SUMMARY
Clofarabine and cytarabine target different steps in DNA synthesis and replication, are synergistic in vivo, and have non-overlapping toxicities making this combination a potentially promising treatment for acute lymphocytic leukemia (ALL). Study goals were to: (1) evaluate the complete remission (CR) rate with Clo/Cy in patients with relapsed/refractory ALL; and (2) assess expression of connective tissue growth factor (CTGF) and nucleoside transporters in leukemic blasts. Associated with poor outcome in newly diagnosed ALL, CTGF is over-expressed in both pediatric and adult ALL. Transport of nucleoside analogs, such as clofarabine, may determine their cytotoxicity, therefore, nucleoside transporter expression may affect response. Thirty-seven patients were treated and evaluated for response. Median age was 41 years, and 44% of patients were either refractory or in ≥ 2nd relapse. Fifty-nine percent of evaluable patients had poor risk cytogenetics. CR/CRi rate was 17% (95% CI 6–33%) and median overall survival 3 months. Elevated levels of hENT1, hCNT3, and dCK were seen in 54%, 33% and 44% of patients, respectively, but were not significantly correlated with outcome. Higher expression CTGF patients showed a trend for shorter overall survival. This regimen is without sufficient activity to warrant further testing. CTGF expression may predict response and overall survival.
Keywords: Clofarabine, Cytarabine, Acute Lymphocytic Leukemia
INTRODUCTION
The prognosis of childhood acute lymphocytic leukemia (ALL) has improved significantly over the last decade with 80–90% of children being cured. Unfortunately, the prognosis for adults with ALL remains poor. More than half of adults will either be refractory to their initial treatment or will relapse. At the time of relapse, the only curative treatment is allogeneic hematopoietic cell transplant (HCT). However, most patients will require re-induction therapy prior to proceeding to HCT. Currently, no standard regimen for re-induction exists. Most salvage regimens incorporate drugs that are used in the initial treatment and complete remission (CR) rates have generally been disappointing: 37% with high dose cytarabine, 10–15% with etoposide (Hoelzer, 1991; Hoelzer et al, 2002). Therefore, novel treatments are needed.
Clofarabine, a nucleoside analog, was recently FDA-approved for the treatment of pediatric relapsed/refractory ALL based on the results of two phase 2 multi-center trials. The overall response rate [complete remission (CR) + partial remission (PR)] was 31% (Jeha et al, 2004). The median number of prior treatment regimens that patients received was three, and 39% of patients had undergone prior allogeneic HCT. Clofarabine requires intracellular phosphorylation by deoxycytidine kinase to be metabolized to the triphosphate form which is necessary for its cytotoxic effect (Gandhi et al, 2003; Parker et al, 1999). Compared to the other nucleoside analogs (fludarabine and cladribine), clofarabine has a high affinity for deoxycytidine kinase and is a potent inhibitor of both DNA polymerase and ribonucleotide reductase (Gandhi et al, 2003; Parker et al, 1991; Parker et al, 1988; Zie et al, 1995; Zie et al, 1996). In a Phase 2 study of clofarabine in adults with active leukemia, 12 patients with ALL were treated (Kantarjian et al, 2003). The response rate was 17%, suggesting modest single agent activity of clofarabine in adults with relapsed/refractory ALL.
Based upon the single agent activity observed with clofarabine in several populations of patients with relapsed or refractory leukemia, efforts were undertaken to combine clofarabine with other antileukemic agents. Cytarabine is active in the treatment of ALL. Clofarabine and cytarabine target different steps in DNA synthesis, are synergistic in vitro, and have non-overlapping toxicities, making this combination potentially promising for the treatment of relapsed/refractory ALL (Gandhi et al 1997; Cooper et al, 2003; Faderl et al, 2005). Clofarabine pre-treatment of cell lines increases cytarabine triphosphate accumulation in cells (Cooper et al, 2005). By inhibiting ribonucleotide reductase, clofarabine causes depletion of normal deoxynucleotides (Parker et al, 1999; Parker et al, 1991; Parker et al, 2988; Xie et al, 1995; Xie et al, 1996). This, in turn, leads to a decrease in feedback inhibition of deoxycytidine kinase and allows for increased production and accumulation of cytarabine triphosphate (Faderl et al, 2005). Faderl and associates previously determined the maximum tolerated dose of clofarabine in combination with cytarabine in a Phase 1/2 study (Faderl et al, 2005). Based on the above observations, we conducted a Phase 2 study of clofarabine/cytarabine in adult patients with relapsed/refractory ALL. In addition to evaluating efficacy, we also assessed connective tissue growth factor (CTGF) expression and nucleoside transporter expression (hENT1, dCK, hCNT3) in pre-treatment samples to see if their expression correlated with response. CTGF is overexpressed in both pediatric and adult ALL and is associated with a poor outcome in de novo ALL (Sala-Torra et al, 2007). Transport of nucleoside analogs such as clofarabine across the plasma membrane of the tumor may determine their cytotoxicity; therefore, nucleoside transporter expression may affect response to therapy.
MATERIALS AND METHODS
Patients were treated at Southwest Oncology Group (SWOG) institutions from February 2007 through July 2008. Clofarabine was supplied by the Genzyme Corporation. All patients provided signed informed consent in accordance with institutional and federal guidelines. This study (ClinicalTrials.govIdentifier: NCT 00337168) was conducted after approval by local institutional review boards, and in accord with an assurance filed with and approved by the Cancer Therapy and Evaluation Program Central Institutional Review Board (CIRB), National Cancer Institute. The study was monitored by the Data and Safety Monitoring Committee of the Southwest Oncology Group.
Eligibility Criteria
Eligibility required age ≥ 16 years, relapsed or refractory ALL (excluding Burkitt or mixed lineage leukemia), Eastern Cooperative Organizational Group (ECOG) performance status 0–2, no evidence of central nervous system involvement, no prior therapy with clofarabine, no evidence of uncontrolled infection, creatinine ≤ 1.5 x institutional upper limits of normal (IULN), aspartate aminotransferase (AST)/alanine aminotransferase (ALT)/bilirubin ≤ 1.5 x IULN, no pregnancy or active lactation, and no chemotherapy within 2 weeks of registration (except for hydroxyurea or maintenance therapy). For this study, refractory ALL was defined as failure to achieve CR with the last chemotherapy received, whereas relapsed patients achieved a CR of any duration before developing recurrence. Patients with only extramedullary disease were not eligible. Philadelphia-chromosome positive patients must have failed therapy with imatinib mesylate. Patients may have received prior allogeneic or autologous HCT. However, the transplant must have been performed more than 90 days prior to registration, and patients could not have evidence of ≥ Grade 2 acute graft versus host disease (GVHD), moderate or severe limited chronic GVHD, or extensive chronic GVHD of any severity.
Statistical Considerations
The study was performed in two stages. If at least 2 of the first 20 patients achieved a CR or complete remission with incomplete count recovery (CRi), 15 additional patients would be accrued. This design has a critical level (probability of falsely concluding that an agent with a 10% true CR rate warrants further study) of 2.0% and power (probability of correctly concluding that an agent with a 30% CR rate warrants further study) of 87%. Logistic regression analysis and Fisher’s exact test (for CR and resistant disease) and proportional hazards regression (for overall survival) were used to test the effect on treatment outcomes of patient and disease characteristics including CTGF expression levels. The overall survival distribution was estimated by the method of Kaplan and Meier (Kaplan and Meier, 1958). Confidence intervals (CIs) were calculated at the 95% confidence level and p-values are two-tailed. Results are based on data available as of December 1, 2009.
CTGF mRNA expression was performed as previously described (Sala-Torra, et al, 2007). Since expression data were skewed, the natural log transformation was used for expression values when non-parametric analyses were performed. In some analyses, the expression data was categorized into two groups for ease of interpretation. These categories were chosen so the groups have approximately equal number of specimens. Tests of significance such as regression and proportional hazards analyses were performed using the continuous variables when possible. CTGF results were analyzed as prognostic factors for CR, residual disease (RD), and overall survival.
Treatment Regimen
Induction therapy consisted of clofarabine 40 mg/m2/day and cytarabine 1 g/m2/day on days 1–5. Response was assessed between days 28–35. Patients with a PR received reinduction therapy with the same regimen. Patients with a CR could receive one cycle of consolidation therapy with clofarabine 40 mg/m2/day and cytarabine 1 g/m2/day on Days 1–4. Cytarabine was administered four hours after the completion of clofarabine.
Treatment Interruption/Dose Modification
For patients developing tachypnea or unexplained tachycardia or hypotension during clofarabine infusion, clofarabine was held until symptoms resolved and was re-initiated with the addition of pre-treatment steroids. Patients with Grade 2 and ≥ Grade 3 non-hematologic toxicity (including life-threatening infections) during induction therapy had a 25% and 50% reduction in their re-induction therapy, respectively. Patients with ≥ Grade 3 non-hematologic toxicity (including life-threatening infections) during induction or re-induction therapy had a 25% reduction in their consolidation therapy.
Definition of Outcomes
Complete remission (CR) was defined as < 5% marrow blasts, neutrophils ≥ 1,000/μlatelets ≥ 100,000/μL, and no evidence of extramedullary disease. CR with incomplete count recovery (CRi) was defined as CR but with a platelet count < 100,000/μL. Partial remission (PR) required at least a 50% decrease in the marrow blast percentage with blasts ≥ 5% and < 25%, no circulating blasts, no increase in extramedullary disease, neutrophils ≥ 1,000/μL, and platelets > 100,000/μL. Overall survival was measured from the date of registration until death from any cause, with observation censored at the date of last contact for patients last known to be alive. Toxicities were defined and grade according to the NCI Common Toxicity Criteria for Adverse Events version 3.0.
Cytogenetics/Fluorescence in situ hybridization (FISH)
The SWOG Cytogenetics Committee reviewed all pre-treatment cytogenetic studies included in this analysis. Cytogenetic studies were considered acceptable if they met the following criteria: processing by at least two different culture methods and at least 20 metaphase cells had to be analyzed, unless an abnormal clone was detected with fewer cells. Patients were grouped into 4 cytogenetic risk levels (standard risk, intermediate risk, high risk, and very high risk) as described previously (Pullarkat et al, 2008). For the purposes of this study, Philadelphia chromosome positive patients were considered very high risk (Pullarkat et al, 2008).
Pre-treatment samples of peripheral blood or bone marrow were analyzed centrally with either a B-cell or T-cell specific FISH panel as previously described (Bobadilla et al, 2007; Moore el al, 2008).
Nucleoside transporter expression
Expression of nucleoside transporters was examined in paraffin-embedded tissue. The development and immunohistochemical application of anti-hENT1, anti-hCNT3, and anti-dCK have been previously described (Dabbagh et al 2001; Mackey et al, 2002). Negative controls were provided by omitting the primary antibodies. The immunostaining intensities were scored on a 0–2+ scale (with 0=negative, 1+=weakly positive, 2+=strongly positive).
CTGF expression
CTGF expression was analyzed by triplicate RT-PCR on pre-treatment samples using standard TaqMan conditions on the ABI PRIZM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) as previously described (Tse et al (2004); Stirewalt et al, 2008). Using the 2-ΔΔ CT method, fold expression difference for CTGF was computed for each sample relative to its expression in SupB13 (Livak et al, 2001).
RESULTS
Four of the first 20 patients responded (2 CRs and 2 CRis), thus the study proceeded to the second stage. Thirty-seven patients were enrolled. One patient was excluded from the analysis because treatment was not started due to elevated liver function tests after registration. All other patients met eligibility criteria.
Patient Characteristics
Patient characteristics are listed in Table 1. Of the 36 evaluable patients, the median age was 41 years (range 20–68) and median white blood count 5200/μL (range 900–93,700). Twenty-nine patients (81%) had a B-cell immunophenotype, and 23 patients (64%) were male. The median time from initial diagnosis to registration was 14 months (range 1–52 months). Twenty patients (56%) were in first relapse, 7 (19%) in subsequent relapse, and 9 (25%) were refractory. Two patients had undergone prior allogeneic HCT.
Table 1.
Patient Characteristics (N=36)
| Median WBC | 5200/μL (range 900–93,700) |
| Gender (Male) | 23 patients (64%) |
| Immunophenotype (B-cell) | 29 patients (81%) |
| Median time from diagnosis to registration | 14 months (range 1–52) |
| Disease status at the time of study entry | |
| First relapse | 20 patients (56%) |
| Subsequent relapse | 7 patients (19%) |
| Refractory | 9 patients (25%) |
| Prior allogeneic HCT | 2 patients (6%) |
Cytogenetics/FISH
Pretreatment marrow and/or blood samples were submitted for cytogenetic analysis for 24 patients. Four studies were rejected because too few metaphases were examined, and reports for three others were not submitted for central review. Thus, 17 (47%) of 36 evaluable patients had reviewed, acceptable pre-study conventional cytogenetic karyotypes (Table 2). The risk categories for these patients were as follows: 10 of 17 (59%) were very poor risk [including 2 patients with t(9;22)] and 7 (41%) were intermediate risk including three patients with normal karyotypes. Among the three with normal karyotypes, FISH detected a biallelic deletion of CDKN2A in one, an IGH gene rearrangement in another, and hypodiploidy (loss of chromosomes 7, 12, 14, and 17) in the third. For the 19 evaluable patients without acceptable conventional cytogenetic reports, FISH detected the following aberrations (Table 2): BCR/ABL fusion (2), MLL gene rearrangement (1), hypodiploidy (1), hyperdiploidy (2), CDKN2A/9p deletion (3), TCR α/δ or γ rearrangement (2), CMYC (1), and one patient with CDKN2A/9p deletion also showed the IGH gene rearrangement.
Table 2.
Cytogenetics and FISH
| Cytogenetics (CG) (N=17) | |
| Very poor risk | 10 patients (59%) |
| Intermediate risk | 7 patients (41%) |
| FISH findings with normal CG (N=7) | |
| Biallelic deletion of CDKN2A | 1 patient |
| IGH gene rearrangement | 1 patient |
| Hypodiploidy | 1 patient |
| FISH results with unknown CG (N=19) | |
| BCR ABL fusion | 2 patients |
| Hypodiploidy | 1 patient |
| Hyperdiploidy | 2 patients |
| C-MYC | 1 patient |
| CDKN2A/9p deletion | 3 patients |
| TCR α/δ or γ rearrangement | 2 patients |
| IGH gene rearrangements | 1 patient (also had the CDKN2A/9p deletion and included above) |
| No pre-treatment specimen | 4 patients |
| No abnormality detected or not tested | 4 patients |
Response to Treatment
Three patients achieved CR and another three achieved CRi for a CR/CRi rate of 17% (95% CI 6–33%). There was no significant difference in response rate for patients with previously untreated first relapse [15% (95% CI 3–38%)] compared to those with refractory disease [33% (95% CI 7–70%)]. Eight patients proceeded to allogeneic HCT after completing protocol therapy. Three patients received re-induction, and 1 patient received consolidation therapy. Thirty-three patients have died, and the remaining 3 patients were last known to be alive at 8–13 months. The median overall survival was 3 months.
Toxicities
The most common Grade 3 or greater non-hematologic toxicities were infection (64%) and metabolic/laboratory abnormalities (33%). No unexpected toxicities were reported (Table 3). Ten deaths occurred during protocol treatment; 7 of these deaths were attributable to treatment [infection (3), pleural effusion (1), disseminated intravascular coagulation (1), hypotension/renal failure/cardiac arrhythmia (1), multiorgan failure (1)]. The remaining three deaths were due to pulmonary/renal failure, sepsis, and disease progression.
Table 3.
Grade 3–5 Non-Hematologic Toxicities (N=36)
| Toxicity | Number of Patients with Toxicity |
|---|---|
| Cardiac arrhythmia | 1 |
| Hypotension | 3 |
| Restrictive cardiomyopathy | 1 |
| Coagulation abnormalities | 2 |
| Fatigue | 2 |
| Multi-organ failure | 1 |
| Skin | 3 |
| Anorexia | 1 |
| Ascites | 2 |
| Colitis | 1 |
| Diarrhea | 2 |
| Typhilitis | 1 |
| Liver dysfunction | 1 |
| Infection | 23 |
| Edema | 1 |
| Metabolic/laboratory | 12 |
| Neurologic | 2 |
| Pain | 3 |
| Pulmonary | 4 |
| Renal Failure | 3 |
CTGF Expression
CTGF was analyzed on marrow or peripheral blood samples from 33 patients. Because there was a significant difference in CTGF expression levels between the marrow and blood specimens, blood specimens were requested for additional CTGF testing to create pairs with the original CTGF testing on marrow. As expected, the correlation between the 18 paired bone marrow and peripheral blood specimens was statistically significant (Spearman correlation coefficient=0.81, p< 0.001). CTGF expression in peripheral blood cells differed markedly between the original 15 cases (median=0.26, min-max 0–112) and the additional 18 cases (median=52, min-max 0–2892). This raises a question of whether the original and new expression levels are in fact comparable. Therefore, analyses of the effects of peripheral blood CTGF expression on outcomes were performed separately for the old and new data sets. To obtain a combined analysis that included all peripheral blood CTGF expression data, expression within each case was represented by the standardized value S= (E - MG)/SDG where E is the case’s log expression level, and MG and SDG, are the group-specific mean and standard deviation of the log-expression levels. CTGF expression was not predictive of CR or resistant disease. However, these analyses were inconclusive due to the small number of patient specimens available. For CTGF expression using peripheral blood specimens, there was a trend for patients with higher expression of CTGF having an inferior overall survival. However, this association was not statistically significant (log rank of standardized scores, p=0.11). The analysis of the two sets separately showed a marginally significant increased survival for patients with lower CTGF expression from the new peripheral blood specimens (log rank, p=0.04).
Nucleoside Transporter Expression
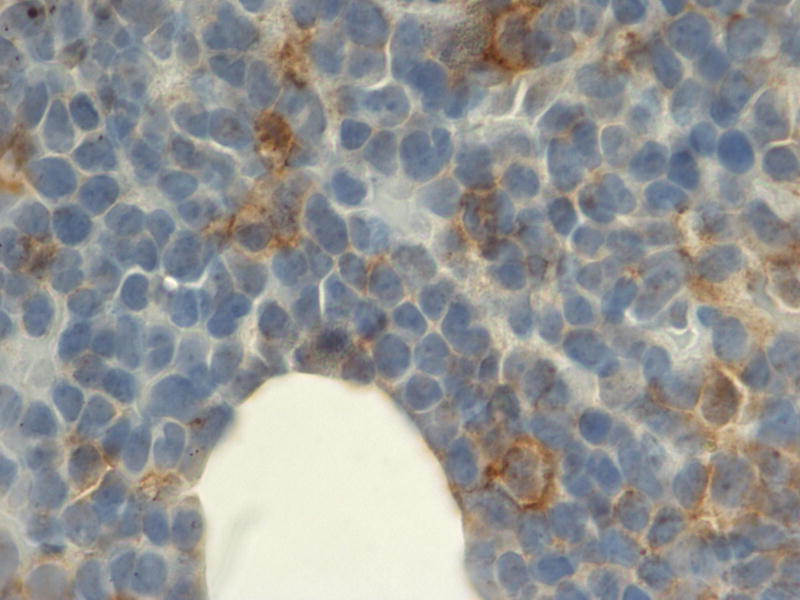
High expression (2+ by immunohistochemistry) of hENT1, hCNT3, dCK cytoplasmic, and dCK nuclear were noted in 54%, 44%, 33%, and 33% of patients respectively (Table 4). Two representative patient samples with negative hENT1 expression and high ENT1 expression are shown in Figures 1A and 1B, respectively. The total number of patients assessed for expression was low (13 for hENT1 expression and 9 for dCK and hCNT3 expression) since blocks were not available for immunohistochemical analysis on all patients. Included patients were more likely to be younger, and to be men. No other variables showed a correlation with the excluded patients (i.e. baseline disease status, time since prior treatment, treatment outcome, immunophenotype, lab values pre-treatment, or cytogenetic risk group).
Table 4.
Nucleoside Transporter Expression (N=13 for hENT1 and N=9 for dCK and hCNT3; High expression was defined as 2+ by immunohistochemistry)
| High dCK Cytoplasmic Expression | 3 patients (33%) |
| High hENT1 Expression | 7 patients (54%) |
| High hCNT3 Expression | 4 patients (44%) |
Figure 1.
Representative immunohistochemistry demonstrating a patient with low (0) hENT1 expression (A) and high (2+) hENT1 expression (magnification × 400).
DISCUSSION
Relapsed/refractory ALL remains difficult to treat. Two drugs have recently been FDA approved for the treatment of relapsed/refractory ALL: clofarabine was approved for pediatric ALL and nelarabine for T-cell ALL. Both drugs demonstrate single agent response rates in the 20–30% range, emphasizing the poor prognosis of this disease (Jeha et al, 2004; Larson, 2007).
In this study, the response rate to clofarabine/cytarabine was low (17%). Admittedly, this was a poor risk group of patients, but even among the group studied in untreated first relapse, the CR rate was only 15%.
Transport of nucleoside analogs, such as clofarabine, across the plasma membrane of the tumor cell may determine their cytotoxicity; therefore, nucleoside transporter expression patterns may be of clinical relevance. In chronic lymphocytic leukemia, expression of the nucleoside transporter hENT2 correlates with ex-vivo sensitivity to fludarabine (Molina-Arcas et al, 2005). Thirty-three to 54% of the patients evaluated in this study had high expression of the nucleoside transporters measured, thus making this a possible mechanism of resistance to chemotherapy. Although the number of patients studied was small and an association could have been missed, in our study, there was no significant difference in CR rate between patients with low versus high expression of the nucleoside transporters. High CTGF expression was associated with a trend towards a decreased overall survival. High CTGF expression has previously been associated with a decreased overall survival in ALL patients (Sala-Torra et al, 2007). The mechanism underlying this poor prognosis likely has to do with the role of CTGF in adhesion, cell cycle control, proliferation, and regulation of the TGFs and WNT pathways (Gao et al, 2003; Kothapalli et al, 2000; Ivkovic et al, 2003; Igarashi et al, 1993; Luo et al, 2004; Mercurio et al, 2004). Evaluating CTGF expression in a larger study will be required to further elucidate its prognostic effects.
Based on the results of this study, the clofarabine/cytarabine regimen tested here does not exhibit sufficient activity to warrant further investigation. Clofarabine remains an attractive drug to study in adults with recurrent ALL, given its single agent activity in treating recurrent ALL in pediatric patients and its absence from upfront regimens used for adult ALL. However, to improve on its efficacy, clofarabine will need to be combined with other agents or its use will need to be restricted to patient subsets more likely to respond. Antibodies, such as Epratuzumab, are attractive agents to combine with clofarabine, given their toxicity profile. If a nucleoside transporter expression profile is found to correlate with response rate, such a profile may impact therapeutic decisions regarding the use of nucleoside analogs. In addition, drugs modulating nucleoside transporter and CTGF expression may have potential therapeutic value.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, NIH, DHHS: CA32102, CA38926, CA073590, CA46282, CA04919, CA27057, CA35090, CA76132, CA11083, CA45450, CA35119, CA58861, CA20319 and supported in part by the Genzyme Corporation
Footnotes
DISCLOSURES: Dr. Advani received honoraria and research funding from Genzyme Corporation.
ClinicalTrials.govIdentifier: NCT00337168
References
- Bobadilla D, Enriquez E, Alvarez E, Gaytan P, Smith D, Slovak ML. An interphase fluorescence in situ hybridization (FISH) assay for the detection of 3q26.2/EVI1 rearrangements in myeloid malignancies. British Journal of Haematology. 2007;136:806–813. doi: 10.1111/j.1365-2141.2007.06505.x. [DOI] [PubMed] [Google Scholar]
- Cooper T, Nowak B, Gandhi V. Biochemical modulation of cytarabine triphosphate by clofarabine. Proceedings of the American Association for Cancer Research. 2003;44:142. doi: 10.1007/s00280-004-0906-y. [DOI] [PubMed] [Google Scholar]
- Cooper T, Ayres M, Nowak B, Gandhi V. Biochemical modulation of cytarabine triphosphate by clofarabine. Cancer Chemotherapy and Pharmacology. 2005;55:361–368. doi: 10.1007/s00280-004-0906-y. [DOI] [PubMed] [Google Scholar]
- Dabbagh L, Coupland RW, Cass CE, Mackey JR. Immunohistochemical variation of human nucleoside transporters in primary breast cancer. Biochemical and Biophysical Research Communications. 2001;280:951–959. [Google Scholar]
- Faderl S, Gandhi V, O’Brien S, Bonate P, Cortes J, Estey E, Beran M, Wierda W, Garcia-Manero G, Ferrajoli A, Estrov Z, Giles FJ, Du M, Swari M, Keating M, Plunkett W, Kantarjian H. Results of a Phase I–II study of clofarabine in combination with cytarabine (ara-c) in relapsed and refractory leukemias. Blood. 2005;105:940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- Gandhi V, Huang P, Chapman AJ, Chen F, Plunkett W. Incorporation of fludarabine- and arabinosylcytosine-5′-triphosphates by DNA polymerase α: affinity, interactions, and consequences. Clinical Cancer Research. 1997;3:1347–1355. [PubMed] [Google Scholar]
- Gandhi V, Kantarjian H, Faderl S, Bonate P, Du M, Ayres M, Rios MB, Keating MJ, Plunkett W. Pharmacokinetics of pharmacodynamics of plasma clofarabine and cellular clofarabine triphosphate in patients with acute leukemia. Clinical Cancer Research. 2003;9:6335–6442. [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Low density lipoprotein receptor-related protein (LRP) is a heparin-dependent adhesion receptor for connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatology Research. 2003;27:214–220. doi: 10.1016/s1386-6346(03)00241-9. [DOI] [PubMed] [Google Scholar]
- Hoelzer D. High-dose chemotherapy in adult acute lymphoblastic leukemia. Seminars in Hematology. 1991;28:84–89. [PubMed] [Google Scholar]
- Hoelzer D, Gokbuget N. Acute lymphoblastic leukemia in adults. In: Henderson, Lister, Greaves, editors. Leukemia. Saunders; New York: 2002. pp. 621–656. [Google Scholar]
- Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Molecular Biology of the Cell. 1993;4:637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeha S, Gandhi V, Chan KW, McDonald L, Ramirez I, Madden R, Rytting M, Brandt M, Keating M, Plunkett W, Kantarjian H. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–789. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Gandhi V, Cortes J, Verstovsek S, Du M, Garcia-Manero G, Giles F, Faderl S, O’Brien S, Jeha S, Davis J, Shaked Z, Craig A, Keating M, Plunkett W, Freireich EJ. Phase II clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Kothapalli D, Grotendorst GR. CTGF modulates cell cycle progression in cAMP- arrested NRK fibroblasts. Journal of Cellular Physiology. 2000;182:119–126. doi: 10.1002/(SICI)1097-4652(200001)182:1<119::AID-JCP13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Larson RA. Three new drugs for acute lymphoblastic leukemia: nelarabine, clofarabine, and forodesine. Seminars in Oncology. 2007;6(Suppl 5):S13–S20. doi: 10.1053/j.seminoncol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real- time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, He TC. Connective tissue growth factor (CTGF) is regulated by WNT and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. Journal of Biological Chemistry. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- Mackey JR, Jennings LL, Clarke ML, Santos C, Dabbagh L, Vsianska M, Koski S, Coupland R, Baldwin S, Young J, Cass C. Immunohistochemical variation of human nucleoside transporters in primary breast cancers. Clinical Cancer Research. 2002;8:110–116. [PubMed] [Google Scholar]
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signaling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- Molina-Arcas M, Marce S, Villamor N, Huber-Ruano I, Casado FJ, Bellosillo B, Montserrat E, Gil J, Colomer D, Pastor-Anglada M. Equilibrative nucleoside transporter-2 (hENT2) protein expression correlates with ex vivo sensitivity to fludarabine in chronic lymphocytic leukemia cells. Leukemia. 2005;19:64–68. doi: 10.1038/sj.leu.2403582. [DOI] [PubMed] [Google Scholar]
- Moore SR, Person DL, Sosman JA, Bobadilla D, Bedell V, Smith DD, Wolman SR, Tuthill RJ, Moon J, Sondak VK, Slovak ML. Detection of copy number alteration in malignant melanoma by both a panel of 16 DNA FISH probes and array comparative genomic hybridization: A Southwest Oncology Group Study (S9431) Clinical Cancer Research. 2008;14:2927–2935. doi: 10.1158/1078-0432.CCR-07-4068. [DOI] [PubMed] [Google Scholar]
- Parker WB, Bapat AR, Shen JX, Townsend AJ, Cheng YC. Interaction of 2-halogenated dATP analogs (F, Cl, and Br) with human polymerases, DNA primase, and ribonucleotide reductase. Molecular Pharmacology. 1988;34:485–491. [PubMed] [Google Scholar]
- Parker WB, Shaddix SC, Chang CH, White EL, Rose LM, Brockman RW, Shortnacy AT, Montgomery JA, Secrist JA, 3rd, Bennett LL. Effects of 2-chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl) adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5′-triphosphate. Cancer Research. 1991;51:2386–2394. [PubMed] [Google Scholar]
- Parker WB, Shaddix SC, Rose LM, Shewach DS, Hertel LW, Secrist JA, 3rd, Montgomery JA, Bennett LL., Jr Comparison of the mechanism of cytotoxicity of 2-chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl) adenine, 2-chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl) adenine, 2-chloro-9-(2-deoxy-2, 2-difluoro-β-D-ribofuranosyl) adenine in CEM cells. Molecular Pharmacology. 1999;55:515–520. [PubMed] [Google Scholar]
- Pullarkat V, Slovak ML, Kopecky KJ, Forman SJ, Appelbaum FR. Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of the Southwest Oncology Group 9400 Study. Blood. 2008;111:2563–2572. doi: 10.1182/blood-2007-10-116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Torra O, Gundacker H, Stirewalt D, Ladne P, Pogosova-Agadjanyan E, Slovak M, Willman C, Heimfeld S, Boldt D, Radich J. Connective tissue growth factor (CTGF) expression and outcome in adult patients with acute lymphoblastic leukemia. Blood. 2007;109:3080–3083. doi: 10.1182/blood-2006-06-031096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirewalt DL, Meschinchi S, Kopecky KJ, Fan W, Pogosova-Agadjanyan EL, Engel JH, Cronk MR, Dorcy KS, McQuary AR, Hockenbery D, Wood B, Heimfeld S, Radich JP. Identification of genes with abnormal expression changes in acute myeloid leukemia. Genes, Chromosomes and Cancer. 2008;47:8–20. doi: 10.1002/gcc.20500. [DOI] [PubMed] [Google Scholar]
- Tse W, Meshinchi S, Alonzo TA, Stirewalt DL, Gerbing RB, Woods WG, Appelbaum FR, Radich JP. Elevated expression of the AF1q gene, an MLL fusion partner, is an independent adverse prognostic factor in pediatric acute myeloid leukemia. Blood. 2004;104:3058–3063. doi: 10.1182/blood-2003-12-4347. [DOI] [PubMed] [Google Scholar]
- Xie KC, Plunkett W. Metabolism and actions of 2-chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl) adenine in human lymphoblastoid cells. Cancer Research. 1995;55:2847–2852. [PubMed] [Google Scholar]
- Xie KC, Plunkett W. Deoxynucleotide pool deletion and sustained inhibition of ribonucleotide reductase and DNA synthesis after treatment of human lymphoblastoid cells with 2-chloro-(2-deoxy-fluoro-β-arabinofuranosyl) adenine. Cancer Research. 1996;56:3030–3037. [PubMed] [Google Scholar]