Abstract
Using real-time gene expression imaging and behavioral analysis, we found that perinatal photoperiod has lasting effects on the circadian rhythms expressed by clock neurons as well as on mouse behavior, and sets the responsiveness of the biological clock to subsequent changes in photoperiod. These developmental gene x environment interactions tune circadian clock responses to subsequent seasonal photoperiods and may contribute to the influence of season on neurobehavioral disorders in humans.
Keywords: light, circadian, entrainment, seasonality, development, epigenetics, imprinting, birth season, suprachiasmatic nuclei, SCN
Environmental factors, and in particular light, can dramatically influence neural development1-5. It is known that seasonal light input can acutely reorganize the mature biological clock located in the suprachiasmatic nuclei (SCN)6, but whether development under different seasonal photoperiods can imprint the mammalian circadian clock is unknown. In this study, we exposed mice to different seasonal developmental photoperiods (short day LD 8:16; long day 16:8) until weaning, followed by four weeks of a matching or counter-balanced continuation seasonal photoperiod, and then at approximately 7 weeks of age assayed either the properties of their SCN circadian clocks by ex vivo imaging of a dynamic fluorescent reporter (as in 7, see Supp. Methods), or recorded wheel-running behavior in constant darkness (Supp. Fig. 1). All animal care was conducted in accordance with Vanderbilt University IACUC guidelines. In mature mice raised on LD 12:12 light cycles, seasonal photoperiod has been shown to be encoded by altering the relative peak times of individual neuronal electrical or molecular rhythms within the SCN, with long days eliciting more dispersed timing of neuronal rhythms and a broadened overall rhythmic waveform, and short days eliciting an increased degree of neuronal synchrony and a narrowed SCN waveform8-10. Analysis of the main effects of the proximal continuation photoperiod with our mouse line and reporter imaging method revealed similar findings, with long days eliciting SCN molecular waveform broadening primarily due to increased variation in the phases of individual clock neurons, but with significant changes in neuronal waveform and period as well (see Supp. Results, Supp. Fig. 2 and Supp. Table 1).
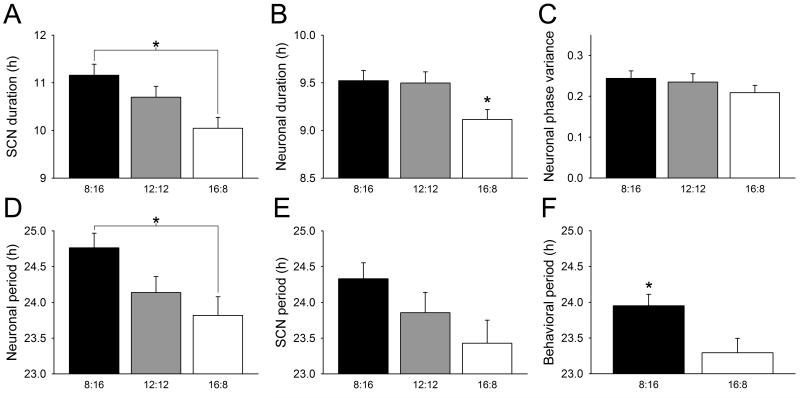
To determine if there were persistent effects of perinatal exposure to seasonal photoperiods on the SCN clock, we analyzed the main effects on SCN and neuronal properties grouped by developmental photoperiod regardless of ensuing continuation photoperiods (Fig. 1, Supp. Table 2). In contrast to encoding of proximal photoperiod in mature mice, perinatal exposure to seasonal photoperiods persistently altered the waveform of the SCN ensemble molecular rhythm solely via alterations in the waveforms and periods of individual SCN neurons, rather than by network-level relative timing of neuronal rhythms. SCN from long-day developed mice were imprinted with narrow Per1::GFP rhythm waveforms (Fig. 1a), compared with those developed on short days, regardless of whether mice had been subsequently continued on long days or on short days. These developmental effects on SCN molecular waveform occurred exclusively at the level of individual neurons and were due to waveform narrowing of individual SCN neurons in long-day developed mice (Fig. 1b), rather than any changes in the variance of neuronal peak times (Fig. 1c). In addition, SCN neurons in the long-day developed SCN were imprinted with shortened rhythmic period (Fig. 1d), and there was a similar trend in the period of SCN at the tissue level (Fig. 1e). The developmental imprinting of neuronal circadian period was reflected in circadian behavior as well: mice developed in the long photoperiod had a significantly shorter free-running period than mice developed in the short photoperiod (Fig. 1f). Of note, the LD 12:12 developed group showed similar trends in most SCN and neuronal parameters, but with values intermediate between the short- and long-day developed groups. These results demonstrate developmental influence of perinatal seasonal photoperiods on circadian neuronal rhythms that persist even in the face of weeks of alternate photoperiodic input, and furthermore demonstrate that these effects are manifest in circadian behavior.
Figure 1.
Persistent effects of perinatal seasonal photoperiod on SCN slice, on SCN neuronal rhythms, and on behavior. Duration of the SCN peak (a) and neuronal peak (b) in Per1::GFP expression, measured as the time from 50% maximum on the rising phase to 50% maximum on the falling phase of the molecular circadian rhythm. c, Neuronal phase variance calculated from the peak times of individual SCN neurons using Rayleigh circular statistics. d, Neuronal period calculated on the first full circadian cycle recorded ex vivo. e, SCN period calculated from the first full circadian cycle recorded ex vivo. f, Behavioral period calculated from the first three days in DD. Short-day developmental photoperiod (black bar), equinox developmental photoperiod (grey bar), long-day developmental photoperiod (white bar); asterisks (*) denote significance (two-way ANOVA, main effect for perinatal photoperiod, p < 0.05).
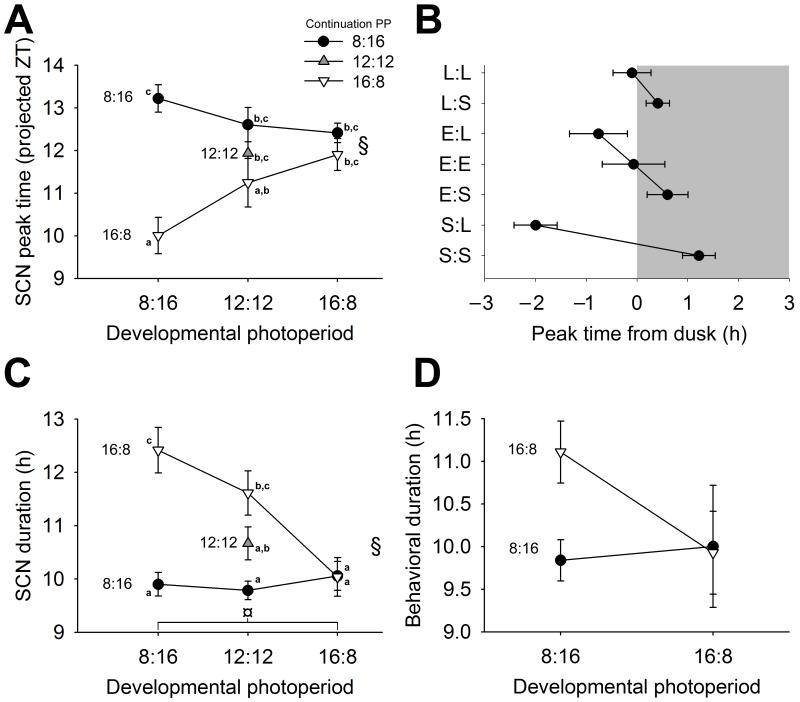
This developmental imprinting of clock neurons by seasonal photoperiods dramatically altered the functional response of the circadian pacemaker to subsequent photoperiodic stimuli (Fig. 2, Supp. Table 3). SCN from long-day developed mice exhibited a stable timing relationship to the nocturnal light/dark transition on both long-day and short-day light cycles, with their molecular rhythms closely tracking dusk on both long-day and short-day continuation photoperiods (Fig. 2 a,b). In contrast, SCN from short-day developed mice exhibited large changes in their timing relative to dusk in different continuation photoperiods, with their molecular rhythm peaking significantly after dusk when continued in short days, but peaking significantly before dusk when continued on long days (Fig. 2 a,b). Similarly, SCN from long-day developed mice were imprinted with a stable, narrow waveform that persisted in either continuation photoperiod, whereas short-day developed SCN exhibited narrow waveforms when continued on short days, but responded to subsequent lengthening of the photoperiod with greatly broadened waveforms (Fig. 2c). The duration of behavioral activity on each cycle strikingly followed a similar trend (Fig. 2d, p = 0.06, Supp. Table 3).
Figure 2.
Interactions of perinatal seasonal photoperiod with subsequent seasonal photoperiod. a, Two-way ANOVA interaction plot for peak times of Per1::GFP expression in SCN. Developmental photoperiod on X-axis; continuation photoperiod: short-day (LD 8:16) represented by black circles, equinox (LD 12:12) represented by grey inverted triangles and long-day (LD 16:8) represented by open triangles (see inset legend). b, Timing of SCN molecular rhythm peaks relative to dusk. White background represents “lights-on,” while grey background represents “lights-off.” Photoperiodic paradigm represented as “Developmental:Continuation” with “L” representing long-day photoperiod, “E” representing equinox photoperiod and “S” representing short-day photoperiod. c, Two-way ANOVA interaction plot for waveform duration from 50% rise to 50% fall of the first peak ex vivo. d, Behavioral duration of activity per circadian cycle in the first three days of constant darkness. Error bars represent SEM. Significance is indicated by letters such that means sharing a letter are not significantly different. Significance ascribed at p < 0.05.
These results demonstrate an environmental imprinting of the mammalian circadian clock and its response to subsequent seasonal change under seasonal light cycles. Perinatal exposure to long days imprints the SCN clock and its constituent neurons with narrowed waveforms of Per1 promoter activation that manifest as shorter behavioral activity duration in constant darkness. SCN from long-day developed mice also exhibit shortened neuronal circadian period, reflected in behavioral free running period, and in a consistent timing relationship to dusk. All of these properties are then stable in the face of subsequent exposure to seasonally changing photoperiod. In contrast, perinatal exposure to short-days imprints lengthened neuronal waveforms and circadian periods (neuronal and behavioral), which then results in high amplitude changes in SCN rhythm timing and waveform when exposed to subsequent seasonally changing photoperiod. SCN neuronal waveform changes, in particular, may manifest as a change in the duration of behavioral activity, depending on the proximal photoperiod. Development in LD 12:12, which has been used in previous studies of SCN seasonal encoding8-10, induces an intermediate state in which subsequent photoperiods evoke waveform changes of larger amplitude than those evoked in long day developed mice, but of smaller amplitude than those evoked in short day developed mice. Taken together, these results show that perinatal seasonal light cycles modulate the neural network encoding of seasonal light cycles by imprinting the molecular pacemaker within individual clock neurons.
The precise molecular mechanisms by which photoperiodic imprinting modifies clock properties are unknown. Our finding that this imprinting occurs at the level of individual clock neuron gene rhythms, rather than at the level of neuronal phase variance suggests a cell autonomous mechanism. Whereas there are many potential control points by which photoperiod could modify molecular circadian rhythms, genetic and pharmacological manipulation of casein kinase 1 isoforms (δ and ε) has been shown to have profound effects on circadian period and waveform11, and expression of these kinases in the SCN is affected by light12 making them likely targets for further study. Future studies will be needed to determine whether the imprinting we note here is due to direct epigenetic modification of clock genes (i.e. gene methylation or acetylation), or occurs at another level of organization.
Our results, as well as a recent report on the epigenetic effects of illumination during the development of dopamine neurons in the frog SCN3, suggest that circadian visual pathways are capable of the kind of developmental plasticity demonstrated in cortical visual pathways2, 4, 5. A limitation of our study is that while we have shown these developmentally-induced changes in the SCN and its constituent neurons persist for weeks, and are not reversed by altered photoperiods, we do not know how long they may persist. However, alterations in the circadian pacemaker properties of cockroaches, induced by perinatal exposure to non-24 hour days, have been shown to persist for a significant portion of total lifespan13.
The seasonal imprinting of the biological clock we have demonstrated likely has further implications for circadian-influenced neurobehavioral disorders. Perinatal exposure to winter-like seasonal light cycles identical to our paradigm induces persistent elevated depressive and anxiety-like behaviors in rodents14, and winter-born humans exhibit enhanced rates and severity of Seasonal Affective Disorder (SAD) as well as elevated risk of bipolar disorder and schizophrenia6. In addition, SAD patients also exhibit enhanced circadian responses to seasonal light cycles15, similar to the short-day matured mice in our study. An understanding of the mechanisms by which seasonal stimuli shape the circadian system during development may contribute to understanding the neural basis of seasonality and the influence of season on neurobehavioral disorders.
Supplementary Material
Acknowledgements
The authors would like to thank Jeremiah Y. Cohen for kindly providing custom software programming. This work was supported by NIH grants P50 MH078028 (DGM), T32 MH64913 and F31 MH080547 (CMC).
References
- 1.Ohta H, et al. Pediatr Res. 2006;60:304–308. doi: 10.1203/01.pdr.0000233114.18403.66. [DOI] [PubMed] [Google Scholar]
- 2.LeVay S, et al. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- 3.Dulcis D, Spitzer NC. Nature. 2008;456:195–201. doi: 10.1038/nature07569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesel TN, Hubel DH. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- 5.Wiesel TN, Hubel DH. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 6.Foster RG, Roenneberg T. Curr Biol. 2008;18:R784–R794. doi: 10.1016/j.cub.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Ciarleglio CM, et al. J Neurosci. 2009;29:1670–1676. doi: 10.1523/JNEUROSCI.3801-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inagaki N, et al. Proc Natl Acad Sci U S A. 2007;104:7664–7669. doi: 10.1073/pnas.0607713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanderLeest HT, et al. Curr Biol. 2007;17:468–473. doi: 10.1016/j.cub.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 10.Naito E, et al. J Biol Rhythms. 2008;23:140–149. doi: 10.1177/0748730408314572. [DOI] [PubMed] [Google Scholar]
- 11.Meng QJ, et al. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida Y, et al. J Neurosci Res. 2001;64:612–616. doi: 10.1002/jnr.1114. [DOI] [PubMed] [Google Scholar]
- 13.Barrett RK, Page TL. J Comp Physiol A. 1989;165:41–49. doi: 10.1007/BF00613798. [DOI] [PubMed] [Google Scholar]
- 14.Pyter LM, Nelson RJ. Behavioral neuroscience. 2006;120:125–134. doi: 10.1037/0735-7044.120.1.125. [DOI] [PubMed] [Google Scholar]
- 15.Wehr TA, et al. Arch Gen Psychiatry. 2001;58:1108–1114. doi: 10.1001/archpsyc.58.12.1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.