Abstract
BACKGROUND
HLA antibodies are a possible cause of transfusion-related acute lung injury (TRALI), and fluorescent bead assays are often used for antibody detection. Serum is the manufacturer’s recommended sample, but plasma may be easier to obtain for studies of HLA antibody prevalence and TRALI case investigations.
STUDY DESIGN AND METHODS
Specimens were obtained from 44 multiparous females positive for HLA antibodies by lymphocytotoxicity testing at least 13 years prior, and from 1,000 contemporary blood donors. Screening tests were performed using a Luminex-based assay. In addition to comparing results obtained with paired plasma and serum samples, the effects of storage at 4 °C for one week and of multiple freeze-thaw cycles were evaluated.
RESULTS
Of 42 evaluable subjects with HLA antibodies documented >13 years earlier, only 1 showed loss of detectable antibodies, with 39 (93%) positive in the screening assay for class I and 24 (57%) positive in the screening assay for HLA class II antibodies. In 968 evaluable contemporary donors, 291 screened positive for HLA class I and 206 for HLA class II antibodies using a low assay cut-off. Screening test concordance using paired plasma and serum samples was high, particularly for subjects with higher level antibodies. Refrigeration of samples for one week did not significantly affect assay results, while repeated freeze-thaw cycles caused a decrement in signal level.
CONCLUSION
Serum and plasma samples gave concordant results in the majority of cases, particularly for specimens with higher-level antibodies. High-level HLA antibodies were present in most individuals for over 13 years.
INTRODUCTION
HLA antibodies represent allo-reactivity against non-self antigens and have implications for organ and bone marrow transplantation and transfusion. In the field of blood transfusion, HLA antibodies play a role in refractoriness to platelet transfusions and may contribute to the pathogenesis of TRALI, which has several proposed etiologies. Though often not diagnosed in the acute setting, the clinical syndrome known as TRALI represented the third leading cause of transfusion-related mortality in the period spanning 1997 to 2002 (1) and has since emerged as the first leading cause (2). It is thought that HLA antibodies present in blood products may react with white blood cells in the lungs in subjects whose HLA type matches the infused antibody type. In the first series of TRALI cases characterized in 1985 by Popovsky et al., 65% of the implicated donors possessed HLA antibodies (3). The specificity of these antibodies matched the patient HLA type in 10 of 17 cases. It appears that plasma components carry the highest risk for induction of TRALI. In one series of fatal reactions, fresh-frozen plasma (FFP) was implicated in half of cases, red blood cell units in one-third of cases, followed by platelets and cryoprecipitate reduced plasma (1). Look-back studies targeting recipients of blood products derived from donors implicated in TRALI reactions have revealed some previously unrecognized or unreported episodes of acute lung injury, supporting the notion that TRALI cases are frequently unrecognized or unreported in clinical practice (4–6). Interestingly, while blood donor HLA antibodies appear to be associated with TRALI cases, the rate is much lower than would be expected if every recipient whose HLA type matched the offending antibody developed TRALI (4, 5), and several look-back studies have shown that not all blood product recipients whose HLA type matches infused HLA antibody develop TRALI.
TRALI has been described most commonly in association with HLA class I antibodies (4, 6). However, TRALI reactions have also been described with HLA class II (7). Infusion of non-cytotoxic HLA DR antibody into a volunteer induced a TRALI-like illness with rapid appearance of infiltrates on CXR and disappearance of monocytes from the peripheral blood (8). In addition to HLA antibodies, neutrophil antibodies have been independently implicated in TRALI pathogenesis (6, 9). While HLA or neutrophil antibodies appear to play a key role in TRALI induction, they do not explain all cases of TRALI since many case have been described in which no antibody has been detected. To explain the lack of exact correlation between HLA or neutrophil antibodies and TRALI, one proposed hypothesis is that TRALI is caused by factors released on prolonged storage of blood products. In a rat model, infusion of plasma from 42 day-old stored red cells into rats pretreated with lipopolysaccharide (LPS) induced acute lung injury reminiscent of TRALI (10). Notably, neither day 42 plasma alone nor day zero plasma in the presence of LPS induce lung damage, implying that a two-hit insult is required in the rat model of acute lung injury. Silliman et al. have documented a series of human cases in which donors did not have leukocyte antibodies and in which some of the transfused blood components contained elevated levels of lysophosphatidylcholines (11). In summary, a series of studies have detected donor HLA antibodies in blood products implicated in the development of TRALI in transfusion recipients, but these antibodies appear to be neither universally necessary nor sufficient for the development of TRALI.
HLA antibodies have long been recognized, first as “leukoagglutinins”. An early study of pregnant women revealed no primigravidae women with HLA antibodies, but showed frequent development of these antibodies in subsequent pregnancies (12). The specificity of the antibodies that developed matched the serological types of both the first and second children in all cases tested, implying that the first pregnancy resulted in sensitization, with the second evoking an anamnestic response. Other sources of HLA antibodies include prior receipt of blood transfusion and organ or bone marrow transplantation. Additionally, a small fraction of nulliparous women and non-transfused men have detectable HLA antibodies, implying that these antibodies can arise spontaneously or through unrecognized environmental stimuli (13).
In light of the increasing importance of TRALI and the potential connection between HLA antibodies and TRALI, there is serious consideration of screening donors of some types of blood products (e.g., fresh frozen plasma or apheresis platelets) for such antibodies prior to donation. Multiparous females show a higher rate of HLA antibodies than other segments of the population, and targeted screening of female parous apheresis donors is under serious consideration as a TRALI risk reduction measure. HLA antibodies have been detected until fairly recently using cumbersome cytotoxicity assays, relying on validated panels of target cells with known HLA types (14). More recently, ELISA, flow cytometric and fluorescent bead based assays have been developed to detect HLA antibodies, as well as antibodies directed against MHC class I chain-related gene A (MICA) (15, 16), with improved sensitivity and standardization compared to traditional cytotoxicity assays (17). While MICA antibodies have not been correlated with TRALI incidence, they have been correlated with rejection of solid organs and survival after bone marrow transplant and were included in the assay kit used in this study (18–21). For most HLA antibody assays the recommended sample for screening is serum, with very limited data on performance of assays on plasma. In many blood bank settings plasma is routinely obtained operationally for infectious disease screening and blood typing assays, facilitating use of plasma as opposed to serum for HLA Antibody screening. Furthermore, anticoagulated whole blood or plasma is often retained as retention aliquots for several months following donations or in long-term frozen donation archives or research repositories which can be accessed to investigate donations implicated in TRALI cases or conduct large scale studies of rates, determinants and consequences of transfusion of HLA antibodies in donors (22, 23). We therefore evaluated whether a plasma sample could be substituted for serum in an HLA antibody screening assay. In the current study we have compared the performance of a fluorescent bead-based assay using the manufacturer’s recommended sample of serum compared to matched plasma aliquots. While the current study utilized a fluorescent bead-based assay to maximize assay throughput and sensitivity, other modalities such as ELISA and flow cytometry analysis represent viable testing methodologies and are being compared in an ongoing study. Additionally, the effects of storage at 4°C and multiple freeze-thaw cycles on assay performance have been characterized. Finally, we have evaluated the long-term persistence of high-level HLA antibodies in a cohort of multiparous women for over 13 years.
MATERIALS AND METHODS
Study subjects
Forty-four females with a history of three or more pregnancies who tested positive for HLA antibodies at least thirteen years prior to the current study were recalled for repeat testing. Prior testing was performed using the lymphocytotoxicity assay with a panel of cryopreserved T and B cells from approximately 50 to 75 donors (14). An additional 1,000 contemporary donors (905 women and 95 men) were selected for the current study from a larger pool of approximately 8,200 blood donors participating in a study of the prevalence of HLA antibodies. Five 1 ml aliquots each of plasma and serum were prepared from each study subject on the same day as phlebotomy and frozen. All samples were sent to the Retrovirus Epidemiology Donor Study-II (REDS II) Central Laboratory in a blinded fashion under code. Research was performed under exemptions issued by the University of California, San Francisco Committee on Human Subjects Research and the BloodCenter of Wisconsin Institutional Review Board.
HLA antibody testing
Screening tests for HLA class I, II or MICA antibodies were performed with One Lambda (Canoga Park, CA) LabScreen LSM12 (LabScreen Mixed) kits according to manufacturer’s instructions. The assay measures the binding of antibody to fluorescently tagged microbeads. Briefly, 5 μl microbeads were incubated with 20 μl serum or plasma in a 96-well V-bottomed polystyrene plate (Whattman, Brentford, UK) for 30 minutes in the dark at 25C, then washed three times. PE-conjugated human IgG was added for a second 30 minute incubation, followed by two more washes. Negative control serum provided by One Lambda was included in each batch of specimens. Samples were analyzed on a Luminex luminometer (Austin, TX), able to discriminate up to 100 unique beads in one reaction.
Plasma aliquots obtained at blood donation are often stored for up to one week at 4 °C to facilitate repeat testing, for use in confirmatory testing (e.g. for the presence of viral pathogens) or to investigate discordant screening test results. We investigated whether refrigerated storage would affect the level of signal in the antibody screening test by studying a selected subset of paired “fresh” and refrigerated samples from the above larger study (these samples had been frozen and thawed once). We obtained results from 15 paired serum and plasma samples, including 5 subjects from our cohort of 44 multiparous women with known pre-existing HLA antibodies and 10 of the randomly selected contemporary blood donor samples that had demonstrated HLA reactivity. We also investigated whether additional freeze-thaw cycles would affect the test signal level, given potential interest in repeatedly accessing frozen archived specimen sets for HLA antibody testing and quality control. We tested five samples that had been frozen and thawed two additional cycles, with valid results obtained for four of the sample pairs.
Antibody test results were reported as the normalized background (NBG) ratio for each bead in the assay, which corresponds to the strength of each HLA reaction. The NBG ratio and assay validity were calculated using the following set of abbreviations:
| S#N | Sample-specific fluorescent value for bead #N; |
| SNC bead | Sample-specific fluorescent value for Negative Control bead; |
| SPC bead | Sample-specific fluorescent value for Positive Control bead |
| BG#N | Background NC Serum fluorescent value for bead #N |
| BGNC bead | Background NC Serum fluorescent value for Negative Control bead |
| NC Serum | Negative Control Serum validated for a given lot of LABScreen® beads |
For an assay to be interpreted as valid, the following criteria needed to be met: SNC ≤ 1500, SPC ≥ 500, and SPC ≥ 2*(SNC).
The NBG value for each bead was calculated as: NBG = (S#N - SNC bead)/(BG#N - BGNC bead)
If any bead for a given sample yielded an NBG ratio greater than the chosen cut-off the test was interpreted as positive. For this study we analyzed the data using two different NBG ratio cut-offs. First an NBG ratio of 2.2 using a serum sample was used as the assay cut-off, as this is a highly sensitive cut-off commonly used in tissue transplant settings. The data were also analyzed using an NBG ratio of 13 for the serum sample, as this was approximately the mean plus three standard deviations of a log transformed distribution of values for plasma samples for over 1,000 non-transfused males enrolled in the same study, multiplied by the serum-plasma conversion factor defined below (D. Triulzi, personal communication).
Statistical analysis
Comparisons of NBG ratios were made using two-tailed t-tests using Excel software (Microsoft Corporation, Redmond, WA). Linear regression analyses were performed using Excel software. Standard error was calculated as the standard deviation (using Excel software) divided by the square-root of the number of subjects tested.
RESULTS
HLA antibody persistence and frequency
Forty-two of 44 multiparous women yielded valid tests using either serum or plasma samples or both. Forty of the 42 subjects with valid tests showed positive tests for HLA class I antibodies based on the LSM12 screening test of serum samples. The average NBG ratio for serum samples positive for class I antibodies was 60.8, an order of magnitude above the lower threshold of 2.2 and well above the higher threshold of 13. Twenty-four of these subjects also showed evidence of HLA class II antibodies, and the average NBG ratio for these positive serum samples was 65.0 (Table 1). Only 1 of the 42 evaluable subjects with a history of HLA antibodies tested negative on both class I and class II screening tests on both serum and plasma samples using the NBG ratio ≥ 2.2 for serum, while 3 subjects were negative using the higher cut-off of NBG ratio ≥ 13 for serum.
TABLE 1.
Average NBG ratios of positive serum samples
| N | Average | St. error | |
|---|---|---|---|
| Pre-selected class I | 39 | 60.8 | 6.4 |
| Contemporary class I | 291 | 23.4 | 2.0 |
| Pre-selected class II | 24 | 65.0 | 8.0 |
| Contemporary class II | 206 | 36.5 | 3.3 |
From the cohort of 1,000 contemporary donors, 30 females and 2 males had invalid assay results on either the plasma or serum sample. The most common reason for an invalid assay result was a high sample negative control bead value, accounting for approximately 70% of invalid tests in both plasma and serum samples. Other reasons for invalid tests included low sample positive control bead values or a low ratio of the positive/negative sample control bead values. Of the 968 subjects with valid screening tests, 273 females (31.2%) and 18 males (19.4%) screened positive for HLA class I antibodies using serum samples at the NBG ratio assay cut-off of 2.2. The contemporary donor cohort also revealed that 200 females (22.9%) and 6 males (6.4%) screened positive for HLA class II antibodies. The NBG ratios of positive serum samples were markedly lower in the reactive contemporary donor population compared to the recalled lymphocytoxicity test-pedigreed female donor population (p < 0.0001 for class I and p = 0.005 for class II). These data show that tests for HLA antibodies can remain positive for over a decade and will generate signals in this assay that are much higher than those in a previously unscreened and unselected population.
Plasma samples yield comparable invalid test rates and lower NBG ratios compared to serum samples, with good test concordance
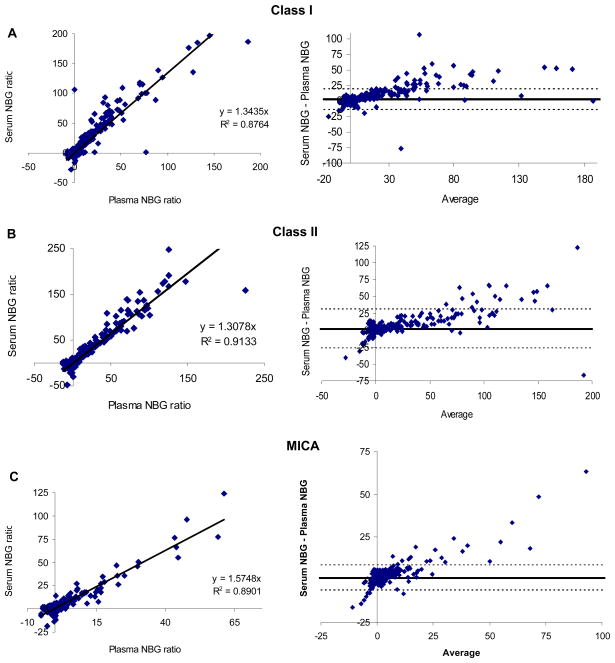
From the entire contemporary cohort of 1,000 subjects, 23 plasma samples and 14 serum samples from a total of 32 individuals yielded invalid assay results. The rates of invalid results were comparable for plasma and serum aliquots (23 vs. 14, respectively, p = 0.13). In the remaining 968 subjects screening of serum revealed 285 with HLA class I antibodies and 204 with HLA class II antibodies based on the 2.2 NBG cutoff. The mean NBG ratios of all valid samples are listed in Table 2 for antibodies to HLA class I, class II, and MICA for both serum and plasma. NBG ratios were higher using serum samples for HLA class I, class II, and MICA antibodies (p < 0.0001 for each). When the results of plasma and serum sample testing for the 1,000 contemporary blood donors were compared (Fig. 1), regression analysis revealed that class I and class II antibodies gave NBG values approximately 1.3-fold higher using serum compared to plasma, and MICA antibodies tested about 1.6-fold higher using serum compared to plasma (Fig. 1, left column). R2 values for the two sample types were generally good (0.88, 0.91, and 0.89 for HLA class I, class II, and MICA antibodies, respectively), though if two outlier plasma specimens with high NBG values were included in the analysis of HLA class II antibodies the R2 values fell to 0.69. Bland-Altman plots showed that at NBG ratios below 30 for class I antibodies and below 50 for HLA class II antibodies the results were within 1.96 times the standard deviation of the measurements, implying reasonably good concordance between the two sample types at NBG ratios below 30–50 (Fig. 1, right column). Given that the threshold for interpreting a test as positive will likely be set well below 30 in most screening applications, this implies that the test would give good concordance within the working range of the assay as long as the 1.3 to 1.6-fold adjustment factor for the NBG ratio were included in the analysis.
TABLE 2.
Average NBG ratios for contemporary blood donors, serum vs. plasma
| N = 968 | Average | St. error |
|---|---|---|
| Class I serum | 7.6 | 0.69 |
| Class I plasma | 4.6 | 0.49 |
| Class II serum | 8.0 | 0.86 |
| Class II plasma | 5.6 | 0.78 |
| MICA serum | 2.4 | 0.26 |
| MICA plasma | 1.2 | 0.16 |
Fig. 1. Concordance between plasma and serum screen test results.
Screening tests (LSM12) were performed in singlicate on 1,000 paired plasma and serum samples from the contemporary cohort of blood donors. NBG ratios obtained in serum are plotted vs. the plasma NBG ratio in the left hand column for HLA class I (A), HLA class II (B), and MICA (C) antibodies. The formula for a linear regression curve and R2 are listed in each plot. The right hand column presents Bland-Altman plots of the data, with the difference in the serum and plasma NBG ratios plotted against the mean of the two NBG ratios. The thick line indicates the mean bias and the two dashed lines depict the mean bias +/− 1.96 times the standard deviation of the NBG difference. The two samples with the highest class II plasma NBG ratios (B) were also positive using serum samples and were excluded from the figure and analysis as the outliers distorted the line of best fit.
We also evaluated the concordance of test interpretation (positive vs. negative) obtained using plasma vs. serum specimens. The cut-off used to determine positive samples using a serum sample was 2.2, and since serum NBG ratios were on average 1.3-fold higher than those from corresponding plasma samples, the threshold for a positive plasma sample was set at 1.7 for HLA class I and II antibodies (2.2 divided by the adjustment factor 1.3) and 1.4 for MICA antibodies. Although the majority of donors with positive test results for HLA class I, HLA Class II, and MICA were positive on both sample types, a significant number of donors gave positive results on only one sample type (Table 3). Test concordance improved substantially using the higher assay threshold.
TABLE 3.
Plasma and serum screening test concordance for contemporary blood donors
| NBG 2.2* | Class I † | Class II | MICA | |
|---|---|---|---|---|
| Plasma only | 54 (15.6%) | 30 (12.7%) | 31 (15.7%) | |
| Serum only | 70 (20.3%) | 47 (19.9%) | 51 (25.8%) | |
| Both positive | 221 (64.1%) | 159 (67.4%) | 116 (58.6%) | |
| NBG 13 | ||||
| Plasma only | 3 (2.5%) | 3 (2.9%) | 3 (7.9%) | |
| Serum only | 14 (11.8%) | 4 (3.8%) | 6 (15.8%) | |
| Both positive | 102 (85.7%) | 98 (93.3%) | 29 (76.3%) | |
At each serum cutoff value (2.2 or 13), the comparable plasma cutoff value was determined by dividing by 1.3 for HLA class I or II antibodies and 1.6 for MICA antibodies (see Fig. 1).
Results expressed as number and (percentage) of positive samples using the given cut-off.
To further characterize the samples that gave discordant results in testing of plasma and serum samples, the NBG ratios were plotted for the samples that tested positive on the screening test on only one of the two sample types using the lower assay cut-off of NBG ≥ 2.2 for the serum sample (Fig. 2). Most of the samples that yielded discordant results on plasma vs. serum testing showed low-level positive results on the screening test of the positive sample. Using the higher cut-off of NBG ≥ 13, relatively few samples yielded discordant results using the two sample types. Of 119 subjects screened positive for HLA class I antibodies, 102 tested positive on both sample types and 17 gave discordant results. For 105 HLA class II positive subjects, 98 were concordant and 7 discordant.
Fig. 2. Screen test results for plasma or serum samples that were positive in only one type of sample.
The NBG ratio is shown for all samples that tested positive by screening in only one of the two sample types for HLA class I (A) (plasma n=54, serum n=70) or class II (B) (plasma n=30, serum n=47) antibodies. An assay cut-off of NBG ≥ 1.7 was used for plasma samples and NBG ≥ 2.2 was used for serum samples. Most samples that yielded a positive result in only one sample type were clustered near the assay cut-off, though higher signals were seen in a few samples.
Freeze-thaw cycles decrease signal intensity more than refrigerated storage
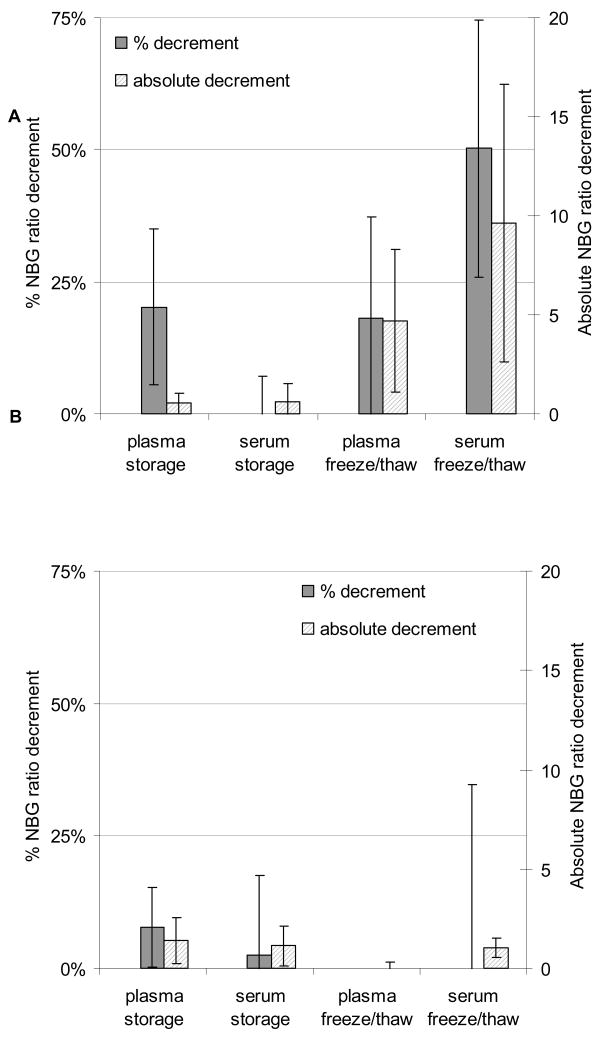
Among the samples tested for the effect of one freeze-thaw cycle, HLA class I antibody NBG ratios fell by 0.6 and 0.4 for plasma and serum samples, respectively (Fig. 3A). The percentage decrement for plasma samples appeared moderate (14%), but was driven by changes in samples with low-level responses. Additional freeze-thaw cycles caused a larger decrement in NBG ratio than refrigerated storage did, with the NBG ratio falling by 4.9 and 11.4 for plasma and serum subjected to two additional freeze-thaw cycles, respectively. For HLA class II antibodies, refrigeration caused an NBG ratio decrement of 1.8 and 1.0 for plasma and serum, respectively (Fig. 3B). Two additional freeze-thaw cycles led to a decrement in NBG ratio of 0.3 for plasma samples and a decrement of 0.6 in serum samples. For all the tests, the magnitude of the effect of refrigerated storage or freeze-thaw cycles varied widely, reflected in the large error bars in Figure 3.
Fig. 3. Effects of refrigerated storage and freeze-thaw cycles on assay signal.
Screening tests (LSM12) are shown for triplicate samples of plasma and serum aliquots from 15 subjects that had been frozen and thawed once and assayed immediately or stored at 4 °C for one week. Results are also shown for four additional samples that were frozen and thawed for two additional cycles and tested in duplicate or triplicate. Results are displayed as percentage and absolute decrement in NBG ratio, and error bars represent the standard error. Panel A shows HLA class I and panel B shows class II results.
DISCUSSSION
As the U.S. blood supply becomes increasingly safe, relatively rare transfusion reactions become responsible for an increased percentage of adverse outcomes associated with transfusion. There has been increasing focus on TRALI as a cause of significant morbidity and mortality among blood products recipients. While the cause of TRALI has not been identified in all reported cases, it appears that at least a subset of cases may be related to HLA antibodies present in plasma of blood donors and infused in implicated blood products. This study demonstrated that HLA antibodies can be detected within an individual at high-levels for over a decade. Factors associated with optimal detection of HLA antibodies using a multiplex bead approach include use of a serum sample and avoiding repeated freeze-thaw cycles of stored specimens.
Our study of 44 multiparous females with known prior HLA antibodies based on lymphocytoxicity assays revealed persistence of positive HLA antibody tests over 13 years, with most subjects still showing strongly positive screening test results as well as antibody specificity (data not shown). Prior studies have revealed that about half of women with HLA antibodies in the post-partum period lose these antibodies over the ensuing six months to five years (12, 24, 25). Rates of HLA antibody prevalence appear to decline more slowly after the initial decrease over the first five years (24). Our data support this view, in that a pre-selected cohort of women with pre-existing, strong HLA antibodies maintained high antibody levels for over 13 years. We were not able to address the persistence of specific HLA antibodies as long-term frozen aliquots from the time of lymphocytotoxicity testing were not available and the samples were tested in an unlinked fashion. The testing technique used in the current analysis is more sensitive than older cytotoxicity based techniques, but the samples from multiparous females scored strongly positive in our study, indicating that the continued positivity on HLA antibody testing was not merely due to the use of a highly sensitive assay. In the future studies of serially collected specimens from alloreactive donors, optimally collected over years or even decades, are warranted to more precisely define the rate and correlates of waning HLA antibody reactivity using contemporary assays
While this study represents a relatively small sample size, the rate of positive HLA antibodies we found in our predominantly female study population of 1,000 blood donors is higher than reported in the published literature. HLA antibody rates in women have been shown to vary with parity, with rates increasing from 1.6% for nulliparous women to around 20% for multiparous women (13, 24). Untransfused men have been reported to have HLA antibody reactivity rates comparable to nulliparous women (13). Most studies performed to date have been performed on relatively small sample sizes using the cytotoxicity method for antibody detection. The assay used in this study is more sensitive than the classic cytotoxicity assay (17), and the assay cut-off to be used for blood bank operations would likely need to be adjusted upwards from the NBG ratio of 2.2 that was used in the current study if the aim were to detect only donors with stronger HLA antibodies and thereby exclude a smaller subset of currently eligible plasma and platelet apheresis donors. The data we have generated using 1,000 paired plasma and serum samples represents a subset of approximately 8,200 subjects who were recently tested for the prevalence of HLA antibodies in the REDS II Leukocyte Antibody Prevalence Study (LAPS). The full study dataset will give a much better estimate of the prevalence, specificities, and signal strength of HLA antibodies in the blood donor population and the associations of these antibodies with gender, parity, and other risk factors for allo-immunization (26).
In summary, we have explored the application of newer, multiplex bead-based technology to the detection of HLA antibodies in blood donors. We identified limitations of the assay associated with sample type and processing, and confirmed that high-level HLA antibodies are detectable over long periods. These findings will have implications for sample selection and processing, assay cutoffs and development of testing algorithms for screening donors to reduce the risk of TRALI.
Acknowledgments
This work was supported by NHLBI contracts N01-HB-47168, -47169, -47170, -47171, -47172, -47174, -47175 and -57181.
This work was supported by NHLBI contract N01 HB57181, the Retrovirus Epidemiology Donor Study-II (REDS-II). The authors thank the staff at all six participating blood centers. Without their help, this study would not have been possible. Dr. Jar-How Lee is an employee of One Lambda Corporation, the manufacturer of the test kits used in this study.
Appendix
The Retrovirus Epidemiology Donor Study - II (REDS-II Study Group) iswas the responsibility of the following persons:
Blood Centers
American Red Cross Blood Services, New England Region
R. Cable, J. Rios and, R. Benjamin
American Red Cross Blood Services, Southern Region/Department of Pathology and Laboratory Medicine, Emory University School of Medicine
C.D. Hillyer, K.L. Hillyer and, J.D. Roback
Hoxworth Blood Center, University of Cincinnati Academic Health Center
R.A. Sacher, S.L. Wilkinson and, P.M. Carey
Blood Centers of the Pacific, Regents of the University of California
E.L. Murphy (University of California San Francisco,), M.P. Busch (Blood Systems Research Institute)
E.L. Murphy, B. Custer and N. Hirschler
The Institute for Transfusion Medicine
D. Triulzi, R. Kakaiya and, J. Kiss
Blood Center of Wisconsin
J. Gottschall and, A. Mast
Coordinating Center
Westat, Inc
.: G.B. Schreiber and, M. King
National Heart, Lung, and Blood Institute, NIH:
G.J. Nemo and, T. Mondoro
Central Laboratory:
Blood Systems Research Institute
M.P. Busch and, P. Norris
References
- 1.Holness L, Knippen MA, Simmons L, Lachenbruch PA. Fatalities caused by TRALI. Transfus Med Rev. 2004;18:184–188. doi: 10.1016/j.tmrv.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Starkey J, Kapler R. Transfusion Recipient Fatalities Reported to the Food and Drug Administration, FY2004–2006. ABC Newsletter; Washington, DC: 2007. p. 21. [Google Scholar]
- 3.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooling L. Transfusion-related acute lung injury. Jama. 2002;288:315–316. doi: 10.1001/jama.288.3.315. author reply 316. [DOI] [PubMed] [Google Scholar]
- 5.Nicolle AL, Chapman CE, Carter V, Wallis JP. Transfusion-related acute lung injury caused by two donors with anti-human leucocyte antigen class II antibodies: a look-back investigation. Transfus Med. 2004;14:225–230. doi: 10.1111/j.0958-7578.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 6.Win N, Ranasinghe E, Lucas G. Transfusion-related acute lung injury: a 5-year look-back study. Transfus Med. 2002;12:387–389. doi: 10.1046/j.1365-3148.2002.00401_1.x. [DOI] [PubMed] [Google Scholar]
- 7.Win N, Brown C, Navarrete C. TRALI associated with HLA class II antibodies. Transfusion. 2003;43:545–546. doi: 10.1046/j.1537-2995.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 8.Flesch BK, Neppert J. Transfusion-related acute lung injury caused by human leucocyte antigen class II antibody. Br J Haematol. 2002;116:673–676. doi: 10.1046/j.0007-1048.2001.03305.x. [DOI] [PubMed] [Google Scholar]
- 9.Kopko PM, Marshall CS, MacKenzie MR, Holland PV, Popovsky MA. Transfusion-related acute lung injury: report of a clinical look-back investigation. Jama. 2002;287:1968–1971. doi: 10.1001/jama.287.15.1968. [DOI] [PubMed] [Google Scholar]
- 10.Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–1467. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silliman CC, Boshkov LK, Mehdizadehkashi Z, Elzi DJ, Dickey WO, Podlosky L, Clarke G, Ambruso DR. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–462. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 12.Payne R. The development and persistence of leukoagglutinins in parous women. Blood. 1962;19:411–424. [PubMed] [Google Scholar]
- 13.MacLennan S, Lucas G, Brown C, Evans R, Kallon D, Brough S, Contreras M, Navarrete C. Prevalence of HLA and HNA antibodies in donors: correlation with pregnancy and transfusion history. Vox Sang. 2004;87(Suppl 3):S2–S16. [Google Scholar]
- 14.Mittal KK, Mickey MR, Singal DP, Terasaki PI. Serotyping for homotransplantation. 18. Refinement of microdroplet lymphocyte cytotoxicity test. Transplantation. 1968;6:913–927. doi: 10.1097/00007890-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 16.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 17.Pei R, Lee J, Chen T, Rojo S, Terasaki PI. Flow cytometric detection of HLA antibodies using a spectrum of microbeads. Hum Immunol. 1999;60:1293–1302. doi: 10.1016/s0198-8859(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 18.Kitcharoen K, Witt CS, Romphruk AV, Christiansen FT, Leelayuwat C. MICA, MICB, and MHC beta block matching in bone marrow transplantation: relevance to transplantation outcome. Hum Immunol. 2006;67:238–246. doi: 10.1016/j.humimm.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Mizutani K, Terasaki P, Rosen A, Esquenazi V, Miller J, Shih RN, Pei R, Ozawa M, Lee J. Serial ten-year follow-up of HLA and MICA antibody production prior to kidney graft failure. Am J Transplant. 2005;5:2265–2272. doi: 10.1111/j.1600-6143.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- 20.Mizutani K, Terasaki PI, Shih RN, Pei R, Ozawa M, Lee J. Frequency of MIC antibody in rejected renal transplant patients without HLA antibody. Hum Immunol. 2006;67:223–229. doi: 10.1016/j.humimm.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Zwirner NW, Marcos CY, Mirbaha F, Zou Y, Stastny P. Identification of MICA as a new polymorphic alloantigen recognized by antibodies in sera of organ transplant recipients. Hum Immunol. 2000;61:917–924. doi: 10.1016/s0198-8859(00)00162-2. [DOI] [PubMed] [Google Scholar]
- 22.Allain JP, Busch MP. Donation archives and prospective donor-recipient repositories: indispensable tools for monitoring blood safety. Transfusion. 2007;47:1110–1114. doi: 10.1111/j.1537-2995.2007.01323.x. [DOI] [PubMed] [Google Scholar]
- 23.Reesink HW, Engelfriet CP, Hyland CA, Coghlan P, Tait B, Wsolak M, Keller AJ, Henn G, Mayr WR, Thomas I, Osselaer JC, Lambermont M, Beaten M, Wendel S, Qiu Y, Georgsen J, Krusius T, Maki T, Andreu G, Morel P, Lefrere JJ, Rebulla P, Giovanelli S, Butti B, Lecchi L, Mozzi F, van Hilten JA, Zwaginga JJ, Flanagan P, Flesland O, Brojer E, Letowska M, Akerblom O, Norda R, Prowse C, Dow B, Jarvis L, Davidson F, Kleinman S, Bianco C, Stramer SL, Dodd RY, Busch MP. Biobanks of blood from donors and recipients of blood products. Vox Sang. 2008;94:242–260. doi: 10.1111/j.1423-0410.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 24.Densmore TL, Goodnough LT, Ali S, Dynis M, Chaplin H. Prevalence of HLA sensitization in female apheresis donors. Transfusion. 1999;39:103–106. doi: 10.1046/j.1537-2995.1999.39199116901.x. [DOI] [PubMed] [Google Scholar]
- 25.Nymand G. Complement fixing and lymphocytotoxic antibodies in serum of pregnant women at delivery. IV. Serology. Vox Sang. 1975;28:34–41. doi: 10.1111/j.1423-0410.1975.tb02738.x. [DOI] [PubMed] [Google Scholar]
- 26.Triulzi DJ, Kakaiya R, Schreiber G. Donor risk factors for white blood cell antibodies associated with transfusion-associated acute lung injury: REDS-II leukocyte antibody prevalence study (LAPS) Transfusion. 2007;47:563–564. doi: 10.1111/j.1537-2995.2007.01184.x. [DOI] [PubMed] [Google Scholar]