pon.1469. [DOI] [PubMed] [Google Scholar]
- 27.Lehto US, Ojanen M, Dyba T, et al. Baseline psychosocial predictors of survival in localised breast cancer. Br J Cancer. 2006;94:1245–1252. doi: 10.1038/sj.bjc.6603091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodwin PJ, Ennis M, Bordeleau LJ, et al. Health-related quality of life and psychosocial status in breast cancer prognosis: Analysis of multiple variables. J Clin Oncol. 2004;22:4184–4192. doi: 10.1200/JCO.2004.12.091. [DOI] [PubMed] [Google Scholar]
- 29.Tross S, Herdon J, 2nd, Korzun A, et al. Psychological symptoms and disease-free and overall survival in women with stage II breast cancer. J Natl Cancer Inst. 1996;88:661–667. doi: 10.1093/jnci/88.10.661. [DOI] [PubMed] [Google Scholar]
- 30.Phillips KA, Osborne RH, Giles GG, et al. Psychosocial factors and survival of young women with breast cancer: A population-based prospective cohort study. J Clin Oncol. 2008;26:4666–4671. doi: 10.1200/JCO.2007.14.8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52:106–111. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Efficace F, Therasse P, Piccart MJ, et al. Health-related quality of life parameters as prognostic factors in a nonmetastatic breast cancer population: An international multicenter study. J Clin Oncol. 2004;22:3381–3388. doi: 10.1200/JCO.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 33.Efficace F, Biganzoli L, Piccart M, et al. Baseline health-related quality-of-life data as prognostic factors in a phase III multicentre study of women with metastatic breast cancer. Eur J Cancer. 2004;40:1021–1030. doi: 10.1016/j.ejca.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Watson M, Haviland JS, Greer S, et al. Influence of psychological response on survival in breast cancer: A population-based cohort study. Lancet. 1999;354:1331–1336. doi: 10.1016/s0140-6736(98)11392-2. [DOI] [PubMed] [Google Scholar]
- 35.Hjerl K, Andersen EW, Keiding N, et al. Depression as a prognostic factor for breast cancer mortality. Psychosomatics. 2003;44:24–30. doi: 10.1176/appi.psy.44.1.24. [DOI] [PubMed] [Google Scholar]
- 36.Groenvold M, Petersen MA, Idler E, et al. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 37.Jamison RN, Burish TG, Wallston KA. Psychogenic factors in predicting survival of breast cancer patients. J Clin Oncol. 1987;5:768–772. doi: 10.1200/JCO.1987.5.5.768. [DOI] [PubMed] [Google Scholar]
- 38.Levy SM, Herberman RB, Lippman M, et al. Immunological and psychosocial predictors of disease recurrence in patients with early-stage breast cancer. Behav Med. 1991;17:67–75. doi: 10.1080/08964289.1991.9935161. [DOI] [PubMed] [Google Scholar]
- 39.Penninx BW, Guralnik JM, Pahor M, et al. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst. 1998;90:1888–1893. doi: 10.1093/jnci/90.24.1888. [DOI] [PubMed] [Google Scholar]
- 40.Derogatis LR, Abeloff MD, Melisaratos N. Psychological coping mechanisms and survival time in metastatic breast cancer. JAMA. 1979;242:1504–1508. [PubMed] [Google Scholar]
- 41.Weihs KL, Enright TM, Simmens SJ, et al. Negative affectivity, restriction of emotions, and site of metastases predict mortality in recurrent breast cancer. J Psychosom Res. 2000;49:59–68. doi: 10.1016/s0022-3999(00)00143-4. [DOI] [PubMed] [Google Scholar]
- 42.Dean C, Surtees PG. Do psychological factors predict survival in breast cancer? J Psychosom Res. 1989;33:561–569. doi: 10.1016/0022-3999(89)90063-9. [DOI] [PubMed] [Google Scholar]
- 43.Jensen MR. Psychobiological factors predicting the course of breast cancer. J Pers. 1987;55:317–342. doi: 10.1111/j.1467-6494.1987.tb00439.x. [DOI] [PubMed] [Google Scholar]
- 44.Gidron Y, Perry H, Glennie M. Does the vagus nerve inform the brain about preclinical tumours and modulate them? Lancet Oncol. 2005;6:245–248. doi: 10.1016/S1470-2045(05)70096-6. [DOI] [PubMed] [Google Scholar]
- 45.Sephton SE, Dhabhar FS, Keuroghlian AS, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23:1148–1155. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Posener JA, DeBattista C, Williams GH, et al. 24-Hour monitoring of cortisol and corticotropin secretion in psychotic and nonpsychotic major depression. Arch Gen Psychiatry. 2000;57:755–760. doi: 10.1001/archpsyc.57.8.755. [DOI] [PubMed] [Google Scholar]
- 47.Raison CL, Miller AH. The neuroimmunology of stress and depression. Semin Clin Neuropsychiatry. 2001;6:277–294. doi: 10.1053/scnp.2001.0060277. [DOI] [PubMed] [Google Scholar]
- 48.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 49.DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: A meta-analysis. Med Care. 2007;45:521–528. doi: 10.1097/MLR.0b013e318032937e. [DOI] [PubMed] [Google Scholar]
- 50.Raison CL, Giese-Davis J, Miller AH, et al. Depression in cancer: Mechanisms, consequences and treatment. In: Evans DL, Charney DS, Lewis L, editors. The Physician's Guide to Depression and Bipolar Disorders. New York, NY: McGraw-Hill; 2006. pp. 377–410. [Google Scholar]
- 51.Zucker TL, Samuelson KW, Muench F, et al. The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: A pilot study. Appl Psychophysiol Biofeedback. 2009;34:135–143. doi: 10.1007/s10484-009-9085-2. [DOI] [PubMed] [Google Scholar]
- 52.Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: Implications for therapy. J Affect Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- 53.Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J Clin Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giese-Davis J, Koopman C, Butler LD, et al. Change in emotion-regulation strategy for women with metastatic breast cancer following supportive-expressive group therapy. J Consult Clin Psychol. 2002;70:916–925. doi: 10.1037//0022-006x.70.4.916. [DOI] [PubMed] [Google Scholar]
- 55.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 56.Contrada RJ, Boulifard DA, Idler EL, et al. Course of depressive symptoms in patients undergoing heart surgery: Confirmatory analysis of the factor pattern and latent mean structure of the Center for Epidemiologic Studies Depression Scale. Psychosom Med. 2006;68:922–930. doi: 10.1097/01.psy.0000244391.56598.10. [DOI] [PubMed] [Google Scholar]
- 57.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: Evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 58.Classen C, Butler LD, Koopman C, et al. Supportive-expressive group therapy and distress in patients with metastatic breast cancer: A randomized clinical intervention trial. Arch Gen Psychiatry. 2001;58:494–501. doi: 10.1001/archpsyc.58.5.494. [DOI] [PubMed] [Google Scholar]
- 59.Butler LD, Koopman C, Neri E, et al. Effects of supportive-expressive therapy on pain in women with metastatic breast cancer. Health Psychol. 2009;28:579–587. doi: 10.1037/a0016124. [DOI] [PubMed] [Google Scholar]
- 60.Gibbons RD, Hedeker D, Waternaux C, et al. Some conceptual and statistical issues in the analysis of longitudinal psychiatric data. Arch Gen Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 61.Kraemer HC, Blasey CM. Centring in regression analyses: A strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butler LD, Koopman C, Cordova MJ, et al. Psychological distress and pain significantly increase before death in metastatic breast cancer patients. Psychosom Med. 2003;65:416–426. doi: 10.1097/01.psy.0000041472.77692.c6. [DOI] [PubMed] [Google Scholar]
- 63.Thombs BD, Hudson M, Schieir O, et al. Reliability and validity of the Center for Epidemiologic Studies Depression Scale in patients with systemic sclerosis. Arthritis Rheum. 2008;59:438–443. doi: 10.1002/art.23329. [DOI] [PubMed] [Google Scholar]
- 64.Frasure-Smith N, Lespérance F. Depression and cardiac risk: Present status and future directions. Heart. 2010;96:173–176. doi: 10.1136/hrt.2009.186957. [DOI] [PubMed] [Google Scholar]
- 65.Frasure-Smith N, Lespérance F, Irwin MR, et al. The relationships among heart rate variability, inflammatory markers and depression in coronary heart disease patients. Brain Behav Immun. 2009;23:1140–1147. doi: 10.1016/j.bbi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 66.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gidron Y, Ronson A. Psychosocial factors, biological mediators, and cancer prognosis: A new look at an old story. Curr Opin Oncol. 2008;20:386–392. doi: 10.1097/CCO.0b013e3282fbcd0d. [DOI] [PubMed] [Google Scholar]
- 68.Gidron Y, Kupper N, Kwaijtaal M, et al. Vagus-brain communication in atherosclerosis-related inflammation: A neuroimmunomodulation perspective of CAD. Atherosclerosis. 2007;195:e1–e9. doi: 10.1016/j.atherosclerosis.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Riordan WP, Jr, Norris PR, Jenkins JM, et al. Early loss of heart rate complexity predicts mortality regardless of mechanism, anatomic location, or severity of injury in 2178 trauma patients. J Surg Res. 2009;156:283–289. doi: 10.1016/j.jss.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 70.Mravec B, Gidron Y, Kukanova B, et al. Neural-endocrine-immune complex in the central modulation of tumorigenesis: Facts, assumptions, and hypotheses. J Neuroimmunol. 2006;180:104–116. doi: 10.1016/j.jneuroim.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Mravec B, Gidron Y, Hulin I. Neurobiology of cancer: Interactions between nervous, endocrine and immune systems as a base for monitoring and modulating the tumorigenesis by the brain. Semin Cancer Biol. 2008;18:150–163. doi: 10.1016/j.semcancer.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 72.Abercrombie HC, Giese-Davis J, Sephton S, et al. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Sephton SE, Sapolsky RM, Kraemer HC, et al. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 74.Pruessner M, Hellhammer DH, Pruessner JC, et al. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom Med. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- 75.Spiegel D, Giese-Davis J, Taylor CB, et al. Stress sensitivity in metastatic breast cancer: Analysis of hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology. 2006;31:1231–1244. doi: 10.1016/j.psyneuen.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyers CA. Mood and cognitive disorders in cancer patients receiving cytokine therapy. Adv Exp Med Biol. 1999;461:75–81. doi: 10.1007/978-0-585-37970-8_5. [DOI] [PubMed] [Google Scholar]
- 77.Van Gool AR, Kruit WHJ, Cornelissen JJ, et al. Management of psychiatric adverse events with immunotherapy with interferon-alfa. Acta Neuropsychiatr. 1999;11:120–124. doi: 10.1017/S0924270800035857. [DOI] [PubMed] [Google Scholar]
- 78.Bonaccorso S, Meltzer H, Maes M. Psychological and behavioural effects of interferon-alpha. Curr Opin Psychiatry. 2000;13:673–677. [Google Scholar]
- 79.Yirmiya R. Depression in medical illness: The role of the immune system. West J Med. 2000;173:333–336. doi: 10.1136/ewjm.173.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maes M, Bosmans E, Meltzer HY. Immunoendocrine aspects of major depression: Relationships between plasma interleukin-6 and soluble interleukin-2 receptor, prolactin and cortisol. Eur Arch Psychiatry Clin Neurosci. 1995;245:172–178. doi: 10.1007/BF02193091. [DOI] [PubMed] [Google Scholar]
- 81.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 82.Warr D, Street J, Carides A. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: Analysis of phase 3 trial of aprepitant in patients receiving Adriamycin-cyclophosphamide-based chemotherapy. Support Care Cancer. doi: 10.1007/s00520-010-0899-5. [epub ahead of print on May 13, 2010] [DOI] [PubMed] [Google Scholar]
- 83.Sephton S, Spiegel D. Circadian disruption in cancer: A neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17:321–328. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 84.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007;21:953–961. doi: 10.1016/j.bbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitlock FA, Sisking M. Depression and cancer: A follow-up study. Psychol Med. 1979;9:747–752. doi: 10.1017/s0033291700034061. [DOI] [PubMed] [Google Scholar]
- 86.Gill D, Hatcher S. Antidepressants for depression in people with physical illness. Cochrane Database Syst Rev. 2000;2 doi: 10.1002/14651858.CD001312. CD001312. [DOI] [PubMed] [Google Scholar]
- 87.Barsevick AM, Sweeney C, Haney E, et al. A systematic qualitative analysis of psychoeducational interventions for depression in patients with cancer. Oncol Nurs Forum. 2002;29:73–84. doi: 10.1188/02.ONF.73-87. [DOI] [PubMed] [Google Scholar]
- 88.Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: A randomized trial. Ann Intern Med. 2007;146:689–698. doi: 10.7326/0003-4819-146-10-200705150-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akechi T, Okuyama T, Onishi J, et al. Psychotherapy for depression among incurable cancer patients. Cochrane Database Syst Rev. 2008;2 doi: 10.1002/14651858.CD005537.pub2. CD005537. [DOI] [PMC free article] [PubMed] [Google Scholar]